The Effect of Hydrophobic Modified Block Copolymers on Water–Oil Interfacial Properties and the Demulsification of Crude Oil Emulsions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Hydrophobic Modification Copolymers
2.3. Preparation of Block Copolymer Solution
2.4. Water-Containing Crude Oil Treatment
2.5. Preparation and Demulsification of O/W Emulsions
2.6. Preparation and Demulsification of W/O Emulsions
2.7. Oil–Water Interfacial Tension Measurements
2.8. Oil–Water Interfacial Shear Viscosity Measurements
2.9. Microscope Image Test of Emulsions
2.10. Zeta Potential Measurements
2.11. Oil Content Measurements
3. Results and Discussion
3.1. Demulsification Effect of Block Copolymers on O/W Emulsions
3.2. Demulsification Effect of Block Copolymers on W/O Emulsions
3.3. Interfacial Shear Viscosity between Oil and Block Copolymer Solution
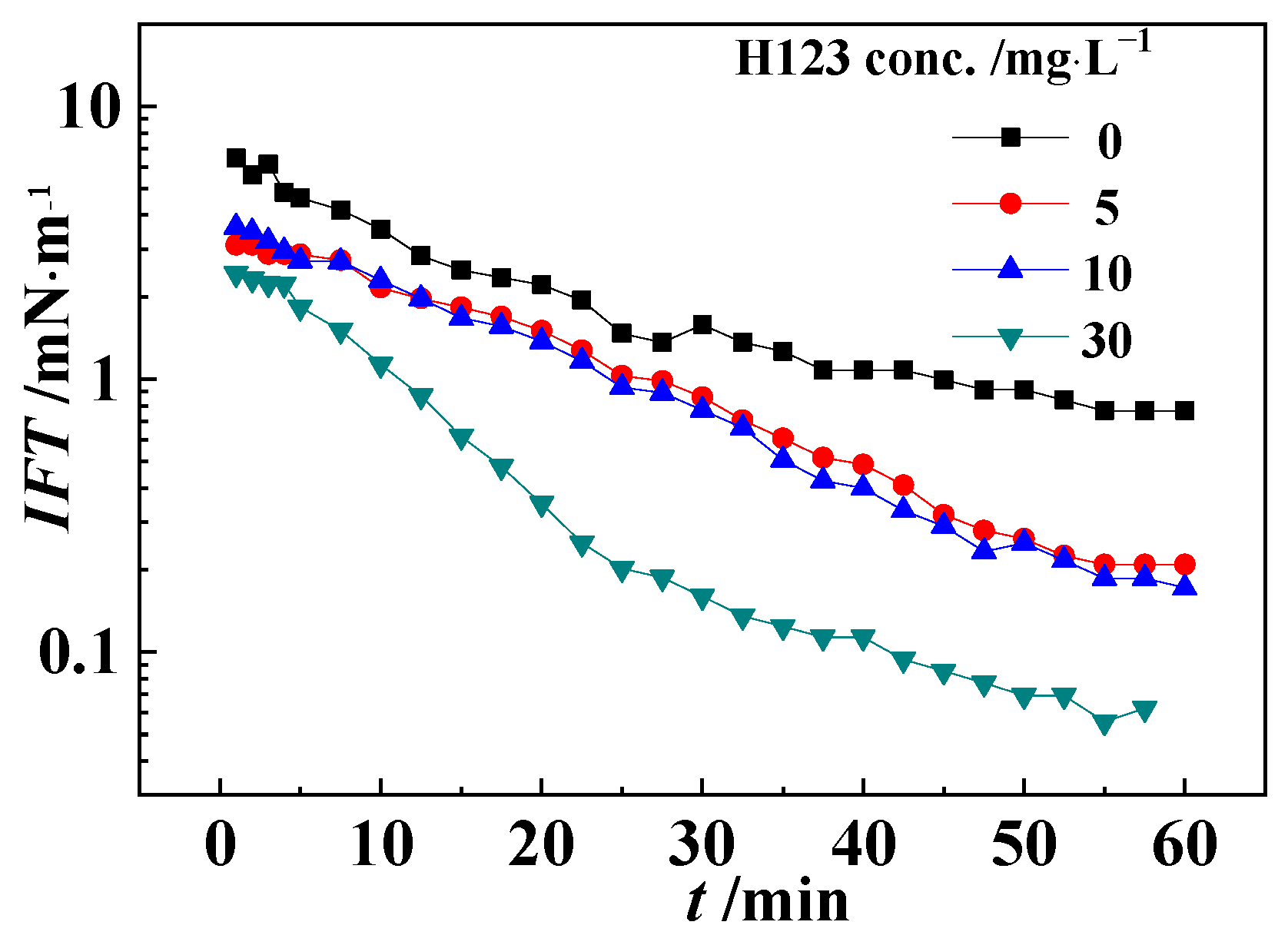
3.4. Interfacial Tension between Oil and Block Copolymer Solution
3.5. The Mechanism of Demulsification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, L.X.; Lu, S.W.; Cao, G. Salt-assisted microwave demulsification. Chem. Eng. Commun. 2004, 191, 1053. [Google Scholar] [CrossRef]
- Wu, X. Investigating the Stability Mechanism of Water-in-Diluted Bitumen Emulsions through Isolation and Characterization of the Stabilizing Materials at the Interface. Energy Fuels 2002, 17, 179. [Google Scholar] [CrossRef]
- Xia, L.; Lu, S.; Cao, G. Stability and demulsification of emulsions stabilized by asphaltenes or resins. J. Colloid Interface Sci. 2004, 271, 504. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.L.; Liu, S.R.; Xu, B.; Wang, X.Z.; Zhang, B.T.; Bai, B.J. Study on Demulsification of a Demulsifier at Low Temperature and Its Field Application. Pet. Sci. Technol. 2013, 31, 572. [Google Scholar] [CrossRef]
- Yonguep, E.; Kapiamba, K.F.; Kabamba, K.J.; Chowdhury, M. Formation, stabilization and chemical demulsification of crude oil-in-water emulsions: A review. Pet. Res. 2022, 7, 459. [Google Scholar] [CrossRef]
- Dhandhi, Y.; Chaudhari, R.K.; Naiya, T.K. Development in separation of oilfield emulsion toward green technology—A comprehensive review. Sep. Sci. Technol. 2022, 57, 1642. [Google Scholar] [CrossRef]
- Akbari, N.; Biria, D. Investigation of the activity of Acinetobacter calcoaceticus biodemulsifier to break stable water in oil emulsions. J. Environ. Chem. Eng. 2018, 6, 4144. [Google Scholar] [CrossRef]
- Jabbari, M.; Izadmanesh, Y.; Ghavidel, H. Synthesis of ionic liquids as novel emulsifier and demulsifiers. J. Mol. Liq. 2019, 293, 111512. [Google Scholar] [CrossRef]
- Yang, X.G.; Tan, W.; Tan, X.F. Demulsification of Crude Oil Emulsion via Ultrasonic Chemical Method. Pet. Sci. Technol. 2009, 27, 2010. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Tade, M.O.; Ali, H.A.; Alao, K.T. Demulsifier: An important agent in breaking crude oil emulsions. Chem. Eng. Technol. 2022, 45, 1707. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Kandile, N.G.; Noor El-Din, M.R. Functions of Demulsifiers in the Petroleum Industry. Sep. Sci. Technol. 2011, 46, 1144. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, H.; Zhao, Y.X.; Deng, X.Y.; Ke, S.; Li, Y.; Tian, M.G. Implementation of fluidized-bed Fenton as tertiary treatment of nitro-aromatic industrial wastewater. Process Saf. Environ. Prot. 2021, 146, 490. [Google Scholar] [CrossRef]
- Otaibi, A.; Elkamel, A.; Al-Sahhaf, T.; Ahmed, A.S. Experimental investigation of crude oil desalting and dehydration. Chem. Eng. Commun. 2003, 190, 65. [Google Scholar] [CrossRef]
- Xu, X.Z.; Cao, D.; Liu, J.; Gao, J.; Wang, X.Y. Research on ultrasound-assisted demulsification/dehydration for crude oil. Ultrason. Sonochemistry 2019, 57, 185. [Google Scholar] [CrossRef]
- Jiang, W.M.; Chen, Y.M.; Chen, M.C.; Liu, X.L.; Liu, Y.; Wang, T.Y.; Yang, J. Removal of emulsified oil from polymer-flooding sewage by an integrated apparatus including EC and separation process. Sep. Purif. Technol. 2019, 211, 259. [Google Scholar] [CrossRef]
- Duan, M.; He, J.; Li, D.J.; Wang, X.J.; Jing, B.; Xiong, Y.; Fang, S.W. Synthesis of a novel copolymer of block polyether macromonomer and diallyldimethylammonium chloride and its reverse demulsification performance. J. Pet. Sci. Eng. 2019, 175, 317. [Google Scholar] [CrossRef]
- Alexandridis, P. Poly (ethylene oxide)/poly (propylene oxide) block copolymer surfactants. Curr. Opin. Colloid Interface Sci. 1997, 2, 478. [Google Scholar] [CrossRef]
- Kadam, Y.; Bharatiya, B.; Hassan, P.A.; Verma, G.; Aswal, V.K.; Bahadur, P. Effect of an amphiphilic diol (Surfynol) on the micellar characteristics of PEO–PPO–PEO block copolymers in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 110. [Google Scholar] [CrossRef]
- Wang, J.; Hu, F.L.; Li, C.Q.; Li, J.; Yang, Y. Synthesis of dendritic polyether surfactants for demulsification. Sep. Purif. Technol. 2010, 73, 349. [Google Scholar] [CrossRef]
- Wang, C.Y.; An, S.G.; Li, Z.W.; Chen, H.; Yan, Z.H.; Tan, Y.B. Novel epigallocatechin gallate-based polyether surfactants: Synthesis, characterization and demulsification properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126757. [Google Scholar] [CrossRef]
- Watanabe, K.; Watanabe, Y.; Masuda, K.; Yang, Z.; Kaneshima, A.; Motoyama, A.; Shima, T.; Tsuchiya, K.; Sakai, H. Key factor of sponge phase formation in commercial polyethoxylated nonionic surfactant/cosurfactant/water systems and its unique feature at interface. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128405. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Z.; Zhao, S.; Yin, S.; Tan, G.; Jing, B.; Tan, Y. Synthesis and Properties of a Novel Branched Polyether Surfactant. J. Surfactants Deterg. 2016, 19, 1107–1120. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, G.Y.; Wang, F.; Dong, S.; Chen, Y. Demulsification by amphiphilic dendrimer copolymers. J. Colloid Interface Sci. 2005, 282, 1–4. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, G.Y.; Wang, F.; Dong, S.L.; Li, Y.M. Characterization and demulsification of poly (ethylene oxide)-block-poly (propylene oxide)-block-poly (ethylene oxide) copolymers. J. Colloid Interface Sci. 2004, 277, 464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.Q.; Wei, L.; Li, C.Y. Research on Demulsification Features of Polymer Flooding Produced Fluids of O/W with High Water Content for Oilfield and the Demulsification Efficacy. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 4443–4444. [Google Scholar]
- Gong, H.; Xu, G.; Liu, T.; Xu, L.; Zhai, X.; Zhang, J.; Lv, X. Aggregation behaviors of PEO-PPO-ph-PPO-PEO and PPO-PEO-ph-PEO-PPO at an air/water interface: Experimental study and molecular dynamics simulation. Langmuir 2012, 28, 13590. [Google Scholar] [CrossRef] [PubMed]
- Paeng, K.; Choi, J.; Park, Y.; Sohn, D. Temperature effect of hydrophobically modified polyethylene oxide at the air–water interface. Colloids Surf. A 2003, 220, 1–7. [Google Scholar] [CrossRef]
- Chang, D.; Du, C.; Liu, J.; Sun, W.; Su, Y.; Zang, D.; Liu, T. Effect of hydrophobic modification of block copolymers on the self-assembly, drug encapsulation and release behavior. J. Mol. Liq. 2022, 368, 120635. [Google Scholar] [CrossRef]
- Tong, K.; Zhang, Y.; Chu, P.K. Evaluation of calcium chloride for synergistic demulsification of super heavy oil wastewater. Colloids Surf. A 2013, 419, 46. [Google Scholar] [CrossRef]
- Abdelrahman, O.E.; Ahmed, M.T.; Hamad, A.A. Synthesis and application of novel gemini pyridinium ionic liquids as demulsifiers for arabian heavy crude oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 634, 127961. [Google Scholar]
- Sun, T.; Lu, Z.; Wang, Y.; Sui, Z.; Bo, P.; Li, M.; Yu, J. Influence of demulsifiers of different structures on interfacial dilational properties of an oil–water interface containing surface-active fractions from crude oil. J. Colloid Interface Sci. 2002, 255, 241. [Google Scholar] [CrossRef] [PubMed]
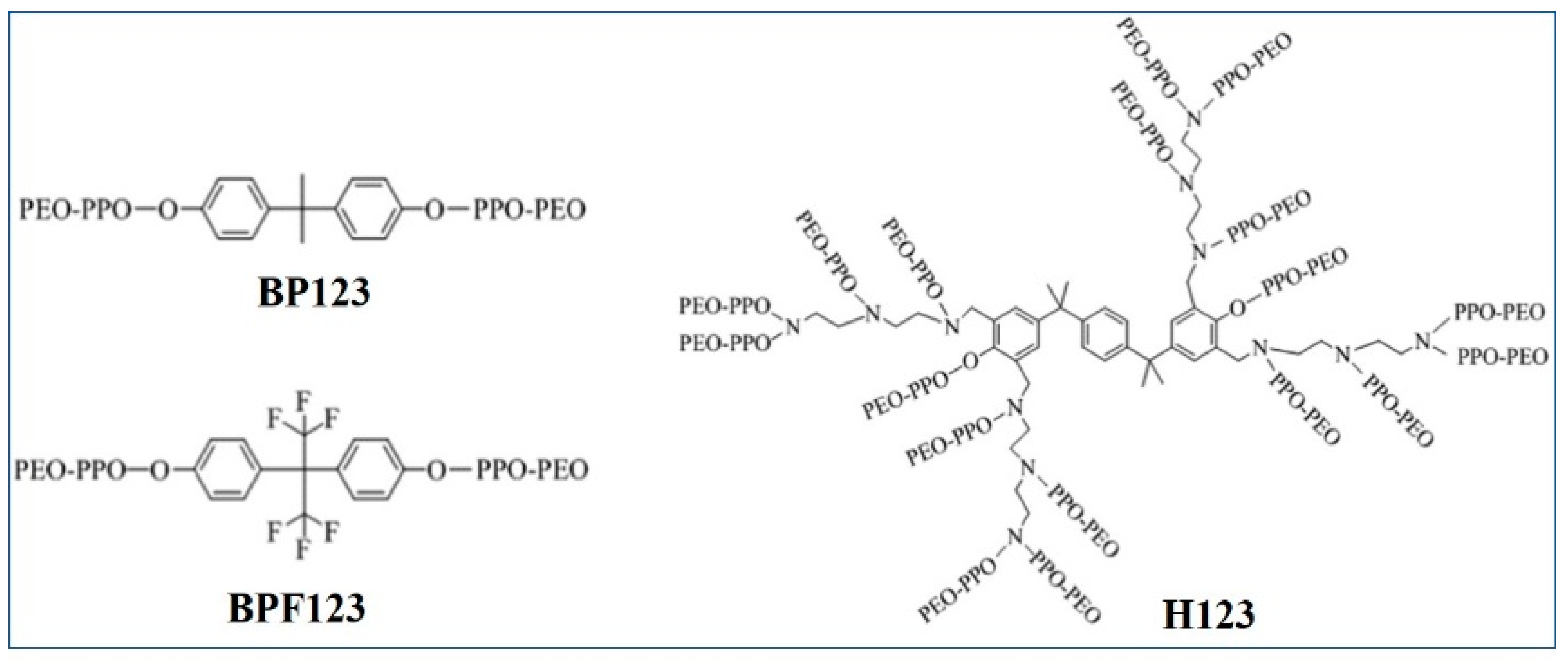
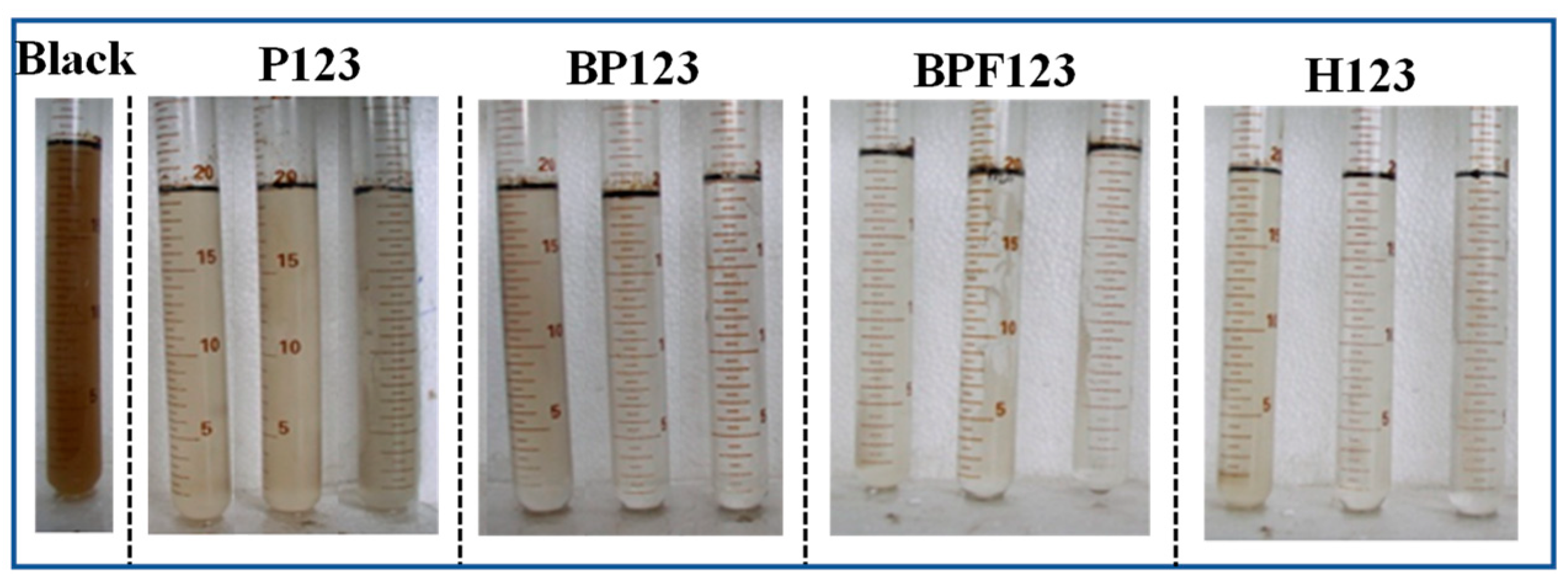


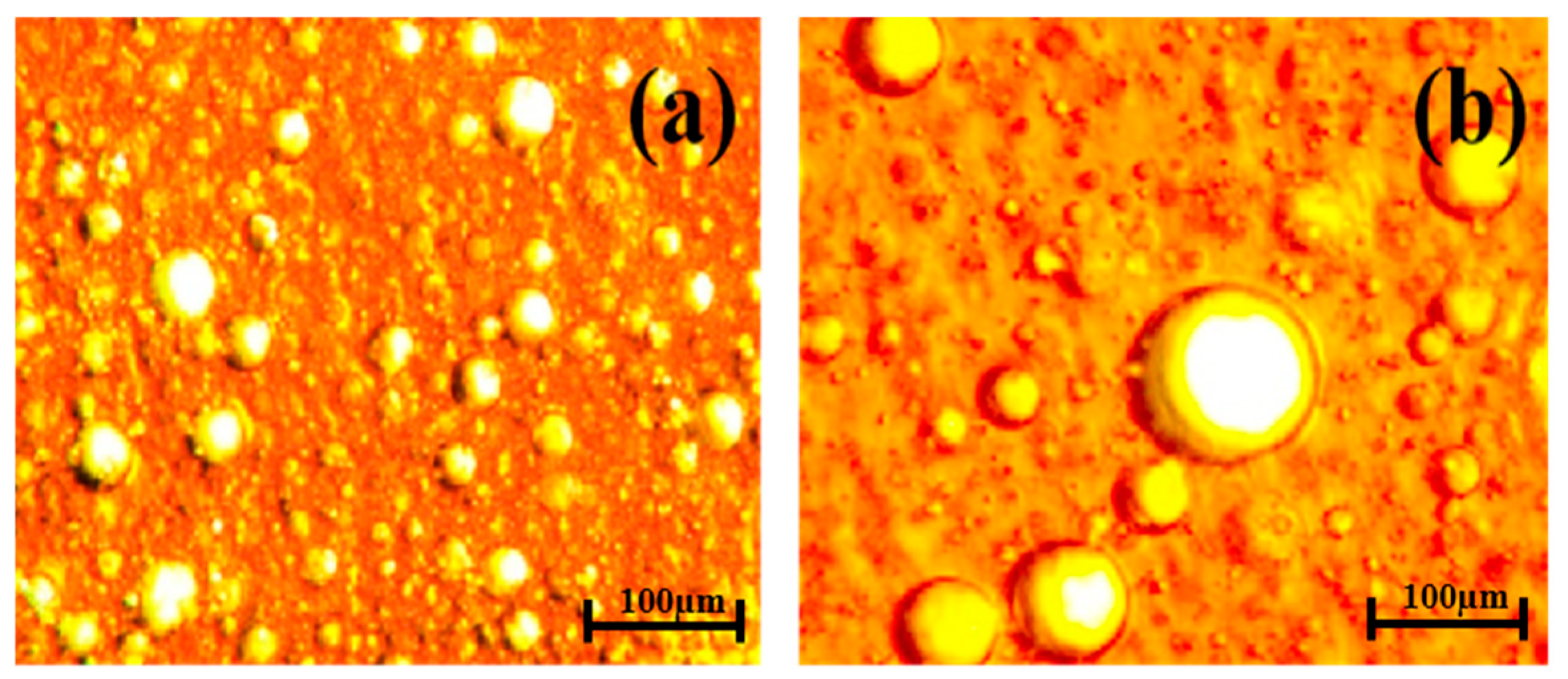
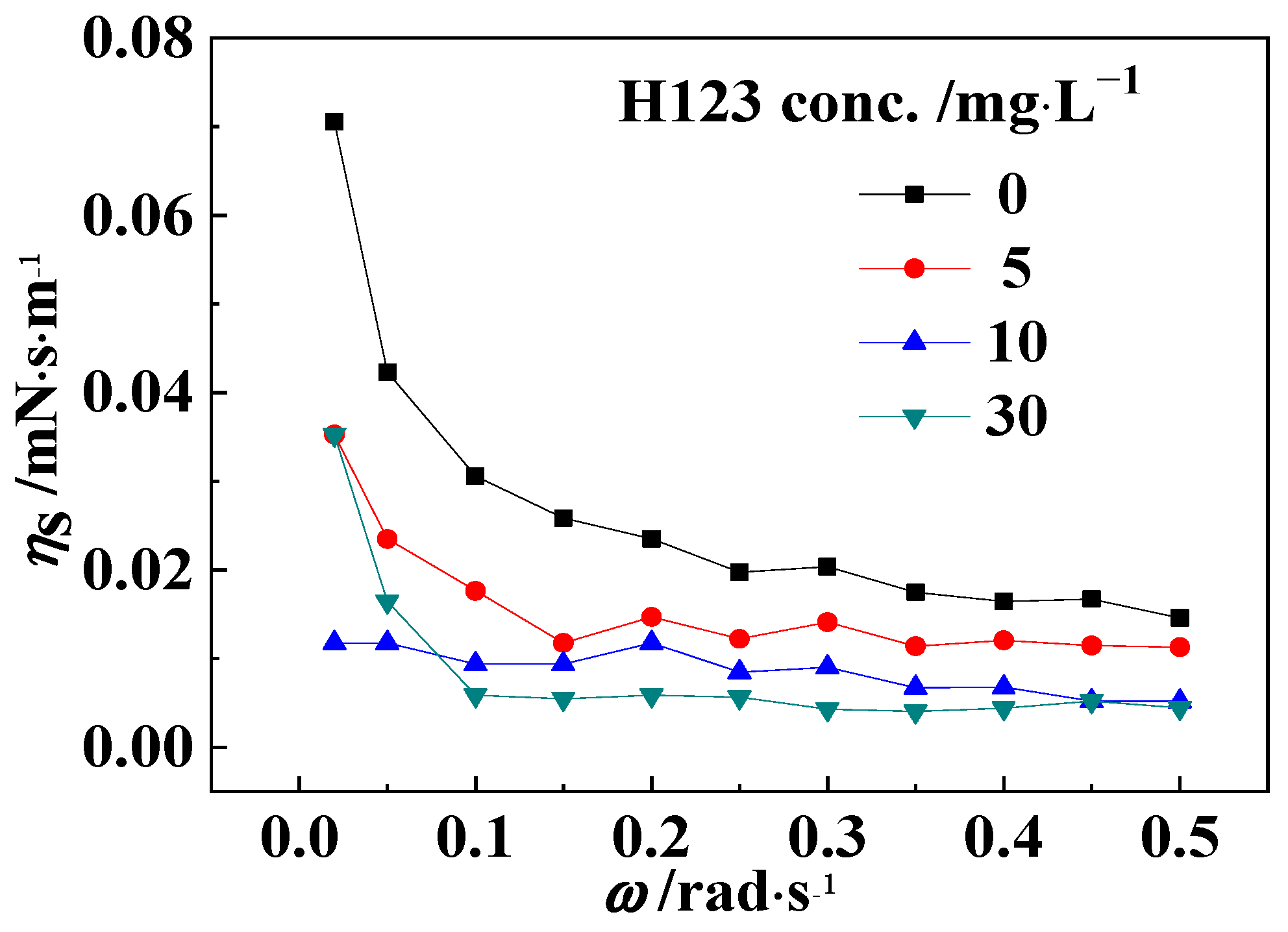
| Ion | Cl− | SO42− | HCO−3 | Mg2+ | Ca2+ | Na+, K+ |
|---|---|---|---|---|---|---|
| Concentration (mg/L) | 3140.0 | 78.3 | 598.0 | 300 | 340 | 2430 |
| Polymers | P123 | BP123 | BPF123 | H123 |
|---|---|---|---|---|
| nPPO/PEO | 1.75 | 1.76 | 1.76 | 1.76 |
| M | 5820 | 5980 | 6090 | 6510 |
| Cloud point/°C | 38 | 41 | 46 | 61 |
| Polymer Conc. (mg·L−1) | Oil Content (mg·L−1) | ||||
|---|---|---|---|---|---|
| P123 | BP123 | BPF123 | H123 | Blank | |
| 5 | 523 | 261 | 19 | 6 | 768 |
| 10 | 302 | 192 | 10 | 4 | |
| 30 | 248 | 92 | 9 | 4 | |
| Polymer Conc. (mg·L−1) | ζ (mV) | ||||
|---|---|---|---|---|---|
| P123 | BP123 | BPF123 | H123 | Blank | |
| 5 | −30.28 | −26.87 | −5.80 | −5.05 | −43.24 |
| 10 | −26.60 | −21.69 | −5.25 | −4.86 | |
| 30 | −20.55 | −20.83 | −5.15 | −4.77 | |
| Polymer Conc. (mg·L−1) | Dewatering Rate (%) | ||||
|---|---|---|---|---|---|
| P123 | BP123 | BPF123 | H123 | Blank | |
| 5 | 60 | 65 | 80 | 88 | 0 |
| 10 | 65 | 70 | 85 | 95 | |
| 30 | 66 | 72 | 89 | 97 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, P.; Gao, Y.; Yu, Q. The Effect of Hydrophobic Modified Block Copolymers on Water–Oil Interfacial Properties and the Demulsification of Crude Oil Emulsions. Polymers 2024, 16, 2392. https://doi.org/10.3390/polym16172392
Zhang J, Liu P, Gao Y, Yu Q. The Effect of Hydrophobic Modified Block Copolymers on Water–Oil Interfacial Properties and the Demulsification of Crude Oil Emulsions. Polymers. 2024; 16(17):2392. https://doi.org/10.3390/polym16172392
Chicago/Turabian StyleZhang, Juan, Ping Liu, Yuan Gao, and Qingping Yu. 2024. "The Effect of Hydrophobic Modified Block Copolymers on Water–Oil Interfacial Properties and the Demulsification of Crude Oil Emulsions" Polymers 16, no. 17: 2392. https://doi.org/10.3390/polym16172392






