Abstract
Biopolymer-based films are a promising alternative for the food packaging industry, in which petrochemical-based polymers like low-density polyethylene (LDPE) are commanding attention because of their high pollution levels. In this research, a biopolymer-based film made of chitosan (CS), gelatin (GEL), and glycerol (GLY) was designed. A Response Surface Methodology (RSM) analysis was performed to determine the chitosan, gelatin, and glycerol content that improved the mechanical properties selected as response variables (thickness, tensile strength (TS), and elongation at break (EAB). The content of CS (1.1% w/v), GEL (1.1% w/v), and GLY (0.4% w/v) in the film-forming solution guarantees an optimized film (OPT-F) with a 0.046 ± 0.003 mm thickness, 11.48 ± 1.42 mPa TS, and 2.6 ± 0.3% EAB. The OPT-F was characterized in terms of thermal, optical, and biodegradability properties compared to LDPE films. Thermogravimetric analysis (TGA) revealed that the OPT-F was thermally stable at temperatures below 300 °C, which is relevant to thermal processes in the food industry of packaging. The reduced water solubility (WS) (24.34 ± 2.47%) and the improved biodegradability properties (7.1%) compared with LDPE suggests that the biopolymer-based film obtained has potential applications in the food industry as a novel packaging material and can serve as a basis for the design of bioactive packaging.
1. Introduction
Nowadays, most of the materials used in food packaging are petrochemical-based polymers due to their low cost and excellent barrier properties. However, petrochemical-based polymers are non-biodegradable, increasing environmental concerns regarding long-term pollution [1]. LDPE is broadly used as packaging for food products due to its excellent barrier and mechanical properties [2]. Based on this, the development of biomaterials for food packaging has attracted the attention of government regulations [3]. As a consequence, the production of biodegradable films that can be used as packaging material has potentially increased, with biopolymers like carbohydrates, lipids, proteins, or their blends being commonly used [4].
Gelatin-based films are widely used because of their excellent film-forming capability and good barrier properties to CO2 and O2. However, gelatin’s direct application in food matrices is limited due to its swelling properties and its dissolution upon contact with food. To avoid these problems, blending gelatin with lipids or another film-forming material [5] or with an opposite-charge polymer improves the physical and mechanical properties of the gelatin-based films, resulting in a film with a higher performance [3]. Chitosan is a polycationic polymer obtained from the deacetylation of chitin, the second-most prominent polysaccharide in nature [6]. Chitosan has attractive characteristics like film-forming ability, biodegradability, and antioxidant and antimicrobial activity, and it is an excellent barrier to the permeation of O2. It is broadly used in food preservation against spoilage deterioration [7].
On the other hand, the degree of deacetylation of chitosan highly influences the biodegradability properties of the hydrogels formed by crosslinking with gelatin [8]. In addition, gelatin-based films require plasticizers, which make the film stretch and soften. Glycerol is the most common plasticizer used, and its small hydrophilic molecule penetrates the gelatine protein chain, promoting the distances between the protein chains, and, consequently, direct interactions between protein chains turn weaker. That is why glycerol content directly affects mechanical properties [5]. As well, biodegradability is an essential parameter for an ideal food packaging material [9]; mechanical, thermal, and barrier properties are correlated with the packaging performance according to its protective functionality [10]. In addition, the manufacturing method of the film elaboration results in a variation in its physical and mechanical properties [11]. The casting method has broadly been used for film production due to its simplicity, in which simple steps are involved. Although it is not an industrial-scale method, it allows an approach to achieving the desired physical and mechanical properties of the films by shifting the concentrations of their main constituents [9]. Several studies are based on biodegradable gelatin–chitosan films [12,13], and their antimicrobial activity [14] has been reported. Furthermore, in recent years the focus of chitosan and gelatin films has been the addition of additives to improve their bioactive and barrier properties; without going into depth about the optimization of polymer concentrations to obtain an optimal film that can compete both in cost/efficiency and in mechanical properties with the packaging currently used by the food industry, such as low-density polyethylene (Table 1).

Table 1.
Chitosan–gelatin films in food packaging.
In addition, many types of biodegradable packaging have been investigated, focusing on the food industry, as a possible alternative to traditional packaging, which is based on low-density polyethylene. However, these proposed types of biodegradable packaging have mechanical and barrier properties that, in many cases, do not allow the replacement of LDPE for use in the food packaging industry. In this context, recent research by Hoque and Janaswamy propose packaging with a degradability rate greater than 90% in 30 days, although the high TS values and low EAB values do not allow its use as an alternative to polyethylene [23]. Also, research related to packaging based on biopolymers assumes that these are biodegradable, and associates this mainly with the origin of the components (Table 1), without this property being verified. On the other hand, and to the best of our knowledge, there is a lack of information allowing us to analyze the biodegradability of chitosan–gelatin based-films through an optimization of their components and considering how their properties are compromised, including the mechanical as well as barrier properties, considering LDPE as a reference material.
According to what was stated above, this research aims to verify the biodegradability of films made with CS/GEL/GLY, guaranteeing mechanical and barrier properties like LDPE, based on the optimization of their constituents. The film obtained under the optimal concentrations will be characterized in terms of its mechanical (TS and EAB) and barrier properties (swelling and solubility). The physicochemical properties were studied by Fourier-transform infrared spectroscopy, thermogravimetric analysis, scanning electron microscopy, and color. The biodegradability tests were carried out at the soil surface level, through a rapid methodology, as an approximation of a practical method that allows studying of the interactions of the film components over time, highlighting potential application in the food industry, as an alternative to non-biodegradable materials such as LDPE.
2. Materials and Methods
2.1. Materials
Food-grade chitosan (CS) of crustacean origin was used with a degree of deacetylation of ≥95% and a molecular weight between 150,000 and 300,000 g/mol (Quitoquímica, Concepción, Chile). The gelatin (GEL) used was from the skin of cold-water fish (Sigma Aldrich, Santiago, Chile), while the glycerol (GLY) had a 1.25 g/mL density (Sigma Aldrich, Santiago, Chile). The acetic acid used for the CS dilution had a molar mass of 60.05 g/mol (Emsure, Merck, Darmstadt, Germany). A low-density polyethylene bag (100 mm × 140 mm), approved for direct contact with food, was used as a control.
2.2. Experimental Design
A Response Surface Methodology (RSM) analysis was performed to optimize the content of CS, GEL, and GLY. A Box–Behnken design (BBD) was built with 15 runs, including three central points; the concentrations of CS (0.5, 1.0, and 1.5% w/v), GEL (0.5, 1.0, and 1.5% w/v) and GLY (0.25, 0.5 and 0.75% w/v) were selected as independent variables. The response variables were thickness, tensile strength (TS), and elongation at break (EAB). A polynomial model was fitted to the experimental data, and coefficients of fit (R2, adjusted R2, and predicted R2) were obtained. The experimental parameters were optimized following the desirability criteria (minimum thickness, maximum TS, and EAB) analyzed by Design-Expert statistical software (Version 11.0, StatEase, Inc., Minneapolis, MN, USA). The model was validated by preparing the sample under optimal conditions, which were subsequently characterized.
2.3. Film-Forming Solution (FFS)
The FFSs were formulated by casting method according to Liu et al., with some modifications [24]. Briefly, an amount of chitosan, according to the experimental design, was dissolved in acetic acid solution (1% v/v). The CS solution was stirred for 2 h at 3000 rpm until complete homogenization. After that, the corresponding amount of GEL and GLY was incorporated and further homogenized. The final FFS had a pH = 4.0 ± 0.1; this was measured using the following equipment: Adwa 1030, L Tech, Chile. Each FFS was prepared separately for each trial. Then, 15 g of FFS was cast on a square acrylic plate 10 cm wide and long, 3 mm thick, and with a 1 cm margin. Each film was recovered after solvent evaporation in an oven (Memmert UM400, Büchenbach, Germany) for 2 h at 60 ± 1 °C. The obtained films were stored in Petri dishes at room temperature (25 ± 1 °C) and 45% relative humidity (RH) until their characterization.
2.4. Attenuated Total Reflection—Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
Fourier-transform infrared (FTIR) spectroscopy was recorded from wavenumber 4000 to 400 cm−1, using a PerkinElmer Spectrum Two (Bruker Banner Lane, Coventry, Germany) with an attenuated total reflectance (ATR) accessory. The analyses were recorded at room temperature (25 ± 2 °C) with 16 scans and a resolution of 4 cm−1.
2.5. Color of Film
The color of the films was measured according to the methodology proposed by Prus-Walendziak and Kozlowska [25] using a colorimeter (BCM 200, BIOBASE, Shandong, China) at room temperature with D65 illuminant. Before the measurements, the colorimeter was calibrated with a white standard (L* = 96.19, a* = +0.06, b* = −1.97). Results were expressed as L* (sample lightness), a* (−green to +red), and b* (−blue to +yellow) parameters. The total color difference (ΔE) and the whiteness index (WI) of the films were calculated using the following equations [26]:
where ΔL*, Δa*, and Δb* are the differences between the corresponding color parameters of the samples and that of a standard white plate used as the film background. Five readings were taken for each film at different points, and the average values were determined from the top and bottom sides.
∆E = [(∆L*)2 + (∆a*)2 + (∆b*)2]0.5
WI = 100 − [(100 − L*)2 + (a*)2 + (b*)2]0.5
2.6. Microstructure of Film
The film’s cross-sectional scanning electron microscopy (SEM) was obtained using a scanning electron microscope (JEOL, JSM-7401F, Tokyo, Japan). The film samples were measured at an acceleration voltage of 10 kV with a resolution of 1.0 nm. The films were freeze-breaking, using nitrogen, before the SEM examination.
2.7. Film Thickness
The thickness of the films (mm) was measured using a digital micrometer (E5010109, VETO & Co., Santiago, Chile). Five random locations around each film sample were used for the thickness determination.
2.8. Mechanical Properties
The mechanical tests of the films for the determination of the tensile strength (TS) and the elongation at break (EAB) were carried out using an Instron 3342 universal testing machine (Instron, Norwood, MA, USA) with a 500 N load cell (precision of 0.025 N). The films were approximately cut into 5 mm × 20 mm strips and stretched with a constant displacement speed of 0.5 mm/min. The tensile strain (also referred to as elongation) is computed as ε = l/L, where l is the instantaneous length, and L is the initial length (20 mm). The elongation can also be expressed as a percentage %ε = ε × 100. An approximation of real stress, due the absence of Poisson’s ratio data, is obtained assuming incompressibility of the material, and it is calculated as σ = F/A × (1 + ε), where F is the instantaneous force and A is the initial cross-sectional area of the samples. Measurements were performed in quintuplicate, and the average of each value was reported.
2.9. Swelling
The swelling of the film was assessed following the procedure outlined by Tagrida et al. [27]. The film was cut into pieces of 2 cm × 2 cm and then dried in an oven (Memmert UM400, Büchenbach, Germany) at 105 ± 1 °C for 24 h. Next, the dried film was weighed and submerged in 50 mL of distilled water for 1 h. After that, the excess water was removed by placing the film on a filter paper for 5 min and re-weighing it. Finally, the swelling percentage was calculated using the following formula:
where W1 and W2 are the weight of the dried film and the weight of the film after eliminating the excess water, respectively.
Swelling (%) = (W2 − W1/W1) × 100
2.10. Water Solubility
The solubility of the films was carried out at 25 °C according to the methodology described by Liu et al. [24]. Before the test, the films were dried as described in Section 2.9. The dried films were weighed and added to 50 mL of distilled water with continuous stirring for 24 h. Then, the film samples were filtered through filter paper (size 150 mm) and dried in an oven at 105 °C for 24 h. After this, the samples were weighed. The water solubility of the films was calculated according to the following equation:
where Wi refers to the initial weight of the dry film and Wr is the weight of the dry film after immersion in water.
Water solubility (%) = (Wi − Wr/Wi) × 100
2.11. Thermogravimetric Analysis (TGA)
The Mettler Toledo TGA/DSC Model 2 StaRe System, fitted with a microbalance with ±0.1 μg precision, was used to examine the thermal stability, degradation, and alterations in the chemical composition of the interpenetrating network. Samples varying in weight from 4 to 8 mg were heated from 25 to 600 °C in a 70 μL alumina crucible with a scan rate of 10 °C min−1 under standard temperature and pressure conditions, controlling the nitrogen atmosphere and with a 40 mL min−1 flow rate.
2.12. Biodegradability Test
The biodegradability testing of the films was carried out according to the methodology proposed by Trejo-Caballero et al. [28]. Mentha piperita was used as an environmental model because it is a plant commonly found in Mexican gardens (where the trial was conducted) and because plastic waste is often inappropriately disposed of in its environment. The films were cut into dimensions of 1 cm2 and placed on the surface of the soil of the Mentha piperita pots for 31 days, with a 27–31 °C temperature and ca. 50–65% humidity. The physical and chemical changes of the films were determined for 31 days. For this, daily photographic monitoring of the samples was carried out, and the chemical composition of the samples was determined both at the beginning and end of the test using the FTIR methodology. LDPE was used as a control sample. Additionally, the weight loss percentage after 31 days of exposure was calculated. The tests were carried out in duplicate.
2.13. Statistical Analysis
Data were presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) tests and the Tukey method considered a significance level set at p < 0.05, using Statgraphics CENTURION XVI. I (Manugistics Inc., Statistical Graphics Corporation, Rockville, MD, USA). All measurements were performed in triplicate.
3. Results and Discussion
3.1. Film Production: Optimization of CS, GEL, and GLY Content
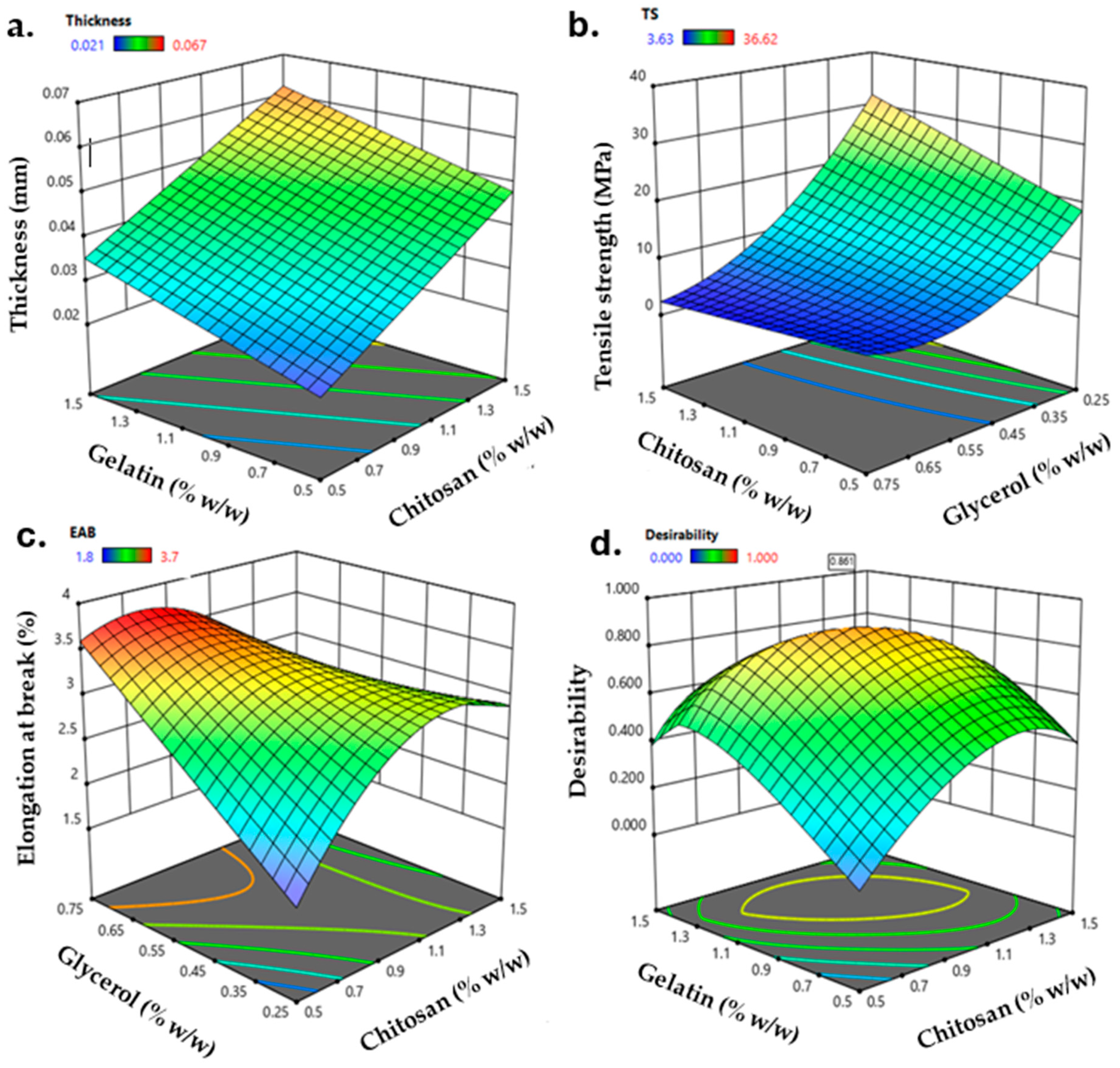
The film production was carried out by casting, and a BBD was used to optimize the CS, GEL, and GLY content in the final film formulation. Mechanical properties are relevant parameters to preserve its integrity and reflect its tolerance of external stress during handling or transportation [3]. The thickness, TS, and EAB were selected as critical parameters associated with the film’s mechanical properties for the dependent variables. Figure 1 shows the surface response of the thickness (mm, Figure 1a), TS (MPa, Figure 1b), and EAB (%, Figure 1c) of the obtained films. The thickness fits a linear model in which the three independent variables are statistically significant (p < 0.05). The thickness shifted from 0.021 to 0.067 mm and, opposite to what was reported by Jridi et al. [29], the increment in CS content primarily increased the thickness of the film due to the interactions between GEL and CS molecules, resulting in a protruded network [27]. Also, the thickness was affected by the content of GLY, in which increasing the content of glycerol increases the thickness of the film because molecules of glycerol act as a filler in the resulting matrix, interacting with the film-forming polymers [30]. The TS (Figure 1b) ranged from 3.63 to 36.62 MPa, where the three independent variables have statistical significance (p < 0.05) and were fitted to a quadratic model. A higher TS contributes to the enhanced mechanical integrity of the packaging, and the TS obtained agrees with [30], confirming that the content of GEL and GLY highly influences TS. Based on this, mixing CS and GEL improves mechanical properties. While GEL promotes a more flexible film, the interaction with the semi-crystalline structure of CS promotes the formation of a more rigid structure. Therefore, incorporating GLY reduces the interactions between the polymer chains, resulting in a more flexible film [27]. The EAB of the obtained films ranged between 1.8% and 3.7%. EAB is highly influenced by the content of GLY (p < 0.05). The addition of GLY increases the capability of a film to stretch. However, a decrease in the EAB was observed for the higher content of GLY. This trend was argued for by Siripatrawan and Vitchayakitti, suggesting that a decrease in the EAB should be attributable to the crystalline structure formed by an excess of GLY in the CS structure, leading to a reduction in the flexibility of the film [31]. In addition, they refer to possible interactions between CS and a higher GLY content, leading to a crosslinking effect and decreasing the free volume and mobility of the chitosan chain, promoting a decrease in the EAB.
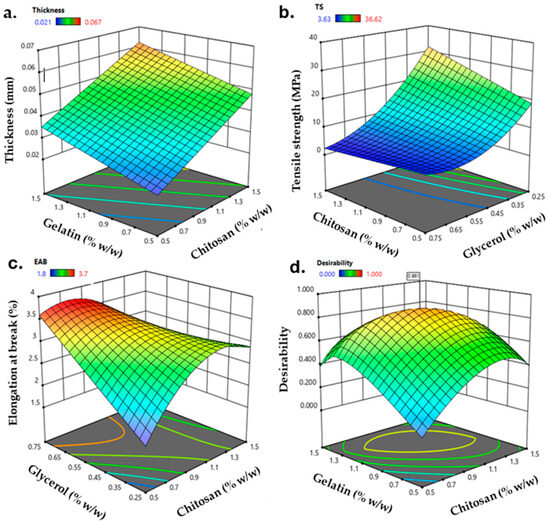
Figure 1.
Response surface plots for (a) thickness (mm), (b) tensile strength (MPa), (c) elongation at break (%), and (d) contour curve for desirability as a function of the content of CS, GEL, and GLY.
The optimization and validation of the model were conducted through numerical optimization for the desirability function. The overall desirability function (Figure 1d) was used to analyze the optimal conditions that led to the optimized film formulation (OPT-F). In this order, the maximum global desirability function was reached (0.86) when CS, GEL, and GLY content were 1.1%, 1.1%, and 0.4% (w/v), respectively. Under these conditions, the predicted variable values are shown in Table 2.

Table 2.
Predicted and experimental response values of the designed CS/GEL/GLY film.
The predicted and experimental data of the optimized conditions were compared as an essential step to validate the model. The differences between the predicted and the experimental values ranged from 6.98% for thickness to 18.75% for EAB, guaranteeing successful responses in the region where they were optimized.
3.2. Chemical Composition of the Films
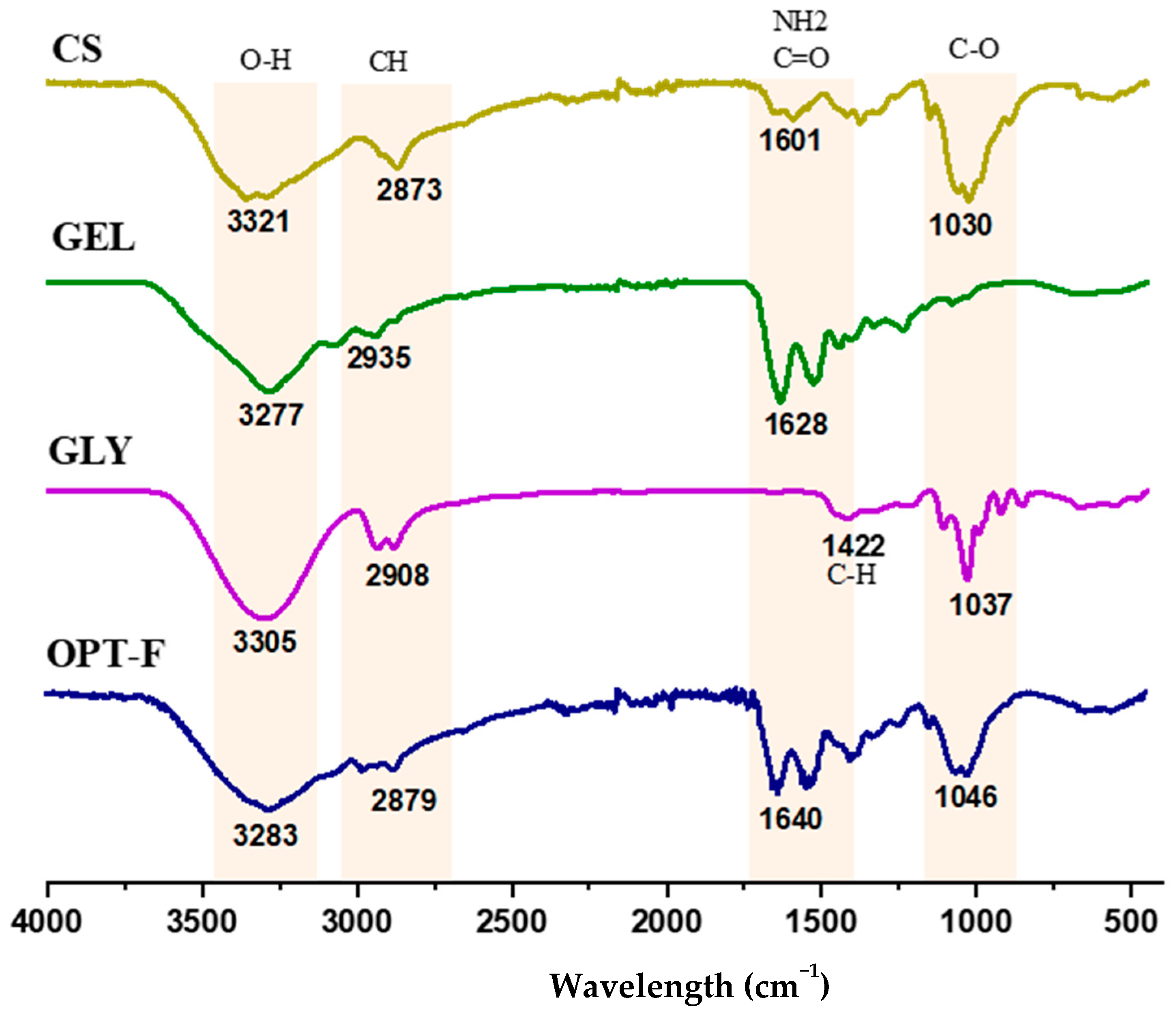
Fourier-transform infrared spectroscopy (ATR-FTIR) analyses were carried out to evaluate possible structural changes and physical or chemical interactions by CS, GEL, and GLY functional groups for a deeper understanding of the interactions of the CS and GEL polymer chains and GLY and their role in the modification of the mechanical properties of the film. Hence, the relevant transmittance peaks at wavenumbers from 4000 cm−1 to 400 cm−1 for the optimized film (OPT-F) were compared with individual components to examine shifting and interaction among its constituents (Figure 2). The position of the most relevant peaks in the spectra agrees with previous studies reported by other authors [18,32,33]. The bands in the wavelength range between 1030 and 1046 cm−1 in the CS and GLY, and the OPT-F samples correspond to the compounds’ fingerprint, referring to the C-O stretching groups [32,34]. In the spectrum of GLY, a peak is observed at 1422 cm−1 related to the stretching of CH, specifically with the movement of CH2 from proline and glycine [32]. Additionally, the OPT-F and the compounds CS, GEL, and GLY displayed a large peak in the 3305–3321 cm−1 range that was attributed to O–H stretching vibrations [18,32]. Likewise, in the range of 2873 to 2935 cm−1, the presence of the Amida B group is observed where the CH groups of antisymmetric and symmetric stretching are found [32,34]. Finally, in the region located at 1601–1640 cm−1, the interaction between the CS and GEL is observed. Relevant peaks in the region 1500–1700 cm−1 were associated with electrostatic interaction between the amino and carbonyl groups [33]. The above is confirmed since in the OPT-F, it is observed that the peak increases to 1640 cm−1 concerning the CS and GEL, which are 1601 and 1628 cm−1, respectively, indicating the formation of electrostatic interactions between the polymers used to formulate the optimized film, forming a homogeneous layer.
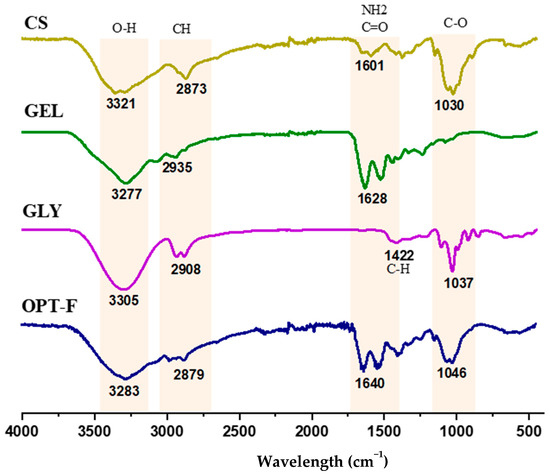
Figure 2.
FTIR spectra of chitosan-based film (OPT-F) and comparison with its single constituents.
3.3. Analysis of Film Color
Optical properties such as color and transparency are relevant for film used as packaging in the food industry due to it altering the appearance of the coated product [29]. Also, sensory properties and consumer acceptance are affected by the color of the packaging [32]. In the present study, the appearance of the optimized film was compared with LDPE. For the OPT-F and LDPE (Table 3), the lightness has statistically significant differences (p < 0.05), with a clear tendency for a slight appearance in the optimized film. This trend agrees with reports by Jridi et al. for films containing GEL [29]. Relative to redness (a*), no statistical significance (p > 0.05) was observed. At the same time, OPT-F’s yellowness (b*) did present a statistical significance compared to the LDPE, indicating that OPT-F has a greater tendency to yellowness than LDPE. Similar behavior was reported by Chu et al. for bi-layer films of CS and GEL, where the yellowish tendency is associated with the presence of β-1-4 linked 2-amino-2-deoxy-d-glucopyranose repeated units [33]. The whiteness index (WI) measures the amount of light reflected by a surface through the visible light spectrum, which helps determine how white a surface appears to the human eye. It is expressed as a percentage on a scale of 1–100%, with 100% being the value that should correspond to a perfect white. The WI values of the OPT-F presented statistically significant differences with LDPE (as seen in Table 3), where OPT-F has a more reflective surface than LDPE, and this is associated with the interaction between CS and the other constituents [35].

Table 3.
Color parameters (L*, a*, and b*), total color difference (ΔE*), and whiteness index of the OPT-F film and their comparison with LDPE.
3.4. Morphology and Microstructure of Designed Chitosan-Based OPT-F Film
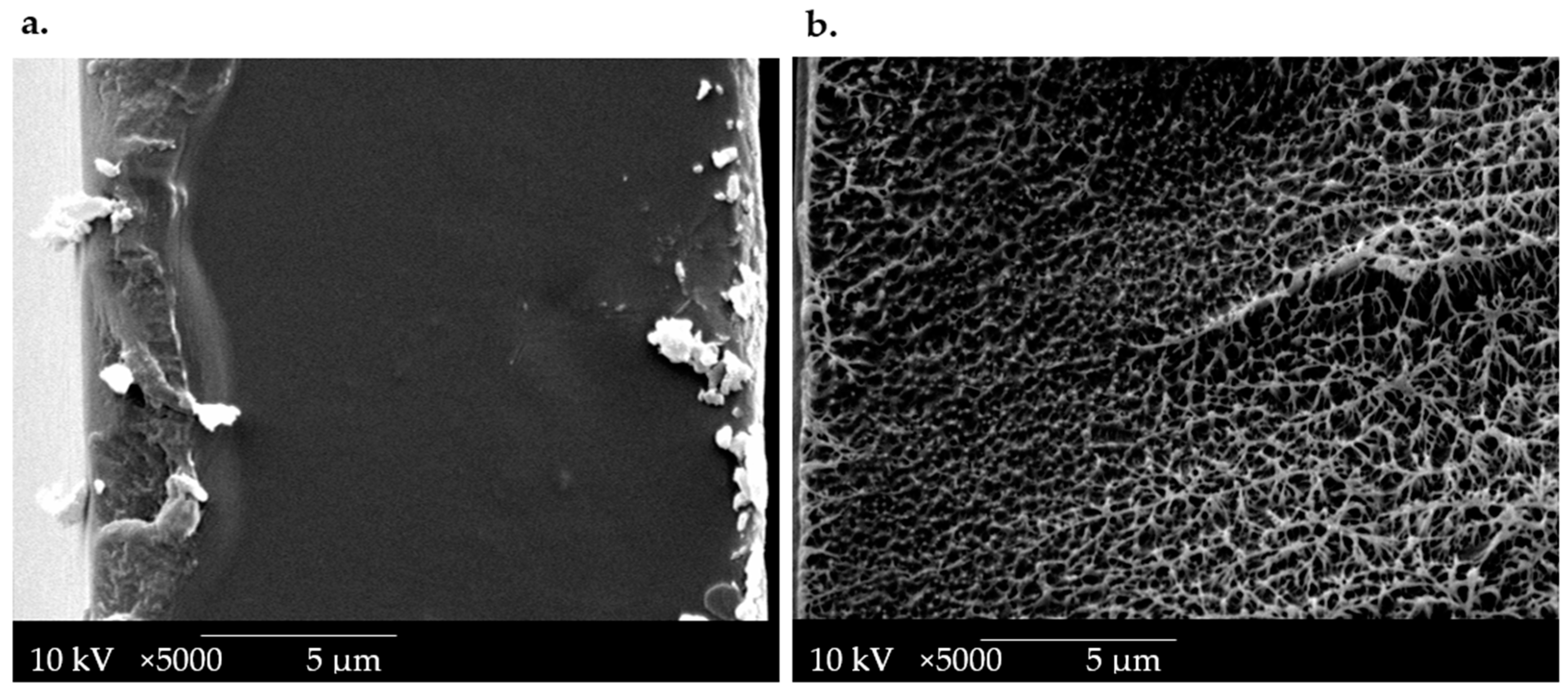
Scanning electron microscopy (SEM) micrographs of cross-sections of the OPT-F film were performed to evaluate the designed material’s microstructure and compare it with LDPE film, as presented in Figure 3. It can be seen that the LDPE cross-sections at 5000× (Figure 3b) revealed a heterogeneous morphology, with porosity. This behavior reveals that significant morphological irregularities were more common in LDPE films with a greater thickness, according to the report by Szlachetka et al. [36]. The OPT-F observed at 5000× (Figure 3a) shows a homogeneous, non-porous structure with good structural integrity due to the high compatibility of each of the components. These attributes, mainly attributable to the hydrophilic nature of the main components, correlate with the good compatibility and compact morphology of the obtained films [18,33]. Even though the microstructures of LDPE and OPT-F cannot be compared due to their different production processes, a homogenous film is generated for possible use as leading food packaging by combining CS, GEL, and GLY.
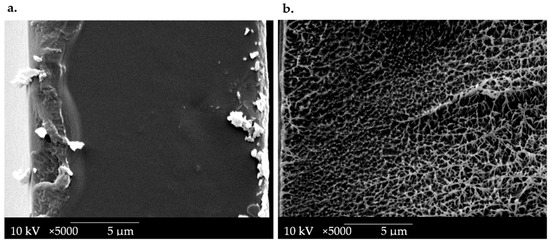
Figure 3.
SEM image of the cross-section of (a) OPT-F and (b) LDPE.
3.5. Mechanical Properties
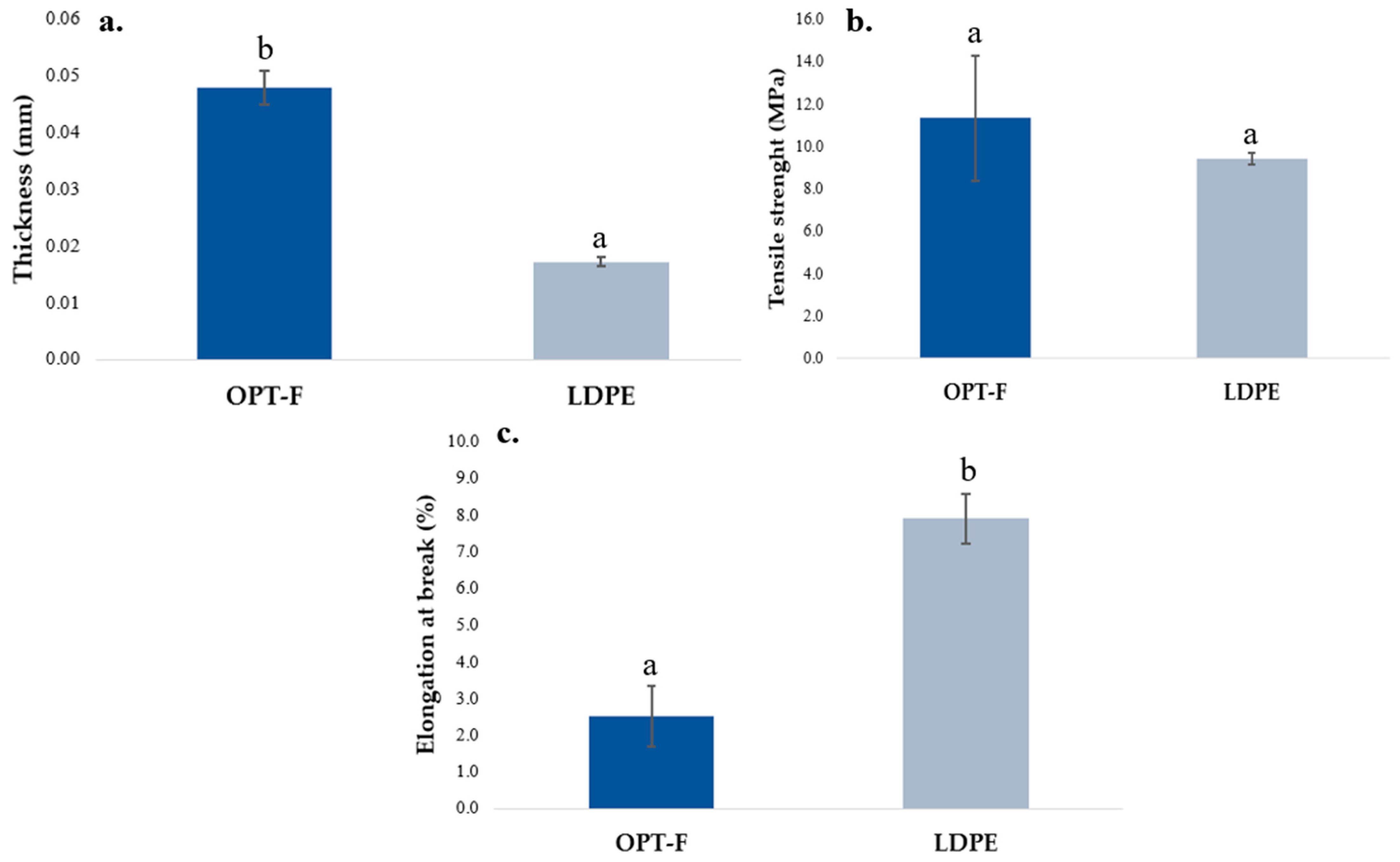
The adequate performance of the mechanical properties of the films is relevant and desirable when they are used as food packaging [29]. The mechanical properties of OPT-F and the LDPE film as control are compared in Figure 4. A significant difference (p < 0.05) is observed for thickness, OPT-F being thicker than the LDPE film (Figure 4a). Jridi et al. obtained films by casting using GEL and CS with a thickness closer to 0.050 ± 0.003 mm and concluded that adding CS to the matrix reduces the thickness of the film [29]. However, the differences between the OPT-F and LDPE films are highly influenced by the film production methods in which extrusion, for LDPE, considerably reduces the thickness of the film [37]. For TS (Figure 4b), no significant differences were observed for both the OPT-F and LDPE films. In addition, the TS obtained for the biodegradable OPT-F agrees with reports by Luzi et al. for LDPE (8–31 MPa) [38]. This result supports the idea of using biodegradable materials for film production able to be used as food packaging, providing a similar mechanical integrity as LDPE films that guarantees the integrity of the content, avoiding food deterioration in transportation, handling, or storage operations [39]. Khan, Ezati, and Rhim obtained chitosan–gelatin-based film with a TS higher than 62 MPa for 2% (w/w) of chitosan, 2% (w/w) of gelatin and 30% (w/w) of glycerol [16]. A similar trend in TS was reported by Haghighi et al. [40], who formulated films based on chitosan–gelatin blends, and they reported a TS up to five times higher than that obtained in this research, which is considered by them as very high for application in the packaging industry. Consequently, the optimized concentrations of chitosan and gelatin for OPT-F guarantee properties similar to LDPE, and being higher than 3 MPa, they can be used in the packaging industry, ensuring the integrity of the film.
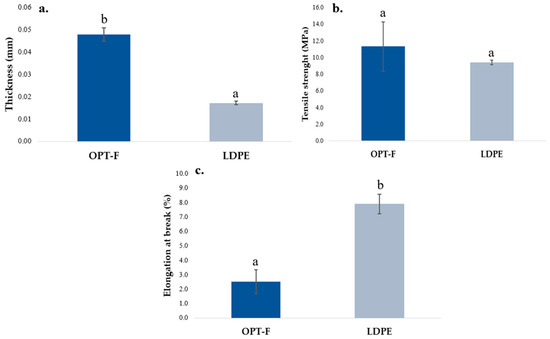
Figure 4.
Comparison of the mechanical properties of chitosan-based OPT-F with LDPE: (a) thickness, (b) TS, and (c) EAB. Different letters indicate significant differences (p < 0.05).
For OPT-F, the EAB was lower than LDPE (p < 0.05), as observed in Figure 4c. Films with a higher TS and lower EAB show the typical behavior of non-plasticized films [41]. The EAB results agree with those reported by Chang et al., and could be attributable to the lower content of GLY as a plasticizer [42]. Despite this, the OPT-F film could be applied as alternative natural-based food packaging, considering some limitations in its application according to the nature of the food matrix.
3.6. Swelling
The degree of swelling is a relevant parameter within the barrier properties of the films, because it determines the ability of the polymer matrix to maintain its structure in the presence of water [43]. Generally, a low percentage of swelling is desirable for films with a packaging application [44]. Table 4 shows that the degree of swelling in OPT-F and LDPE are statistically different. This is due to the chemical nature of OPT-F obtaining a higher swelling degree compared to LDPE, these being 75.95% and 2.68%, respectively. The high degree of swelling in OPT-F is caused by the hydrophilic nature of gelatin and glycerol, since they react with water molecules through hydrogen bonds [45]. In the study carried out by Zhang, Han, and Zhou, they found that the swelling degree of chitosan and gelatin films was 6611.4% and they reduced it to 314.1% by adding 15% wt of citric acid [18]. Therefore, in this study we reduced the swelling degree by optimizing the concentrations of chitosan, gelatin, and glycerol, obtaining a film with greater stability to water contact. Furthermore, the formulation proposed in this study serves as a basis for the future addition of crosslinking agents that will allow the % of swelling to be further reduced, without compromising the degradability of the film.

Table 4.
Swelling and solubility of OPT-F film and comparison with LDPE.
3.7. Water Solubility (WS)
Table 4 shows the WS of the OPT-F and LDPE samples, obtaining values of 24.3% and 0.9% respectively. From this, it was determined that the WS of the analyzed samples are statistically different from each other. In the same way as discussed in Section 3.6. this is due to the hydrophilic nature of gelatin and glycerol. However, in this study, a lower percentage of WS was obtained compared to other studies of chitosan and gelatin films. For example, Reyes Mendez et al. reported a WS of 33.7% and added eugenol essential oil to improve the solubility, reaching a WS of 35.8% [17]. The chitosan and gelatin film obtained here, OPT-F, led with a WS of 39.8% [18]. Therefore, the optimization of the concentrations of chitosan, gelatin, and glycerol proposed in this study allowed the WS values to be reduced.
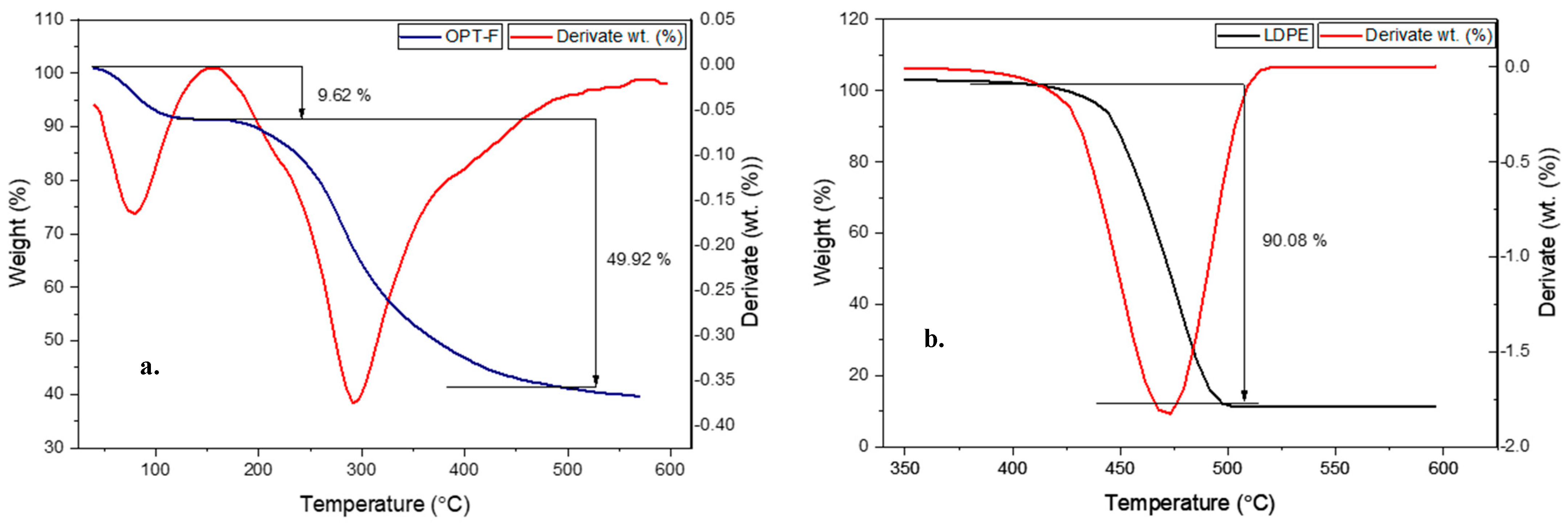
3.8. Thermogravimetric Analysis
Thermogravimetric analysis evaluates a material’s thermal stability, including the sample’s moisture or volatile compound content. In this regard, a TGA measurement was performed to evaluate the thermal stability of the designed CS/GEL/GLY film and compare it with the thermal stability of LDPE, as shown in Figure 5. The OPT-F exhibits two main mass losses closer to 100 °C and 300 °C, respectively (Figure 5a). On the other hand, for the LDPE, mass losses ca. 90.08 wt. % were observed at 450 °C (Figure 5b), suggesting high thermal stability under environmental conditions, a key factor of its poor biodegradability. According to the TGA and DTA curves for LDPE films (Figure 5b) a single decomposition step closer to 450 °C is attributable to polymers in which C-C bonds prevail in the main chain, promoting a break in the polymeric chain and further thermal decomposition [46]. For CS/GEL films, the first mass losses were observed for temperatures lower than 150 °C and could be attributable to physisorbed water and traces of acetic acid [18], and they agree with the temperature obtained for the OPT-F film in this research. A similar trend was observed in both the CS and GEL thermograms (Supporting Information, Figure S1). The second relevant mass loss was closer to 300 °C in the OPT-F (49.9%) and was closer to the temperature in which CS (300 °C) and GEL (320 °C) had the most relevant mass losses. Since no intermediate peaks were observed at temperatures above 150 °C, it can be stated that the system behaves as a homogeneous mixture with a good miscibility of its single constituents [29], also confirmed by the FT-IR analysis, in which intermolecular hydrogen bonds formed by the interaction of CS, GEL, and GLY promoted the thermal stability of the film. As the OPT-F degradation temperature was higher than 300 °C, it is feasible to confirm that the obtained film can be subjected to packaging and thermal processing temperatures below 300 °C, which are characteristic in the food industry, without causing thermal decomposition of the film [47], as occurs with materials such as LDPE.
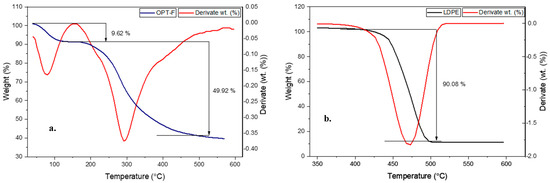
Figure 5.
Thermal gravimetric analysis (TGA) and differential thermal analysis (DTA) curves for (a) OPT-F and (b) LDPE.
3.9. Biodegradability Test of Designed OPT-F
The OPT-F and LDPE films were exposed to the soil surface of Mentha piperita pots for 31 days (Figure 6) to study the change in the samples’ color, mass, dimensions, and composition. After 31 days, significant physical changes were observed for the OPT-F film, since the sample wrinkled and small holes formed, indicating the beginning of degradation [48]. In contrast, no morphological changes were observed for the LDPE film when it was compared for day 0 and day 31. Regarding the color of the samples, OPT-F presented changes in color from day 0 to 31, becoming more yellowish, while LDPE did not present color changes. A similar situation occurred with the mass loss percentage, where the OPT-F had 7.08%, while LDPE did not present a mass loss. Therefore, the LDPE film did not show signs of degradation (Table 5). Also, it is important to mention that the mass values for the OPT-F sample include the pot soil’s residues adhered to the sample’s surface, which affected the weight measurements, and possibly the mass loss on day 31 was higher. In a study carried out by Oberlintner et al. on CS films, a mass loss of more than 80% was obtained in 7 days [49], while in a study carried out by Martucci and Ruseckaite on GEL films, a mass loss of 18% was obtained in 3 days [50].

Figure 6.
Biodegradability tests for both OPT-F and LDPE films when exposed to the soil surface of a Mentha piperita pot as an environmental model.

Table 5.
OPT-F and LDPE weight loss in the soil of a Mentha piperita pot.
This study obtained a lower degradation percentage than those reported in the literature, possibly due to the homogeneous structure that is formed from the mixture of CS and GEL. Furthermore, many factors such as soil type and microbial community also influenced the degradation of the proposed film [51]. However, the OPT-F sample shows a more significant mass loss compared to LDPE.
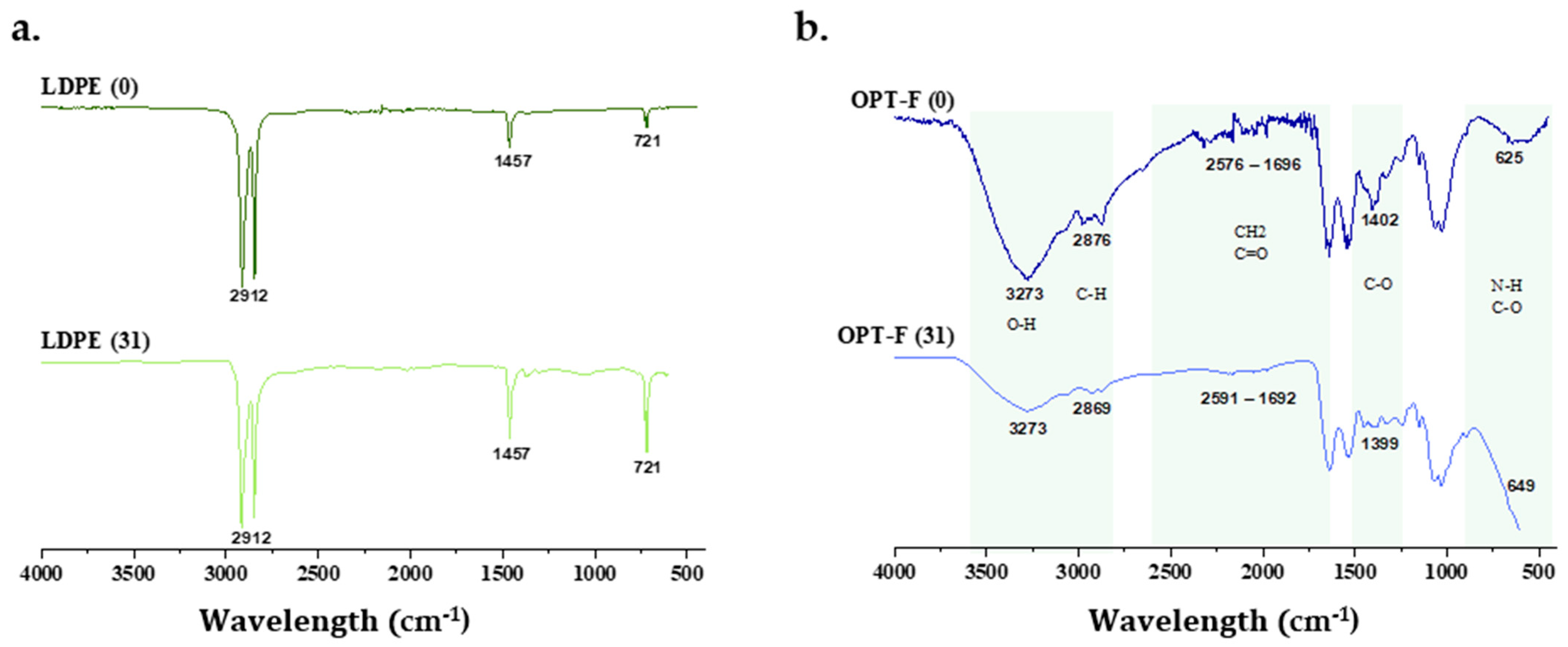
There are studies that analyze the percentage of degradation of films that are based on biopolymers, by calculating the mass loss that the film suffers when exposed to environmental conditions [23,52]. While this study also analyzed the changes in the structure of OPT-F and LDPE films after 31 days of exposure to the soil surface by FTIR spectroscopy. In Figure 7, it is observed that the structure of LDPE (Figure 7b) on day 31 had no structural changes compared to day 0, indicating that this material, when exposed to the soil surface, maintains its chemical structure, indicating that its polymer chain does not break and, consequently, there is no degradation on the soil surface. However, OPT-F did present changes in its chemical structure on day 31 of being exposed to the soil surface (Figure 7a), demonstrating that after 31 days of exposure, a decrease in the O–H groups (3273 cm−1) and the C-H groups (2869 cm−1) is presented, suggesting the biodegradation of the designed film. Also, significant changes in CH2, C=O, NH, and C-O bonds were observed [53]. The above indicates that polymer chain breakdown through chemical hydrolysis forms microdoses of O2, CH4, water, biomass, humic matter, and other natural substances [54]. As observed in Figure 6, it could be inferred that with the degradation of OPT-F, no qualitative changes are observed for the Mentha piperita, suggesting that the growth integrity of the plant is not compromised.
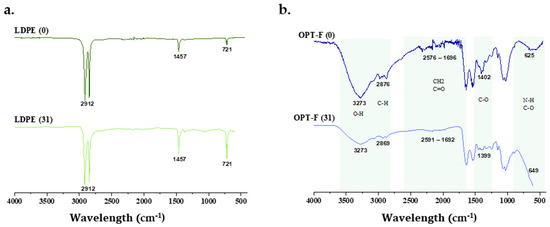
Figure 7.
FTIR spectra of (a) chitosan-based OPT-F and comparison with (b) LDPE after 31 days of exposure to the soil surface of Mentha piperita pots.
4. Conclusions
An eco-friendly and biodegradable chitosan-based film (OPT-F) was obtained by optimizing the content of CS, GEL, and GLY using a Response Surface Methodology, with a similar mechanical performance compared with non-degradable LDPE. As a result, for the optimized CS (1.1% w/v), GEL (1.1% w/v), and GLY (0.4% w/v), the thickness, TS, and EAB obtained were 0.046 mm, 11.48 MPa, and 2.6%, respectively. Under these conditions, the OPT-F was thermally stable and desirable for packaging submitted to a high thermal process. The biodegradability test revealed that OPT-F, after 31 days of exposure in the soil surface, suffered chemical changes in the CH2, C=O, NH, and C-O bonds, suggesting the biodegradation of the film, while LDPE did not present any chemical changes in its structure. The optical properties suggested the optimized film’s homogeneity, enhancing the protected food’s visual properties. This research demonstrates the potential applicability of biopolymer-based films as a novel alternative in the food packaging industry, avoiding pollution and offering mechanical, thermal, and biodegradability properties that promote food preservation, handling, and storage. However, a long-term study is required to determine the complete degradation time of the films. With this starting point, an optimized formulation could include crosslinking or the incorporation of bioactive compounds, to improve the activity, mechanical properties, and swelling of the film without compromising its degradability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym16172471/s1, Figure S1. TGA measurements of CS, GEL, and GLY components to design of chitosan-based OPT-F.
Author Contributions
Investigation, formal analysis, J.F.-N.; original draft preparation, J.F.-N. and Y.C.; writing—review and editing, B.L.E.-S. and L.M.V.; methodology, G.C.-B., C.M.G.-H. and M.E.; visualization, J.F.-N. and M.I.; supervision, B.L.E.-S. and L.M.V.; funding acquisition, L.M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by funding from the COPEC-UC Project 2022.R2.009, Bolsolution.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the support of Centro de Investigación y Desarrollo Tecnológico en Electroquímica (CIDETEQ) for the experimental development. Acknowledgments to R.A. Mauricio-Sánchez (CINVESTAV-Qro), Clara María Rodríguez (CIDETEQ), Susana Citlali Gaucín (CIDETEQ), and Gerardo Antonio Fonseca (CFATA-UNAM) for their technical support in materials characterization. Gustavo Cabrera-Barjas acknowledges Fondecyt Regular Project 1221609. Claudio García-Herrera and Matías Inostroza acknowledge the Dicyt Project 052316GH_Ayudante, funded by the Universidad de Santiago de Chile.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Wang, Q. Advances in Using Nanotechnology Structuring Approaches for Improving Food Packaging. Annu. Rev. Food Sci. Technol. 2020, 11, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, J.; Hosseini, S.F.; Keyvani, N.; Gómez-Guillén, M.C. Polymer Blending Effects on the Physicochemical and Structural Features of the Chitosan/Poly(Vinyl Alcohol)/Fish Gelatin Ternary Biodegradable Films. Food Hydrocoll. 2019, 95, 122–132. [Google Scholar] [CrossRef]
- Rathod, N.B.; Bangar, S.P.; Šimat, V.; Ozogul, F. Chitosan and Gelatine Biopolymer-Based Active/Biodegradable Packaging for the Preservation of Fish and Fishery Products. Int. J. Food Sci. Technol. 2023, 58, 854–861. [Google Scholar] [CrossRef]
- Lou, L.; Chen, H. Functional Modification of Gelatin-Based Biodegradable Composite Films: A Review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2023, 40, 928–949. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- La Fuente Arias, C.I.; Kubo, M.T.K.-N.; Tadini, C.C.; Augusto, P.E.D. Bio-Based Multilayer Films: A Review of the Principal Methods of Production and Challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 2260–2276. [Google Scholar] [CrossRef]
- Dilruba Öznur, K.G.; Ayşe Pınar, T.D. Statistical Evaluation of Biocompatibility and Biodegradability of Chitosan/Gelatin Hydrogels for Wound-Dressing Applications. Polym. Bull. 2024, 81, 1563–1596. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-Based Biodegradable Functional Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, H.; Nishinari, K. (Eds.) Food Hydrocolloids: Functionalities and Applications; Springer: Singapore, 2021; ISBN 9789811603198. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of Essential Oils in Active Food Packaging: Recent Advances and Future Trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Liu, Y.; Kai, Y.; Yang, H. Biodegradable Fish Gelatin/Chitosan-Based Active Films Alter Chill-Stored Golden Pomfret (Trachinotus blochii) Metabolites Mainly through Modulating Four Metabolic Pathways. Food Packag. Shelf Life 2023, 36, 101046. [Google Scholar] [CrossRef]
- Said, N.S.; Howell, N.K.; Sarbon, N.M. A Review on Potential Use of Gelatin-Based Film as Active and Smart Biodegradable Films for Food Packaging Application. Food Rev. Int. 2023, 39, 1063–1085. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Wen, H.; Tang, D.; Lin, Y.; Zou, J.; Liu, Z.; Zhou, P.; Wang, X. Enhancement of Water Barrier and Antimicrobial Properties of Chitosan/Gelatin Films by Hydrophobic Deep Eutectic Solvent. Carbohydr. Polym. 2023, 303, 120435. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ezati, P.; Rhim, J.-W. Chitosan/Gelatin-Based Multifunctional Film Integrated with Green Tea Carbon Dots to Extend the Shelf Life of Pork. Food Packag. Shelf Life 2023, 37, 101075. [Google Scholar] [CrossRef]
- Reyes Méndez, L.M.; Méndez Morales, P.A.; López-Córdoba, A.; Ortega-Toro, R.; Gutiérrez, T.J. Active Chitosan/Gelatin-Based Films and Coatings Containing Eugenol and Oregano Essential Oil for Fresh Cheese Preservation. J. Food Process Eng. 2023, 46, e14396. [Google Scholar] [CrossRef]
- Zhang, A.; Han, Y.; Zhou, Z. Characterization of Citric Acid Crosslinked Chitosan/Gelatin Composite Film with Enterocin CHQS and Red Cabbage Pigment. Food Hydrocoll. 2023, 135, 108144. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, Q.; Yu, L.; Xie, F. Thermomechanically Processed Chitosan:Gelatin Films Being Transparent, Mechanically Robust and Less Hygroscopic. Carbohydr. Polym. 2021, 272, 118522. [Google Scholar] [CrossRef] [PubMed]
- Naseri, H.R.; Beigmohammadi, F.; Mohammadi, R.; Sadeghi, E. Production and Characterization of Edible Film Based on Gelatin–Chitosan Containing Ferulago Angulate Essential Oil and Its Application in the Prolongation of the Shelf Life of Turkey Meat. J. Food Process. Preserv. 2020, 44, e14558. [Google Scholar] [CrossRef]
- Rodrigues, M.Á.V.; Bertolo, M.R.V.; Marangon, C.A.; Martins, V.d.C.A.; de Guzzi Plepis, A.M. Chitosan and Gelatin Materials Incorporated with Phenolic Extracts of Grape Seed and Jabuticaba Peel: Rheological, Physicochemical, Antioxidant, Antimicrobial and Barrier Properties. Int. J. Biol. Macromol. 2020, 160, 769–779. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Abdin, M.; Hashim, M.H.; Ahmed, W. Preparation and Characterization of Chitosan/Gelatin-Based Active Food Packaging Films Containing Apple Peel Nanoparticles. J. Polym. Environ. 2020, 28, 411–420. [Google Scholar] [CrossRef]
- Hoque, M.; Janaswamy, S. Biodegradable Packaging Films from Banana Peel Fiber. Sustain. Chem. Pharm. 2024, 37, 101400. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J.; et al. Preparation and Characterization of Chitosan Films with Three Kinds of Molecular Weight for Food Packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Prus-Walendziak, W.; Kozlowska, J. Design of Sodium Alginate/Gelatin-Based Emulsion Film Fused with Polylactide Microparticles Charged with Plant Extract. Materials 2021, 14, 745. [Google Scholar] [CrossRef]
- Boun, H.R.; Huxsoll, C.C. Control of Minimally Processed Carrot (Daucus Carota) Surface Discoloration Caused by Abrasion Peeling. J. Food Sci. 1991, 56, 416–418. [Google Scholar] [CrossRef]
- Tagrida, M.; Nilsuwan, K.; Gulzar, S.; Prodpran, T.; Benjakul, S. Fish Gelatin/Chitosan Blend Films Incorporated with Betel (Piper betle L.) Leaf Ethanolic Extracts: Characteristics, Antioxidant and Antimicrobial Properties. Food Hydrocoll. 2023, 137, 108316. [Google Scholar] [CrossRef]
- Trejo-Caballero, M.E.; Díaz-Patiño, L.; González-Reynac, M.; Molina, G.A.; López-Miranda, J.L.; Esparza, R.; España-Sánchez, B.L.; Arjona, N.; Estevez, M. Biopolymeric Hydrogel Electrolytes Obtained by Using Natural Polysaccharide–Poly(Itaconic Acid-Co-2-Hydroxyethyl Methacrylate) in Deep Eutectic Solvents for Rechargeable Zn–Air Batteries. Green Chem. 2023, 25, 6784–6796. [Google Scholar] [CrossRef]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, Structural, Antioxidant and Antimicrobial Properties of Gelatin–Chitosan Composite Edible Films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Fathiraja, P.; Gopalrajan, S.; Karunanithi, M.; Nagarajan, M.; OBAIAH, M.; Sukumar, D.; Neethiselvan, N. Development of a Biodegradable Composite Film from Chitosan, Agar and Glycerol Based on Optimization Process by Response Surface Methodology. Cellul. Chem. Technol. 2021, 55, 849–865. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving Functional Properties of Chitosan Films as Active Food Packaging by Incorporating with Propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Fathiraja, P.; Gopalrajan, S.; Karunanithi, M.; Obaiah, M.; Rajasekaran, B.; Vedhi, C. Process Optimization and Characterization of Composite Biopolymer Films Obtained from Fish Scale Gelatin, Agar and Chitosan Using Response Surface Methodology. Polym. Bull. 2022, 80, 10775–10807. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-Gelatin Composites and Bi-Layer Films with Potential Antimicrobial Activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Chu, M.; Feng, N.; An, H.; You, G.; Mo, C.; Zhong, H.; Pan, L.; Hu, D. Design and Validation of Antibacterial and pH Response of Cationic Guar Gum Film by Combining Hydroxyethyl Cellulose and Red Cabbage Pigment. Int. J. Biol. Macromol. 2020, 162, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Liu, C. Film Transparency and Opacity Measurements. Food Anal. Methods 2022, 15, 2840–2846. [Google Scholar] [CrossRef]
- Szlachetka, O.; Witkowska-Dobrev, J.; Baryła, A.; Dohojda, M. Low-Density Polyethylene (LDPE) Building Films—Tensile Properties and Surface Morphology. J. Build. Eng. 2021, 44, 103386. [Google Scholar] [CrossRef]
- Peled, A.; Shah, S.P. Processing Effects in Cementitious Composites: Extrusion and Casting. J. Mater. Civ. Eng. 2003, 15, 192–199. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio- and Fossil-Based Polymeric Blends and Nanocomposites for Packaging: Structure–Property Relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef]
- Fathiraja, P.; Gopalrajan, S.; Kumar, K.; Obaiah, M.C. Augmentation of Bioactivity with Addition of Clove Essential Oil into Fish Scale Gelatin, Agar and Chitosan Composite Film and Biodegradable Features. Polym. Bull. 2024, 81, 5329–5357. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive Characterization of Active Chitosan-Gelatin Blend Films Enriched with Different Essential Oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Pérez-Córdoba, L.J.; Norton, I.T.; Batchelor, H.K.; Gkatzionis, K.; Spyropoulos, F.; Sobral, P.J.A. Physico-Chemical, Antimicrobial and Antioxidant Properties of Gelatin-Chitosan Based Films Loaded with Nanoemulsions Encapsulating Active Compounds. Food Hydrocoll. 2018, 79, 544–559. [Google Scholar] [CrossRef]
- Chang, W.; Liu, F.; Sharif, H.R.; Huang, Z.; Goff, H.D.; Zhong, F. Preparation of Chitosan Films by Neutralization for Improving Their Preservation Effects on Chilled Meat. Food Hydrocoll. 2019, 90, 50–61. [Google Scholar] [CrossRef]
- Lian, H.; Peng, Y.; Shi, J.; Wang, Q. Effect of Emulsifier Hydrophilic-Lipophilic Balance (HLB) on the Release of Thyme Essential Oil from Chitosan Films. Food Hydrocoll. 2019, 97, 105213. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Ciannamea, E.M.; Castillo, L.A.; Barbosa, S.E.; De Angelis, M.G. Barrier Properties and Mechanical Strength of Bio-Renewable, Heat-Sealable Films Based on Gelatin, Glycerol and Soybean Oil for Sustainable Food Packaging. React. Funct. Polym. 2018, 125, 29–36. [Google Scholar] [CrossRef]
- Babaghayou, M.I.; Mourad, A.-H.I.; Ochoa, A.; Beltrán, F.; Cherupurakal, N. Study on the Thermal Stability of Stabilized and Unstabilized Low-Density Polyethylene Films. Polym. Bull. 2021, 78, 5225–5241. [Google Scholar] [CrossRef]
- Tavares, L.; Barros, H.L.B.; Vaghetti, J.C.P.; Noreña, C.P.Z. Microencapsulation of Garlic Extract by Complex Coacervation Using Whey Protein Isolate/Chitosan and Gum Arabic/Chitosan as Wall Materials: Influence of Anionic Biopolymers on the Physicochemical and Structural Properties of Microparticles. Food Bioprocess. Technol. 2019, 12, 2093–2106. [Google Scholar] [CrossRef]
- Medina Jaramillo, C.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and Plasticizing Effect of Yerba Mate Extract on Cassava Starch Edible Films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef]
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability Study of Active Chitosan Biopolymer Films Enriched with Quercus Polyphenol Extract in Different Soil Types. Environ. Technol. Innov. 2021, 21, 101318. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation Behavior of Three-Layer Sheets Based on Gelatin and Poly (Lactic Acid) Buried under Indoor Soil Conditions. Polym. Degrad. Stab. 2015, 116, 36–44. [Google Scholar] [CrossRef]
- Sawaguchi, A.; Ono, S.; Oomura, M.; Inami, K.; Kumeta, Y.; Honda, K.; Sameshima-Saito, R.; Sakamoto, K.; Ando, A.; Saito, A. Chitosan Degradation and Associated Changes in Bacterial Community Structures in Two Contrasting Soils. Soil Sci. Plant Nutr. 2015, 61, 471–480. [Google Scholar] [CrossRef]
- Hasan, M.; Gopakumar, D.A.; Olaiya, N.G.; Zarlaida, F.; Alfian, A.; Aprinasari, C.; Alfatah, T.; Rizal, S.; Khalil, H.P.S.A. Evaluation of the Thermomechanical Properties and Biodegradation of Brown Rice Starch-Based Chitosan Biodegradable Composite Films. Int. J. Biol. Macromol. 2020, 156, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus Modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).