Effect of Graphene Oxide Nanoparticles Incorporation on the Mechanical Properties of a Resin-Modified Glass Ionomer Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Resin-Modified Glass Ionomer Cements with Graphene Oxide
2.2. Flexural Strength Test
2.3. Elastic Modulus Test
2.4. Vickers Microhardness Test (VHN)
2.5. Surface Roughness Test
2.6. Statistical Analysis
3. Results
3.1. Powder Characterization (SEM/EDS)
3.2. Flexural Strength and Elastic Modulus
3.3. Vickers Microhardness
3.4. Surface Roughness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, A.D.; Kent, B.E. A New Translucent Cement for Dentistry. The Glass Ionomer Cement. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef]
- Ngo, H.C.; Mount, G.; McIntyre, J.; Do, L. An in Vitro Model for the Study of Chemical Exchange between Glass Ionomer Restorations and Partially Demineralized Dentin Using a Minimally Invasive Restorative Technique. J. Dent. 2011, 39 (Suppl. S2), S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.G.P.; Ogasawara, T. Estudos comparativos de alguns cimentos ionoméricos convencionais. Matéria (Rio J.) 2006, 11, 297–305. [Google Scholar] [CrossRef][Green Version]
- Berg, J.H.; Croll, T.P. Glass Ionomer Restorative Cement Systems: An Update. Pediatr. Dent. 2015, 37, 116–124. [Google Scholar] [PubMed]
- Spezzia, S. Cimento de ionômero de vidro: Revisão de literatura. J. Oral Investig. 2017, 6, 74–88. [Google Scholar] [CrossRef]
- Wilson, A.D. Resin-Modified Glass-Ionomer Cements. Int. J. Prosthodont. 1990, 3, 425–429. [Google Scholar] [PubMed]
- Tyas, M.J. Clinical Evaluation of Glass-Ionomer Cement Restorations. J. Appl. Oral. Sci. 2006, 14, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, H.M.C.; Butler, S.; Santos, M.J.C. Evaluation of the Mechanical Properties of Three Resin-Modified Glass-Ionomer Materials. Biomed. Res. Int. 2022, 2022, 4690656. [Google Scholar] [CrossRef]
- Malhotra, S.; Bhullar, K.K.; Kaur, S.; Malhotra, M.; Kaur, R.; Handa, A. Comparative Evaluation of Compressive Strength and Flexural Strength of GC Gold Hybrid, GIC Conventional and Resin-Modified Glass-Ionomer Cement. J. Pharm. Bioallied Sci. 2022, 14, S214–S216. [Google Scholar] [CrossRef]
- Chadwick, B.L.; Evans, D.J.P. Restoration of Class II Cavities in Primary Molar Teeth with Conventional and Resin Modified Glass Ionomer Cements: A Systematic Review of the Literature. Eur. Arch. Paediatr. Dent. 2007, 8, 14–21. [Google Scholar] [CrossRef]
- Dülgergil, C.T.; Soyman, M.; Civelek, A. Atraumatic Restorative Treatment with Resin-Modified Glass Ionomer Material: Short-Term Results of a Pilot Study. Med. Princ. Pract. 2005, 14, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Moberg, M.; Brewster, J.; Nicholson, J.; Roberts, H. Physical Property Investigation of Contemporary Glass Ionomer and Resin-Modified Glass Ionomer Restorative Materials. Clin. Oral. Investig. 2019, 23, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Balkaya, H.; Arslan, S.; Pala, K. A Randomized, Prospective Clinical Study Evaluating Effectiveness of a Bulk-Fill Composite Resin, a Conventional Composite Resin and a Reinforced Glass Ionomer in Class II Cavities: One-Year Results. J. Appl. Oral. Sci. 2019, 27, e20180678. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.B.; Ershad, F.; Ellakwa, A.; Kruzic, J.J. Fiber Reinforcement of a Resin Modified Glass Ionomer Cement. Dent. Mater. 2020, 36, 1516–1523. [Google Scholar] [CrossRef]
- Valanezhad, A.; Odatsu, T.; Udoh, K.; Shiraishi, T.; Sawase, T.; Watanabe, I. Modification of Resin Modified Glass Ionomer Cement by Addition of Bioactive Glass Nanoparticles. J. Mater. Sci. Mater. Med. 2016, 27, 3. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-Dimensional Gas of Massless Dirac Fermions in Graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Rosa, V.; Xie, H.; Dubey, N.; Madanagopal, T.T.; Rajan, S.S.; Morin, J.L.P.; Islam, I.; Castro Neto, A.H. Graphene Oxide-Based Substrate: Physical and Surface Characterization, Cytocompatibility and Differentiation Potential of Dental Pulp Stem Cells. Dent. Mater. 2016, 32, 1019–1025. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-Based Dental Adhesive with Anti-Biofilm Activity. J. Nanobiotechnol. 2017, 15, 89. [Google Scholar] [CrossRef]
- Li, X.; Liang, X.; Wang, Y.; Wang, D.; Teng, M.; Xu, H.; Zhao, B.; Han, L. Graphene-Based Nanomaterials for Dental Applications: Principles, Current Advances, and Future Outlook. Front. Bioeng. Biotechnol. 2022, 10, 804201. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Noroozi Pesyan, N.; Fathi, M.; Omidi, Y. Injectable Thermosensitive Hybrid Hydrogel Containing Graphene Oxide and Chitosan as Dental Pulp Stem Cells Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Miyaji, H.; Nishida, E.; Miyata, S.; Kato, A.; Tateyama, A.; Furihata, T.; Shitomi, K.; Iwanaga, T.; Sugaya, T. Characterization and Evaluation of Graphene Oxide Scaffold for Periodontal Wound Healing of Class II Furcation Defects in Dog. Int. J. Nanomed. 2018, 13, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, P.; Li, X.; Liu, H.; Ge, S. Bioactivity of Periodontal Ligament Stem Cells on Sodium Titanate Coated with Graphene Oxide. Sci. Rep. 2016, 6, 19343. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Qiu, J.; Su, J.; Liu, X. Minocycline Hydrochloride Loaded on Titanium by Graphene Oxide: An Excellent Antibacterial Platform with the Synergistic Effect of Contact-Killing and Release-Killing. Biomater. Sci. 2018, 6, 304–313. [Google Scholar] [CrossRef]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.-S.; Rosa, V. Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the Mechanical, Tribological and Antibacterial Properties of Glass Ionomer Cements by Fluorinated Graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef]
- Sharafeddin, F.; Farhadpour, H.; Hefzollah, R. Evaluation of the Effect of Nanoparticle Graphene Oxide on Flexural Strength of Glass Ionomer Cements. Int. J. Dent. 2023, 2023, 8183167. [Google Scholar] [CrossRef]
- Sari, F.; Ugurlu, M. Reinforcement of Resin-Modified Glass-Ionomer Cement with Glass Fiber and Graphene Oxide. J. Mech. Behav. Biomed. Mater. 2023, 142, 105850. [Google Scholar] [CrossRef]
- Ghodrati, P.; Sharafeddin, F. Evaluation of the Effect of Nano-Graphene Oxide on Shear Bond Strength of Conventional and Resin-Modified Glass Ionomer Cement. Clin. Exp. Dent. Res. 2023, 9, 851–858. [Google Scholar] [CrossRef]
- ISO 9917-2:2017; Dentistry—Water-Based Cements Part 2: Resin-Modified Cements. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Xie, H.; Cao, T.; Rodríguez-Lozano, F.J.; Luong-Van, E.K.; Rosa, V. Graphene for the Development of the Next-Generation of Biocomposites for Dental and Medical Applications. Dent. Mater. 2017, 33, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Al-Qahtani, Y.M. Impact of Graphene Oxide and Silver Diamine Fluoride in Comparison to Photodynamic Therapy on Bond Integrity and Microleakage Scores of Resin Modified Glass Ionomer Cement to Demineralized Dentin. Photodiagnosis Photodyn. Ther. 2021, 33, 102163. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Q.; Peng, J.; Yang, X.; Yu, D.; Zhao, W. Antibacterial and Mechanical Properties of Reduced Graphene-Silver Nanoparticle Nanocomposite Modified Glass Ionomer Cements. J. Dent. 2020, 96, 103332. [Google Scholar] [CrossRef]
- Liu, R.; Wang, E.; Guo, Y.; Zhou, Q.; Zheng, Y.; Zhai, J.; Zhang, K.; Zhang, B. Enhanced Antibacterial Properties and Promoted Cell Proliferation in Glass Ionomer Cement by Modified with Fluorinated Graphene-Doped. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211037487. [Google Scholar] [CrossRef]
- McLean, J.W. Cermet Cements. J. Am. Dent. Assoc. 1990, 120, 43–47. [Google Scholar] [CrossRef]
- Baig, M.S.; Fleming, G.J.P. Conventional Glass-Ionomer Materials: A Review of the Developments in Glass Powder, Polyacid Liquid and the Strategies of Reinforcement. J. Dent. 2015, 43, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.P.; Singh, S.; Sood, S.; Sharma, N. A Comparative Evaluation of Sorption, Solubility, and Compressive Strength of Three Different Glass Ionomer Cements in Artificial Saliva: An in Vitro Study. Int. J. Clin. Pediatr. Dent. 2017, 10, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kerby, R.E.; Bleiholder, R.F. Physical Properties of Stainless-Steel and Silver-Reinforced Glass-Ionomer Cements. J. Dent. Res. 1991, 70, 1358–1361. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Enhancing the Mechanical Properties of Glass-Ionomer Dental Cements: A Review. Materials 2020, 13, 2510. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium Dioxide Nanoparticles Addition to a Conventional Glass-Ionomer Restorative: Influence on Physical and Antibacterial Properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Khademolhosseini, M.; Barounian, M.; Eskandari, A.; Aminzare, M.; Zahedi, A.; Ghahremani, D. Development of New Al2O3/TiO2 Reinforced Glass-Ionomer Cements (GICs) Nano-Composites. Basic Appl. Sci. Res. 2012, 2, 7526–7529. [Google Scholar]
- Semyari, H.; Sattari, M.; Atai, M.; Pournasir, M. The Effect of Nanozirconia Mixed with Glass-Ionomer on Proliferation of Epithelial Cells and Adhesive Molecules. J. Periodontol. Implant. Dent. 2011, 3, 63–68. [Google Scholar]
- Alatawi, R.A.S.; Elsayed, N.H.; Mohamed, W.S. Influence of Hydroxyapatite Nanoparticles on the Properties of Glass Ionomer Cement. J. Mater. Res. Technol. 2019, 8, 344–349. [Google Scholar] [CrossRef]
- Malekhoseini, Z.; Rezvani, M.B.; Niakan, M.; Atai, M.; Bassir, M.M.; Alizade, H.S.; Siabani, S. Effect of Zinc Oxide Nanoparticles on Physical and Antimicrobial Properties of Resin-Modified Glass Ionomer Cement. Dent. Res. J. 2021, 18, 73. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Nicholson, J.W.; Gabrić, D.; Guclu, Z.A.; Miletić, I.; Coleman, N.J. Assessment of the Impact of the Addition of Nanoparticles on the Properties of Glass–Ionomer Cements. Materials 2020, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Sharafeddin, F.; Jowkar, Z.; Bahrani, S. Comparison between the Effect of Adding Microhydroxyapatite and Chitosan on Surface Roughness and Microhardness of Resin Modified and Conventional Glass Ionomer Cements. J. Clin. Exp. Dent. 2021, 13, e737–e744. [Google Scholar] [CrossRef]
- Soygun, K.; Soygun, A.; Dogan, M.C. The Effects of Chitosan Addition to Glass Ionomer Cement on Microhardness and Surface Roughness. J. Appl. Biomater. Funct. Mater. 2021, 19, 2280800021989706. [Google Scholar] [CrossRef]

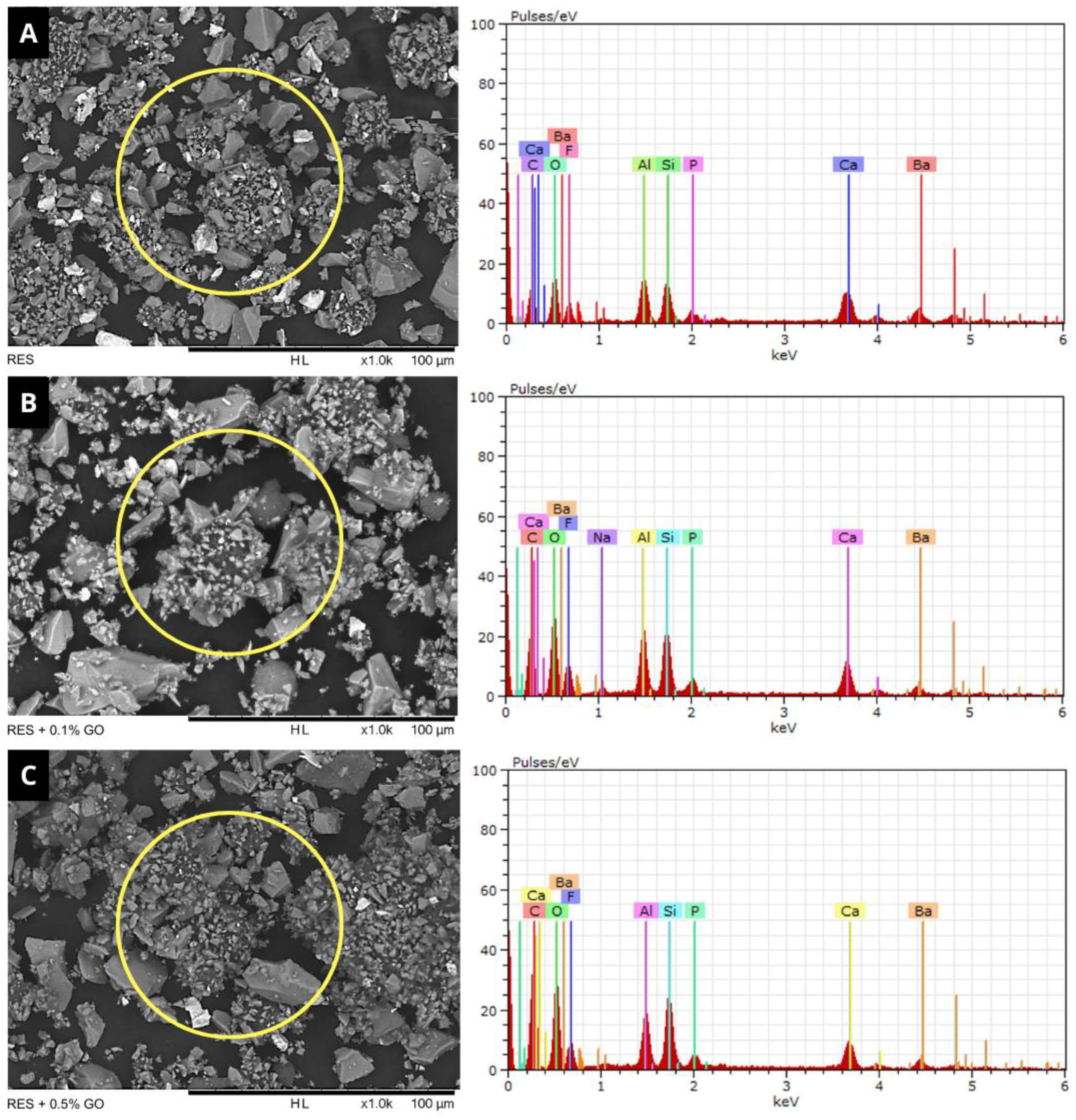



| Elements | RES | RES + 0.1% GO | RES + 0.5% GO |
|---|---|---|---|
| Carbon | 27.52% | 31.91% | 40.30% |
| Oxygen | 26.81% | 30.73% | 31.22% |
| Fluorine | 10.27% | 11.99% | 9.19% |
| Barium | 8.65% | 4.95% | 3.00% |
| Aluminum | 8.52% | 5.58% | 4.80% |
| Calcium | 8.06% | 6.63% | 3.91% |
| Silicon | 7.91% | 6.07% | 6.28% |
| Phosphorus | 2.26% | 1.40% | 1.30% |
| Sodium | - | 0.75% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira e Moraes, R.U.; Abreu, M.A.P.; Frazão, M.C.A.; Ferreira, P.V.C.; Bauer, J.; Carvalho, C.N.; Carvalho, E.M. Effect of Graphene Oxide Nanoparticles Incorporation on the Mechanical Properties of a Resin-Modified Glass Ionomer Cement. Polymers 2024, 16, 2401. https://doi.org/10.3390/polym16172401
Moreira e Moraes RU, Abreu MAP, Frazão MCA, Ferreira PVC, Bauer J, Carvalho CN, Carvalho EM. Effect of Graphene Oxide Nanoparticles Incorporation on the Mechanical Properties of a Resin-Modified Glass Ionomer Cement. Polymers. 2024; 16(17):2401. https://doi.org/10.3390/polym16172401
Chicago/Turabian StyleMoreira e Moraes, Rafael Ubaldo, Marcos Andre Pinheiro Abreu, Mayara Cristina Abas Frazão, Paulo Vitor Campos Ferreira, José Bauer, Ceci Nunes Carvalho, and Edilausson Moreno Carvalho. 2024. "Effect of Graphene Oxide Nanoparticles Incorporation on the Mechanical Properties of a Resin-Modified Glass Ionomer Cement" Polymers 16, no. 17: 2401. https://doi.org/10.3390/polym16172401
APA StyleMoreira e Moraes, R. U., Abreu, M. A. P., Frazão, M. C. A., Ferreira, P. V. C., Bauer, J., Carvalho, C. N., & Carvalho, E. M. (2024). Effect of Graphene Oxide Nanoparticles Incorporation on the Mechanical Properties of a Resin-Modified Glass Ionomer Cement. Polymers, 16(17), 2401. https://doi.org/10.3390/polym16172401









