Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Information
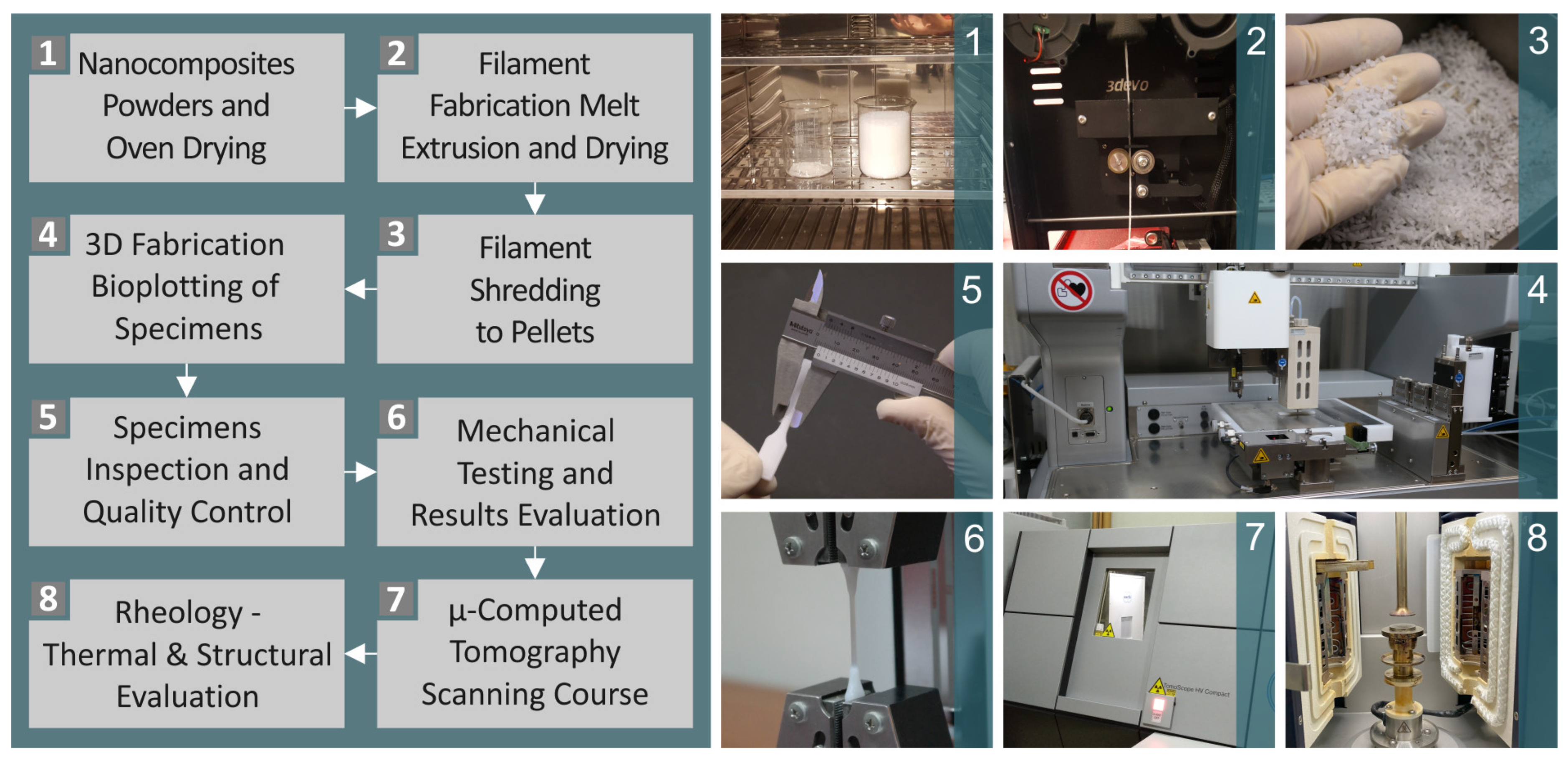
2.2. Composite Preparation, Mixtures, Filaments and Pellets, and Testing of Filaments
2.3. Manufacturing of Three-Dimensional Printing Samples
2.4. Morphological and Elemental Examination of the Parts
2.5. Raman Spectral Evaluation
2.6. Conduction of Thermal Analysis
2.7. μ-CT Analysis
2.8. Mechanical Equipment and Settings
3. Results
3.1. Raman Spectroscopy and Spectral Differences
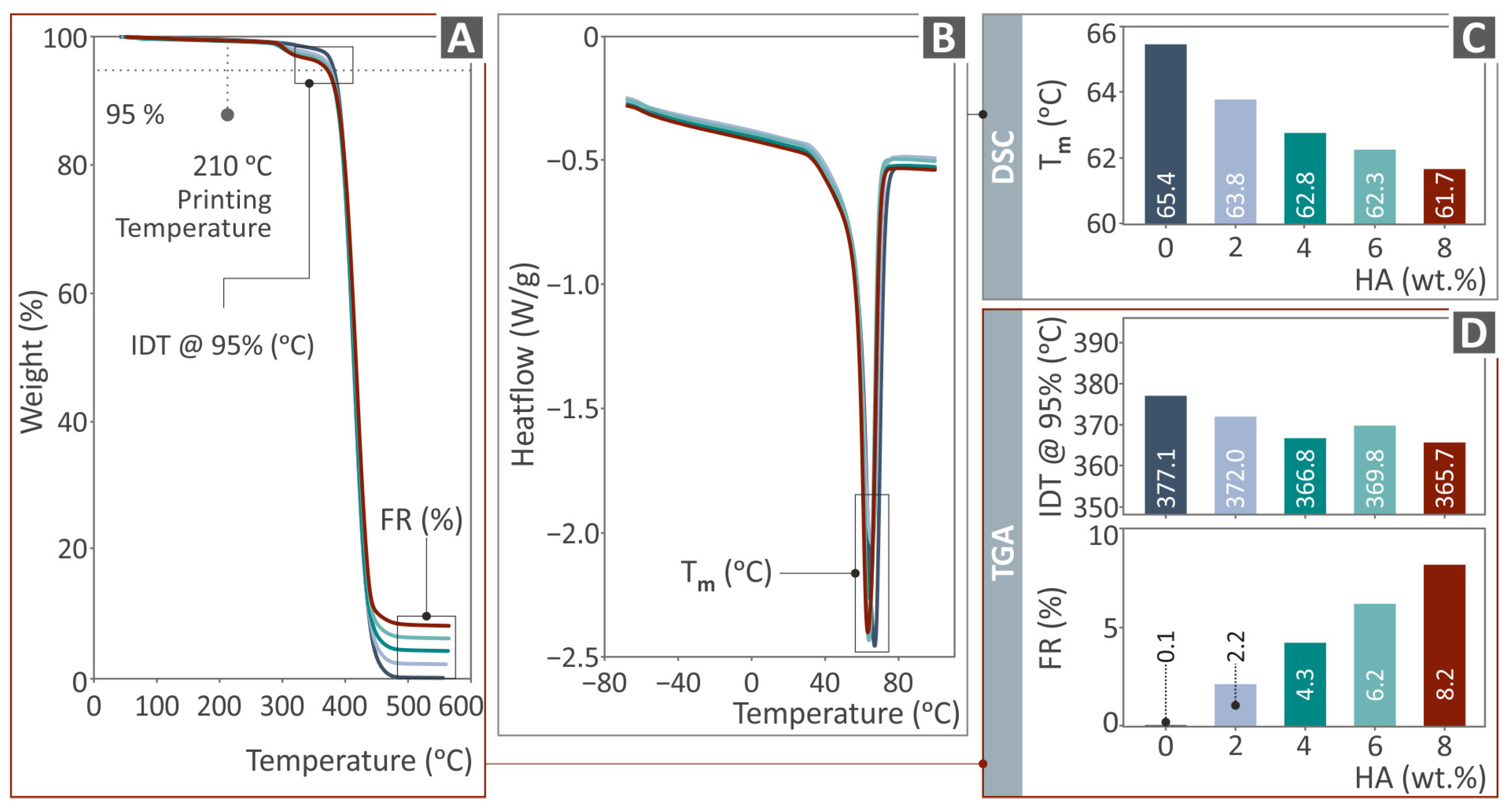
3.2. Thermal Characterization
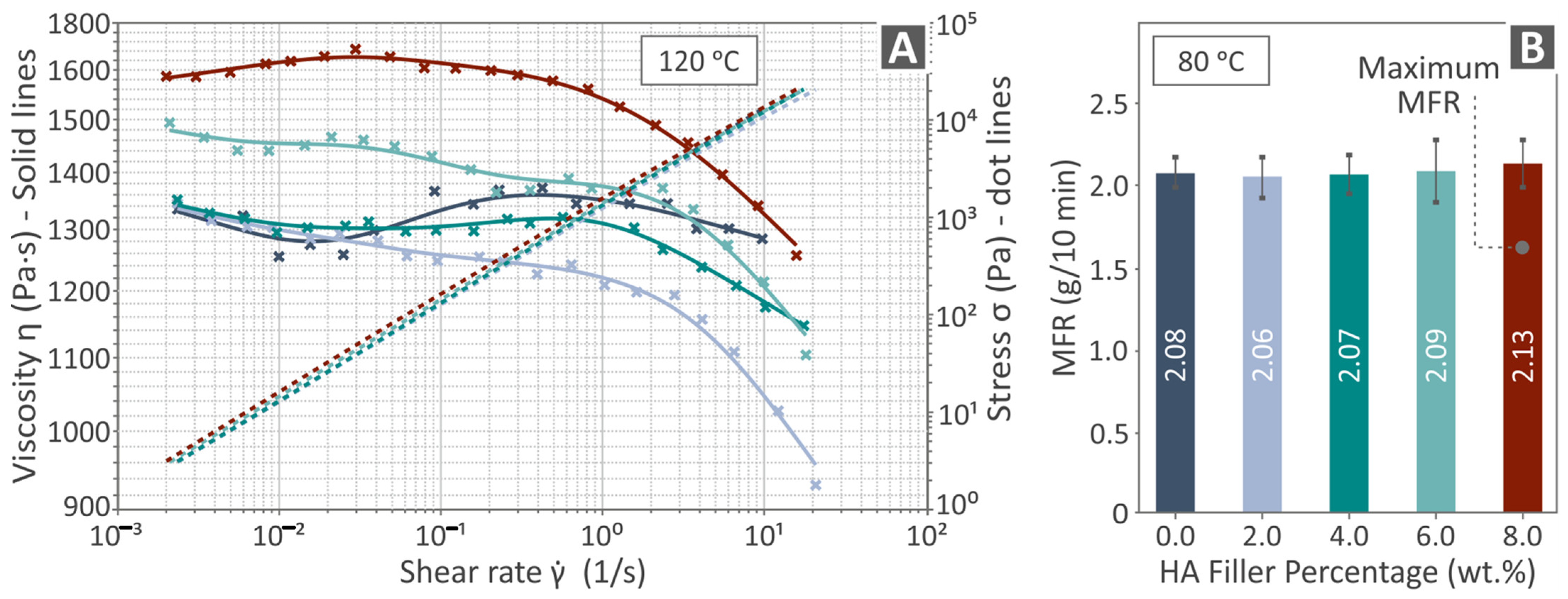
3.3. Viscosity and MFR (Rheological Properties)
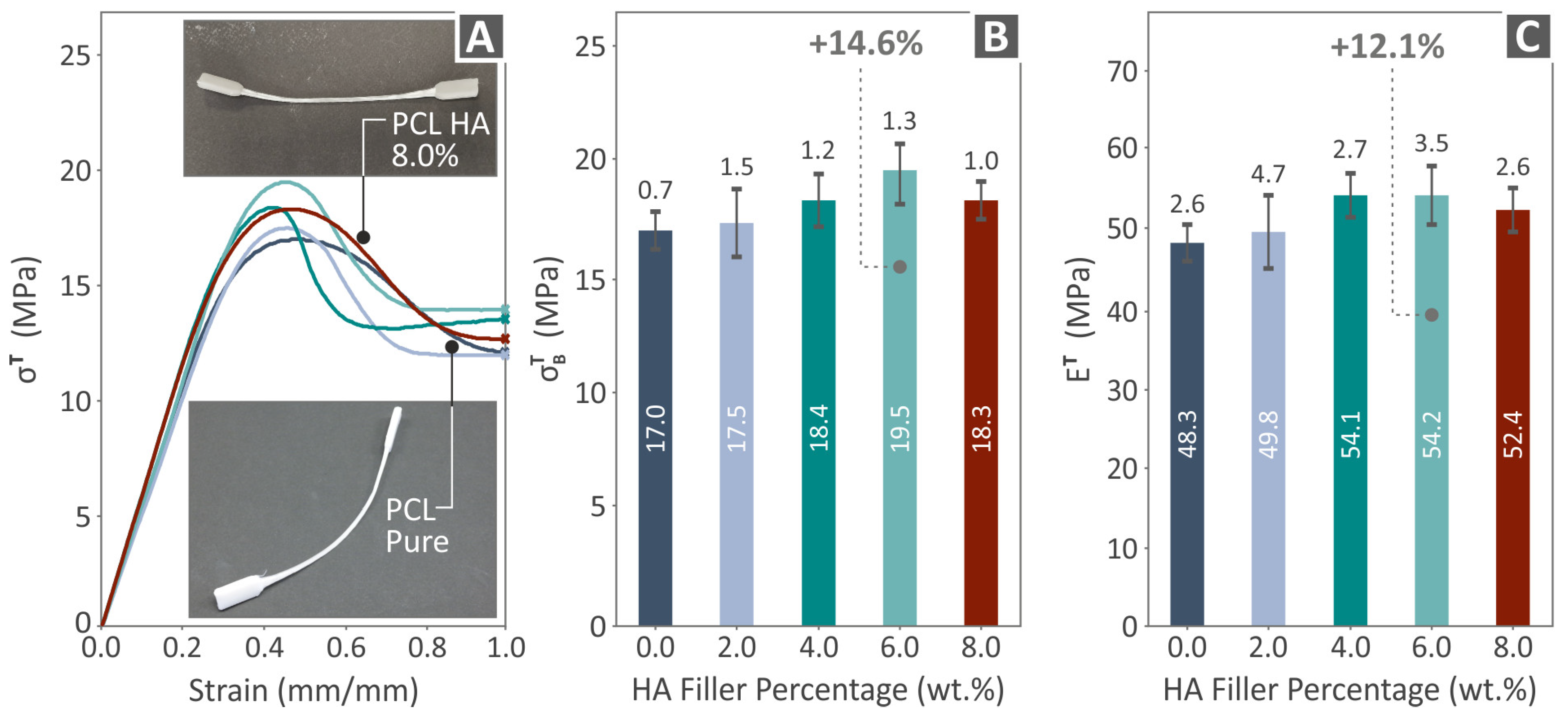
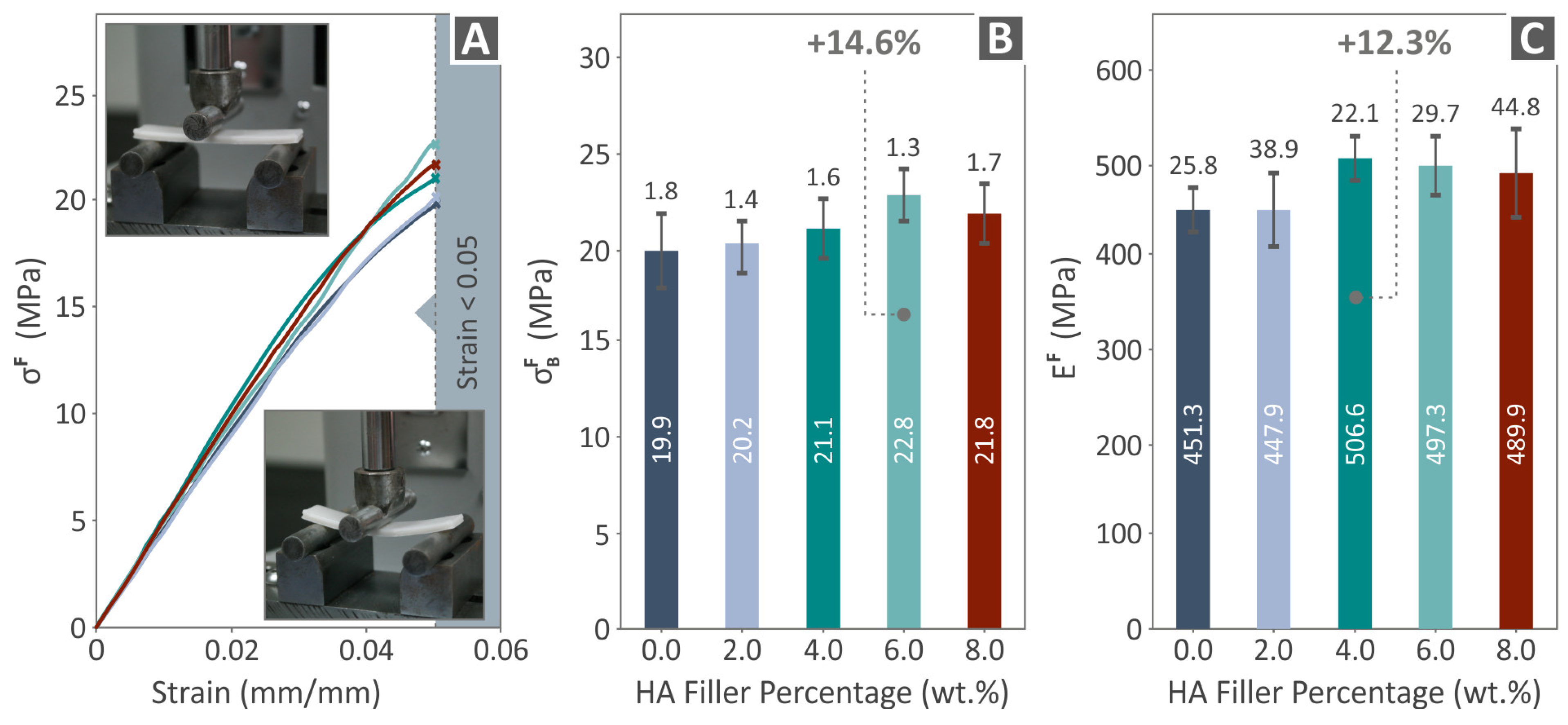
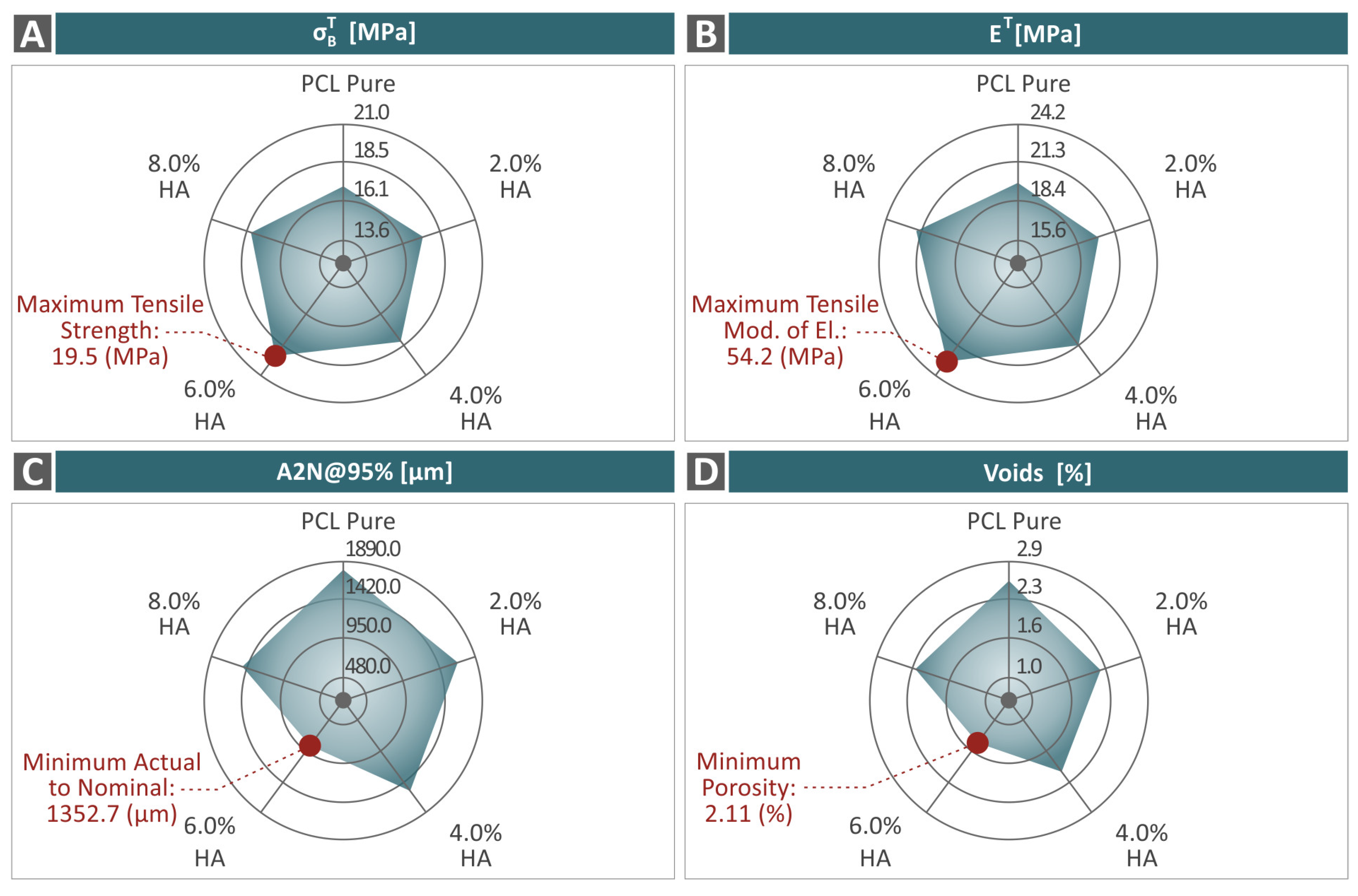
3.4. Mechanical Response of 3D-Printed Examples
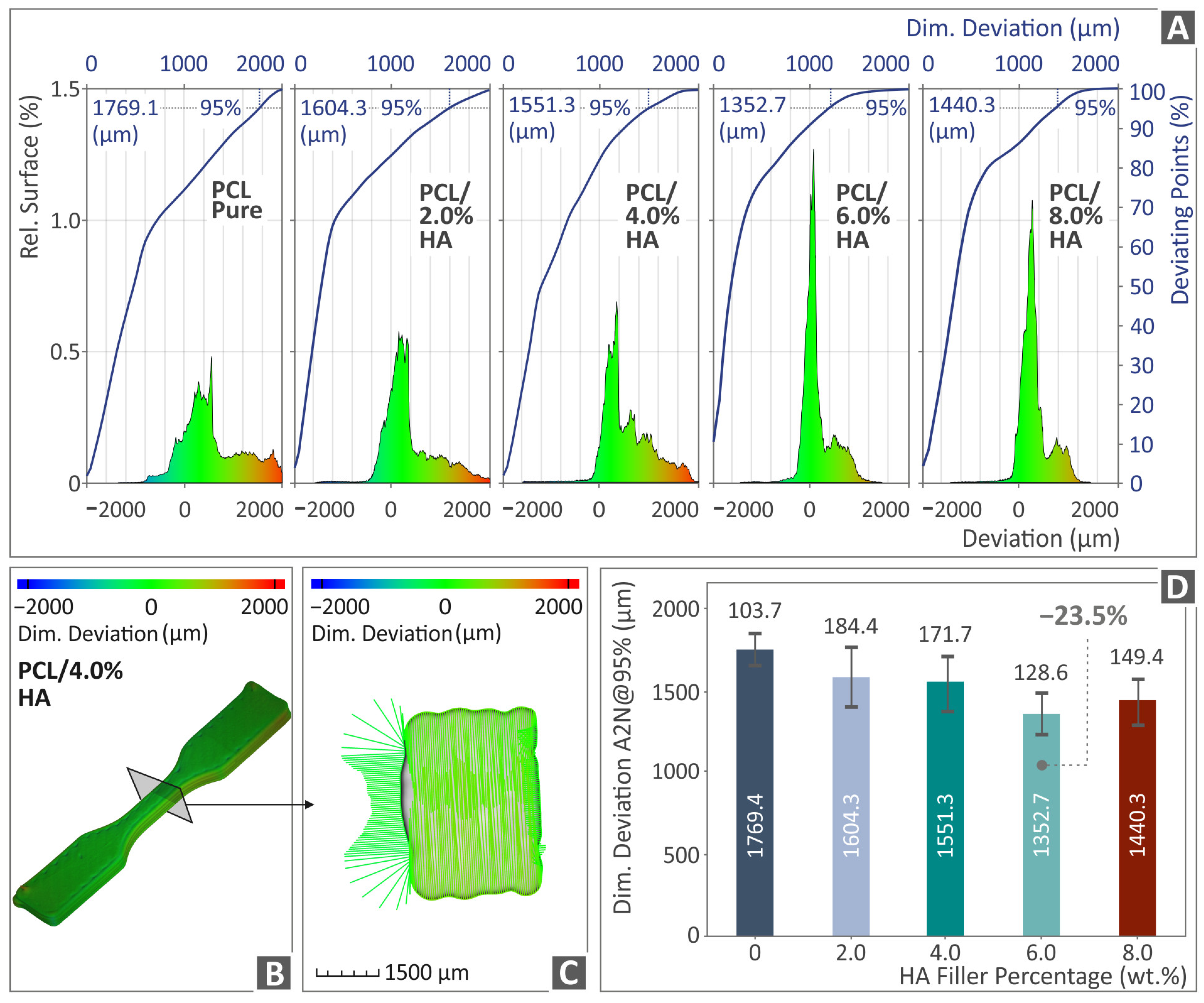
3.5. Tomography Results of 3D-Bioplotted Specimens
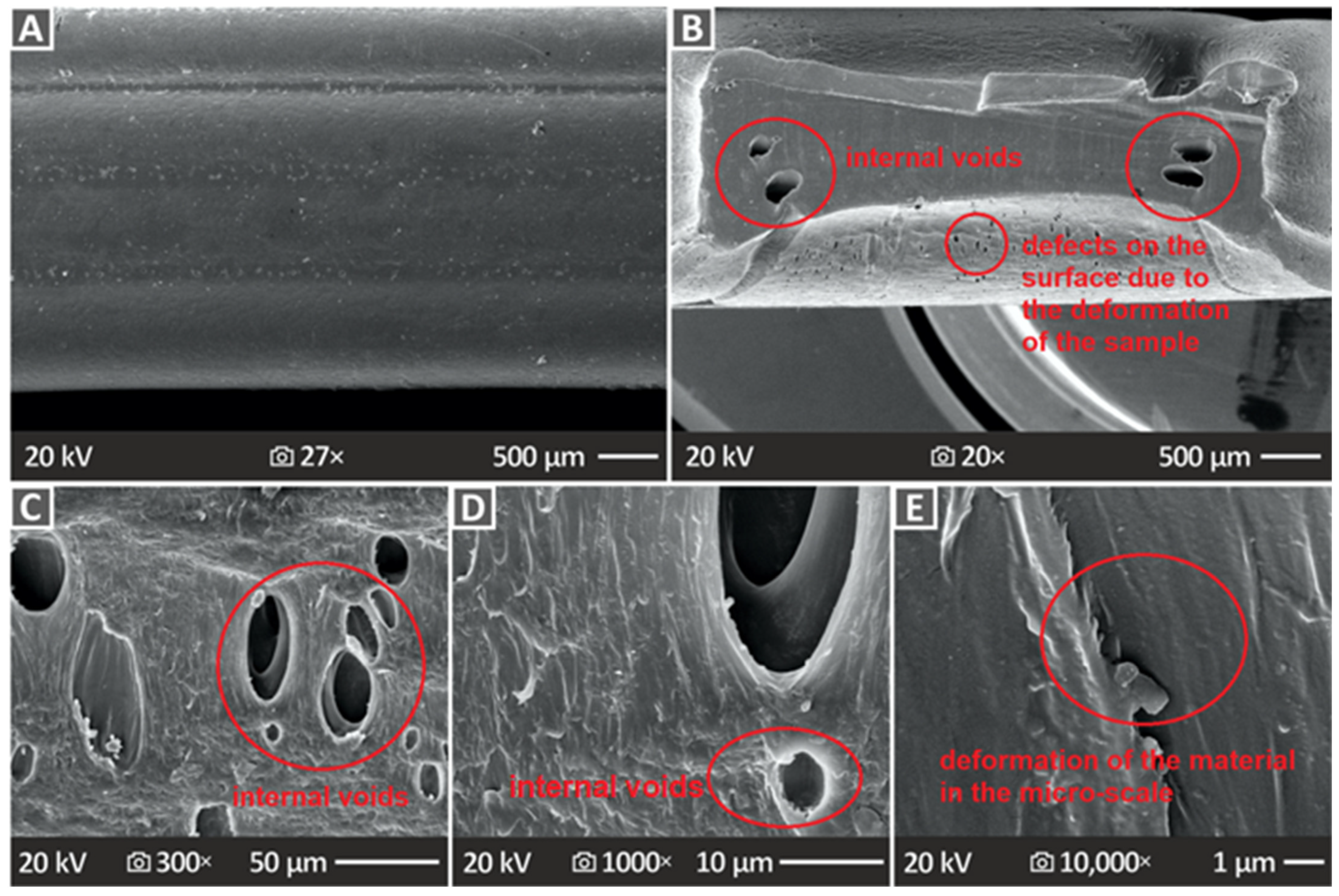
3.6. Three-Dimensional-Printed Samples’ SEM Morphological Analysis
4. Discussion
5. Conclusions
- The introduction of HAp in the PCL matrix did not negatively affect the behavior of the polymer, as the characterization process revealed;
- The 6.0 wt. % composite overall was the optimum loading in the study in terms of mechanical reinforcement;
- There was a connection found between the mechanical performance and both the porosity and dimensional accuracy of the samples;
- The study proposed a biocompatible PCL/HAp composite for bioplotting, with robust mechanical performance, which can be an asset for medical-related applications in tissue engineering and scaffolding.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gharibshahian, M.; Salehi, M.; Beheshtizadeh, N.; Kamalabadi-Farahani, M.; Atashi, A.; Nourbakhsh, M.-S.; Alizadeh, M. Recent Advances on 3D-Printed PCL-Based Composite Scaffolds for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1168504. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.; Quinn, A.; Hsieh, T.; Strong, E.B.; Steele, T. Optimization of 3D Print Material for the Recreation of Patient-Specific Temporal Bone Models. Ann. Otol. Rhinol. Laryngol. 2018, 127, 338–343. [Google Scholar] [CrossRef] [PubMed]
- El-Habashy, S.E.; El-Kamel, A.H.; Essawy, M.M.; Abdelfattah, E.-Z.A.; Eltaher, H.M. Engineering 3D-Printed Core–Shell Hydrogel Scaffolds Reinforced with Hybrid Hydroxyapatite/Polycaprolactone Nanoparticles for in Vivo Bone Regeneration. Biomater. Sci. 2021, 9, 4019–4039. [Google Scholar] [CrossRef]
- Michailidis, N.; Petousis, M.; Moutsopoulou, A.; Argyros, A.; Ntintakis, I.; Papadakis, V.; Nasikas, N.K.; Vidakis, N. Engineering Response of Biomedical Grade Isotactic Polypropylene Reinforced with Titanium Nitride Nanoparticles for Material Extrusion Three-Dimensional Printing. Eur. J. Mater. 2024, 4, 2340944. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Mountakis, N.; Papadakis, V.; Argyros, A.; Charou, C. Medical Grade Polyamide 12 Silver Nanoparticle Filaments Fabricated with In-Situ Reactive Reduction Melt-Extrusion: Rheological, Thermomechanical, and Bactericidal Performance in MEX 3D Printing. Appl. Nanosci. 2024, 14, 69–88. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Mountakis, N.; Papadakis, V.; Argyros, A.; Charou, C. Polyethylene Glycol and Polyvinylpyrrolidone Reduction Agents for Medical Grade Polyamide 12/Silver Nanocomposites Development for Material Extrusion 3D Printing: Rheological, Thermomechanical, and Biocidal Performance. React. Funct. Polym. 2023, 190, 105623. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Grammatikos, S.; David, C.N.; Mountakis, N.; Argyros, A.; Boura, O. Development and Optimization of Medical-Grade MultiFunctional Polyamide 12-Cuprous Oxide Nanocomposites with Superior Mechanical and Antibacterial Properties for Cost-Effective 3D Printing. Nanomaterials 2022, 12, 534. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Papadakis, V.; Mountakis, N.; Argyros, A.; Dimitriou, E.; Charou, C.; Moutsopoulou, A. Polylactic Acid/Silicon Nitride Biodegradable and Biomedical Nanocomposites with Optimized Rheological and Thermomechanical Response for Material Extrusion Additive Manufacturing. Biomed. Eng. Adv. 2023, 6, 100103. [Google Scholar] [CrossRef]
- Vidakis, N.; Moutsopoulou, A.; Petousis, M.; Michailidis, N.; Charou, C.; Mountakis, N.; Argyros, A.; Papadakis, V.; Dimitriou, E. Medical-Grade PLA Nanocomposites with Optimized Tungsten Carbide Nanofiller Content in MEX Additive Manufacturing: A Rheological, Morphological, and Thermomechanical Evaluation. Polymers 2023, 15, 3883. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Saltas, V.; Sagris, D.; Spiridaki, M.; Argyros, A.; Mountakis, N.; Papadakis, V. Interpretation of the Optimization Course of Silicon Nitride Nano-Powder Content in Biomedical Resins for Vat Photopolymerization Additive Manufacturing. Ceram. Int. 2024, 50, 14919–14935. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Moutsopoulou, A.; Mountakis, N.; Grammatikos, S.; Papadakis, V.; Tsikritzis, D. Biomedical Engineering Advances Cost-Effective Bi-Functional Resin Reinforced with a Nano-Inclusion Blend for Vat Photopolymerization Additive Manufacturing: The Effect of Multiple Antibacterial Nanoparticle Agents. Biomed. Eng. Adv. 2023, 5, 100091. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Mountakis, N.; Papadakis, V.; Moutsopoulou, A. Mechanical Strength Predictability of Full Factorial, Taguchi, and Box Behnken Designs: Optimization of Thermal Settings and Cellulose Nanofibers Content in PA12 for MEX AM. J. Mech. Behav. Biomed. Mater. 2023, 142, 105846. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; David, C.N.; Sagris, D.; Mountakis, N. Biomedical Resin Reinforced with Cellulose Nanofibers (CNF) in VAT Photopolymerization (VPP) Additive Manufacturing (AM): The Effect of Filler Loading and Process Control Parameters on Critical Quality Indicators (CQIs). J. Manuf. Process 2023, 101, 755–769. [Google Scholar] [CrossRef]
- Liu, F.; Vyas, C.; Poologasundarampillai, G.; Pape, I.; Hinduja, S.; Mirihanage, W.; Bartolo, P. Structural Evolution of PCL during Melt Extrusion 3D Printing. Macromol. Mater. Eng. 2018, 303, 1700494. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/Carboxymethyl Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Thadavirul, N.; Pavasant, P.; Supaphol, P. Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. J. Biomed. Mater. Res. A 2013, 102, 3379–3392. [Google Scholar] [CrossRef]
- Carter, P.; Rahman, S.M.; Bhattarai, N. Facile Fabrication of Aloe Vera Containing PCL Nanofibers for Barrier Membrane Application. J. Biomater. Sci. Polym. Ed. 2016, 27, 692–708. [Google Scholar] [CrossRef]
- Rashidi, H.; Yang, J.; Shakesheff, K.M. Surface Engineering of Synthetic Polymer Materials for Tissue Engineering and Regenerative Medicine Applications. Biomater. Sci. 2014, 2, 1318–1331. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic Biopolymer Nanocomposites for Tissue Engineering Scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Synthetic Biodegradable Functional Polymers for Tissue Engineering: A Brief Review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.H.; Harb, S.V.; Beatrice, C.A.G.; Shimomura, K.M.B.; Passador, F.R.; Costa, L.C.; Pessan, L.A. Polycaprolactone Usage in Additive Manufacturing Strategies for Tissue Engineering Applications: A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1479–1503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Liu, J.; Zhou, W.; Peng, S. Recent Progress of Preparation of Branched Poly(Lactic Acid) and Its Application in the Modification of Polylactic Acid Materials. Int. J. Biol. Macromol. 2021, 193, 874–892. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent Advances in 3D-Printed Polylactide and Polycaprolactone-Based Biomaterials for Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Haryńska, A.; Kucinska-Lipka, J.; Sulowska, A.; Gubanska, I.; Kostrzewa, M.; Janik, H. Medical-Grade PCL Based Polyurethane System for FDM 3D Printing—Characterization and Fabrication. Materials 2019, 12, 887. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-Caprolactone (PCL), a Promising Polymer for Pharmaceutical and Biomedical Applications: Focus on Nanomedicine in Cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Augustine, R.; Nethi, S.K.; Kalarikkal, N.; Thomas, S.; Patra, C.R. Electrospun Polycaprolactone (PCL) Scaffolds Embedded with Europium Hydroxide Nanorods (EHNs) with Enhanced Vascularization and Cell Proliferation for Tissue Engineering Applications. J. Mater. Chem. B 2017, 5, 4660–4672. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Covalently Linked Biocompatible Graphene/Polycaprolactone Composites for Tissue Engineering. Carbon 2013, 52, 296–304. [Google Scholar] [CrossRef]
- Kamath, M.S.; Ahmed, S.S.; Dhanasekaran, M.; Santosh, S.W. Polycaprolactone Scaffold Engineered for Sustained Release of Resveratrol: Therapeutic Enhancement in Bone Tissue Engineering. Int. J. Nanomed. 2013, 9, 183–195. [Google Scholar] [CrossRef]
- Plivelic, T.S.; Cassu, S.N.; do Carmo Gonçalves, M.; Torriani, I.L. Structure and Morphology of Poly(ε-Caprolactone)/Chlorinated Polyethylene (PCL/PECl) Blends Investigated by DSC, Simultaneous SAXS/WAXD, and Elemental Mapping by ESI-TEM. Macromolecules 2007, 40, 253–264. [Google Scholar] [CrossRef]
- Guo, T.; Holzberg, T.R.; Lim, C.G.; Gao, F.; Gargava, A.; Trachtenberg, J.E.; Mikos, A.G.; Fisher, J.P. 3D Printing PLGA: A Quantitative Examination of the Effects of Polymer Composition and Printing Parameters on Print Resolution. Biofabrication 2017, 9, 024101. [Google Scholar] [CrossRef] [PubMed]
- Bas, O.; De-Juan-Pardo, E.M.; Meinert, C.; D’Angella, D.; Baldwin, J.G.; Bray, L.J.; Wellard, R.M.; Kollmannsberger, S.; Rank, E.; Werner, C.; et al. Biofabricated Soft Network Composites for Cartilage Tissue Engineering. Biofabrication 2017, 9, 025014. [Google Scholar] [CrossRef]
- Koch, F.; Thaden, O.; Conrad, S.; Tröndle, K.; Finkenzeller, G.; Zengerle, R.; Kartmann, S.; Zimmermann, S.; Koltay, P. Mechanical Properties of Polycaprolactone (PCL) Scaffolds for Hybrid 3D-Bioprinting with Alginate-Gelatin Hydrogel. J. Mech. Behav. Biomed. Mater. 2022, 130, 105219. [Google Scholar] [CrossRef]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of GelMA/PCL and DECM/PCL Resins for 3D Printing of Acellular in Vitro Tissue Scaffolds by Stereolithography. Mater. Sci. Eng. C 2020, 112, 110958. [Google Scholar] [CrossRef] [PubMed]
- Eosoly, S.; Vrana, N.E.; Lohfeld, S.; Hindie, M.; Looney, L. Interaction of Cell Culture with Composition Effects on the Mechanical Properties of Polycaprolactone-Hydroxyapatite Scaffolds Fabricated via Selective Laser Sintering (SLS). Mater. Sci. Eng. C 2012, 32, 2250–2257. [Google Scholar] [CrossRef]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic Characterization of 3D-Printed PCL/β-TCP Scaffolds for Biomedical Devices and Bone Tissue Engineering: Influence of Composition and Porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef]
- Cengiz, B.; Gokce, Y.; Yildiz, N.; Aktas, Z.; Calimli, A. Synthesis and Characterization of Hydroxyapatite Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 29–33. [Google Scholar] [CrossRef]
- Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Hassan, I.; Sajad, P.; Majid, S.; Hassan, T. Serum Antioxidant Status in Patients with Systemic Sclerosis. Indian. J. Dermatol. 2013, 58, 239. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, W.; Guo, X.; Zhang, Y.; Wei, Q.; Du, B. Synthesis of Strontium Hydroxyapatite Embedding Ferroferric Oxide Nano-Composite and Its Application in Pb2+ Adsorption. J. Mol. Liq. 2014, 197, 40–47. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. A Study on the Bioactivity of Chitosan/Nano-Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Eur. Polym. J. 2006, 42, 3171–3179. [Google Scholar] [CrossRef]
- Degirmenbasi, N.; Kalyon, D.M.; Birinci, E. Biocomposites of Nanohydroxyapatite with Collagen and Poly(Vinyl Alcohol). Colloids Surf. B Biointerfaces 2006, 48, 42–49. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, C.; Li, Y.; Yan, Y.; Hou, C.; Wang, H.; Yang, Y.; Guan, G.; Feng, Q. The Preparation and In Vitro Evaluations of a Nanoscaled Injectable Bone Repair Material. J. Nanomater. 2015, 2015, 858493. [Google Scholar] [CrossRef]
- Marchi, J.; Dantas, A.C.S.; Greil, P.; Bressiani, J.C.; Bressiani, A.H.A.; Müller, F.A. Influence of Mg-Substitution on the Physicochemical Properties of Calcium Phosphate Powders. Mater. Res. Bull. 2007, 42, 1040–1050. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Ramay, H.R.; Zhang, M. Preparation of Porous Hydroxyapatite Scaffolds by Combination of the Gel-Casting and Polymer Sponge Methods. Biomaterials 2003, 24, 3293–3302. [Google Scholar] [CrossRef]
- Parhi, P.; Ramanan, A.; Ray, A.R. A Convenient Route for the Synthesis of Hydroxyapatite through a Novel Microwave-Mediated Metathesis Reaction. Mater. Lett. 2004, 58, 3610–3612. [Google Scholar] [CrossRef]
- Rodriguez, G.; Dias, J.; d’Ávila, M.A.; Bártolo, P. Influence of Hydroxyapatite on Extruded 3D Scaffolds. Procedia Eng. 2013, 59, 263–269. [Google Scholar] [CrossRef]
- Farag, M.M.; Yun, H. Effect of Gelatin Addition on Fabrication of Magnesium Phosphate-Based Scaffolds Prepared by Additive Manufacturing System. Mater. Lett. 2014, 132, 111–115. [Google Scholar] [CrossRef]
- Leukers, B.; Gülkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Schieker, M.; Seitz, H. Hydroxyapatite Scaffolds for Bone Tissue Engineering Made by 3D Printing. J. Mater. Sci. Mater. Med. 2005, 16, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D Printing of Porous Hydroxyapatite Scaffolds Intended for Use in Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Bogala, M.R. Three-Dimensional (3D) Printing of Hydroxyapatite-Based Scaffolds: A Review. Bioprinting 2022, 28, e00244. [Google Scholar] [CrossRef]
- Han, Y.; Wei, Q.; Chang, P.; Hu, K.; Okoro, O.V.; Shavandi, A.; Nie, L. Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application. Crystals 2021, 11, 353. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Zhang, X.; Xie, S.; Zhao, G.; Zhang, L. Biocompatibility and Physicochemical Characteristics of Poly(Ɛ-Caprolactone)/Poly(Lactide-Co-Glycolide)/Nano-Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Mater. Des. 2017, 114, 149–160. [Google Scholar] [CrossRef]
- Lee, J.-S.; Seol, Y.-J.; Sung, M.; Moon, W.; Kim, S.W.; Oh, J.-H.; Cho, D.-W. Development and Analysis of Three-Dimensional (3D) Printed Biomimetic Ceramic. Int. J. Precis. Eng. Manuf. 2016, 17, 1711–1719. [Google Scholar] [CrossRef]
- Jakus, A.E.; Shah, R.N. Multi and Mixed 3D-printing of Graphene-hydroxyapatite Hybrid Materials for Complex Tissue Engineering. J. Biomed. Mater. Res. A 2017, 105, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a Novel Alginate-polyvinyl Alcohol-hydroxyapatite Hydrogel for 3D Bioprinting Bone Tissue Engineered Scaffolds. J. Biomed. Mater. Res. A 2017, 105, 1457–1468. [Google Scholar] [CrossRef]
- Slots, C.; Jensen, M.B.; Ditzel, N.; Hedegaard, M.A.B.; Borg, S.W.; Albrektsen, O.; Thygesen, T.; Kassem, M.; Andersen, M.Ø. Simple Additive Manufacturing of an Osteoconductive Ceramic Using Suspension Melt Extrusion. Dent. Mater. 2017, 33, 198–208. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.; Liu, W.; He, R.; Zhou, M.; Wu, S.; Song, X.; Chen, Y. Effect of the Particle Size and the Debinding Process on the Density of Alumina Ceramics Fabricated by 3D Printing Based on Stereolithography. Ceram. Int. 2016, 42, 17290–17294. [Google Scholar] [CrossRef]
- Kim, I.-S.; Kumta, P.N. Sol–Gel Synthesis and Characterization of Nanostructured Hydroxyapatite Powder. Mater. Sci. Eng. B 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, G.; Wang, Z.; Guo, H. Synthesis of Single-Crystal HAP Nanorods. Ceram. Int. 2006, 32, 951–954. [Google Scholar] [CrossRef]
- Koumoulidis, G.C.; Katsoulidis, A.P.; Ladavos, A.K.; Pomonis, P.J.; Trapalis, C.C.; Sdoukos, A.T.; Vaimakis, T.C. Preparation of Hydroxyapatite via Microemulsion Route. J. Colloid. Interface Sci. 2003, 259, 254–260. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wei, K.; Zhao, N.; Chen, J.; Wang, X. Hydrothermal Synthesis of Hydroxyapatite Nanopowders Using Cationic Surfactant as a Template. Mater. Lett. 2006, 60, 1484–1487. [Google Scholar] [CrossRef]
- Han, J.-K.; Song, H.-Y.; Saito, F.; Lee, B.-T. Synthesis of High Purity Nano-Sized Hydroxyapatite Powder by Microwave-Hydrothermal Method. Mater. Chem. Phys. 2006, 99, 235–239. [Google Scholar] [CrossRef]
- Wang, F.; Li, M.-S.; Lu, Y.-P.; Qi, Y.-X.; Liu, Y.-X. Synthesis and Microstructure of Hydroxyapatite Nanofibers Synthesized at 37°C. Mater. Chem. Phys. 2006, 95, 145–149. [Google Scholar] [CrossRef]
- Cüneyt Tas, A. Synthesis of Biomimetic Ca-Hydroxyapatite Powders at 37°C in Synthetic Body Fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar] [CrossRef]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D Printing of HA / PCL Composite Tissue Engineering Scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-Printed PCL/PLA Composite Stents: Towards a New Solution to Cardiovascular Problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef]
- Ródenas-Rochina, J.; Ribelles, J.L.G.; Lebourg, M. Comparative Study of PCL-HAp and PCL-Bioglass Composite Scaffolds for Bone Tissue Engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1293–1308. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Rahmani Del Bakhshayesh, A.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The Efficiency of PCL/HAp Electrospun Nanofibers in Bone Regeneration: A Review. J. Med. Eng. Technol. 2021, 45, 511–531. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Mozafari, M.; Nezafati, N.; Azami, M.; Hadipour, H. A Proposed Fabrication Method of Novel PCL-GEL-HAp Nanocomposite Scaffolds for Bone Tissue Engineering Applications. Adv. Compos. Lett. 2010, 19, 096369351001900401. [Google Scholar] [CrossRef]
- Qi, H.; Ye, Z.; Ren, H.; Chen, N.; Zeng, Q.; Wu, X.; Lu, T. Bioactivity Assessment of PLLA/PCL/HAP Electrospun Nanofibrous Scaffolds for Bone Tissue Engineering. Life Sci. 2016, 148, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Uma Maheshwari, S.; Samuel, V.K.; Nagiah, N. Fabrication and Evaluation of (PVA/HAp/PCL) Bilayer Composites as Potential Scaffolds for Bone Tissue Regeneration Application. Ceram. Int. 2014, 40, 8469–8477. [Google Scholar] [CrossRef]
- Lebourg, M.; Antón, J.S.; Ribelles, J.L.G. Hybrid Structure in PCL-HAp Scaffold Resulting from Biomimetic Apatite Growth. J. Mater. Sci. Mater. Med. 2010, 21, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/Hydroxyapatite Composite Scaffolds: Preparation, Characterization, and in Vitro and in Vivo Biological Responses of Human Primary Bone Cells. J. Biomed. Mater. Res. A 2010, 94A, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, P.; Mehmanchi, M.; Atai, M.; Omidian, H.; Shokrolahi, F. Effect of Interface on Mechanical Properties and Biodegradation of PCL HAp Supramolecular Nano-Composites. J. Mater. Sci. Mater. Med. 2014, 25, 23–35. [Google Scholar] [CrossRef]
- Motloung, M.P.; Mofokeng, T.G.; Ray, S.S. Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites. Materials 2022, 15, 104. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Desando, G.; Cavallo, C.; Bartolotti, I.; Shelyakova, T.; Goranov, V.; Brucale, M.; Dediu, V.A.; Fini, M.; et al. Multifunctional 3D-Printed Magnetic Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 3825. [Google Scholar] [CrossRef]
- Mystiridou, E.; Patsidis, A.C.; Bouropoulos, N. Development and Characterization of 3D Printed Multifunctional Bioscaffolds Based on PLA/PCL/HAp/BaTiO3 Composites. Appl. Sci. 2021, 11, 4253. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Enhanced Osteogenic Differentiation of Stem Cells by 3D Printed PCL Scaffolds Coated with Collagen and Hydroxyapatite. Sci. Rep. 2022, 12, 12359. [Google Scholar] [CrossRef]
- Kwon, B.-J.; Kim, J.; Kim, Y.H.; Lee, M.H.; Baek, H.S.; Lee, D.H.; Kim, H.-L.; Seo, H.J.; Lee, M.H.; Kwon, S.-Y.; et al. Biological Advantages of Porous Hydroxyapatite Scaffold Made by Solid Freeform Fabrication for Bone Tissue Regeneration. Artif. Organs 2013, 37, 663–670. [Google Scholar] [CrossRef]
- Fox, K.; Tran, P.A.; Tran, N. Recent Advances in Research Applications of Nanophase Hydroxyapatite. ChemPhysChem 2012, 13, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Macuvele, D.L.P.; Nones, J.; Matsinhe, J.V.; Lima, M.M.; Soares, C.; Fiori, M.A.; Riella, H.G. Advances in Ultra High Molecular Weight Polyethylene/Hydroxyapatite Composites for Biomedical Applications: A Brief Review. Mater. Sci. Eng. C 2017, 76, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Jouault, N.; Dalmas, F.; Boué, F.; Jestin, J. Multiscale Characterization of Filler Dispersion and Origins of Mechanical Reinforcement in Model Nanocomposites. Polymer 2012, 53, 761–775. [Google Scholar] [CrossRef]
- Kamatchi, T.; Saravanan, R.; Rangappa, S.M.; Siengchin, S. Effect of Filler Content and Size on the Mechanical Properties of Graphene-Filled Natural Fiber-Based Nanocomposites. Biomass Convers. Biorefin 2023, 13, 11311–11320. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Mountakis, N.; Papadakis, V.; Sfakiotakis, E.; Sagris, D.; Argyros, A. Optimization of Cellulose Nanocrystal (CNC) Concentration in Polycaprolactone Bio-Composites for Bio-Plotting: A Robust Interpretation of the Reinforcement Mechanisms. Cellulose 2024, 31, 3657–3680. [Google Scholar] [CrossRef]
- ASTM D638-02; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Lin, Z.; Guo, X.; He, Z.; Liang, X.; Wang, M.; Jin, G. Thermal Degradation Kinetics Study of Molten Polylactide Based on Raman Spectroscopy. Polym. Eng. Sci. 2020, 61, 201–210. [Google Scholar] [CrossRef]
- Stuart, B.H. Temperature Studies of Polycarbonate Using Fourier Transform Raman Spectroscopy. Polym. Bull. 1996, 36, 341–346. [Google Scholar] [CrossRef]
- Camerlingo, C.; Zenone, F.; Delfino, I.; Diano, N.; Mita, D.G.; Lepore, M. Investigation on Clarified Fruit Juice Composition by Using Visible Light Micro-Raman Spectroscopy. Sensors 2007, 7, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Veluthandath, A.V.; Bisht, P.B. Identification of Whispering Gallery Mode (WGM) Coupled Photoluminescence and Raman Modes in Complex Spectra of MoS2 in Polymethyl Methacrylate (PMMA) Microspheres. J. Lumin. 2017, 187, 255–259. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing Cellulose Structures with Vibrational Spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- ASTM D1238-13; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM International: West Conshohocken, PA, USA, 2020.
- Feng, P.; He, J.; Peng, S.; Gao, C.; Zhao, Z.; Xiong, S.; Shuai, C. Characterizations and Interfacial Reinforcement Mechanisms of Multicomponent Biopolymer Based Scaffold. Mater. Sci. Eng. C 2019, 100, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Roeder, R.K.; Converse, G.L.; Kane, R.J.; Yue, W. Hydroxyapatite-Reinforced Polymer Biocomposites for Synthetic Bone Substitutes. JOM 2008, 60, 38–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Tanner, K.E. Impact Behavior of Hydroxyapatite Reinforced Polyethylene Composites. J. Mater. Sci. Mater. Med. 2003, 14, 63–68. [Google Scholar] [CrossRef]
- Ramier, J.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V.; Renard, E.; Albanese, P.; Grande, D. Biocomposite Scaffolds Based on Electrospun Poly(3-Hydroxybutyrate) Nanofibers and Electrosprayed Hydroxyapatite Nanoparticles for Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2014, 38, 161–169. [Google Scholar] [CrossRef]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K.A. Porous Hydroxyapatite for Artificial Bone Applications. Sci. Technol. Adv. Mater. 2007, 8, 116–123. [Google Scholar] [CrossRef]
- Ma, P.X.; Choi, J.-W. Biodegradable Polymer Scaffolds with Well-Defined Interconnected Spherical Pore Network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.C.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive Ceramics as a Synthetic Alternative to Autologous Bone Grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef]
- ISO 10993; Biocompatibility testing of medical devices. International Organization for Standardization: Geneva, Switzerland, 2018.
- Xu, C.; Liu, Z.; Chen, X.; Gao, Y.; Wang, W.; Zhuang, X.; Zhang, H.; Dong, X. Bone Tissue Engineering Scaffold Materials: Fundamentals, Advances, and Challenges. Chin. Chem. Lett. 2024, 35, 109197. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D Printing of PLA/n-HA Composite Scaffolds with Customized Mechanical Properties and Biological Functions for Bone Tissue Engineering. Compos. B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Feuerbach, T.; Callau-Mendoza, S.; Thommes, M. Development of Filaments for Fused Deposition Modeling 3D Printing with Medical Grade Poly(Lactic-Co-Glycolic Acid) Copolymers. Pharm. Dev. Technol. 2019, 24, 487–493. [Google Scholar] [CrossRef]
- Topsakal, A.; Midha, S.; Yuca, E.; Tukay, A.; Sasmazel, H.T.; Kalaskar, D.M.; Gunduz, O. Study on the Cytocompatibility, Mechanical and Antimicrobial Properties of 3D Printed Composite Scaffolds Based on PVA/ Gold Nanoparticles (AuNP)/ Ampicillin (AMP) for Bone Tissue Engineering. Mater. Today Commun. 2021, 28, 102458. [Google Scholar] [CrossRef]
- Statnik, E.S.; Sorokina, E.A.; Larin, I.I.; Yu, K.; Salimon, A.I.; Kalyaev, V.Y.; Zherebtsov, D.D.; Zadorozhnyy, M.Y.; Korsunsky, A.M. The Characterization of PVA/PHY Hydrogels for 3D Printing Fabrication of Organ Phantoms. Mater. Today Proc. 2020, 33, 1874–1879. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Abifarin, J.K.; Prakash, C.; Singh, S. Optimization and Significance of Fabrication Parameters on the Mechanical Properties of 3D Printed Chitosan/PLA Scaffold. Mater. Today Proc. 2022, 50, 2018–2025. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Mountakis, N.; Papadakis, V.; Sfakiotakis, E.; Sagris, D.; Spiridaki, M.; Argyros, A. Optimized PCL/CNF Bio-Nanocomposites for Medical Bio-Plotted Applications: Rheological, Structural, and Thermomechanical Aspects. Bioprinting 2023, 36, e00311. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Abu Bakar, A.; Tahir, P.M.; Zaidul, I.S.M.; Jawaid, M. Cellulosic Nanocomposites from Natural Fibers for Medical Applications: A Review. In Handbook of Polymer Nanocomposites. Processing, Performance and Application: Volume C: Polymer Nanocomposites of Cellulose Nanoparticles; Pandey, J.K., Takagi, H., Nakagaito, A.N., Kim, H.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 475–511. ISBN 978-3-642-45232-1. [Google Scholar]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Costa, L.M.M.; de Olyveira, G.M.; Kottaisamy, M.; Nagarajan, E.R.; Thomas, S. Cellulose Nanocomposites with Nanofibres Isolated from Pineapple Leaf Fibers for Medical Applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]
- Zimmerer, C.; Matulaitiene, I.; Niaura, G.; Reuter, U.; Janke, A.; Boldt, R.; Sablinskas, V.; Steiner, G. Nondestructive Characterization of the Polycarbonate - Octadecylamine Interface by Surface Enhanced Raman Spectroscopy. Polym. Test 2019, 73, 152–158. [Google Scholar] [CrossRef]
- Gatin, E.; Iordache, S.-M.; Matei, E.; Luculescu, C.-R.; Iordache, A.-M.; Grigorescu, C.; Ilici, R. Raman Spectroscopy as Spectral Tool for Assessing the Degree of Conversion after Curing of Two Resin-Based Materials Used in Restorative Dentistry. Diagnostics 2022, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Resta, V.; Quarta, G.; Lomascolo, M.; Maruccio, L.; Calcagnile, L. Raman and Photoluminescence Spectroscopy of Polycarbonate Matrices Irradiated with Different Energy 28Si+ Ions. Vacuum 2015, 116, 82–89. [Google Scholar] [CrossRef]
- Luiz, B.K.M.; Amboni, R.D.M.C.; Prates, L.H.M.; Roberto Bertolino, J.; Pires, A.T.N. Influence of Drinks on Resin Composite: Evaluation of Degree of Cure and Color Change Parameters. Polym. Test 2007, 26, 438–444. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Łydżba-Kopczyńska, B.; Sentandreu, E. Raman Spectroscopy Coupled to Chemometrics to Discriminate Provenance and Geological Age of Amber. J. Raman Spectrosc. 2018, 49, 842–851. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Y.; Li, W.; Cao, G.; Chen, W. Kinetics of Thermo-Oxidative and Thermal Degradation of Poly(d,l-Lactide) (PDLLA) at Processing Temperature. Polym. Degrad Stab. 2006, 91, 3259–3265. [Google Scholar] [CrossRef]





| Wavenumber (cm−1) | Change | Raman Peak Assignment |
|---|---|---|
| 960 | Gradual increase | Strong increase in O-CH3 rocking |
| 1035 | Gradual increase | Small increase in C-CH3 stretching |
| 1065 | Gradual increase | Small increase in C-O-C stretching |
| 1109 | Gradual increase | Strong increase in C-O-C stretching |
| 1282 | increase | Small increase in C-O-C stretching |
| 1305 | increase | Small increase in C=O bond stretching |
| 1443 | Increase | Small increase in CH3 deformation |
| 2844–3000 | Gradual decrease | Decrease in CH and CH2 stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petousis, M.; Michailidis, N.; Korlos, A.; Papadakis, V.; David, C.; Sagris, D.; Mountakis, N.; Argyros, A.; Valsamos, J.; Vidakis, N. Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course. Polymers 2024, 16, 2400. https://doi.org/10.3390/polym16172400
Petousis M, Michailidis N, Korlos A, Papadakis V, David C, Sagris D, Mountakis N, Argyros A, Valsamos J, Vidakis N. Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course. Polymers. 2024; 16(17):2400. https://doi.org/10.3390/polym16172400
Chicago/Turabian StylePetousis, Markos, Nikolaos Michailidis, Apostolos Korlos, Vassilis Papadakis, Constantine David, Dimitrios Sagris, Nikolaos Mountakis, Apostolos Argyros, John Valsamos, and Nectarios Vidakis. 2024. "Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course" Polymers 16, no. 17: 2400. https://doi.org/10.3390/polym16172400






