An Assessment of Biodegradability and Phytotoxicity of Natural Rubber in a Simulated Soil Condition via CO2 Evolution Measurement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Rubber Compounds and Vulcanizates
2.3. Biodegradation Test
2.3.1. Characterization of the Test Materials
2.3.2. Soil Preparation and Characterization
2.3.3. Experimental Set-Up and Biodegradation Assay
2.4. Plant Toxicity Test
3. Results
3.1. Basic Properties of the Rubber Samples
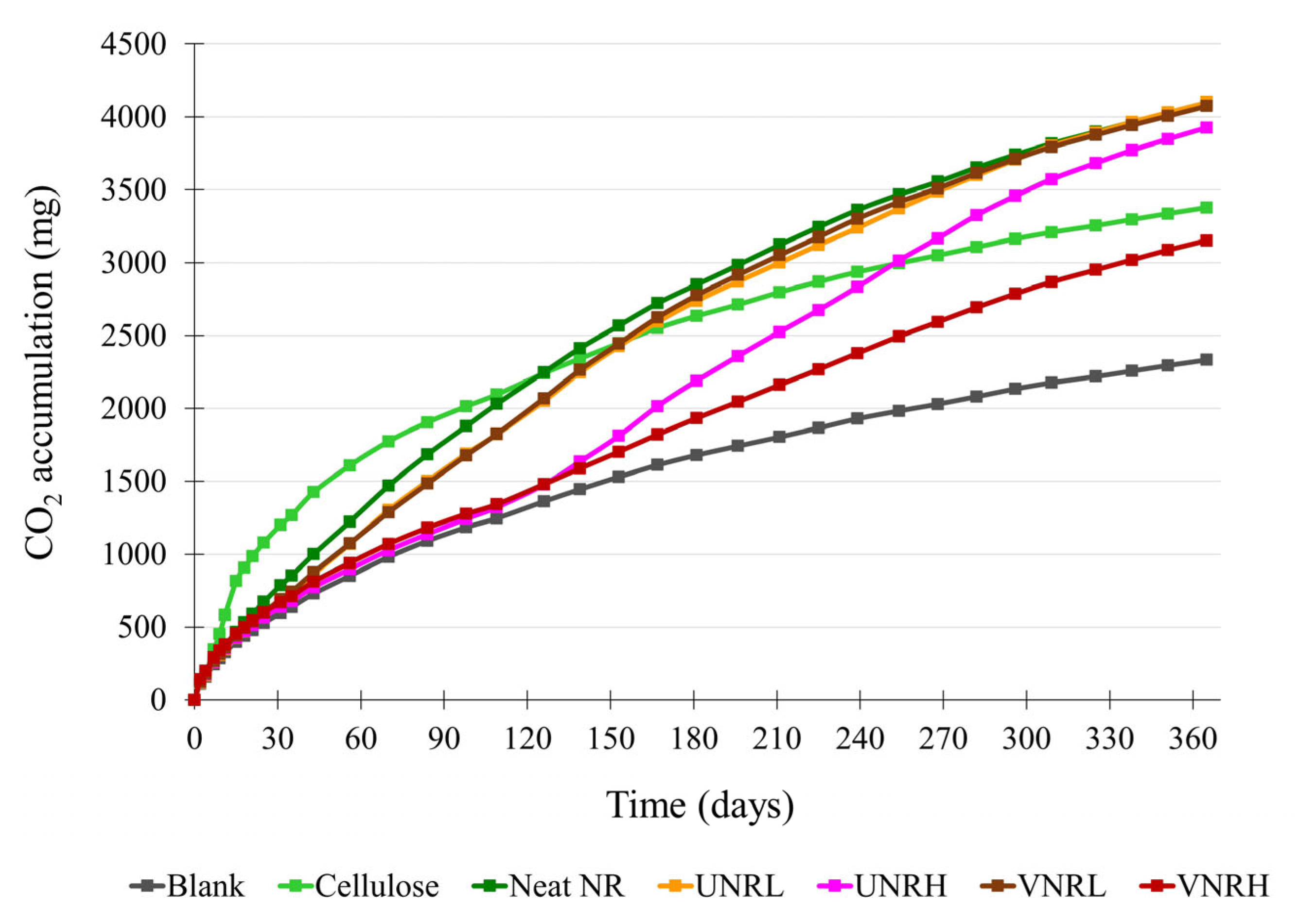
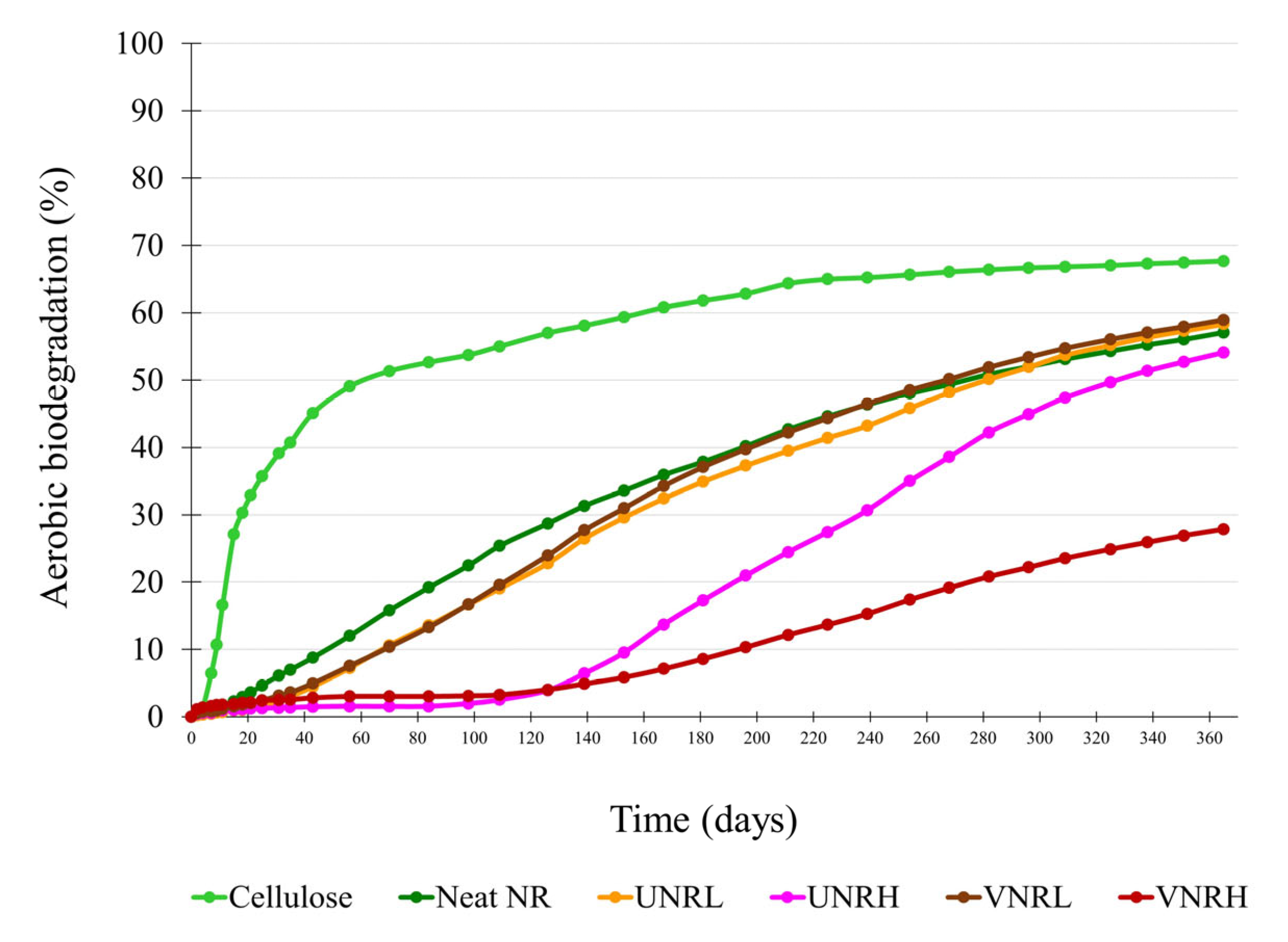
3.2. Biodegradation Results
3.2.1. Basic Properties of the Test Materials
3.2.2. Biodegradation of the Rubber Samples
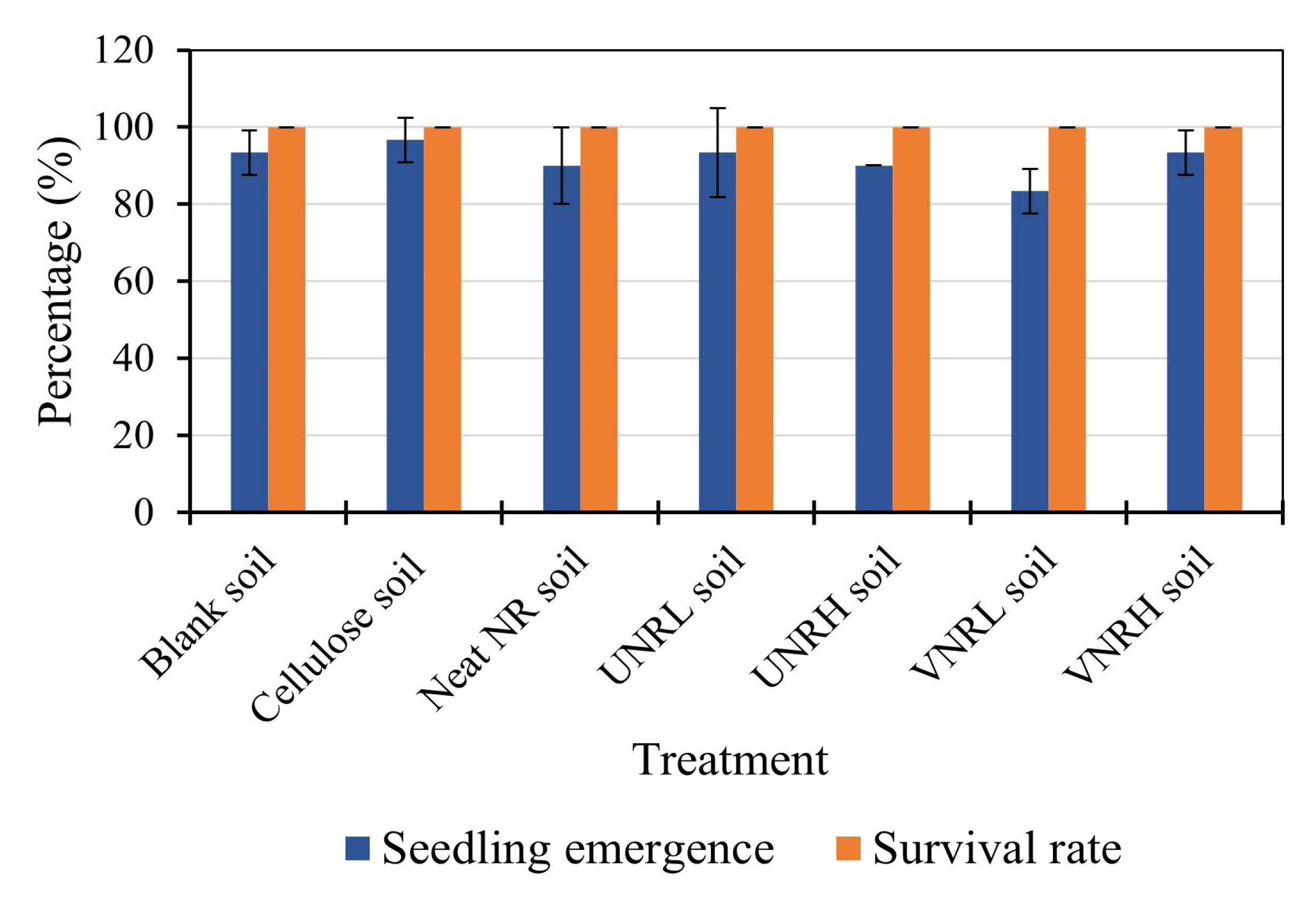
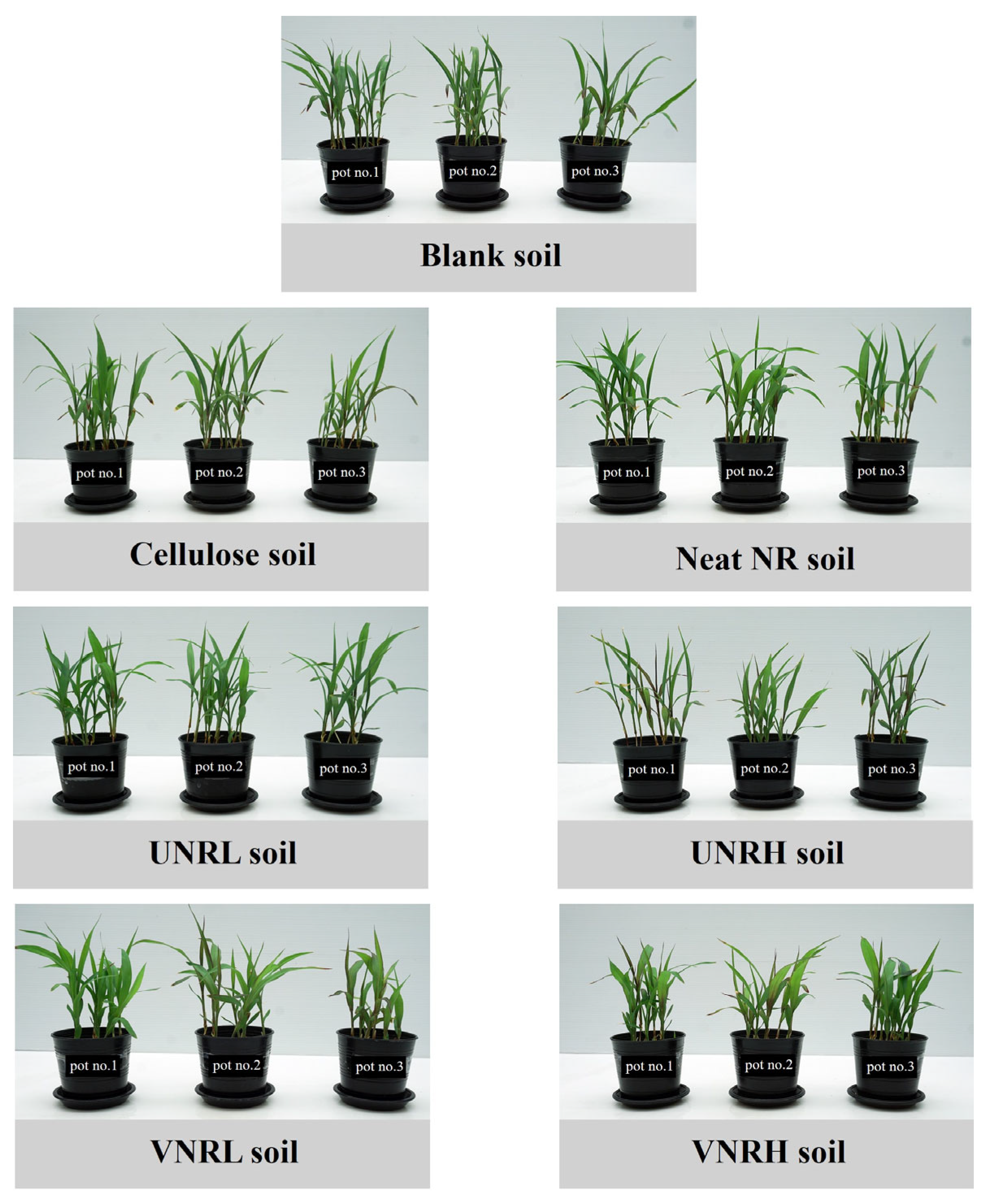
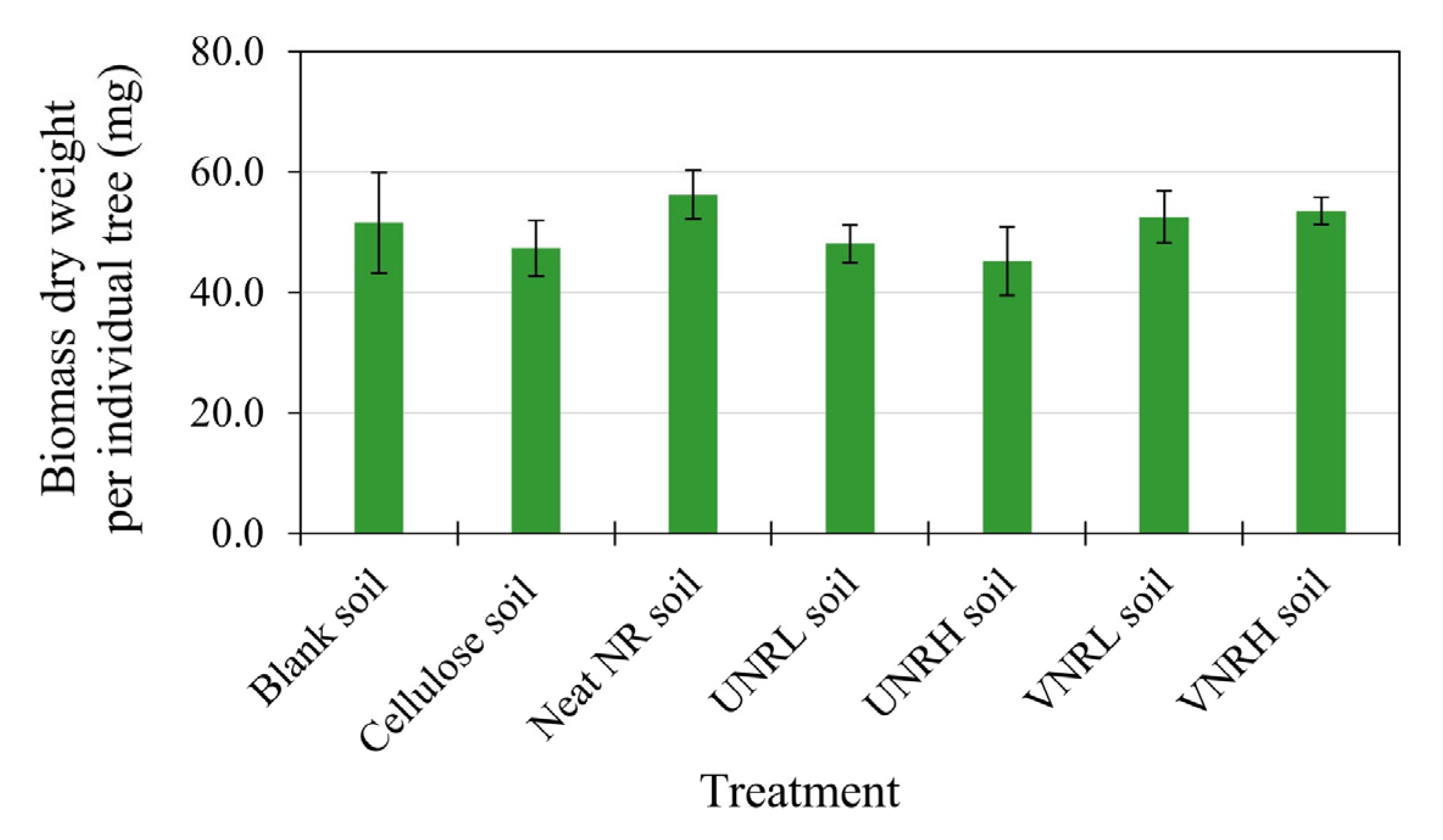
3.3. Plant Toxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rose, K.; Steinbüchel, A. Biodegradation of natural rubber and related compounds: Recent insights into a hardly understood catabolic capability of microorganisms. Appl. Environ. Microbiol. 2005, 71, 2803–2812. [Google Scholar] [CrossRef]
- Vo, M.L.; Plank, J. Evaluation of natural rubber latex as film forming additive in cementitious mortar. Constr. Build. Mater. 2018, 169, 93–99. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Coran, A.Y. Vulcanization. In The Science and Technology of Rubber, 4th ed.; Erman, B., Mark, J.E., Roland, C.M., Eds.; Elsevier: Waltham, MA, USA, 2013; pp. 343–351. [Google Scholar]
- United Nations Environment Programme. Global Waste Management Outlook 2024: Beyond an Age of Waste—Turning Rubbish into a Resource. Nairobi. 2024. Available online: https://wedocs.unep.org/20.500.11822/44939 (accessed on 15 August 2024).
- Arimoro, F.O. Impact of rubber effluent discharges on the water quality and macroinvertebrate community assemblages in a forest stream in the Niger Delta. Chemosphere 2009, 77, 440–449. [Google Scholar] [CrossRef]
- Lao, W.C.; Alves de Toledo, R.; Lu, Q.; Shim, H. Degradation of scrap tyre by Bacillus sp.—Kinetic aspects of major environmental parameters and identification of potential growth substrates. Int. Biodeterior. Biodegrad. 2019, 137, 95–101. [Google Scholar] [CrossRef]
- Leong, S.Y.; Lee, S.Y.; Koh, T.Y.; Ang, D.T. 4R of rubber waste management: Current and outlook. J. Mater. Cycles Waste Manag. 2023, 25, 37–51. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, M.; Meng, X.; Liu, A.; Duo, L.A. Waste rubber—Black pollution reframed as a global issue: Ecological challenges and sustainability initiatives. Environ. Pollut. 2024, 356, 124291. [Google Scholar] [CrossRef] [PubMed]
- Formela, K. Sustainable development of waste tires recycling technologies—Recent advances, challenges and future trends. Adv. Ind. Eng. Polym. Res. 2021, 4, 209–222. [Google Scholar] [CrossRef]
- Tsuchii, A.; Suzuki, T.; Takeda, K. Microbial degradation of natural rubber vulcanizates. Appl. Environ. Microbiol. 1985, 50, 965–970. [Google Scholar] [CrossRef]
- Gallert, C. Degradation of Latex and of Natural Rubber by Streptomyces Strain La 7. Syst. Appl. Microbiol. 2000, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Chia, K.-H.; Nanthini, J.; Thottathil, G.P.; Najimudin, N.; Hakim Mas Haris, M.R.; Sudesh, K. Identification of new rubber-degrading bacterial strains from aged latex. Polym. Degrad. Stab. 2014, 109, 354–361. [Google Scholar] [CrossRef]
- Imai, S.; Ichikawa, K.; Muramatsu, Y.; Kasai, D.; Masai, E.; Fukuda, M. Isolation and characterization of Streptomyces, Actinoplanes, and Methylibium strains that are involved in degradation of natural rubber and synthetic poly(cis-1,4-isoprene). Enzym. Microb. Technol. 2011, 49, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Linh, D.V.; Huong, N.L.; Tabata, M.; Imai, S.; Iijima, S.; Kasai, D.; Anh, T.K.; Fukuda, M. Characterization and functional expression of a rubber degradation gene of a Nocardia degrader from a rubber-processing factory. J. Biosci. Bioeng. 2017, 123, 412–418. [Google Scholar] [CrossRef]
- Jendrossek, D.; Tomasi, G.; Kroppenstedt, R.M. Bacterial degradation of natural rubber: A privilege of actinomycetes? FEMS Microbiol. Lett. 1997, 150, 179–188. [Google Scholar] [CrossRef]
- Arenskötter, M.; Baumeister, D.; Berekaa, M.M.; Pötter, G.; Kroppenstedt, R.M.; Linos, A.; Steinbüchel, A. Taxonomic characterization of two rubber degrading bacteria belonging to the species Gordonia polyisoprenivorans and analysis of hyper variable regions of 16S rDNA sequences. FEMS Microbiol. Lett. 2001, 205, 277–282. [Google Scholar] [CrossRef]
- Basik, A.A.; Sanglier, J.-J.; Yeo, C.T.; Sudesh, K. Microbial Degradation of Rubber: Actinobacteria. Polymers 2021, 13, 1989. [Google Scholar] [CrossRef] [PubMed]
- Ali Shah, A.; Hasan, F.; Shah, Z.; Kanwal, N.; Zeb, S. Biodegradation of natural and synthetic rubbers: A review. Int. Biodeterior. Biodegrad. 2013, 83, 145–157. [Google Scholar] [CrossRef]
- Marchut-Mikołajczyk, O.; Drożdżyński, P.; Januszewicz, B.; Domański, J.; Wrześniewska-Tosik, K. Degradation of ozonized tire rubber by aniline—Degrading Candida methanosorbosa BP6 strain. J. Hazard. Mater. 2019, 367, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Mohamed, N.H.; Shoreit, A.A.M. Degradation of Ficus elastica rubber latex by Aspergillus terreus, Aspergillus flavus and Myceliophthora thermophila. Int. Biodeterior. Biodegrad. 2013, 78, 82–88. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Ismail, M.A.; Abdel-Mageed, W.M.; Shoreit, A.A.M. Biodegradation of Natural Rubber Latex of Calotropis procera by Two Endophytic Fungal Species. J. Bioremediat. Biodegrad. 2017, 8, 380. [Google Scholar] [CrossRef]
- Andler, R.; Hiessl, S.; Yücel, O.; Tesch, M.; Steinbüchel, A. Cleavage of poly(cis-1,4-isoprene) rubber as solid substrate by cultures of Gordonia polyisoprenivorans. New Biotechnol. 2018, 44, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Linos, A.; Reichelt, R.; Keller, U.; Steinbüchel, A. A Gram-negative bacterium, identified as Pseudomonas aeruginosa AL98, is a potent degrader of natural rubber and synthetic cis-1,4-polyisoprene. FEMS Microbiol. Lett. 2000, 182, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Shah, Z.; Mutiullah; Hameed, A. Degradation of polyisoprene rubber by newly isolated Bacillus sp. AF-666 from soil. Appl. Biochem. Microbiol. 2012, 48, 45–50. [Google Scholar] [CrossRef]
- Muralidharan, M.; Krishnaswamy, V.G. Artificial rubber mineralization by co-cultured bacterial strains. Int. J. Biol. Res. 2016, 4, 105–111. [Google Scholar] [CrossRef]
- Nayanashree, G.; Thippeswamy, B. Natural rubber degradation by laccase and manganese peroxidase enzymes of Penicillium chrysogenum. Int. J. Environ. Sci. Technol. 2015, 12, 2665–2672. [Google Scholar] [CrossRef]
- Afiq, M.M.; Azura, A.R. Effect of sago starch loadings on soil decomposition of Natural Rubber Latex (NRL) composite films mechanical properties. Int. Biodeterior. Biodegrad. 2013, 85, 139–149. [Google Scholar] [CrossRef]
- Rosli, N.A.; Karamanlioglu, M.; Kargarzadeh, H.; Ahmad, I. Comprehensive exploration of natural degradation of poly(lactic acid) blends in various degradation media: A review. Int. J. Biol. Macromol. 2021, 187, 732–741. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Boonluksiri, Y.; Sornsri, P.; Duangkhaw, S.; Udompongpaiboon, P.; Johns, J.; Klinnawee, L. Composites of thermoplastic starch/natural rubber blends for fertilizer-releasing in agriculture. Ind. Crops Prod. 2022, 187, 115522. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, T.; Xu, X.; Yin, X.; Zhang, D.; Geng, M.; Pang, C.; Luo, G.; Xiong, L.; Peng, J.; et al. Screening and degradation characteristics of plastic-degrading microorganisms in film-mulched vegetable soil. Int. Biodeterior. Biodegrad. 2024, 186, 105686. [Google Scholar] [CrossRef]
- Briassoulis, D.; Mistriotis, A. Key parameters in testing biodegradation of bio-based materials in soil. Chemosphere 2018, 207, 18–26. [Google Scholar] [CrossRef]
- ISO 17556; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. ISO: Geneva, Switzerland, 2019.
- Bettas Ardisson, G.; Tosin, M.; Barbale, M.; Degli-Innocenti, F. Biodegradation of plastics in soil and effects on nitrification activity. A laboratory approach. Front. Microbiol. 2014, 5, 710. [Google Scholar] [CrossRef] [PubMed]
- Camacho Muñoz, R.; Villada Castillo, H.S.; Hoyos Concha, J.L.; Solanilla Duque, J.F. Aerobic biodegradation of poly(lactic acid) (PLA) in thermoplastic starch (TPS) blends in soil induced by gelatin. Int. Biodeterior. Biodegrad. 2024, 193, 105831. [Google Scholar] [CrossRef]
- Catto, A.L.; Montagna, L.S.; Almeida, S.H.; Silveira, R.M.B.; Santana, R.M.C. Wood plastic composites weathering: Effects of compatibilization on biodegradation in soil and fungal decay. Int. Biodeterior. Biodegrad. 2016, 109, 11–22. [Google Scholar] [CrossRef]
- Kanwal, N.; Shah, A.A.; Qayyum, S.; Hasan, F. Optimization of pH and temperature for degradation of tyre rubber by Bacillus sp. strain S10 isolated from sewage sludge. Int. Biodeterior. Biodegrad. 2015, 103, 154–160. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ionescu, D.; Grossart, H.-P. Tapping into fungal potential: Biodegradation of plastic and rubber by potent Fungi. Sci. Total Environ. 2024, 934, 173188. [Google Scholar] [CrossRef] [PubMed]
- ISO 6502-3; Rubber—Measurement of Vulcanization Characteristics Using Curemeters Part 3: Rotorless Curemeter. ISO: Geneva, Switzerland, 2023.
- ISO 48-4; Rubber, Vulcanized or Thermoplastic—Determination of Hardness Part 4: Indentation Hardness by Durometer method (Shore Hardness). ISO: Geneva, Switzerland, 2018.
- ISO 37; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- Pattanawanidchai, S.; Loykulnant, S.; Sae-oui, P.; Maneevas, N.; Sirisinha, C. Development of eco-friendly coupling agent for precipitated silica filled natural rubber compounds. Polym. Test. 2014, 34, 58–63. [Google Scholar] [CrossRef]
- OECD 208; Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test, OECD Guidelines for the Testing of Chemicals. OECD: Paris, France, 2006.
- Norhazariah, S.; Azura, A.R.; Sivakumar, R.; Azahari, B. Effect of Different Preparation Methods on Crosslink density and Mechanical Properties of Carrageenan filled Natural Rubber (NR) Latex Films. Procedia Chem. 2016, 19, 986–992. [Google Scholar] [CrossRef][Green Version]
- Hiranobe, C.T.; Ribeiro, G.D.; Torres, G.B.; dos Reis, E.A.P.; Cabrera, F.C.; Job, A.E.; Paim, L.L.; dos Santos, R.J. Cross-Linked Density Determination of Natural Rubber Compounds by Different Analytical Techniques. Mater. Res. 2021, 24 (Suppl. S1), e20210041. [Google Scholar] [CrossRef]
- Shao, H.; Guo, Q.; He, A. Strategy for the NR/BR blends with improved thermo-oxidative resistance. Polym. Degrad. Stab. 2021, 191, 109665. [Google Scholar] [CrossRef]
- Ren, T.; Wan, C.; Song, P.; Rodrigue, D.; Zhang, Y.; Wang, S. Thermo-oxidative degradation behavior of natural rubber vulcanized by different curing systems. Chem. Eng. Sci. 2024, 295, 120147. [Google Scholar] [CrossRef]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Adhikari, B. Aerobic biodegradation of starch–polyurethane flexible films under soil burial condition: Changes in physical structure and chemical composition. Int. Biodeterior. Biodegrad. 2019, 145, 104793. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Microbial desulfurization of ground tire rubber by Thiobacillus ferrooxidans. Polym. Degrad. Stab. 2011, 96, 1662–1668. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Microbial Desulfurization of Ground Tire Rubber by Sphingomonas sp.: A Novel Technology for Crumb Rubber Composites. J. Polym. Environ. 2012, 20, 372–380. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Improvement of the properties of natural rubber/ground tire rubber composites through biological desulfurization of GTR. J. Polym. Res. 2012, 19, 9864. [Google Scholar] [CrossRef]
- Yao, C.; Zhao, S.; Wang, Y.; Wang, B.; Wei, M.; Hu, M. Microbial desulfurization of waste latex rubber with Alicyclobacillus sp. Polym. Degrad. Stab. 2013, 98, 1724–1730. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, S.; Li, C.; Wang, B.; Yao, C.; Wang, Y. The influence of different Tween surfactants on biodesulfurization of ground tire rubber by Sphingomonas sp. Polym. Degrad. Stab. 2014, 107, 91–97. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, S.; Wang, B. Microbial desulfurization for ground tire rubber by mixed consortium-Sphingomonas sp. and Gordonia sp. Polym. Degrad. Stab. 2016, 128, 165–171. [Google Scholar] [CrossRef]
- Tatangelo, V.; Mangili, I.; Caracino, P.; Anzano, M.; Najmi, Z.; Bestetti, G.; Collina, E.; Franzetti, A.; Lasagni, M. Biological devulcanization of ground natural rubber by Gordonia desulfuricans DSM 44462T strain. Appl. Microbiol. Biotechnol. 2016, 100, 8931–8942. [Google Scholar] [CrossRef]
- Oldfield, C.; Pogrebinsky, O.; Simmonds, J.; Olson, E.S.; Kulpa, C.F. Elucidation of the metabolic pathway for dibenzothiophene desulphurization by Rhodococcus sp. strain IGTS8 (ATCC 53968). Microbiology 1997, 143, 2961–2973. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, Y.; Wu, H.; Zhang, T.; Li, S.; Zhu, N.; Zhang, Q.; Li, W. Biodegradation of vulcanized natural rubber by enriched bacterial consortia. Chem. Eng. J. 2024, 481, 148685. [Google Scholar] [CrossRef]
- De Wever, H.; Verachtert, H. Biodegradation and toxicity of benzothiazoles. Water Res. 1997, 31, 2673–2684. [Google Scholar] [CrossRef]
- Krainara, S.; Suraraksa, B.; Prommeenate, P.; Thayanukul, P.; Luepromchai, E. Enrichment and characterization of bacterial consortia for degrading 2-mercaptobenzothiazole in rubber industrial wastewater. J. Hazard. Mater. 2020, 400, 123291. [Google Scholar] [CrossRef] [PubMed]
- San-Martín, M.I.; Escapa, A.; Alonso, R.M.; Canle, M.; Morán, A. Degradation of 2-mercaptobenzothizaole in microbial electrolysis cells: Intermediates, toxicity, and microbial communities. Sci. Total Environ. 2020, 733, 139155. [Google Scholar] [CrossRef]
- Umamaheswari, B.; Rajaram, R. Microaerobic degradation of 2-Mercaptobenzothiazole present in industrial wastewater. J. Hazard. Mater. 2017, 321, 773–781. [Google Scholar] [CrossRef]
- El-Bassi, L.; Iwasaki, H.; Oku, H.; Shinzato, N.; Matsui, T. Biotransformation of benzothiazole derivatives by the Pseudomonas putida strain HKT554. Chemosphere 2010, 81, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Mollea, C. Biodegradation of Natural Rubber: Microcosm Study. Water Air Soil Pollut. 2021, 232, 227. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Azhar, N.N.H.; Abdullah, R.; Lee, S.Y.; Ang, D.T.-C. Degradation of oxo-biodegradable rubber and its impact on ecosystem services. Eur. Polym. J. 2023, 190, 112026. [Google Scholar] [CrossRef]
| Ingredients | Content (Parts per Hundred Rubber; phr) | |
|---|---|---|
| NRL | NRH | |
| Natural rubber (STR 5 L) | 100 | 100 |
| Zinc oxide (ZnO) | 3 | 3 |
| Stearic acid | 1 | 1 |
| Mercaptobenzothiazole (MBT) | 0.375 | 1.875 |
| Sulfur | 0.5 | 2.5 |
| Properties | Test Materials | |||
|---|---|---|---|---|
| UNRL | UNRH | VNRL | VNRH | |
| ML (dN·m) | 1.31 | 1.46 | - | - |
| MH (dN·m) | 2.23 | 4.86 | - | - |
| MH–ML (dN·m) | 0.92 | 3.40 | - | - |
| ts2 (min) | 6.82 | 1.53 | - | - |
| tc90 (min) | 14.53 | 3.02 | - | - |
| Hardness (shore A) | - | - | 21.0 ± 0.2 | 35.0 ± 0.4 |
| Stress at 100% strain (MPa) | - | - | 0.29 ± 0.01 | 0.66 ± 0.01 |
| Tensile strength (MPa) | - | - | 1.7 ± 0.0 | 17.6 ± 0.4 |
| Elongation at break (%) | - | - | 854 ± 21 | 726 ± 33 |
| Swelling degree (%) | - | - | 1631 ± 2 | 430 ± 2 |
| Properties | Test Materials | |||||
|---|---|---|---|---|---|---|
| Cellulose | Neat NR | UNRL | UNRH | VNRL | VNRH | |
| Moisture content (% wt.) | 4.6 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 |
| Total dry solid content (TS, % wt.) | 95.4 ± 0.0 | 99.6 ± 0.0 | 99.6 ± 0.0 | 99.8 ± 0.0 | 99.7 ± 0.0 | 99.8 ± 0.0 |
| Volatile solid content (VS, % wt. on TS) | 100.0 ± 0.0 | 99.6 ± 0.0 | 96.7 ± 0.0 | 96.7 ± 0.0 | 96.6 ± 0.0 | 96.8 ± 0.0 |
| Total organic carbon content (TOC, % wt. on TS) | 42.0 | 84.2 | 82.5 | 80.3 | 80.6 | 80.1 |
| Characteristics | Test Soil |
|---|---|
| pH | 7.2 ± 0.0 |
| Electrical conductivity (dS/m) | 0.59 ± 0.05 |
| Initial moisture content (% wt.) | 7.2 ± 0.1 |
| Total dry solid content (TS, % wt.) | 92.8 ± 0.1 |
| Volatile solid content (VS, % wt. on TS) | 3.7 ± 0.0 |
| Water holding capacity (WHC, % wt.) | 57.6 ± 7.4 |
| Total organic carbon content (TOC, % wt. on TS) | 1.3 |
| Total nitrogen content (% wt. on TS) | 0.11 |
| Characteristics | Blank Soil | Test Soils | |||||
|---|---|---|---|---|---|---|---|
| Cellulose | Neat NR | UNRL | UNRH | VNRL | VNRH | ||
| pH | 5.7 ± 0.1 | 6.0 ± 0.1 | 5.9 ± 0.1 | 6.0 ± 0.1 | 5.8 ± 0.0 | 6.0 ± 0.1 | 6.1 ± 0.1 |
| Electrical conductivity (dS/m) | 0.67 ± 0.04 | 0.71 ± 0.03 | 0.68 ± 0.03 | 0.71 ± 0.01 | 0.66 ± 0.03 | 0.66 ± 0.01 | 0.73 ± 0.01 |
| Moisture content (% wt.) | 11.2 ± 0.2 | 11.2 ± 0.4 | 13.2 ± 0.0 | 11.5 ± 0.5 | 11.7 ± 0.2 | 12.3 ± 0.2 | 10.0 ± 0.2 |
| Total dry solid content (TS, % wt.) | 88.8 ± 0.2 | 88.8 ± 0.4 | 86.8 ± 0.0 | 88.5 ± 0.5 | 88.3 ± 0.2 | 87.7 ± 0.2 | 90.0 ± 0.2 |
| Total organic carbon content (% wt. on TS) | 1.2 | 1.3 | 1.2 | 1.3 | 1.3 | 1.1 | 1.1 |
| Total nitrogen content (% wt. on TS) | 0.35 | 0.27 | 0.42 | 0.31 | 0.21 | 0.22 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattanawanidchai, S.; Saeoui, P.; Leejarkpai, T.; Pokphat, P.; Jiangchareon, B.; Thuanboon, S.; Boonyuen, N.; Suriyachadkun, C.; Boonmee, C. An Assessment of Biodegradability and Phytotoxicity of Natural Rubber in a Simulated Soil Condition via CO2 Evolution Measurement. Polymers 2024, 16, 2429. https://doi.org/10.3390/polym16172429
Pattanawanidchai S, Saeoui P, Leejarkpai T, Pokphat P, Jiangchareon B, Thuanboon S, Boonyuen N, Suriyachadkun C, Boonmee C. An Assessment of Biodegradability and Phytotoxicity of Natural Rubber in a Simulated Soil Condition via CO2 Evolution Measurement. Polymers. 2024; 16(17):2429. https://doi.org/10.3390/polym16172429
Chicago/Turabian StylePattanawanidchai, Sirichai, Pongdhorn Saeoui, Thanawadee Leejarkpai, Peeraphong Pokphat, Banphot Jiangchareon, Swieng Thuanboon, Nattawut Boonyuen, Chanwit Suriyachadkun, and Chomnutcha Boonmee. 2024. "An Assessment of Biodegradability and Phytotoxicity of Natural Rubber in a Simulated Soil Condition via CO2 Evolution Measurement" Polymers 16, no. 17: 2429. https://doi.org/10.3390/polym16172429
APA StylePattanawanidchai, S., Saeoui, P., Leejarkpai, T., Pokphat, P., Jiangchareon, B., Thuanboon, S., Boonyuen, N., Suriyachadkun, C., & Boonmee, C. (2024). An Assessment of Biodegradability and Phytotoxicity of Natural Rubber in a Simulated Soil Condition via CO2 Evolution Measurement. Polymers, 16(17), 2429. https://doi.org/10.3390/polym16172429








