Cross-Linking Reaction of Bio-Based Epoxy Systems: An Investigation into Cure Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Epoxy–Novolac Systems
2.2. Differential Scanning Calorimetry
2.3. Kinetic Analysis
2.3.1. Model Free—Isoconversional Method
2.3.2. Model Fitting
2.4. Specimen Manufacturing
2.5. Tensile Test—Digital Image Correlation
3. Results and Discussion
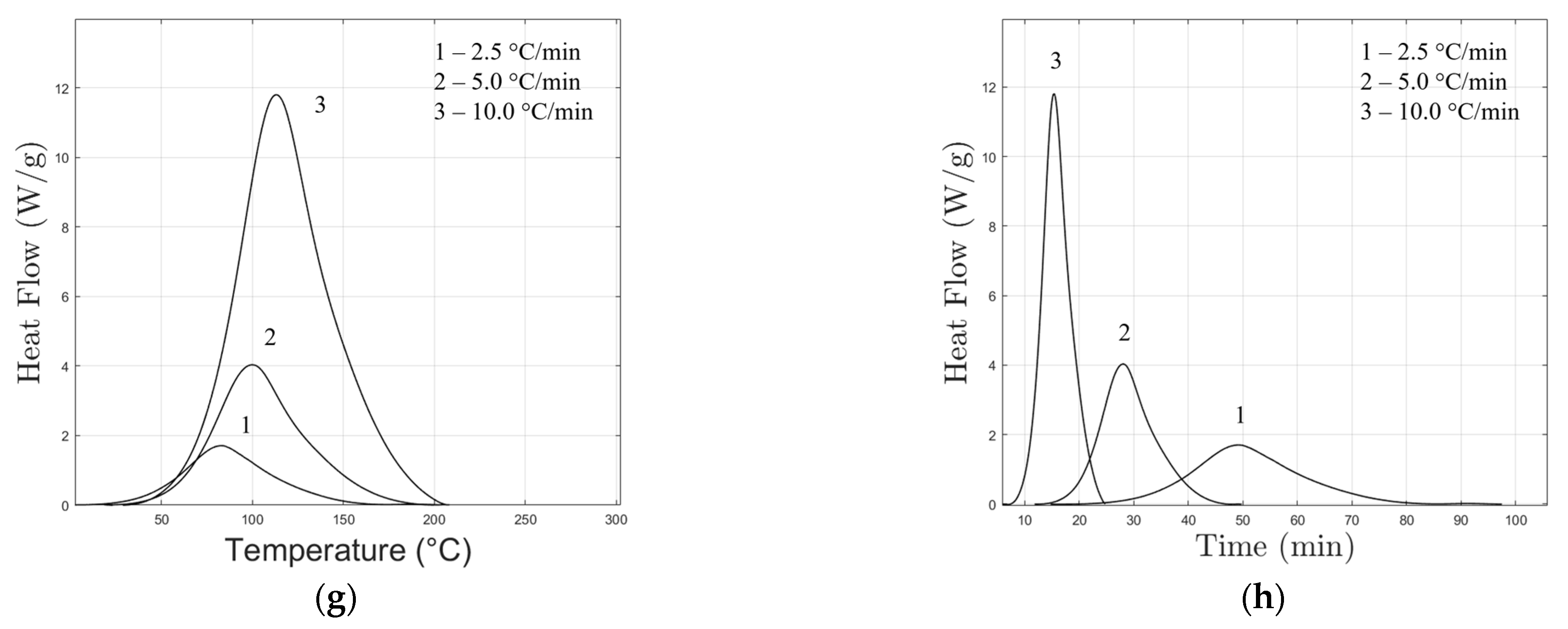
3.1. Non-Isothermal Differential Scanning Calorimetry (DSC)
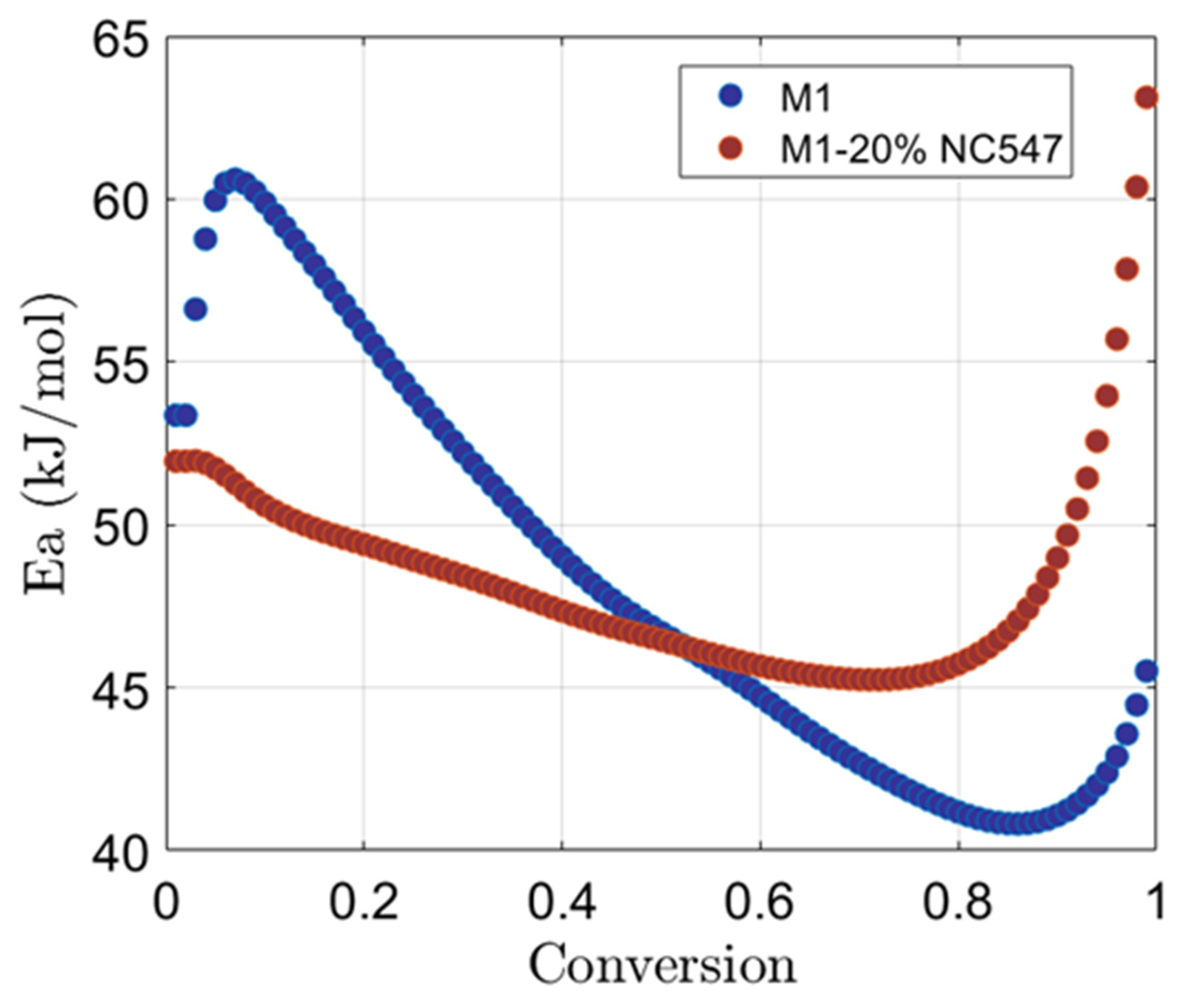
3.2. Isoconversional Analysis
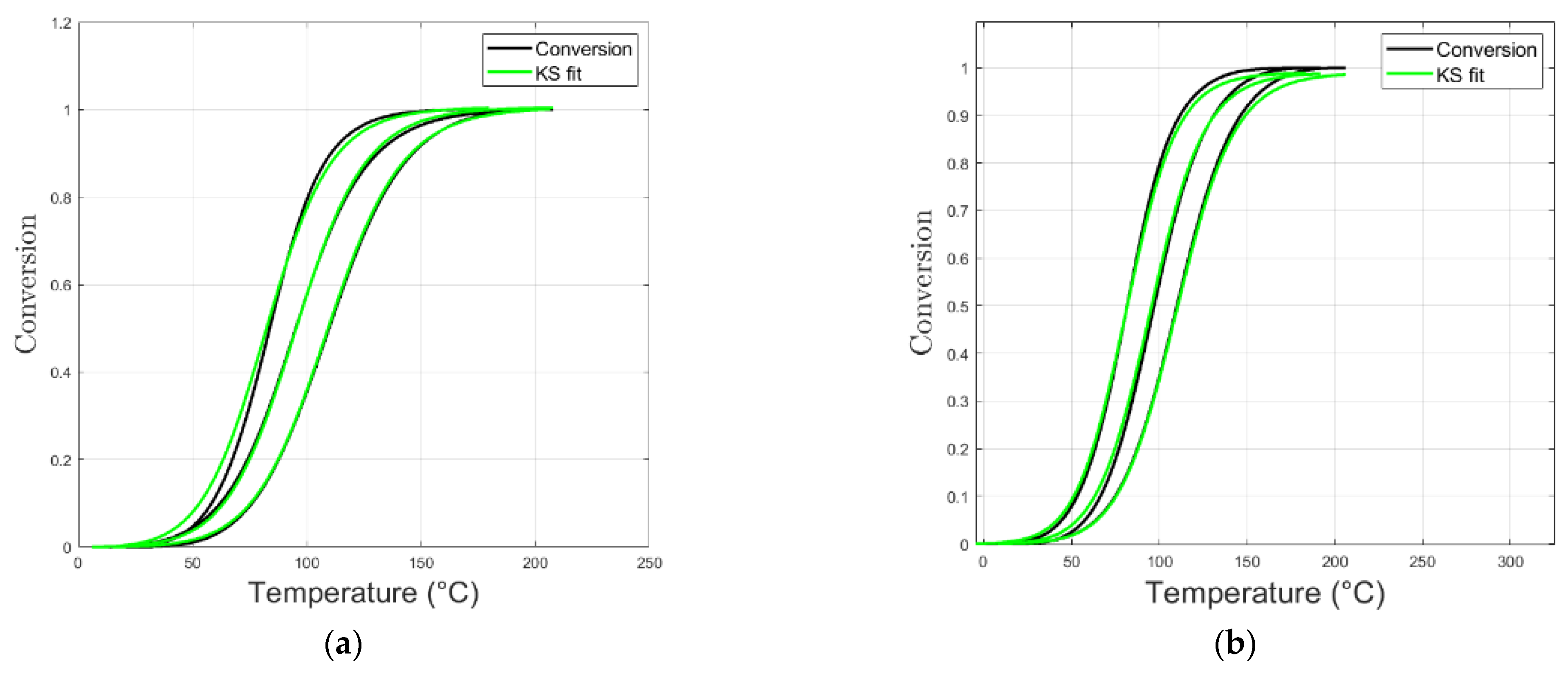
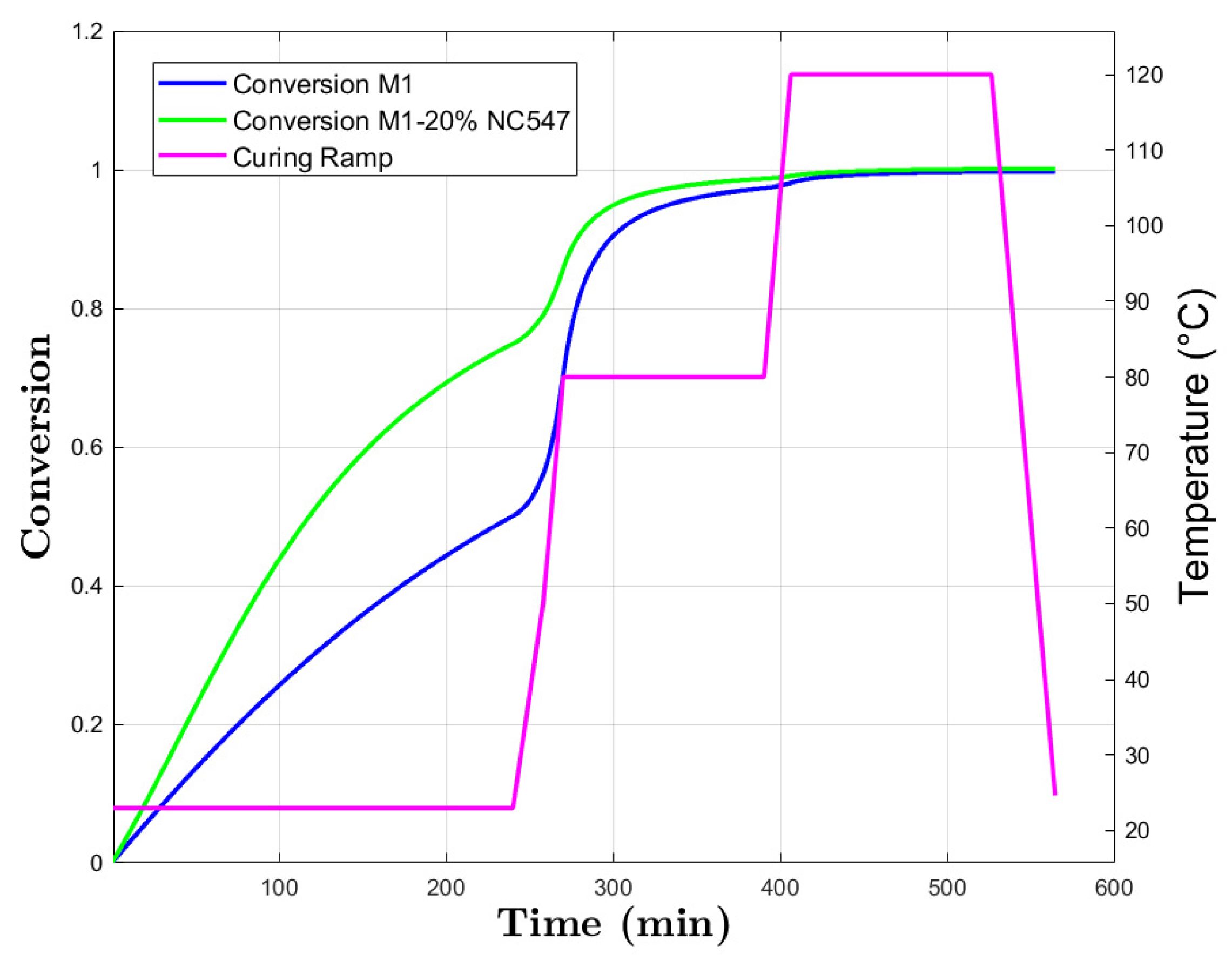
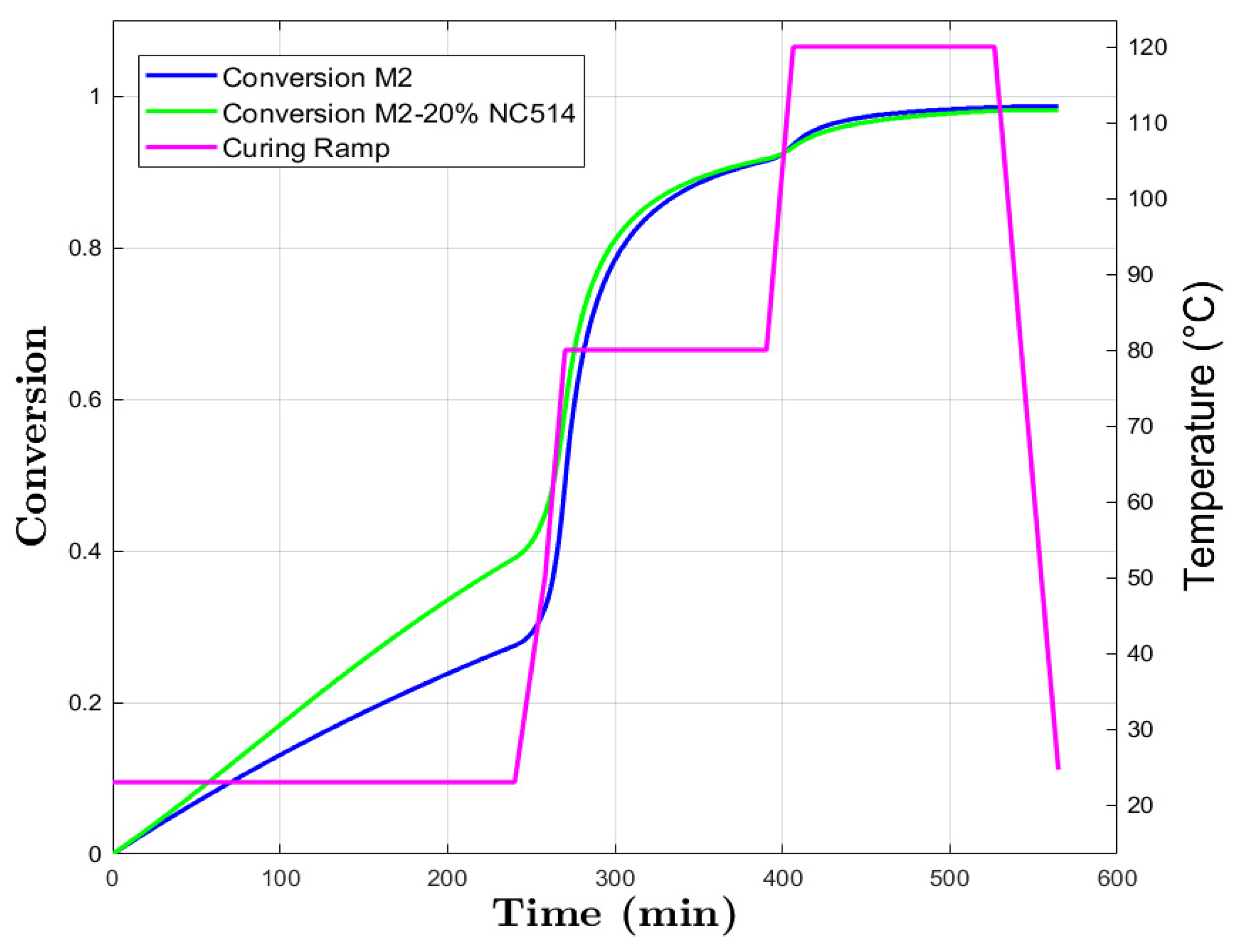
3.3. Kamal–Sourour
Model Curing Prediction
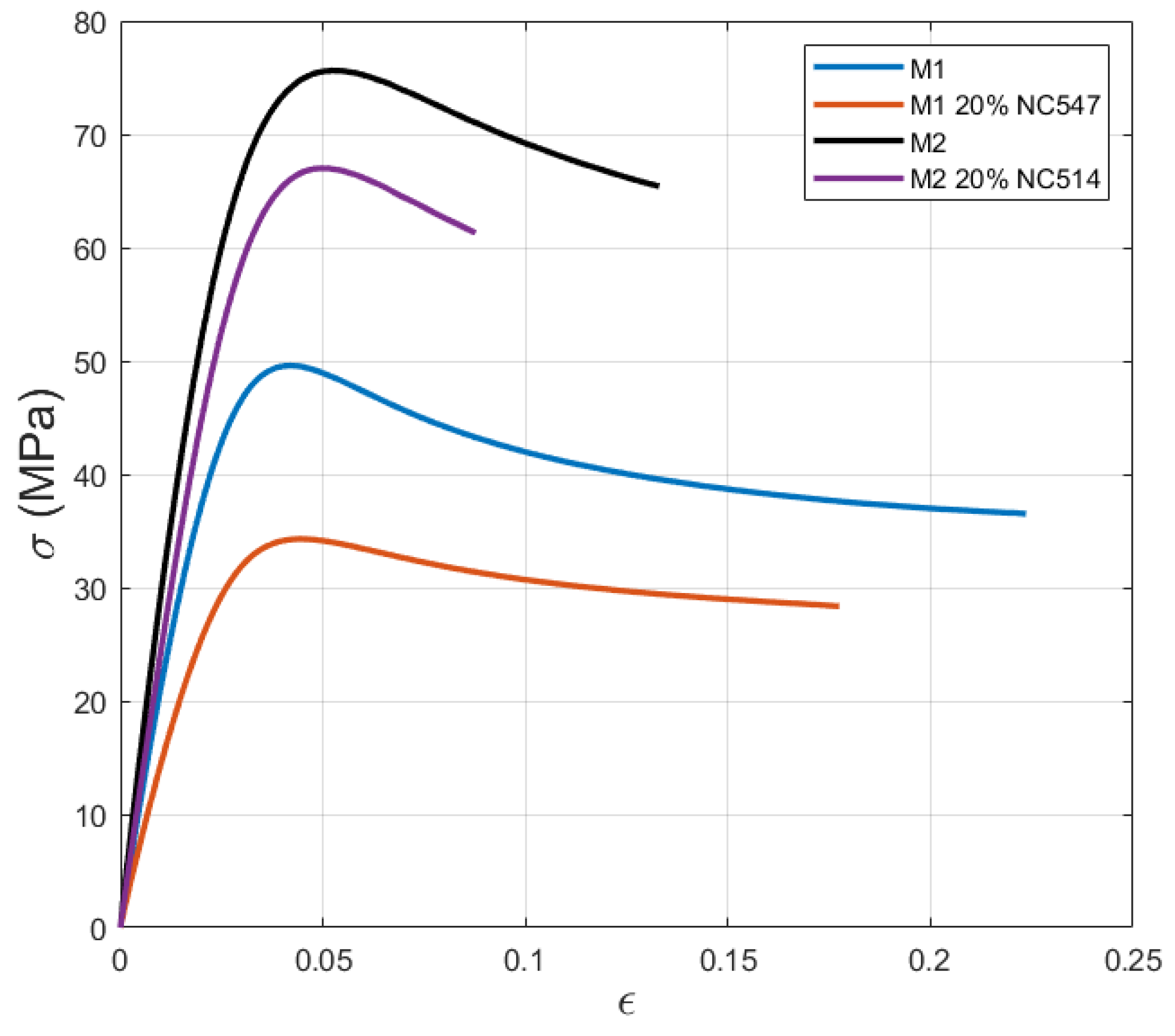
3.4. Tensile Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopinath, A.; Kumar, M.S.; Elayaperumal, A. Experimental investigations on mechanical properties of jute fiber reinforced composites with polyester and epoxy resin matrices. Procedia Eng. 2014, 97, 2052–2063. [Google Scholar]
- Tarafdar, A. Bisphenol A: Toxicity, detection, and remediation. J. Hazard. Mater. 2022, 423, 127097. [Google Scholar] [PubMed]
- Wool, R.; Sun, X.S. Bio-Based Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2011; pp. 56–157. [Google Scholar]
- European Commission. Regulation (EU) 2019/631 of the European Parliament and of the Council of 17 April 2019 setting CO2 emission performance standards for new passenger cars and for new light commercial vehicles, and repealing Regulations (EC) No 443/2009 and (EU) No 510/2011. Off. J. Eur. Union 2019, L 111, 13–53.
- Baroncini, E.A.; Kumar, A.; Eljack, F.; Cavallo, J.A.; Gill, B.J.; Chen, Q.; Zhang, W. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 45. [Google Scholar] [CrossRef]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawde, D.R.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Iadarola, A.; Di Matteo, P.; Ciardiello, R.; Paolino, D.S.; Gazza, F.; Lambertini, V.G.; Brunella, V. Quasi-static and dynamic response of cardanol bio-based epoxy resins: Effect of different bio-contents. Procedia Struct. Integr. 2023, 47, 383–397. [Google Scholar] [CrossRef]
- Loos, A.C.; Springer, G.S. Curing of epoxy matrix composites. J. Compos. Mater. 1983, 17, 135–169. [Google Scholar] [CrossRef]
- Cai, H.; Liu, J.; Yang, C.; Guo, Q.; Zhao, Y.; Yao, S. Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal DSC. Thermochim. Acta 2008, 473, 101–105. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Roşu, D.; Cascaval, C.N.; Mustata, F.; Ciobanu, C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim. Acta 2002, 383, 119–127. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Manić, N.; Janković, B.; Dodevski, V. Model-free and model-based kinetic analysis of Poplar fluff (Populus alba) pyrolysis process under dynamic conditions. J. Therm. Anal. Calorim. 2021, 143, 3419–3438. [Google Scholar] [CrossRef]
- Šimon, P. Isoconversional methods. J. Therm. Anal. Calorim. 2004, 76, 123–132. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Mashouf Roudsari, G.; Mohanty, A.K.; Misra, M. Study of the curing kinetics of epoxy resins with biobased hardener and epoxidized soybean oil. ACS Sustain. Chem. Eng. 2014, 2, 2111–2116. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Rodrigue, D. Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur. Polym. J. 2016, 82, 153–165. [Google Scholar] [CrossRef]
- Mijovic, J.; Fishbain, A.; Wijaya, J. Mechanistic modeling of epoxy-amine kinetics. 1. Model compound study. Macromolecules 1992, 25, 979–985. [Google Scholar] [CrossRef]
- Wan, J.; Lai, S.; Xie, S.; Li, Y.; Lu, H. Model-fitting and model-free nonisothermal curing kinetics of epoxy resin with a low-volatile five-armed starlike aliphatic polyamine. Thermochim. Acta 2011, 525, 31–39. [Google Scholar] [CrossRef]
- ASTM D2196-18; ASTM International. Standard Test Methods for Rheological Properties of Non-Newtonian Materials by Rotational (Brookfield type) Viscometer. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM E3418-99; ASTM International. Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM D638-10; ASTM International. Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- Flammersheim, H.J.; Opfermann, J.R. Investigation of epoxide curing reactions by differential scanning calorimetry–formal kinetic evaluation. Macromol. Mater. Eng. 2001, 286, 143–150. [Google Scholar] [CrossRef]
- Redmann, A.; Berkland, C.; Detamore, M.S. Thermal curing kinetics optimization of epoxy resin in Digital Light Synthesis. Addit. Manuf. 2020, 32, 101018. [Google Scholar] [CrossRef]
- Zvetkov, V.L. Comparative DSC kinetics of the reaction of DGEBA with aromatic diamines: I. Non-isothermal kinetic study of the reaction of DGEBA with m-phenylene diamine. Polymer 2001, 42, 6687–6697. [Google Scholar] [CrossRef]
- Flynn, J.H. Analysis of DSC results by integration. Thermochim. Acta 1993, 217, 129–149. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules 1996, 29, 1867–1873. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Koga, N.; Schick, C. Handbook of Thermal Analysis and Calorimetry: Recent Advances, Techniques and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Turi, E. Thermal Characterization of Polymeric Materials; Academic Press: San Diego, CA, USA, 2012; pp. 250–350. [Google Scholar]
- Sourour, S.; Kamal, M.R. Differential scanning calorimetry of epoxy cure: Isothermal cure kinetics. Thermochim. Acta 1976, 14, 41–59. [Google Scholar] [CrossRef]
- Tran, T.K.N.; Chen, J.; Tsai, C.H.; Chiu, C.F.; Huang, T.W.; Chang, F.C. Hydroxyl telechelic natural rubber-based polyurethane: Influence of molecular weight on non-isothermal cure kinetics. Thermochim. Acta 2015, 620, 51–58. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Y.; Huang, G.; Liu, Y.; Wang, G. Flame retardant, mechanical properties and curing kinetics of DOPO-based epoxy resins. Polym. Degrad. Stab. 2014, 109, 218–225. [Google Scholar] [CrossRef]
- Kamal, M.R.; Sourour, S.K. Kinetics and thermal characterization of thermoset cure. Polym. Eng. Sci. 1973, 13, 59–64. [Google Scholar] [CrossRef]
- Javdanitehran, M.; Naderi, G.; Shendi, H.K.; Kashfi, M. An iterative approach for isothermal curing kinetics modeling of an epoxy resin system. Thermochim. Acta 2016, 623, 72–79. [Google Scholar] [CrossRef]
- Smith, I.T. The mechanism of the crosslinking of epoxide resins by amines. Polymer 1961, 2, 95–108. [Google Scholar] [CrossRef]
- Thomas, R.; Singh, R.P.; Verma, V. Cure kinetics, morphology, and miscibility of modified DGEBA-based epoxy resin—Effects of a liquid rubber inclusion. Polymer 2007, 48, 1695–1710. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional kinetics of polymers: The decade past. Macromol. Rapid Commun. 2017, 38, 1600615. [Google Scholar] [CrossRef]
- Wisanrakkit, G.; Gillham, J.K. The glass transition temperature (Tg) as an index of chemical conversion for a high-Tg amine/epoxy system: Chemical and diffusion-controlled reaction kinetics. J. Appl. Polym. Sci. 1990, 41, 2885–2929. [Google Scholar] [CrossRef]
- Dušek, K.; Dušková-Smrčková, M. Network structure formation during crosslinking of organic coating systems. Prog. Polym. Sci. 2000, 25, 1215–1260. [Google Scholar] [CrossRef]
- Horie, K.; Hiura, H.; Sawada, M.; Oku, T.; Mita, I.; Kambe, H. Calorimetric investigation of polymerization reactions. III. Curing reaction of epoxides with amines. J. Polym. Sci. A 1970, 8, 1357–1372. [Google Scholar] [CrossRef]
- Montserrat, S.; Málek, J. A kinetic analysis of the curing reaction of an epoxy resin. Thermochim. Acta 1993, 228, 47–60. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]



| Epoxy Resin 2501A + 2002B (M1) | Values | Test Method |
|---|---|---|
| Mixing ratio by mass | 100:52 | Internal supplier method |
| Mix Viscosity @ 25 °C [cPs] | 1100 | ASTM D2196 [20] |
| Pot life, 100 g mix @ 23 °C [min] | 58 | Internal supplier method |
| Glass transition temperature [°C] | 73 | ASTM 3418-99 [21] |
| Tensile Strength [MPa] | 52 | ASTM D638-10 [22] |
| Tensile Modulus [MPa] | 2599 | ASTM D638-10 [22] |
| Epoxy Resin 2501A + 2401B (M2) | Values | Test Method |
|---|---|---|
| Mixing ratio by mass | 100:31 | Internal supplier method |
| Mix Viscosity @ 25 °C [cPs] | 905 | ASTM D2196 [20] |
| Pot life, 100 g mix @ 23 °C [min] | 95 | Internal supplier method |
| Glass transition temperature [°C] | 100 | ASTM 3418-99 [21] |
| Tensile Strength [MPa] | 69 | ASTM D638-10 [22] |
| Tensile Modulus [MPa] | 3134 | ASTM D638-10 [22] |
| Name | Cardolite FORMULITE Blends | Bio-Content |
|---|---|---|
| M1 | (2501A + 2002B) + 0% novolac | 45% |
| M1-20% NC547 | (2501A + 2002B) + 20% NC547 | 51% |
| M2 | (2501A + 2401B) + 0% epoxy | 34% |
| M2-20% NC514 | (2501A + 2401B) + 20% NC514 | 39% |
| Fitting Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Ea1 (kJ/mol) | Log Pre Exp (Log 1/s) | React Order (n) | Log Autocat PreExp (Log 1/s) | AutoCat Power (m) | Ea2 (kJ/mol) | R2 |
| M1 | 54.3 | 3.34 | 1.6 | 1.72 | 0.15 | 52.1 | 0.99807 |
| M1-20% NC547 | 48.5 | 2.75 | 1.6 | 1.83 | 0.18 | 48.9 | 0.99960 |
| M2 | 48.3 | 1.58 | 1.9 | 2.72 | 0.29 | 47.0 | 0.99504 |
| M2-20% NC514 | 45.6 | 2.23 | 1.9 | 2.32 | 0.36 | 48.3 | 0.99884 |
| Sample | Total Bio-Content | UTS (MPa) | Elastic Modulus (MPa) | Tg (°C) |
|---|---|---|---|---|
| M1 | 45% | 51 | 2291 | 65 |
| M1+20% NC547 | 51% | 34 | 1558 | 59 |
| M2 | 34% | 76 | 3098 | 92 |
| M2+20% NC514 | 39% | 67 | 2473 | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Matteo, P.; Iadarola, A.; Ciardiello, R.; Paolino, D.S.; Gazza, F.; Lambertini, V.G.; Brunella, V. Cross-Linking Reaction of Bio-Based Epoxy Systems: An Investigation into Cure Kinetics. Polymers 2024, 16, 2499. https://doi.org/10.3390/polym16172499
Di Matteo P, Iadarola A, Ciardiello R, Paolino DS, Gazza F, Lambertini VG, Brunella V. Cross-Linking Reaction of Bio-Based Epoxy Systems: An Investigation into Cure Kinetics. Polymers. 2024; 16(17):2499. https://doi.org/10.3390/polym16172499
Chicago/Turabian StyleDi Matteo, Pietro, Andrea Iadarola, Raffaele Ciardiello, Davide Salvatore Paolino, Francesco Gazza, Vito Guido Lambertini, and Valentina Brunella. 2024. "Cross-Linking Reaction of Bio-Based Epoxy Systems: An Investigation into Cure Kinetics" Polymers 16, no. 17: 2499. https://doi.org/10.3390/polym16172499








