Toward the Production of Hydroxyapatite/Poly(Ether-Ether-Ketone) (PEEK) Biocomposites: Exploring the Physicochemical, Mechanical, Cytotoxic and Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Biocomposites by High-Energy Ball Milling
2.3. Characterization
2.3.1. X-ray Diffraction (XRD)
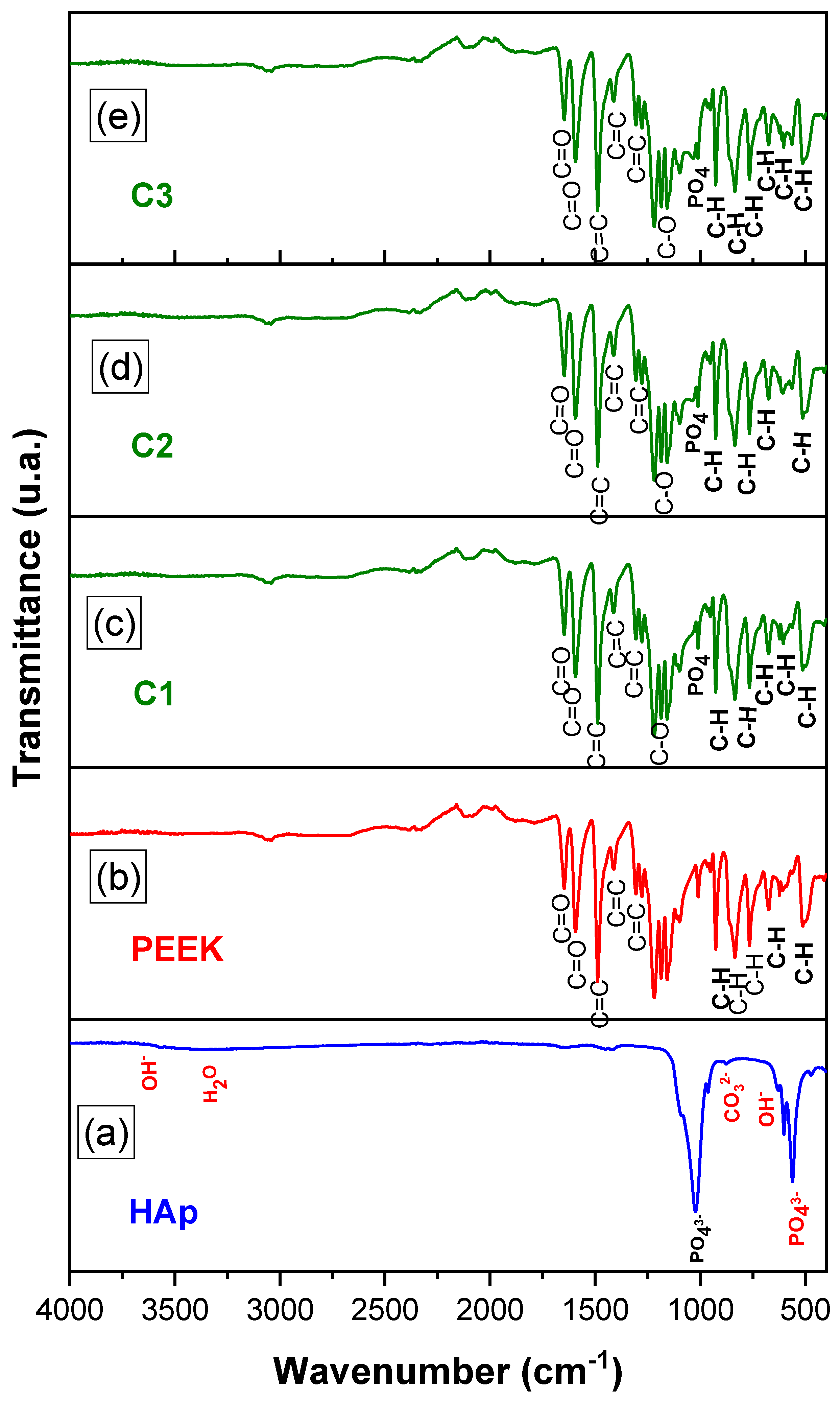
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
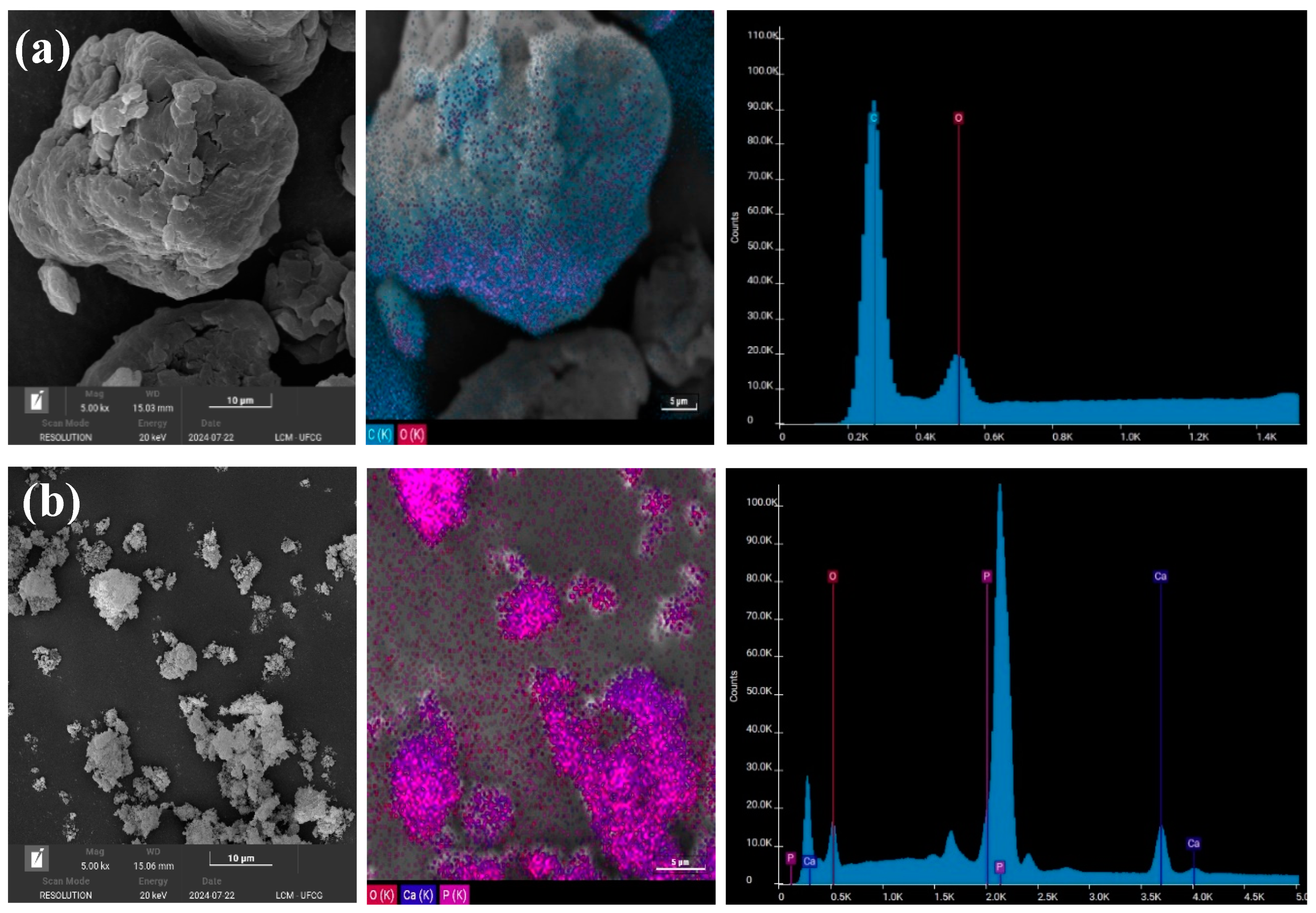
2.3.3. Scanning Electron Microscopy (SEM/EDS)
2.3.4. Apparent Porosity Determination
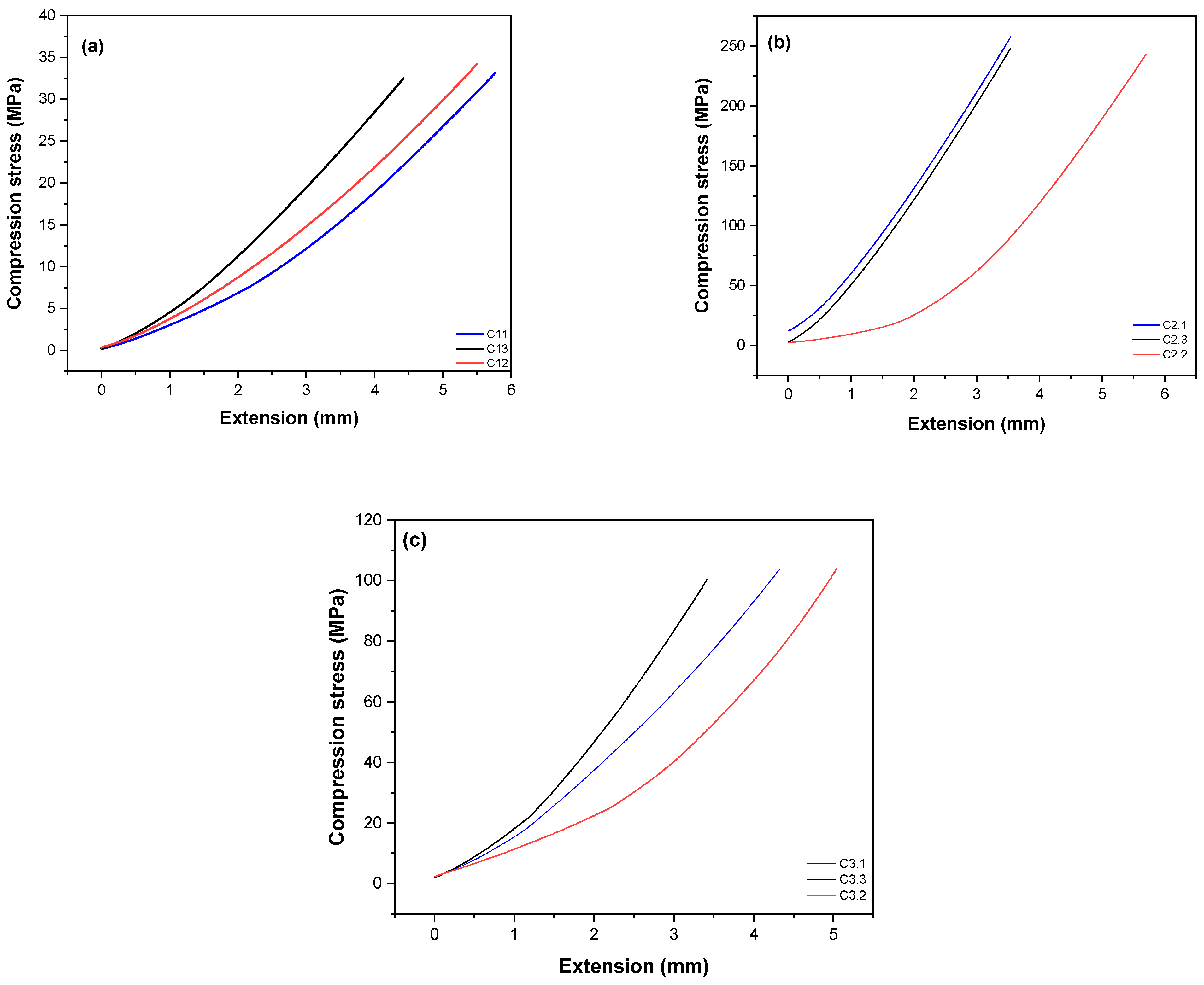
2.3.5. Compressive Strength Mechanical Testing
2.3.6. Cytotoxicity Testing
2.3.7. Determination of Antimicrobial Activity from the Minimum Inhibitory Concentration (MIC)
Preparation of Bacterial Suspension and Inoculum Standardization
Broth Microdilution Method for Minimum Inhibitory Concentration Determination
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Celiński, M.; Cybulski, M.; Fiłon, J.; Muszalik, M.; Goniewicz, M.; Krajewska-Kułak, E.; Ślifirczyk, A. Analysis of medical Management in Geriatric Patients in the hospital emergency department by example of selected cities with county status in Poland: A retrospective cohort study. Int. J. Environ. Res. Public Health 2021, 19, 48. [Google Scholar] [CrossRef]
- Kodama, J.; Wilkinson, K.J.; Iwamoto, M.; Otsuru, S.; Enomoto-Iwamoto, M. The role of hypertrophic chondrocytes in regulation of the cartilage-to-bone transition in fracture healing. Bone Rep. 2022, 17, 101616. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Rahaman, K.A.; Kim, Y.-C.; Jeon, H.; Han, H.-S. Fostering tissue engineering and regenerative medicine to treat musculoskeletal disorders in bone and muscle. Bioact. Mater. 2024, 40, 345–365. [Google Scholar] [CrossRef]
- Tsang, K.Y.; Cheah, K.S.E. The extended chondrocyte lineage: Implications for skeletal homeostasis and disorders. Curr. Opin. Cell Biol. 2019, 61, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Li, C.; Chai, W.; Zhang, L.; Chen, H.; Zhang, W.; Hou, Z.; Chen, B.; Sun, T. Clinical guidelines for the diagnosis and treatment of fragility fractures of the pelvis. Orthop. Surg. 2023, 15, 2195–2212. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Costa, M.M.; Alves, N.; Miranda, G.; Silva, F.S. Additive manufacturing of NiTi-Ti6Al4V multi-material cellular structures targeting orthopedic implants. Opt. Lasers Eng. 2020, 134, 106208. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, X.; Zhu, D.; Zheng, Y. Design, printing, and engineering of regenerative biomaterials for personalized bone healthcare. Prog. Mater. Sci. 2023, 134, 101072. [Google Scholar]
- Ślusarczyk, K.; Flejszar, M.; Spilarewicz, K.; Wytrwal, M.; Awsiuk, K.; Wolski, K.; Raczkowska, J.; Janiszewska, N.; Chmielarz, P. On the way to increase osseointegration potential: Sequential SI-ATRP as promising tool for PEEK-based implant nano-engineering. Eur. Polym. J. 2024, 210, 112953. [Google Scholar] [CrossRef]
- Adhikara, A.G.; Maharani, A.P.; Puspitasari, A.; Nuswantoro, N.F.; Juliadmi, D.; Maras, M.A.J.; Nugroho, D.B.; Saksono, B. Bovine hydroxyapatite for bone tissue engineering: Preparation, characterization, challenges, and future perspectives. Eur. Polym. J. 2024, 214, 113171. [Google Scholar] [CrossRef]
- Daneshvar, A.; Farokhi, M.; Bonakdar, S.; Vossoughi, M. Synthesis and characterization of injectable thermosensitive hydrogel based on Pluronic-grafted silk fibroin copolymer containing hydroxyapatite nanoparticles as potential for bone tissue engineering. Int. J. Biol. Macromol. 2024, 277, 134412. [Google Scholar] [CrossRef]
- Fendi, F.; Abdullah, B.; Suryani, S.; Usman, A.N.; Tahir, D. Development and application of hydroxyapatite-based scaffolds for bone tissue regeneration: A systematic literature review. Bone 2024, 183, 117075. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Naghib, S.M. 3D and 4D printing hydroxyapatite-based scaffolds for bone tissue engineering and regeneration. Heliyon 2023, 9, e19363. [Google Scholar] [CrossRef]
- Safavi, M.S.; Khalil-Allafi, J.; Visai, L. Improved osteogenic activity of NiTi orthopedic implant by HAp-Nb2O5 composite coatings: Materials and biological points of view. Biomater. Adv. 2023, 150, 213435. [Google Scholar] [CrossRef]
- Chopra, V.; Fuentes-Velasco, V.; Nacif-Lopez, S.R.; Melendez-Malpicca, J.; Mendez-Hernandez, A.S.; Ramos-Mendez-Iris, L.F.; Arroyo-Jimenez, D.A.; Reyes-Segura, D.G.; Gonzalez-Y-Mendoza, P.; Sanchez-Hernandez, K.A. Advancements in 3D-4D printing of hydroxyapatite composites for bone tissue engineering. Ceram. Int. 2024, in press. [Google Scholar] [CrossRef]
- Gani, M.A.; Budiatin, A.S.; Shinta, D.W.; Ardianto, C.; Khotib, J. Bovine hydroxyapatite-based scaffold accelerated the inflammatory phase and bone growth in rats with bone defect. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000221149193. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Mahmoud, E.M.; Sayed, M.; Kamel, S.M.; Naga, S.M. In-vitro and in-vivo evaluation for the bio-natural Alginate/nano-Hydroxyapatite (Alg/n-HA) injectable hydrogel for critical size bone substitution. Int. J. Biol. Macromol. 2023, 253, 126618. [Google Scholar] [CrossRef]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 32. [Google Scholar] [CrossRef]
- Qiu, B.; Zhao, C.; Pan, J.; Zhou, Q.; Yao, W. Enhancing osteointegration and antibacterial properties of PEEK implants via AMP/HA dual-layer coatings. Surf. Interfaces 2024, 51, 104761. [Google Scholar] [CrossRef]
- He, M.; Huang, Y.; Xu, H.; Feng, G.; Liu, L.; Li, Y.; Sun, D.; Zhang, L. Modification of polyetheretherketone implants: From enhancing bone integration to enabling multi-modal therapeutics. Acta Biomater. 2021, 129, 18–32. [Google Scholar] [CrossRef]
- Han, X.; Gao, W.; Zhou, Z.; Yang, S.; Wang, J.; Shi, R.; Li, Y.; Jiao, J.; Qi, Y.; Zhao, J. Application of biomolecules modification strategies on PEEK and its composites for osteogenesis and antibacterial properties. Colloids Surf. B Biointerfaces 2022, 215, 112492. [Google Scholar]
- Li, J.; Liu, H.; Wang, Y.; Wang, L.; Liu, G.; Chen, C.; Wei, L.; Li, H. Strength-plasticity synergetic CF/PEEK composites obtained by adjusting melt flow rate. Polymer 2024, 305, 127186. [Google Scholar]
- Hu, Q.; Wang, Y.; Liu, S.; Liu, Q.; Zhang, H. 3D printed polyetheretherketone bone tissue substitute modified via amoxicillin-laden hydroxyapatite nanocoating. J. Mater. Sci. 2022, 57, 18601–18614. [Google Scholar]
- Shimizu, T.; Fujibayashi, S.; Yamaguchi, S.; Yamamoto, K.; Otsuki, B.; Takemoto, M.; Tsukanaka, M.; Kizuki, T.; Matsushita, T.; Kokubo, T.; et al. Bioactivity of sol–gel-derived TiO2 coating on polyetheretherketone: In vitro and in vivo studies. Acta Biomater. 2016, 35, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Fan, X.; Peng, F.; Yan, X.; Song, J.; Deng, C.; Liu, M.; Zeng, D.; Ning, C. Characterization of porous titanium-hydroxyapatite composite biological coating on polyetheretherketone (PEEK) by vacuum plasma spraying. Coatings 2022, 12, 433. [Google Scholar] [CrossRef]
- Yabutsuka, T.; Fukushima, K.; Hiruta, T.; Takai, S.; Yao, T. Effect of pores formation process and oxygen plasma treatment to hydroxyapatite formation on bioactive PEEK prepared by incorporation of precursor of apatite. Mater. Sci. Eng. C 2017, 81, 349–358. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T. 3D printing of PEEK and its composite to increase biointerfaces as a biomedical material-A review. Colloids Surf. B Biointerfaces 2021, 203, 111726. [Google Scholar] [CrossRef]
- Barandehfard, F.; Kianpour Rad, M.; Hosseinnia, A.; Rashidi, A.; Tahriri, M.; Tayebi, L. The evaluation of the mechanical characteristics of the synthesized glass-ionomer cements (GICs): The effect of hydroxyapatite and fluorapatite nanoparticles and glass powders. J. Aust. Ceram. Soc. 2019, 55, 507–517. [Google Scholar] [CrossRef]
- Saeri, M.R.; Afshar, A.; Ghorbani, M.; Ehsani, N.; Sorrell, C.C. The wet precipitation process of hydroxyapatite. Mater. Lett. 2003, 57, 4064–4069. [Google Scholar] [CrossRef]
- Sarkar, C.; Sahu, S.K.; Sinha, A.; Chakraborty, J.; Garai, S. Facile synthesis of carbon fiber reinforced polymer-hydroxyapatite ternary composite: A mechanically strong bioactive bone graft. Mater. Sci. Eng. C 2019, 97, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- ASTM D695-23; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM: West Conshohocken, PA, USA, 2023. [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Pina, H.d.V.; Farias, A.J.A.d.; Barbosa, F.C.; de Lima Souza, W.J.; de Sousa Barros, A.B.; Batista Cardoso, M.J.; Fook, M.V.L.; Wellen, R.M.R. Microbiological and cytotoxic perspectives of active PCL/ZnO film for food packaging. Mater. Res. Express 2020, 7, 025312. [Google Scholar] [CrossRef]
- Wanderley, D.M.S.; Melo, D.F.; Silva, L.M.; Souza, J.W.L.; Pina, H.V.; Lima, D.B.; Amoah, S.K.S.; Borges, S.M.P.; Fook, M.V.L.; Moura, R.O. Biocompatibility and mechanical properties evaluation of chitosan films containing an N-acylhydrazonic derivative. Eur. J. Pharm. Sci. 2020, 155, 105547. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, S.-H.; Kim, K.-N.; Kim, K.-M.; Shim, I.-B.; Lee, Y.-K. Cytotoxicity of ferrite particles by MTT and agar diffusion methods for hyperthermic application. J. Magn. Magn. Mater. 2005, 293, 287–292. [Google Scholar] [CrossRef]
- Puškar, T.; Trifković, B.; Koprivica, D.D.; Kojić, V.; Jevremović, A.; Mirković, S.; Eggbeer, D. In vitro cytotoxicity assessment of the 3D printed polymer based epoxy resin intended for use in dentistry. Vojnosanit. Pregl. 2019, 76. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Li, S.; Li, G.; Lian, X.; Hu, J.; Li, M.; Wang, B.; Zou, Y.; Zhou, Z. Integrated porous polyetheretherketone/hydroxyapatite scaffolds: Design, manufacturing and performance evaluation. Compos. Part A Appl. Sci. Manuf. 2023, 173. [Google Scholar] [CrossRef]
- Asante, N.A.; Wang, Y.; Bakhet, S.; Kareem, S.; Owusu, K.A.; Hu, Y.; Appiah, M. Ambient temperature sulfonated carbon fiber reinforced PEEK with hydroxyapatite and reduced graphene oxide hydroxyapatite composite coating. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2174–2183. [Google Scholar] [CrossRef]
- Qi, D.; Wang, N.; Wang, S.; Liu, L.; Zhu, S.; She, P.; Yue, X. High-strength porous polyetheretherketone/hydroxyapatite composite for the treatment of bone defect. Compos. Commun. 2023, 38, 101473. [Google Scholar] [CrossRef]
- Yusong, P.; Qianqian, S.; Yan, C. Fabrication and characterisation of functional gradient hydroxyapatite reinforced poly (ether ether ketone) biocomposites. Micro Nano Lett. 2013, 8, 357–361. [Google Scholar] [CrossRef]
- Nascimento, M.; Dos Santos Almeida, A.R.; Hirata, M.C.; Elzubair, A.; Navarro da Rocha, D.; Prado da Silva, M.H. Biomineralization of calcium phosphates functionalized with hydroxyapatite-binding peptide. J. Mech. Behav. Biomed. Mater. 2023, 146, 106082. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Tabrizi, B.; Basirun, W.J.; Yeong, C.H.; Walvekar, R.; Phang, S.W. Mechanosynthesis and microstructural characterization of F− and CO32− mono-and co-substituted hydroxyapatite. J. Mol. Struct. 2024, 1315, 138809. [Google Scholar] [CrossRef]
- Xu, H.; Yang, L.; Wang, P.; Liu, Y.; Peng, M. Kinetic research on the sorption of aqueous lead by synthetic carbonate hydroxyapatite. J. Environ. Manag. 2008, 86, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.S.F.; Ferreira, V.P.; Sá, M.D.; Fook, M.V.L. Modificação da superfície do poli (éter-éter-cetona). Matéria (Rio de Janeiro) 2017, 22, e-11883. [Google Scholar] [CrossRef]
- Afshar, A.; Ghorbani, M.; Ehsani, N.; Saeri, M.R.; Sorrell, C.C. Some important factors in the wet precipitation process of hydroxyapatite. Mater. Des. 2003, 24, 197–202. [Google Scholar] [CrossRef]
- Ponciano, R.C.d.O.; de Melo Costa, A.C.F.; Barbosa, R.C.; Fook, M.V.L.; Ponciano, J.J. Chitosan and hydroxyapatite scaffolds with amoxicillin for bone repair. Res. Soc. Dev. 2021, 10, e13410514790. [Google Scholar] [CrossRef]
- Manzoor, F.; Golbang, A.; Jindal, S.; Dixon, D.; McIlhagger, A.; Harkin-Jones, E.; Crawford, D.; Mancuso, E. 3D printed PEEK/HA composites for bone tissue engineering applications: Effect of material formulation on mechanical performance and bioactive potential. J. Mech. Behav. Biomed. Mater. 2021, 121, 104601. [Google Scholar] [CrossRef]
- Zhang, D.; George, O.J.; Petersen, K.M.; Jimenez-Vergara, A.C.; Hahn, M.S.; Grunlan, M.A. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014, 10, 4597–4605. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Hu, J.; Li, G.; Wang, B.; Zhou, Z. A new strategy for PEEK-based biocomposites to achieve porous surface for bioactivity and adjustable mechanical properties for orthopedic stress matching. Compos. Part A Appl. Sci. Manuf. 2024, 177, 107909. [Google Scholar] [CrossRef]
- Bastan, F.E.; Atiq Ur Rehman, M.; Avcu, Y.Y.; Avcu, E.; Ustel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surf. B Biointerfaces 2018, 169, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Landy, B.C.; VanGordon, S.B.; McFetridge, P.S.; Sikavitsas, V.I.; Jarman-Smith, M. Mechanical and in vitro investigation of a porous PEEK foam for medical device implants. J. Appl. Biomater. Funct. Mater. 2013, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Torstrick, F.B.; Lin, A.S.P.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Chen, X.; Zeng, X.; Luo, K. Programmable, biodegradable composite scaffolds with variable pore morphology for minimal invasive bone repair. Compos. Part A Appl. Sci. Manuf. 2022, 162, 107130. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, H.; Dong, E.; Kang, J.; Liu, C.; Sun, C.; Li, D.; Wang, L. Additively-manufactured PEEK/HA porous scaffolds with highly-controllable mechanical properties and excellent biocompatibility. Mater. Sci. Eng. C 2021, 128, 112333. [Google Scholar] [CrossRef]
- Silva, L.A.J. Obtenção e Caracterização de Scaffolds de Hidroxiapatita Utilizando Amido de Milho Como Agente Porogênico. Master’s Thesis, State University of Campinas, Faculty of Mechanical Engineering, Campinas, Brazylia, 2012. [Google Scholar]
- Oladapo, B.I.; Ismail, S.O.; Bowoto, O.K.; Omigbodun, F.T.; Olawumi, M.A.; Muhammad, M.A. Lattice design and 3D-printing of PEEK with Ca10 (OH)(PO4) 3 and in-vitro bio-composite for bone implant. Int. J. Biol. Macromol. 2020, 165, 50–62. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Wang, Z.; Li, S.; Song, L.; Xu, T.; Shen, G.; Wang, Y.; Huang, T.; Dong, X. Surface-activated 3D-printed PEEK implant enhances anti-infection and osteogenesis. Compos. Part B Eng. 2024, 273, 111258. [Google Scholar] [CrossRef]
- Sun, A.A.; Lin, X.; Xue, Z.; Huang, J.; Bai, X.; Huang, L.; Lin, X.; Weng, S.; Chen, M. Facile surface functional polyetheretherketone with antibacterial and immunoregulatory activities for enhanced regeneration toward bacterium-infected bone destruction. Drug Deliv. 2021, 28, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, P.; Zhang, X.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef]
- He, X.; Deng, Y.; Yu, Y.; Lyu, H.; Liao, L. Drug-loaded/grafted peptide-modified porous PEEK to promote bone tissue repair and eliminate bacteria. Colloids Surf. B Biointerfaces 2019, 181, 767–777. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, M.H.; Abdalsalam, A.H.; Bohan, A.J. Influence of chitosan on the antibacterial activity of composite coating (PEEK/HAp) fabricated by electrophoretic deposition. Prog. Org. Coat. 2019, 130, 251–259. [Google Scholar] [CrossRef]
- Resmim, C.M.; Dalpasquale, M.; Vielmo, N.I.C.; Mariani, F.Q.; Villalba, J.C.; Anaissi, F.J.; Caetano, M.M.; Tusi, M.M. Study of physico-chemical properties and in vitro antimicrobial activity of hydroxyapatites obtained from bone calcination. Prog. Biomater. 2019, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]



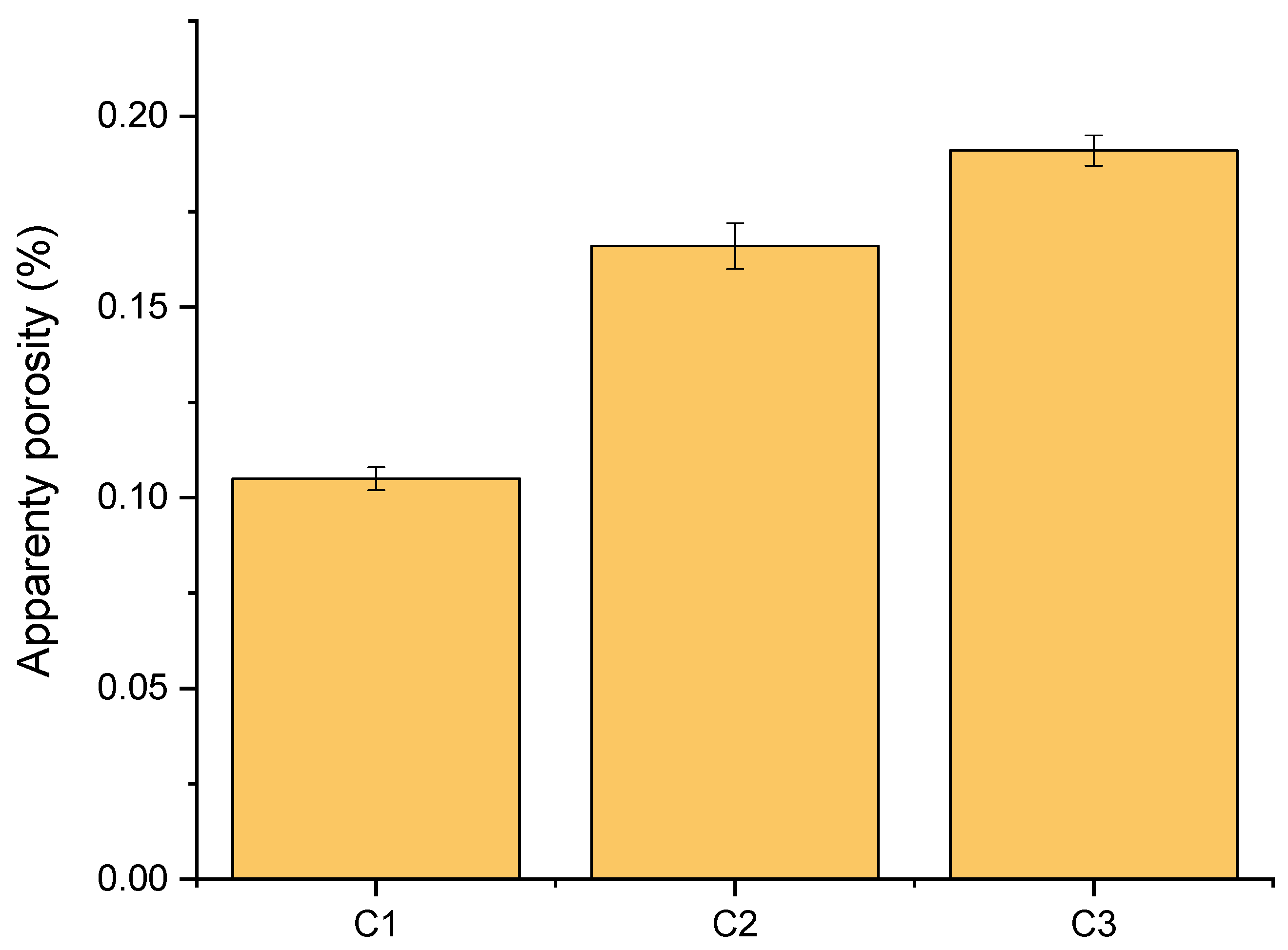
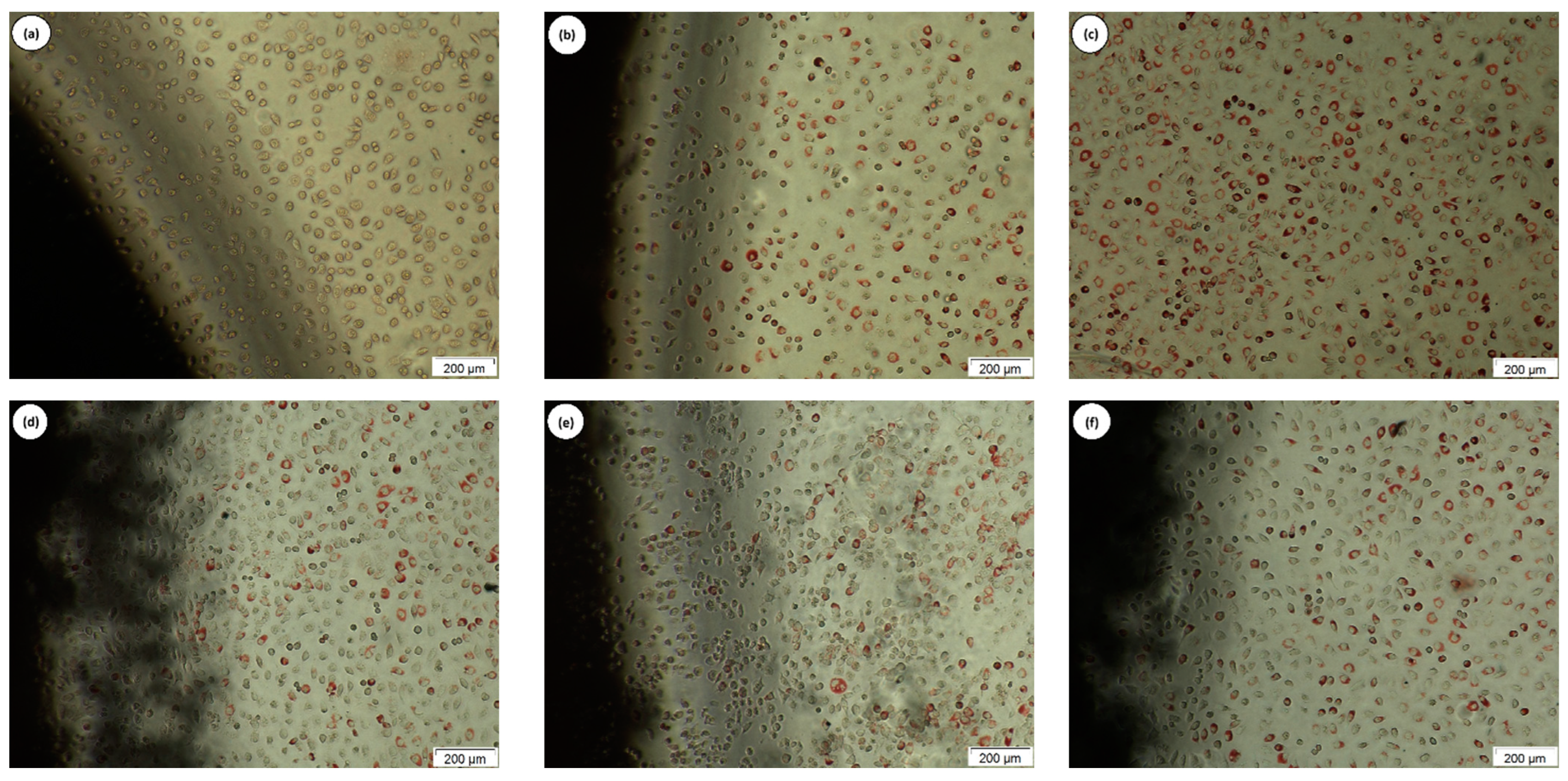
| Degree | Cytotoxicity | Description of the Cytotoxicity Zone |
|---|---|---|
| 0 | Absence | Absence of bleaching around or under the sample. |
| 1 | Light | Bleaching zone limited to the area under the sample |
| 2 | Soft | Sample bleaching zone size less than 0.45 cm. |
| 3 | Moderate | Sample bleaching zone size less than 0.45 cm to 1.0 cm. |
| 4 | Severe | Size of the sample bleaching zone greater than 1.0 cm, but not involving the entire plate. |
| Tested Material | Degree Discoloration | Cell Lysis | Interpretation |
|---|---|---|---|
| Positive control | 3 | 4 | Moderate cytotoxicity |
| Negative control | 0 | 0 | Non-cytotoxic |
| white | 0 | 0 | Non-cytotoxic |
| C1 | 0 | 0 | Non-cytotoxic |
| C2 | 0 | 0 | Non-cytotoxic |
| C3 | 0 | 0 | Non-cytotoxic |
| Microrganisms | MIC/MBC (µg.mL−1) | |||
|---|---|---|---|---|
| C2 | CIPRO | OXA | CFT | |
| S. aureus ATCC (25923) | 1562.5/3125 | 200 | 200 | - |
| P. aeruginosa ATCC (27853) | 1562.5/3125 | 200 | - | 200 |
| E. coli ATCC (25922) | 390.62/781.25 | 200 | - | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.R.; Filho, J.A.C.; Luna, C.B.B.; Dantas, G.M.P.; Costa, A.C.F.d.M.; Oliveira, N.M.d.S. Toward the Production of Hydroxyapatite/Poly(Ether-Ether-Ketone) (PEEK) Biocomposites: Exploring the Physicochemical, Mechanical, Cytotoxic and Antimicrobial Properties. Polymers 2024, 16, 2520. https://doi.org/10.3390/polym16172520
Costa MR, Filho JAC, Luna CBB, Dantas GMP, Costa ACFdM, Oliveira NMdS. Toward the Production of Hydroxyapatite/Poly(Ether-Ether-Ketone) (PEEK) Biocomposites: Exploring the Physicochemical, Mechanical, Cytotoxic and Antimicrobial Properties. Polymers. 2024; 16(17):2520. https://doi.org/10.3390/polym16172520
Chicago/Turabian StyleCosta, Meirilany Rozeno, José Adeilton Carvalho Filho, Carlos Bruno Barreto Luna, Gleydis Manalig Pereira Dantas, Ana Cristina Figueiredo de Melo Costa, and Nadja Maria da Silva Oliveira. 2024. "Toward the Production of Hydroxyapatite/Poly(Ether-Ether-Ketone) (PEEK) Biocomposites: Exploring the Physicochemical, Mechanical, Cytotoxic and Antimicrobial Properties" Polymers 16, no. 17: 2520. https://doi.org/10.3390/polym16172520






