Recent Advances in Fire-Retardant Silicone Rubber Composites
Abstract
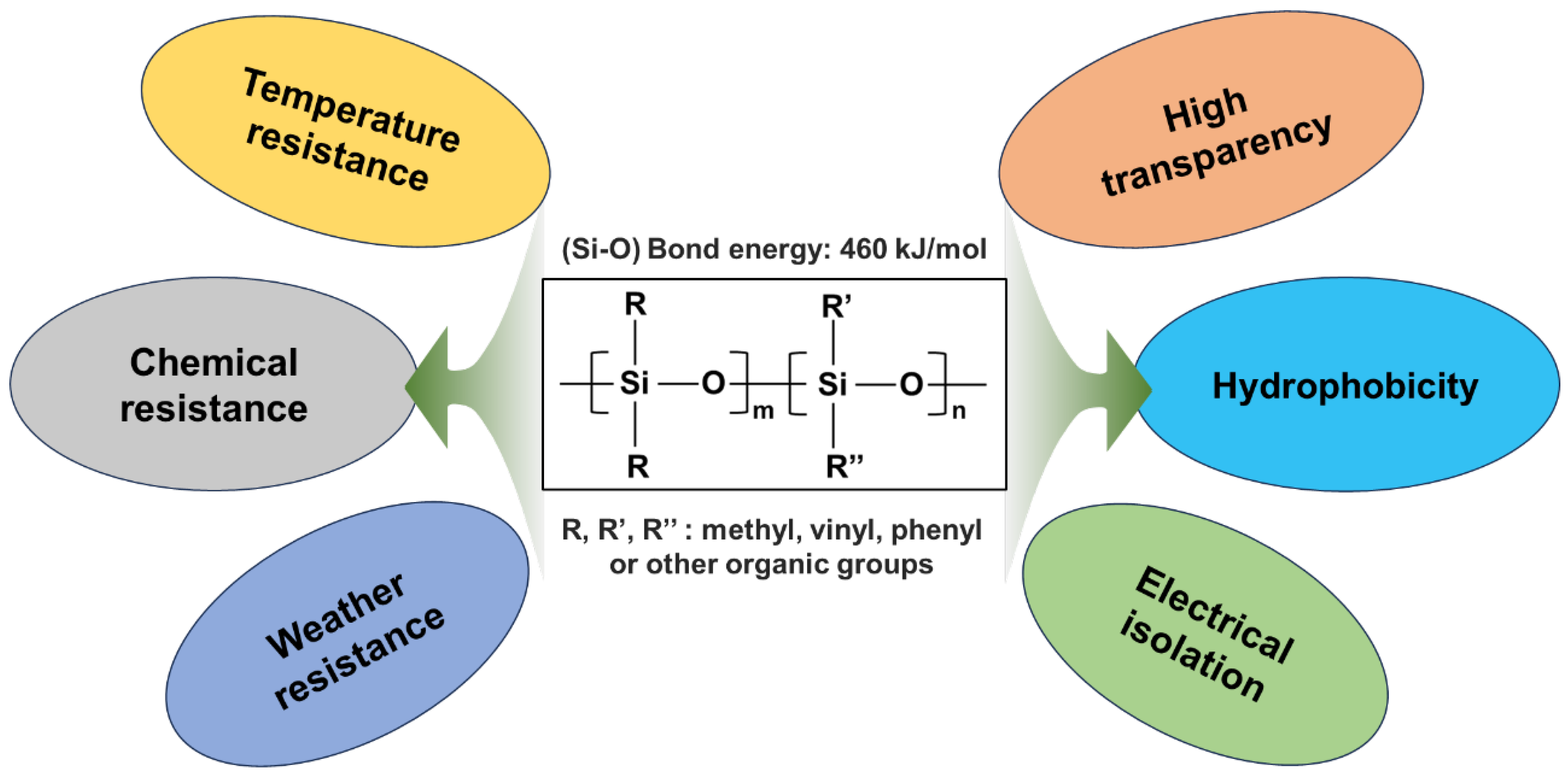
:1. Introduction
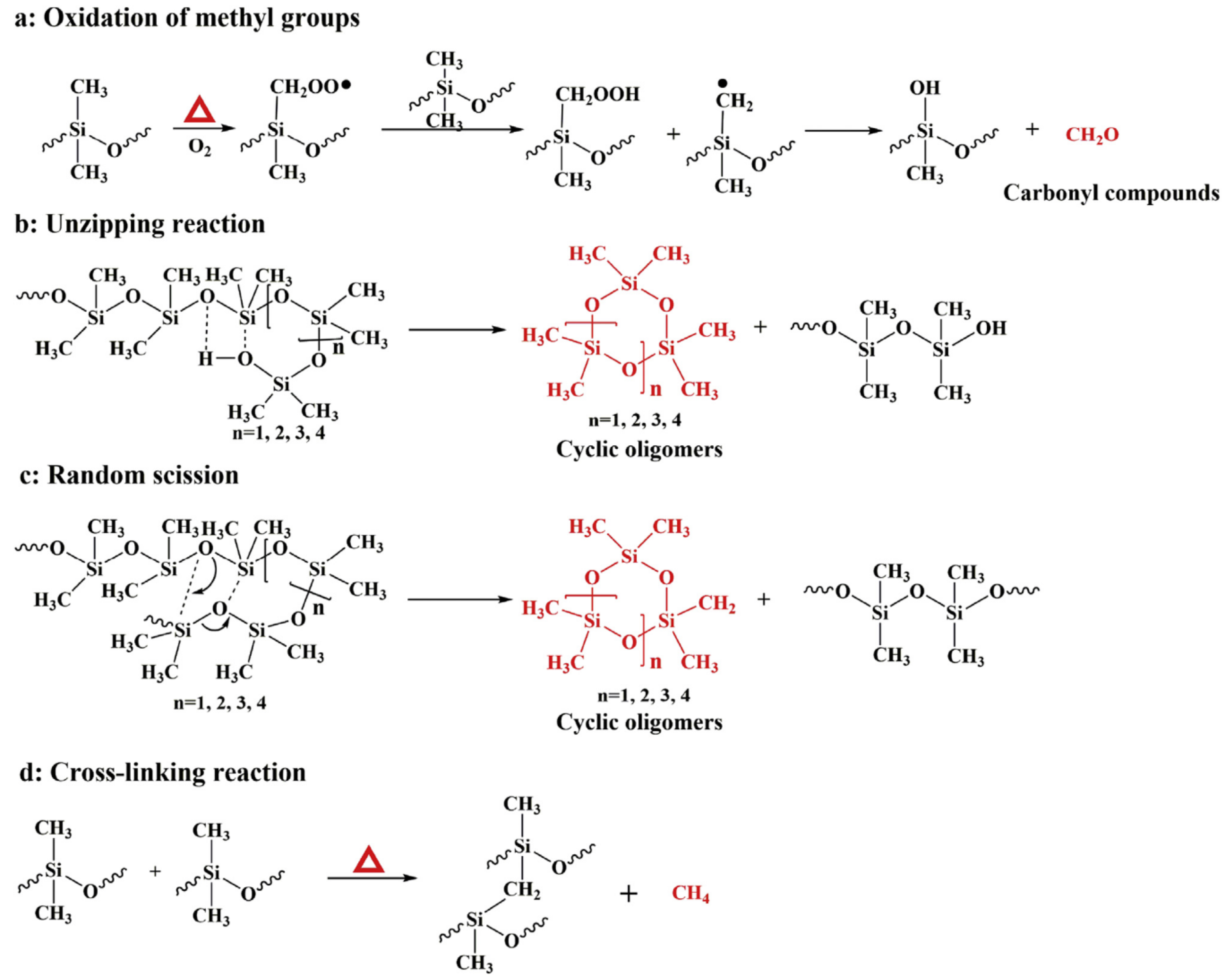
2. Thermal Decomposition Behavior of SR
3. Laboratory Fire Testing Methods
3.1. UL 94 Vertical Burning Test
3.2. Limiting Oxygen Index Test
3.3. Cone Calorimeter Test
4. Flame Retardance of SR
4.1. Intrinsic Flame Retardation
4.2. Additive-Type Flame Retardation
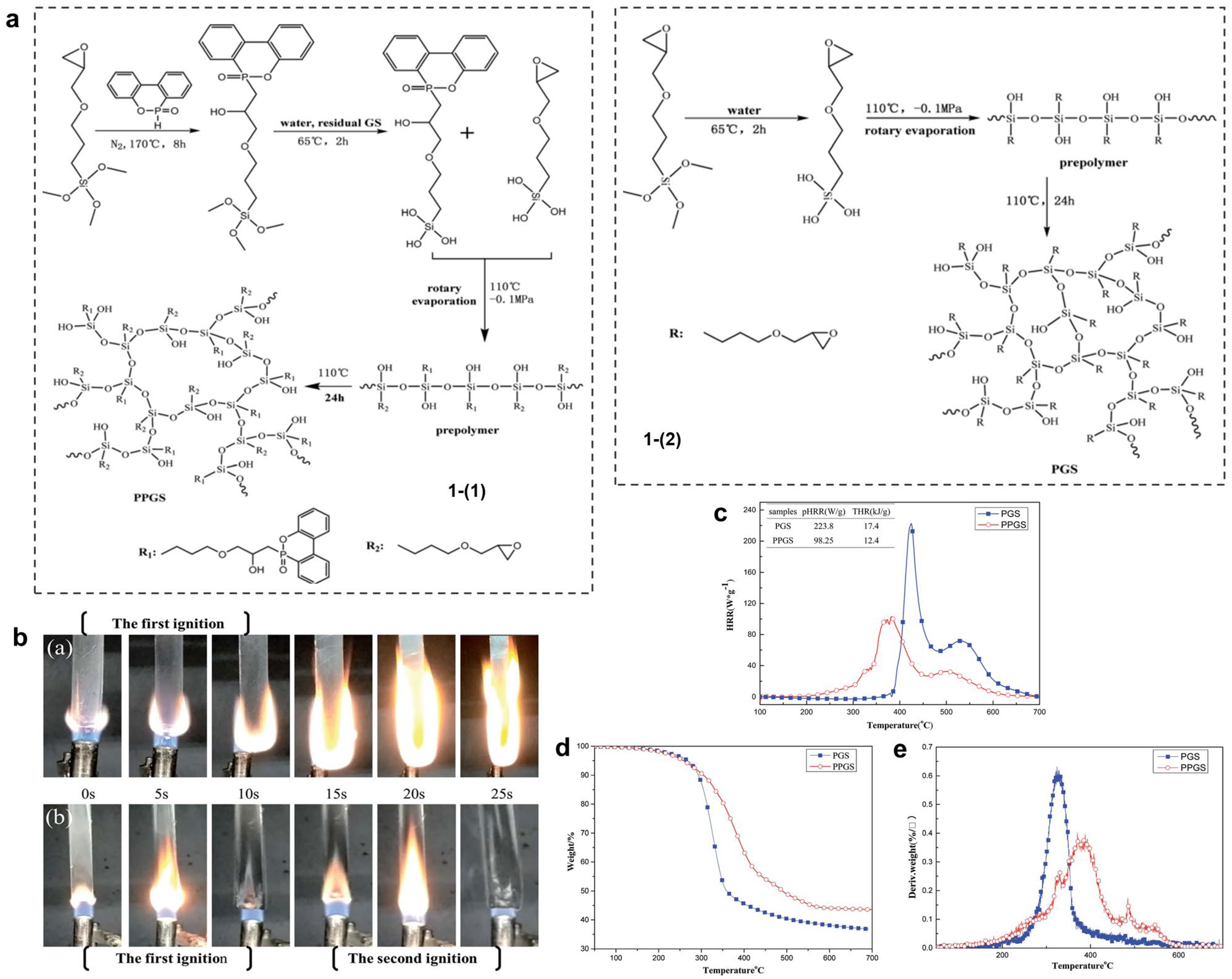
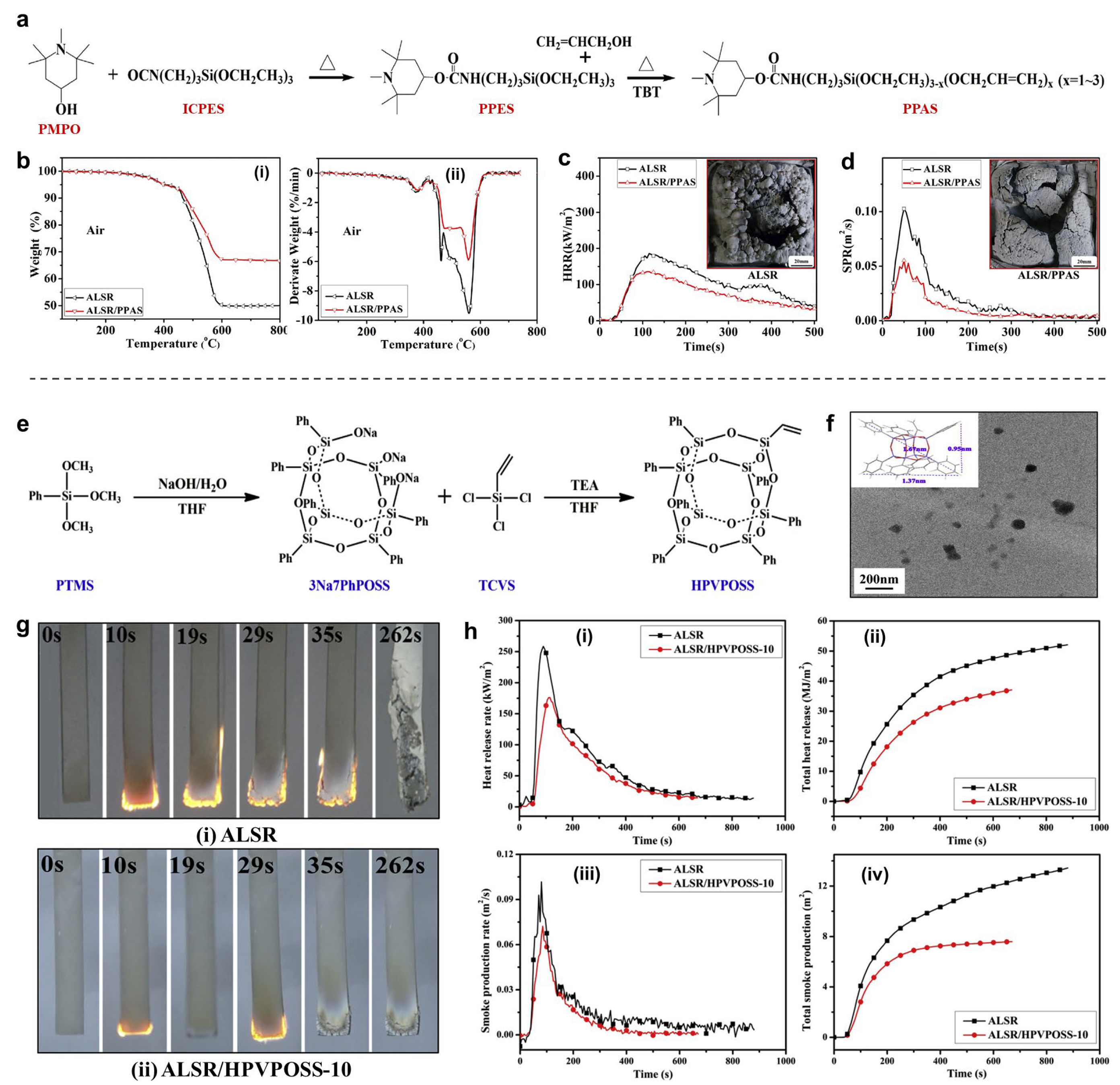
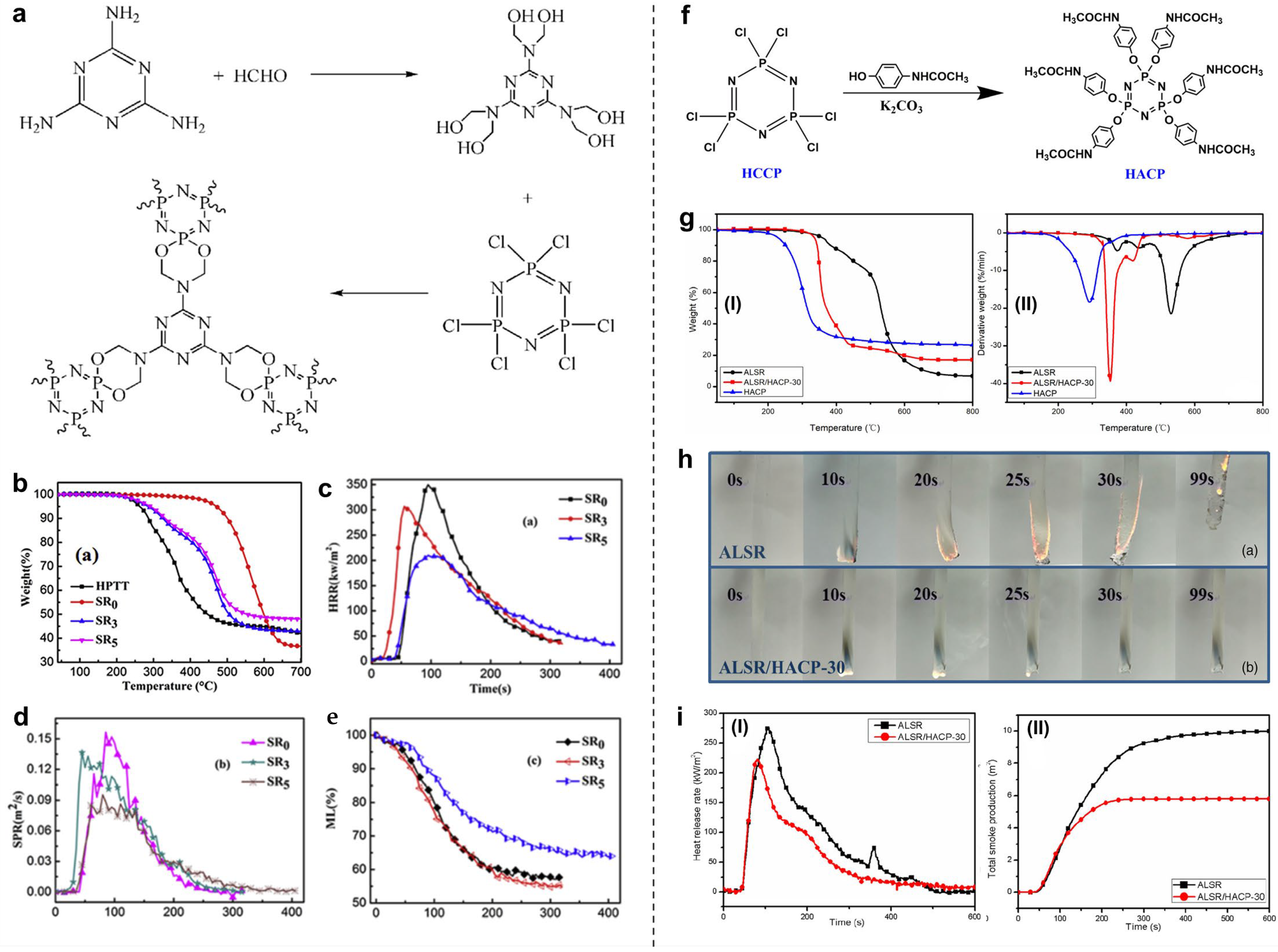
4.2.1. Phosphorus-/Nitrogen-/Silicon-Containing Flame Retardants
4.2.2. Inorganic Flame Retardants
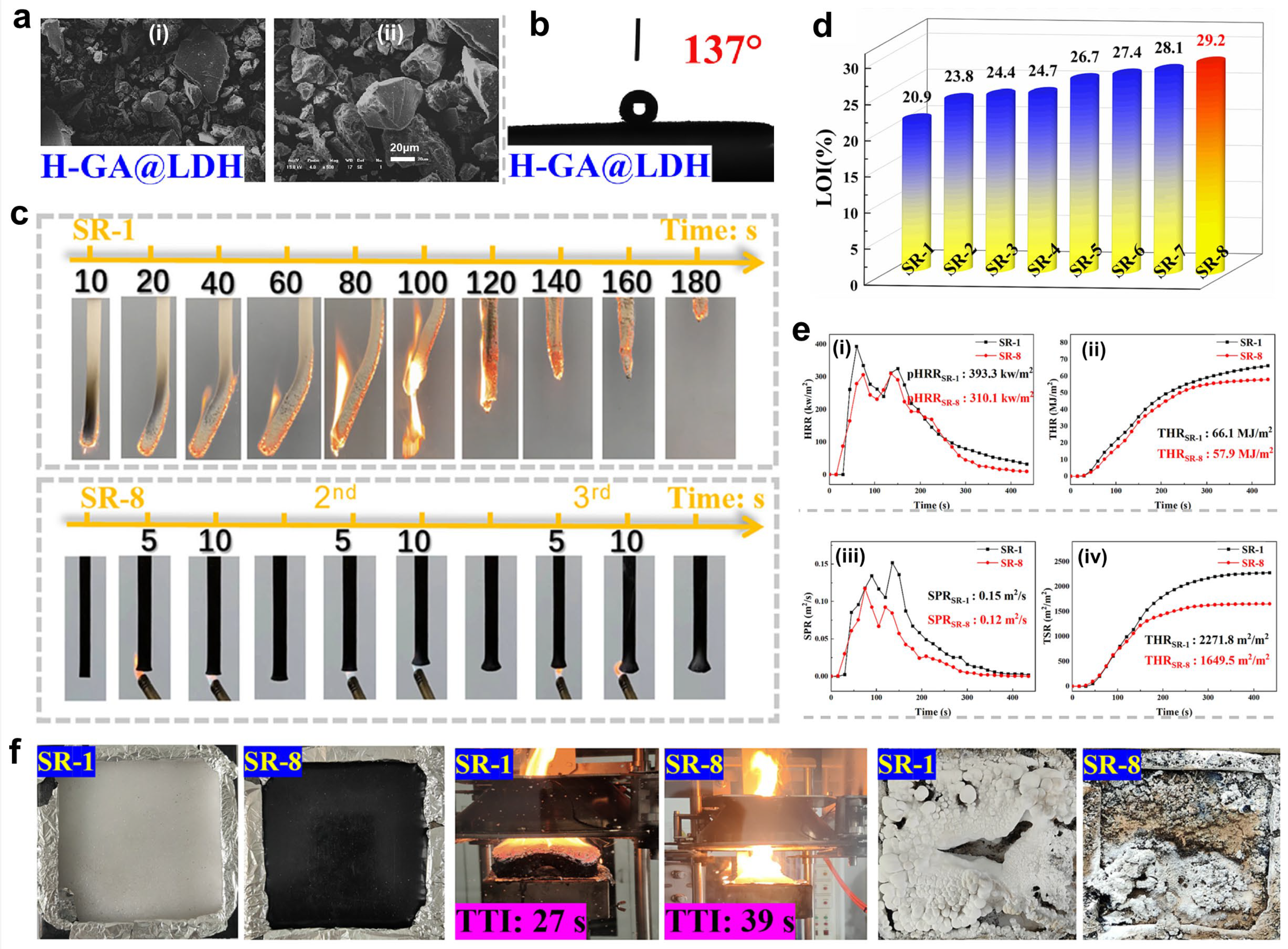
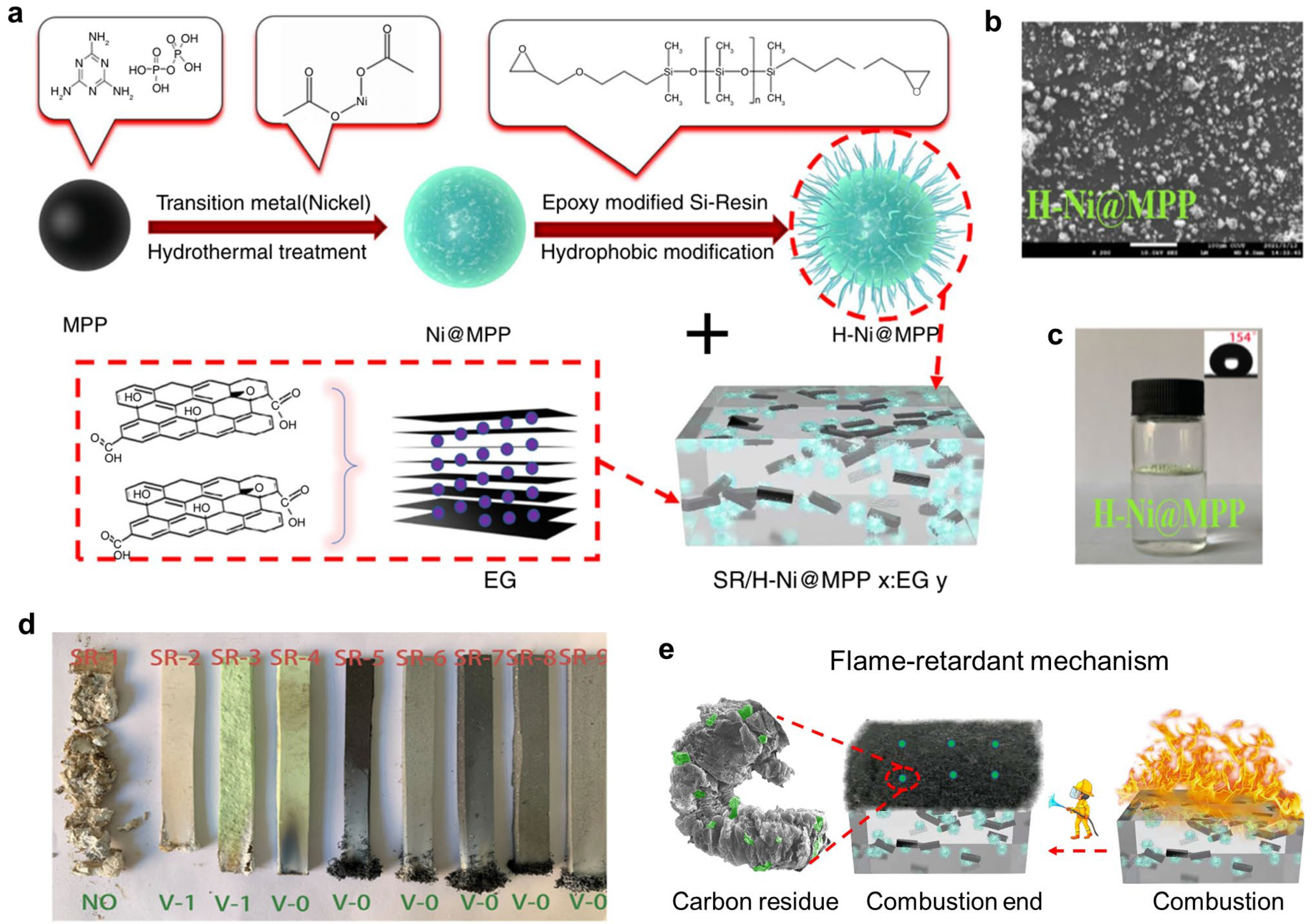
4.2.3. Intumescent Flame Retardants
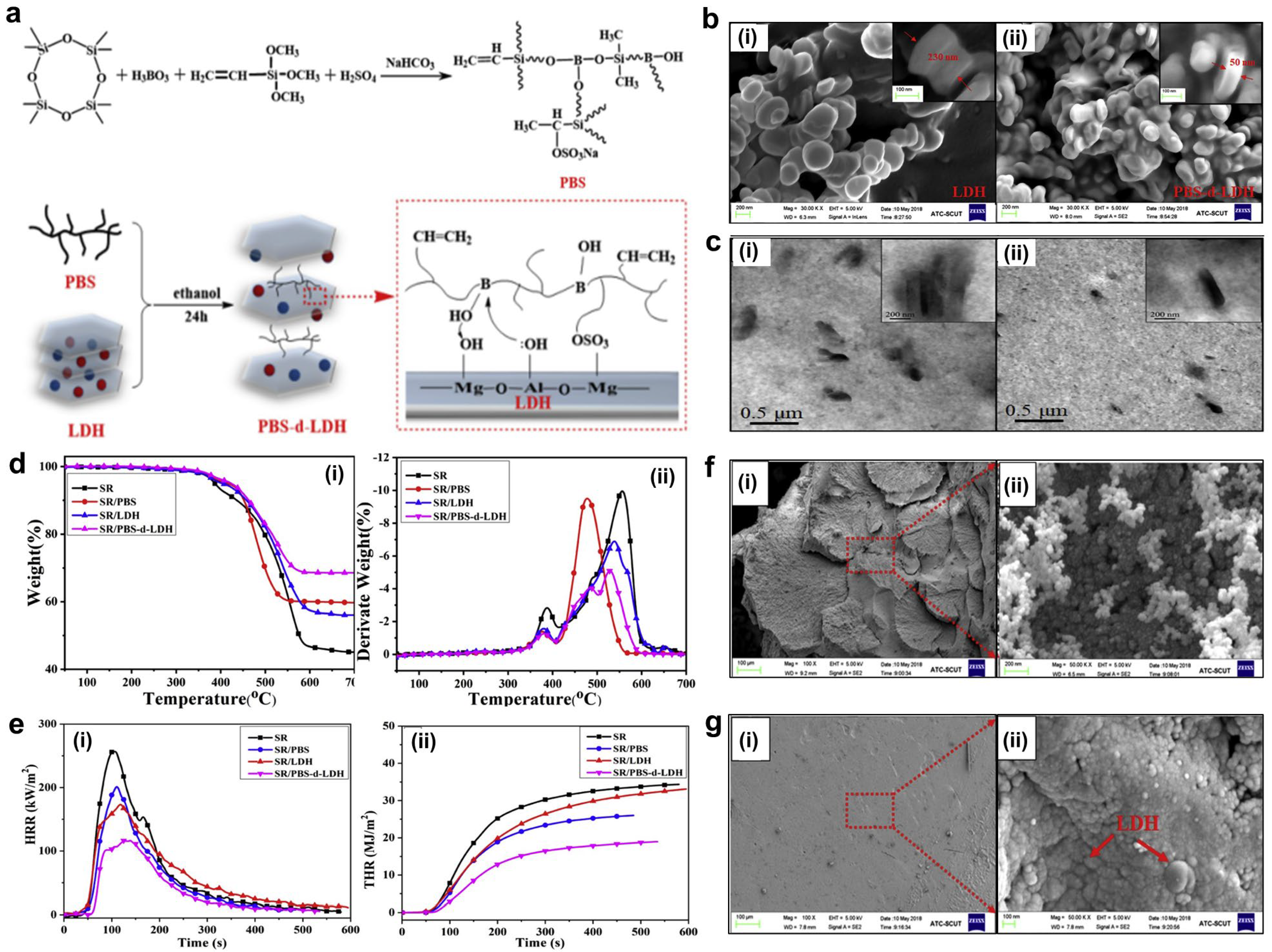
4.2.4. Nano-Flame Retardants
4.2.5. Other Flame Retardants
5. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.-T.; Liu, W.-J.; Shen, F.-X.; Zhang, G.-D.; Gong, L.-X.; Zhao, L.; Song, P.; Gao, J.-F.; Tang, L.-C. Processing, thermal conductivity and flame retardant properties of silicone rubber filled with different geometries of thermally conductive fillers: A comparative study. Compos. Part B Eng. 2022, 238, 109907. [Google Scholar] [CrossRef]
- Nazir, M.T.; Khalid, A.; Kabir, I.; Wang, C.; Baena, J.-C.; Akram, S.; Bhutta, M.S.; Yasin, G.; Phung, B.T.; Yeoh, G.H. Flame Retardancy and Excellent Electrical Insulation Performance of RTV Silicone Rubber. Polymers 2021, 13, 2854. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Hong, T.; Zhao, Y.; Wang, S.; Jing, X. High ablation-resistant silicone rubber composites via nanoscale phenolic resin dispersion. Chem. Eng. J. 2023, 472, 145132. [Google Scholar] [CrossRef]
- Chen, W.J.; Zeng, X.; Lai, X.; Li, H.; Fang, W.Z.; Hou, F. Suppression Effect and Mechanism of Platinum and Nitrogen-Containing Silane on the Tracking and Erosion of Silicone Rubber for High-Voltage Insulation. ACS Appl. Mater. Interfaces 2016, 8, 21039–21045. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.-F.; Wang, P.-H.; Zhang, J.-W.; Guo, K.-Y.; Li, Y.; Xia, Q.-Q.; Zhang, G.-D.; Zhao, L.; Chen, H.; Wang, L.; et al. One-step and green synthesis of lightweight, mechanically flexible and flame-retardant polydimethylsiloxane foam nanocomposites via surface-assembling ultralow content of graphene derivative. Chem. Eng. J. 2020, 393, 124724. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Wu, Z.-H.; Chen, Z.-Y.; Peng, L.-D.; Guan, Z.-Q.; Li, Y.; Cao, C.-F.; Zhang, G.-D.; Gao, J.-F.; Song, P.; et al. Large-scale and facile fabrication of phenyl-containing silicone foam materials with lightweight, wide-temperature flexibility and tunable pore structure for exceptional thermal insulation. Chem. Eng. J. 2024, 492, 152183. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G.; Hu, T.; Li, Q.; Cheng, J.; Liu, D. Fabrication of robust superhydrophobic surface on silicone rubber. Compos. Sci. Technol. 2024, 247, 110401. [Google Scholar] [CrossRef]
- Rius-Bartra, J.M.; Ferrer-Serrano, N.; Agulló, N.; Borrós, S. High-consistency silicone rubber with reduced Young’s modulus. An industrial option to dielectric silicone rubber. J. Appl. Polym. Sci. 2023, 140, e54405. [Google Scholar] [CrossRef]
- Wang, S.; Hou, M.; Ma, K.; Li, Z.; Geng, H.; Zhang, W.; Li, N. Research on the Influence of Extremely Cold Environment on the Performance of Silicone Rubber and Fluorinated Silicone Rubber. Polymers 2022, 14, 1898. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Yue, S.; Jiang, X.; Hu, J. Performance Characteristics of Silicone Rubber for Use in Acidic Environments. Polymers 2023, 15, 3598. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; He, Q.; Zhang, F.; Dai, F. Preparation and analysis of conductive and superhydrophobic silicone rubber. Sens. Actuators A Phys. 2023, 350, 114123. [Google Scholar] [CrossRef]
- Qu, Y.X.; Xia, Q.Q.; Li, L.T.; Cao, C.F.; Zhang, G.D.; Castignolles, P.; Bae, J.; Song, P.; Gao, J.F.; Tang, L.C. Rational Design of Oil-Resistant and Electrically Conductive Fluorosilicone Rubber Foam Nanocomposites for Sensitive Detectability in Complex Solvent Environments. ACS Nano 2024, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chen, Z.Y.; Mao, M.; Wu, Y.Y.; Yang, F.; Gong, L.X.; Zhao, L.; Cao, C.F.; Song, P.; Gao, J.F.; et al. Self-Adhesive Polydimethylsiloxane Foam Materials Decorated with MXene/Cellulose Nanofiber Interconnected Network for Versatile Functionalities. Adv. Funct. Mater. 2023, 33, 2304927. [Google Scholar] [CrossRef]
- Guo, B.-F.; Wang, P.-H.; Cao, C.-F.; Qu, Z.-H.; Lv, L.-Y.; Zhang, G.-D.; Gong, L.-X.; Song, P.; Gao, J.-F.; Mai, Y.-W.; et al. Restricted assembly of ultralow loading of graphene oxide for lightweight, mechanically flexible and flame retardant polydimethylsiloxane foam composites. Compos. Part B Eng. 2022, 247, 110290. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Qi, J.; Wen, Q.; Zhu, W. Research progress on flame-retarded silicone rubber. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 032007. [Google Scholar] [CrossRef]
- Tian, J.F.; Yan, L.W.; Zhang, H.; Wang, Y.; Cai, Y.B.; Huang, Y.S.; Lu, Z.H.; Xia, S.; Chen, Y.; Heng, Z.G.; et al. Enhanced flexibility and ablative performance of silicone rubber by constructing an interpenetrating zirconium-containing polysiloxane double network. Polymer 2023, 270, 125749. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Xu, C.; Liu, Y.; Wang, Q. Synthesis and properties of an intrinsic flame retardant silicone rubber containing phosphaphenanthrene structure. RSC Adv. 2017, 7, 39786–39795. [Google Scholar] [CrossRef]
- Shui-Yu Lu, I.H. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Chao, X.; Feng, Y. Performance of Room Temperature Vulcanized (RTV) Silicone Rubber-Based Composites: DBDPO/RTV and DBDPE/Sb2O3/RTV. Polym.-Plast. Technol. Eng. 2012, 51, 1245–1250. [Google Scholar] [CrossRef]
- Song, J.; Zhang, X.; Wang, J.; Sun, J.; Shi, A. Ceramifiable Flame-Retarded Silicone Rubber Composites Based on Novel Phosphorus/Nitrogen/Silicon-Containing Flame Retardants. Silicon 2023, 15, 5001–5011. [Google Scholar] [CrossRef]
- Shen, S.; Xin, Y.; Niu, Z.; Ma, X.; Zhang, J.; Hou, X.; Ma, X. Phosphazene derivative cross-linked liquid silicone rubber and its mechanical and thermal properties. Polym. Degrad. Stab. 2022, 203, 110086. [Google Scholar] [CrossRef]
- Xu, P.; Yang, S.; Yang, R.; Wang, R.; Chen, G.; Li, Q.; Zhou, Z. RTV silicone rubbers cured by polyhedral oligomeric silsesquioxane and ladder-like polysilsesquioxane: Thermostability, long-term thermal properties, and pyrolysis mechanism. Polymer 2024, 298, 126879. [Google Scholar] [CrossRef]
- Qiu, J.; Lai, X.; Fang, W.; Li, H.; Zeng, X. An efficient strategy for simultaneously improving tracking resistance and flame retardancy of addition-cure liquid silicone rubber. Polym. Degrad. Stab. 2017, 144, 176–186. [Google Scholar] [CrossRef]
- Wang, W.; Yang, F.; Lu, Y.; Luo, Z.; Li, F.; Wu, Y.; Zhang, J.; Xiao, Z.; Li, W.; Qin, C. Application of Magnesium Hydroxide/Diphenoxy Phosphate in Silicone Rubber Flame Retardant Cable Material. Coatings 2023, 13, 934. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Song, X.; Lin, Q.; Wang, Z.; Xue, Y.; Sun, X.; Shi, Y.; Li, Z.; Fu, Y.; et al. Coupling silsesquioxane nanocages into Fe-Mg-Al layered metal hydroxide for enhanced flame retardancy and surface charring of silicone elastomer. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132156. [Google Scholar] [CrossRef]
- Fang, S.; Hu, Y.; Song, L.; Zhan, J.; He, Q. Mechanical properties, fire performance and thermal stability of magnesium hydroxide sulfate hydrate whiskers flame retardant silicone rubber. J. Mater. Sci. 2007, 43, 1057–1062. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Zhuo, J.; Ma, C.; Jiao, C. Influence of Fe2O3 on smoke suppression and thermal degradation properties in intumescent flame-retardant silicone rubber. J. Therm. Anal. Calorim. 2015, 123, 439–448. [Google Scholar] [CrossRef]
- Jiao, C.; Zhuo, J.; Chen, X. Synergistic effects of zinc oxide in intumescent flame retardant silicone rubber composites. Plast. Rubber Compos. 2013, 42, 374–378. [Google Scholar] [CrossRef]
- Qi, J.; Wen, Q.; Zhu, J. Synergistic effect of intumescent flame retardant system consisting of hexophenoxy cyclotriphosphazene and ammonium polyphosphate on methyl ethyl silicone rubber. Mater. Lett. 2019, 249, 62–65. [Google Scholar] [CrossRef]
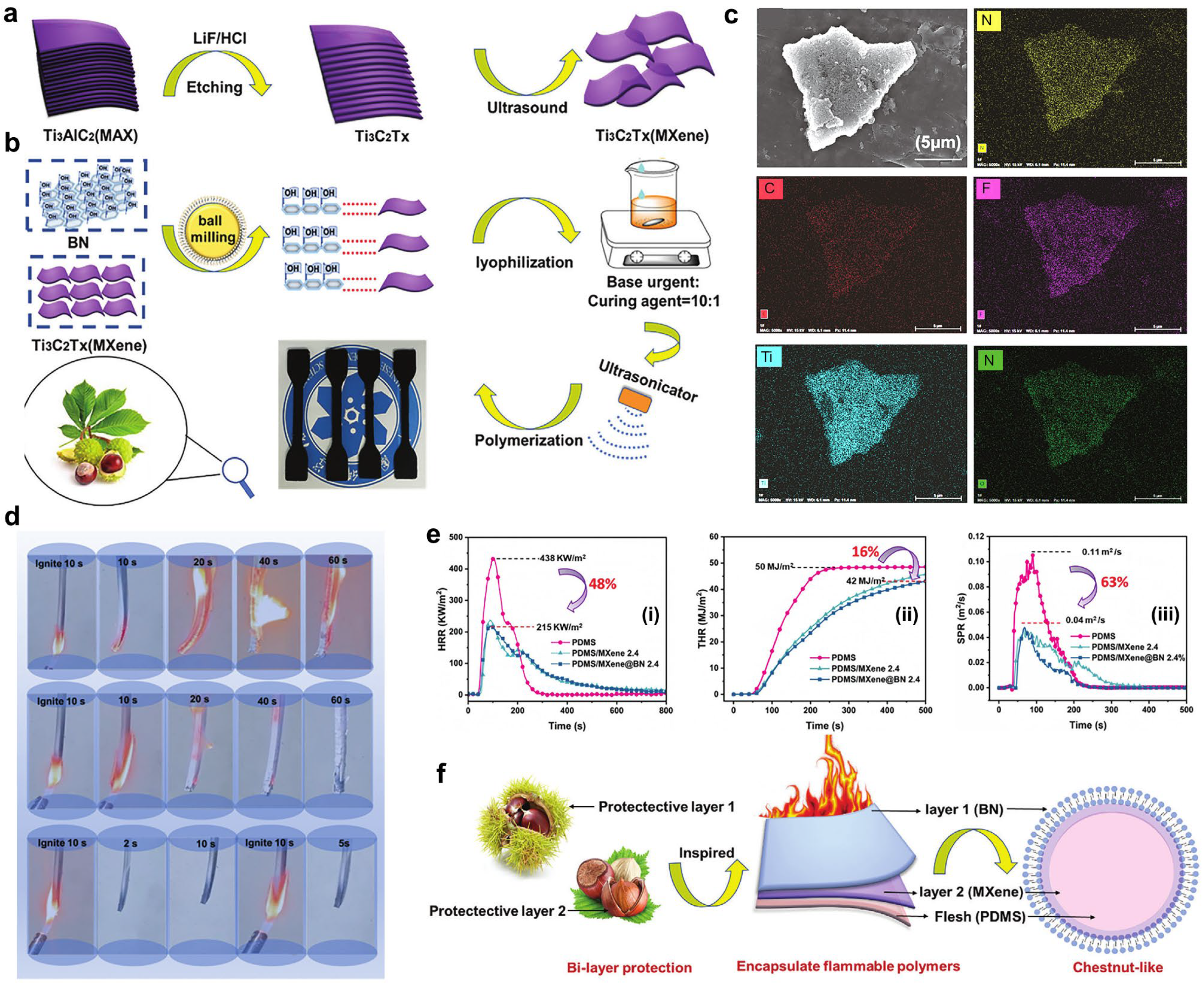
- Bian, F.; Huang, R.; Li, X.; Hu, J.; Lin, S. Facile Construction of Chestnut-Like Structural Fireproof PDMS/Mxene@BN for Advanced Thermal Management and Electromagnetic Shielding Applications. Adv. Sci. 2024, 11, 2307482. [Google Scholar] [CrossRef]
- Bian, Z.; Xu, W.; Yao, L.; Jia, R.; Ding, D.; Zhang, Z. Effect of phytate doped cobalt and copper ion modified carbon nanotubes on flame retardancy and ceramic properties of silicone rubber. J. Appl. Polym. Sci. 2024, 141, e55309. [Google Scholar] [CrossRef]
- Wang, F.; Shen, X.; Wang, Q.; Zhu, H. Preparation and characterization of halloysite nanotube-silica/silicone rubber composites: Effect of HNTs on mechanical and thermal properties. Polym. Compos. 2024, 45, 8759–8769. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Feng, Y.; Yin, J. Recent progress in polymer/two-dimensional nanosheets composites with novel performances. Prog. Polym. Sci. 2022, 126, 101505. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.-T.; Wang, D.-Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Yeoh, G.H.; De Cachinho Cordeiro, I.M.; Wang, W.; Wang, C.; Yuen, A.C.Y.; Chen, T.B.Y.; Vargas, J.B.; Mao, G.; Garbe, U.; Chua, H.T. Carbon-based Flame Retardants for Polymers: A Bottom-up Review. Adv. Mater. 2024, e2403835. [Google Scholar] [CrossRef] [PubMed]
- Soo Kim, E.; Lee, T.H.; Shin, S.H.; Yoon, J.S. Effect of incorporation of carbon fiber and silicon carbide powder into silicone rubber on the ablation and mechanical properties of the silicone rubber-based ablation material. J. Appl. Polym. Sci. 2010, 120, 831–838. [Google Scholar] [CrossRef]
- Lou, F.; Yan, W.; Guo, W.; Wei, T.; Li, Q. Preparation and properties of ceramifiable flame-retarded silicone rubber composites. J. Therm. Anal. Calorim. 2017, 130, 813–821. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, T.; Xu, Y.; Zhang, J.; Shen, Y.; Wang, T. Investigation of the thermal degradation kinetics of ceramifiable silicone rubber-based composite. J. Therm. Anal. Calorim. 2023, 148, 6487–6499. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhang, D.; Li, J.; Huang, G. Preparation and thermal degradation behavior of room temperature vulcanized silicone rubber-g-polyhedral oligomeric silsesquioxanes. Polymer 2013, 54, 6140–6149. [Google Scholar] [CrossRef]
- Liu, J.; Xin, Z. Synergistic flame-retardant effect of sepiolite and magnesium hydroxide/melamine/starch on the properties of silicone rubber composites. Polym. Bull. 2023, 81, 2957–2973. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lageard, M. Polydimethylsiloxane thermal degradation. Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lageard, M. Thermal polydimethylsiloxane degradation. Part 2. The degradation mechanisms. Polymer 2002, 43, 2011–2015. [Google Scholar] [CrossRef]
- Wu, T.; Qiu, J.; Lai, X.; Li, H.; Zeng, X. Effect and mechanism of hepta-phenyl vinyl polyhedral oligomeric silsesquioxane on the flame retardancy of silicone rubber. Polym. Degrad. Stab. 2019, 159, 163–173. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Lai, X.; Li, H.; Fang, W. Effect of the platinum catalyst content on the tracking and erosion resistance of addition-cure liquid silicone rubber. Polym. Test. 2017, 63, 92–100. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- T Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Fenimore, C.P.; Martin, F.J. Flammability of polymers. Combust. Flame 1966, 10, 135–139. [Google Scholar] [CrossRef]
- Cao, C.-F.; Yu, B.; Guo, B.-F.; Hu, W.-J.; Sun, F.-N.; Zhang, Z.-H.; Li, S.-N.; Wu, W.; Tang, L.-C.; Song, P.; et al. Bio-inspired, sustainable and mechanically robust graphene oxide-based hybrid networks for efficient fire protection and warning. Chem. Eng. J. 2022, 439, 134516. [Google Scholar] [CrossRef]
- Cao, C.F.; Yu, B.; Huang, J.; Feng, X.L.; Lv, L.Y.; Sun, F.N.; Tang, L.C.; Feng, J.; Song, P.; Wang, H. Biomimetic, Mechanically Strong Supramolecular Nanosystem Enabling Solvent Resistance, Reliable Fire Protection and Ultralong Fire Warning. ACS Nano 2022, 16, 20865–20876. [Google Scholar] [CrossRef]
- Genovese, A.; Shanks, R.A. Fire performance of poly(dimethyl siloxane) composites evaluated by cone calorimetry. Compos. Part A Appl. Sci. Manuf. 2008, 39, 398–405. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2021, 34, e2107905. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Han, S.T.; Tian, J.F.; Wang, Y.; Yan, L.W.; Zhang, H.; Yuan, Q.; Xia, S.; Chen, Y.; Heng, Z.G.; Zou, H.W.; et al. Significantly improved ablation properties under high heat flux environment based on carbon fiber reinforced Zr-doped silicone rubber. J. Alloys Compd. 2023, 965, 171460. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Li, H.; Liu, T.; Zeng, X. Significantly improve fire safety of silicone rubber by efficiently catalyzing ceramization on fluorophlogopite. Compos. Commun. 2021, 25, 100683. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, S.; Zou, H.; Liang, M. Thermal stability and ablation properties study of aluminum silicate ceramic fiber and acicular wollastonite filled silicone rubber composite. J. Appl. Polym. Sci. 2013, 131, 39700. [Google Scholar] [CrossRef]
- Chai, H.; Tang, X.; Ni, M.; Chen, F.; Zhang, Y.; Chen, D.; Qiu, Y. Preparation and properties of flexible flame-retardant neutron shielding material based on methyl vinyl silicone rubber. J. Nucl. Mater. 2015, 464, 210–215. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, X.Y.; Xue, K.L.; Liu, M.; Huang, Y.D.; Liu, L. Novel hybrid zirconium-silicone resin as high-temperature adhesive and an insight into the thermal resistance mechanism. Chem. Eng. J. 2022, 446, 137350. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, X.; He, X.; Guan, S.; Wu, Q.; Liu, M.; Liu, L.; Huang, Y. Effects of cross-linking degree and steric hindrance on the thermal degradation behavior of novel hybrid zirconium-silicone resin. Polymer 2023, 284, 126306. [Google Scholar] [CrossRef]
- Zhang, Y.-l.; Zang, C.-g.; Shi, L.-p.; Jiao, Q.-j.; Pan, H.-w.; She-li, Y.-f. Preparation of boron-containg hybridized silicon rubber by in-situ polymerization of vinylphenyl-functionalized polyborosiloxane and liquid silicone rubber. Polymer 2021, 219, 123541. [Google Scholar] [CrossRef]
- Su, X.; Chai, W.; Xia, Y.; Gao, M.; Li, Y.; Tang, Z.; Zhang, Z.; Han, Z.; Zheng, Z. Bio-inspired construction of hydrophobic, bio-based and halogen-free flame-retardant strategy for silicone rubber. J. Therm. Anal. Calorim. 2023, 148, 9857–9874. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.L.; Mu, X.W.; Zhou, M.T.; Cai, W.; Song, L.; Xing, W.Y.; Hu, Y. Polyphosphazenes-based flame retardants: A review. Compos. Part B-Eng. 2020, 202, 108397. [Google Scholar] [CrossRef]
- Cai, W.; Lin, B.; Qi, L.; Cui, T.; Li, Z.; Wang, J.; Li, S.; Cao, C.; Ziaur Rahman, M.; Hu, X.; et al. Bio-based and fireproof radiative cooling aerogel film: Achieving higher sustainability and safety. Chem. Eng. J. 2024, 488, 150784. [Google Scholar] [CrossRef]
- Cao, C.-F.; Yu, B.; Chen, Z.-Y.; Qu, Y.-X.; Li, Y.-T.; Shi, Y.-Q.; Ma, Z.-W.; Sun, F.-N.; Pan, Q.-H.; Tang, L.-C.; et al. Fire Intumescent, High-Temperature Resistant, Mechanically Flexible Graphene Oxide Network for Exceptional Fire Shielding and Ultra-Fast Fire Warning. Nano-Micro Lett. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tian, Z.; Zhang, D.; Jing, Q.; Li, J. The synergetic effect of antimony (Sb2O3) and melamine cyanurate (MCA) on the flame-retardant behavior of silicon rubber. Polym. Bull. 2020, 78, 185–202. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, J.; Li, J.; Shi, J. Thermal aging properties of flame retardant silicone rubber based on melamine cyanurate. J. Appl. Polym. Sci. 2020, 138, 49919. [Google Scholar] [CrossRef]
- Wu, Q.; Gong, L.-X.; Li, Y.; Cao, C.-F.; Tang, L.-C.; Wu, L.; Zhao, L.; Zhang, G.-D.; Li, S.-N.; Gao, J.; et al. Efficient Flame Detection and Early Warning Sensors on Combustible Materials Using Hierarchical Graphene Oxide/Silicone Coatings. ACS Nano 2018, 12, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Rybiński, P.; Syrek, B.; Bradło, D.; Żukowski, W. Effect of POSS Particles and Synergism Action of POSS and Poly-(Melamine Phosphate) on the Thermal Properties and Flame Retardance of Silicone Rubber Composites. Materials 2018, 11, 1298. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wei, H.; Zheng, J.; Huang, G. Effect of incompletely condensed tri-silanol-phenyl-POSS on the thermal stability of silicone rubber. Polym. Bull. 2018, 76, 2835–2850. [Google Scholar] [CrossRef]
- He, W.T.; Song, P.A.; Yu, B.; Fang, Z.P.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Yaoke, D.; Yuan, Z.; Wang, J. Synthesis of a phosphorus-containing trisilanol POSS and its application in RTV composites. e-Polymers 2018, 18, 237–245. [Google Scholar] [CrossRef]
- Zhu, C.; Deng, C.; Cao, J.-Y.; Wang, Y.-Z. An efficient flame retardant for silicone rubber: Preparation and application. Polym. Degrad. Stab. 2015, 121, 42–50. [Google Scholar] [CrossRef]
- Konstantinova, A.; Yudaev, P.; Orlov, A.; Loban, O.; Lukashov, N.; Chistyakov, E. Aryloxyphosphazene-Modified and Graphite-Filled Epoxy Compositions with Reduced Flammability and Electrically Conductive Properties. J. Compos. Sci. 2023, 7, 417. [Google Scholar] [CrossRef]
- Bornosuz, N.V.; Gorbunova, I.Y.; Kireev, V.V.; Bilichenko, Y.V.; Chursova, L.V.; Svistunov, Y.S.; Onuchin, D.V.; Shutov, V.V.; Petrakova, V.V.; Kolenchenko, A.A.; et al. Synthesis and Application of Arylaminophosphazene as a Flame Retardant and Catalyst for the Polymerization of Benzoxazines. Polymers 2021, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.; Konstantinova, A.; Korotkov, R.; Yudaev, P.; Mezhuev, Y.; Terekhov, I.; Gurevich, L.; Chistyakov, E. Epoxy Compositions with Reduced Flammability Based on DER-354 Resin and a Curing Agent Containing Aminophosphazenes Synthesized in Bulk Isophoronediamine. Polymers 2022, 14, 3592. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, W.; Hu, D.; Zhang, D.; Wu, L.; Yang, B.; Zhang, S. Facile preparation and flame retardancy mechanism of cyclophosphazene derivatives for highly flame-retardant silicone rubber composites. J. Appl. Polym. Sci. 2020, 138, 50297. [Google Scholar] [CrossRef]
- Chai, W.; Su, X.; Xia, Y.; Gao, M.; Li, Y.; Liao, C.; Zheng, Z. Construction of a novel halogen-free and phosphorus-free and synergistic flame retardant system with low addition in simultaneously improving the flame retardancy, water resistance and mechanical properties for silicone rubber. Appl. Clay Sci. 2023, 238, 106926. [Google Scholar] [CrossRef]
- An, W.; Ma, J.; Xu, Q. Bio-template synthesis of MgAl layered double hydroxide with enhanced flame retardant property for leather finishes. Appl. Surf. Sci. 2021, 551, 149409. [Google Scholar] [CrossRef]
- Ma, J.; Yang, N.; Li, W.; Zhou, Y.; Sun, X. Functional nanocomposite based on waterborne polyurethane and layered double hydroxide as a flame retardant for leather. J. Clean. Prod. 2022, 380, 134966. [Google Scholar] [CrossRef]
- Lyu, B.; Luo, K.; Gao, D.; Wang, Y.; Ma, J. Modified layered double hydroxide/zanthoxylum bungeanum seed oil composites to improve the flame retardant of leather. Polym. Degrad. Stab. 2021, 183, 109430. [Google Scholar] [CrossRef]
- Qiu, J.; Lai, X.; Li, H.; Gao, J.; Zeng, X.; Liao, X. Facile fabrication of a novel polyborosiloxane-decorated layered double hydroxide for remarkably reducing fire hazard of silicone rubber. Compos. Part B Eng. 2019, 175, 107068. [Google Scholar] [CrossRef]
- Wang, T.; Long, M.C.; Zhao, H.B.; An, W.L.; Xu, S.M.; Deng, C.; Wang, Y.Z. Temperature-Responsive Intumescent Chemistry toward Fire Resistance and Super Thermal Insulation under Extremely Harsh Conditions. Chem Mater 2021, 33, 6018–6028. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Z.; Leng, Y.; Li, B.; Xu, M. Synthesis of a novel mono-component intumescent flame retardant and its high efficiency for flame retardant polyethylene. J. Anal. Appl. Pyrolysis 2018, 134, 632–640. [Google Scholar] [CrossRef]
- Zhu, C.; He, M.; Liu, Y.; Cui, J.; Tai, Q.; Song, L.; Hu, Y. Synthesis and application of a mono-component intumescent flame retardant for polypropylene. Polym. Degrad. Stab. 2018, 151, 144–151. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.G.; Lee, J.S.; Ma, B.C. Understanding the Flame Retardant Mechanism of Intumescent Flame Retardant on Improving the Fire Safety of Rigid Polyurethane Foam. Polymers 2022, 14, 4904. [Google Scholar] [CrossRef]
- Hong, L.; Hu, X. Mechanical and Flame Retardant Properties and Microstructure of Expandable Graphite/Silicone Rubber Composites. J. Macromol. Sci. Part B 2016, 55, 175–187. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Yao, J.; Zhang, Y. Organic Nano-Montmorillonite for Simultaneously Improving the Flame Retardancy, Thermal Stability, and Mechanical Properties of Intumescent Flame-Retardant Silicone Rubber Composites. J. Macromol. Sci. Part B 2015, 54, 1282–1296. [Google Scholar] [CrossRef]
- Song, W.; Lan, Y.; Wang, J.; Zhang, C. Synergistic effect of diatomite and intumescent flame retardant on flame retardant properties of silicone rubber composites. J. Rubber Res. 2021, 24, 489–499. [Google Scholar] [CrossRef]
- Chai, W.; Su, X.; Xia, Y.; Liao, C.; Gao, M.; Li, Y.; Zheng, Z. Fabrication of Ni-doped synergistic intumescent flame-retarding silicone rubber system with superior flame retardancy and water resistance. J. Therm. Anal. Calorim. 2022, 148, 1827–1839. [Google Scholar] [CrossRef]
- Liu, L.; Zhuo, J.; Chen, X.; Jiao, C.; Li, S.; Gu, Y. Influence of ferric hydroxide on smoke suppression properties and combustion behavior of intumescent flame retardant silicone rubber composites. J. Therm. Anal. Calorim. 2014, 119, 487–497. [Google Scholar] [CrossRef]
- Chen, X.; Zhuo, J.; Song, W.; Jiao, C.; Qian, Y.; Li, S. Flame retardant effects of organic inorganic hybrid intumescent flame retardant based on expandable graphite in silicone rubber composites. Polym. Adv. Technol. 2014, 25, 1530–1537. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Guo, B.-F.; Peng, L.-D.; Li, Y.; Cao, C.-F.; Zhang, G.-D.; Gao, J.-F.; Song, P.; Shi, Y.-Q.; Cao, K.; et al. Recent progress on MXene-based advanced nanocomposite materials for thermal radiation protection and fire safety. Adv. Nanocomposites 2024, 1, 217–239. [Google Scholar] [CrossRef]
- Shen, Y.-B.; Yu, K.-X.; Wang, Y.-J.; Qu, Y.-H.; Pan, L.-Q.; Cao, C.-F.; Cao, K.; Gao, J.-F.; Shi, Y.; Song, P.; et al. Color adjustable, mechanically robust, flame-retardant and weather-resistant TiO2/MMT/CNF hierarchical nanocomposite coatings toward intelligent fire cyclic warning and protection. Compos. Part B Eng. 2024, 271, 111159. [Google Scholar] [CrossRef]
- Yu, B.; Tawiah, B.; Wang, L.Q.; Yin Yuen, A.C.; Zhang, Z.C.; Shen, L.L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.; Guo, W.; Qiu, S.; Wang, X.; Lo, S.; Hu, Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol–gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Wang, S.; Long, C.; Wang, X.; Li, Q.; Qi, Z. Synthesis and properties of silicone rubber/organomontmorillonite hybrid nanocomposites. J. Appl. Polym. Sci. 1998, 69, 1557–1561. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y.; Lu, H.; Song, L. Morphology, thermal, and mechanical properties of flame-retardant silicone rubber/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2006, 99, 3275–3280. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, F.; Huang, Z.; Dai, J.; Shi, M. Improved Ablation Resistance of Silicone Rubber Composites by Introducing Montmorillonite and Silicon Carbide Whisker. Materials 2016, 9, 723. [Google Scholar] [CrossRef]
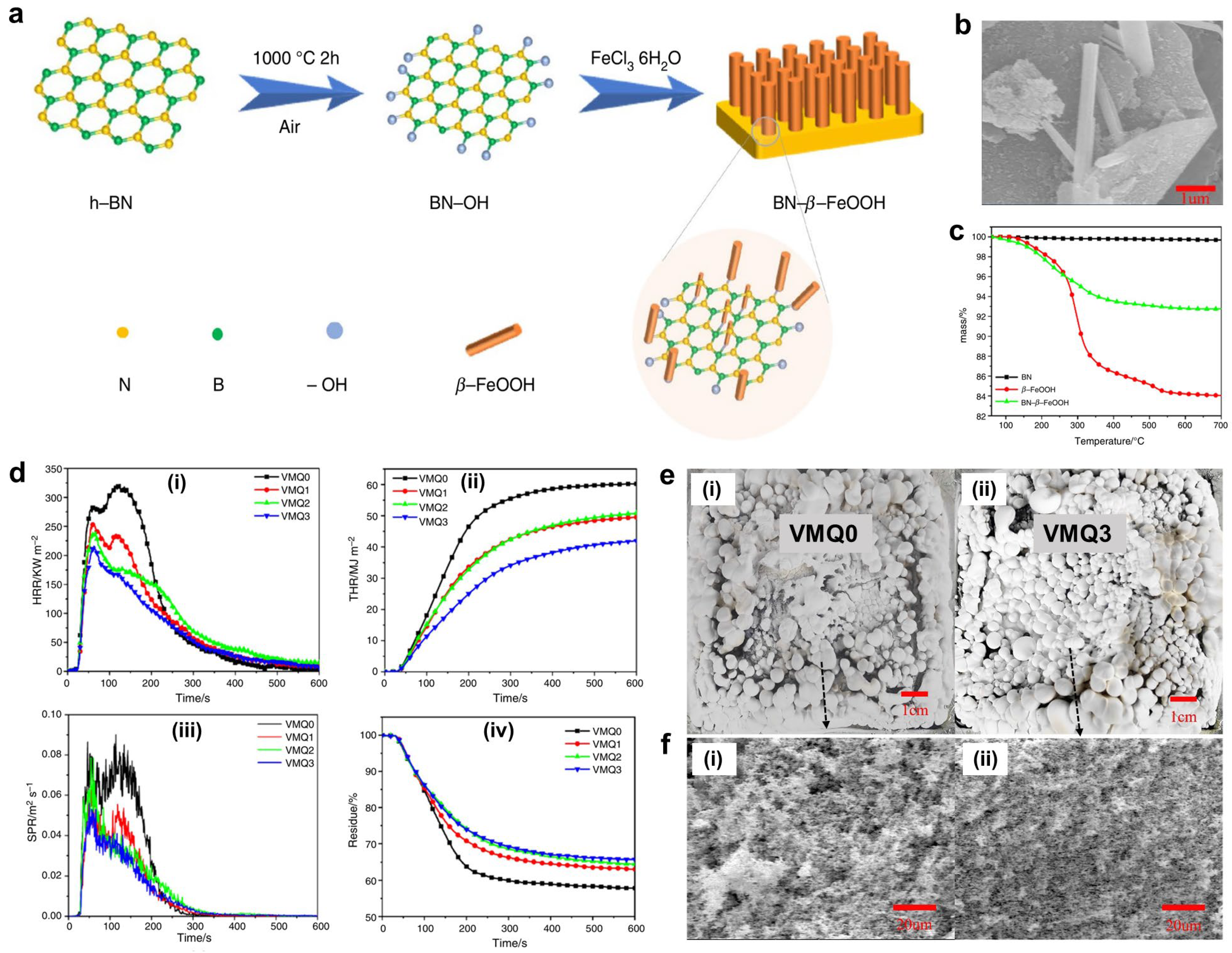
- Qiu, J.; Wu, T.; Qu, J. Fabrication of iron oxide nanoparticle decorated boron nitride nanosheet for flame-retarding silicone rubber. Mater. Lett. 2021, 283, 128712. [Google Scholar] [CrossRef]
- Wu, T.; Qiu, J.-d.; Xu, W.-h.; Du, Y.; Zhou, W.-l.; Xie, H.; Qu, J.-p. Efficient fabrication of flame-retarding silicone rubber/hydroxylated boron nitride nanocomposites based on volumetric extensional rheology. Chem. Eng. J. 2022, 435, 135154. [Google Scholar] [CrossRef]
- Ren, H.; Cunha, E.; Li, Z.; Wang, L.; Kinloch, I.A.; Yi, D.; Kretinin, A.; Sun, Q.; Fan, Z.; Young, R.J. Silane-functionalized graphene nanoplatelets for silicone rubber nanocomposites. J. Mater. Sci. 2022, 57, 2683–2696. [Google Scholar] [CrossRef]
- Li, H.; Tao, S.; Huang, Y.; Su, Z.; Zheng, J. The improved thermal oxidative stability of silicone rubber by using iron oxide and carbon nanotubes as thermal resistant additives. Compos. Sci. Technol. 2013, 76, 52–60. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, H.; Gong, Y.; Wang, F.; Ding, X.; Hu, R.; Zhang, X.; He, J.; Tian, X. Highly Thermally Conductive Polymer Composite Originated from Assembly of Boron Nitride at an Oil-Water Interface. ACS Appl. Mater. Interfaces 2019, 11, 42818–42826. [Google Scholar] [CrossRef] [PubMed]
- Davesne, A.-L.; Lazar, S.; Bellayer, S.; Qin, S.; Grunlan, J.C.; Bourbigot, S.; Jimenez, M. Hexagonal Boron Nitride Platelet-Based Nanocoating for Fire Protection. ACS Appl. Nano Mater. 2019, 2, 5450–5459. [Google Scholar] [CrossRef]
- Chen, S.; Xu, R.; Liu, J.; Zou, X.; Qiu, L.; Kang, F.; Liu, B.; Cheng, H.M. Simultaneous Production and Functionalization of Boron Nitride Nanosheets by Sugar-Assisted Mechanochemical Exfoliation. Adv. Mater. 2019, 31, e1804810. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fan, L.; Wang, J.; Zhang, Y.; Yao, L.; Qiao, M. Preparation of three-dimensional modified boron nitride and its effect on the flame-retardant performance and thermal conductivity of silicone rubber. J. Therm. Anal. Calorim. 2022, 148, 1133–1147. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, X.; Lai, X.; Li, H.; Fang, W.; Liu, T. Synergistic effect and mechanism of platinum catalyst and nitrogen-containing silane on the thermal stability of silicone rubber. Thermochim. Acta 2016, 632, 1–9. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, X.; Lai, X.; Li, H.; Wang, Y. Remarkable improvement of organic-to-inorganic conversion of silicone rubber at elevated temperature through platinum-nitrogen catalytic system. Polym. Degrad. Stab. 2020, 171, 109026. [Google Scholar] [CrossRef]
- Jiang, G.; Zhou, H.; Liao, K. Effect of benzotriazole-protected platinum catalyst on flame retardancy and ceramic-forming property of ceramifiable silicone rubber. Polym. Adv. Technol. 2020, 31, 2687–2700. [Google Scholar] [CrossRef]
- Li, Z.; Liang, W.; Shan, Y.; Wang, X.; Yang, K.; Cui, Y. Study of flame-retarded silicone rubber with ceramifiable property. Fire Mater. 2019, 44, 487–496. [Google Scholar] [CrossRef]
- Nazir, M.T.; Khalid, A.; Wang, C.; Baena, J.C.; Phung, B.T.; Akram, S.; Wong, K.L.; Yeoh, G.H. Enhanced fire retardancy with excellent electrical breakdown voltage, mechanical and hydrophobicity of silicone rubber/aluminium trihydroxide composites by milled glass fibres and graphene nanoplatelets. Surf. Interfaces 2022, 35, 102494. [Google Scholar] [CrossRef]


| Criteria | No Rating | V-2 | V-1 | V-0 |
|---|---|---|---|---|
| Maximum flame burning time for a single sample (t1/t2) | >30 s | ≤30 s | ≤30 s | ≤10 s |
| Total flame combustion time of five samples (t1 + t2) | >250 s | ≤250 s | ≤250 s | ≤50 s |
| Flame and no-flame burning time after second ignition (t2 + t3) | >60 s | ≤60 s | ≤60 s | ≤30 s |
| Whether flaming or flame burning spreads to the fixture | No | No | No | No |
| Whether the dropping ignites the cotton wool | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-H.; Liu, J.; Chen, Z.-Y.; Li, Y.; Cao, C.-F.; Zhang, G.-D.; Tang, L.-C. Recent Advances in Fire-Retardant Silicone Rubber Composites. Polymers 2024, 16, 2442. https://doi.org/10.3390/polym16172442
Tang Y-H, Liu J, Chen Z-Y, Li Y, Cao C-F, Zhang G-D, Tang L-C. Recent Advances in Fire-Retardant Silicone Rubber Composites. Polymers. 2024; 16(17):2442. https://doi.org/10.3390/polym16172442
Chicago/Turabian StyleTang, Yi-Hao, Jun Liu, Zuan-Yu Chen, Yang Li, Cheng-Fei Cao, Guo-Dong Zhang, and Long-Cheng Tang. 2024. "Recent Advances in Fire-Retardant Silicone Rubber Composites" Polymers 16, no. 17: 2442. https://doi.org/10.3390/polym16172442






