Molecularly Imprinted Polypyrrole-Modified Screen-Printed Electrode for Dopamine Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthetic Urine Preparation
2.3. Urine Sample Collection and Pre-Treatment
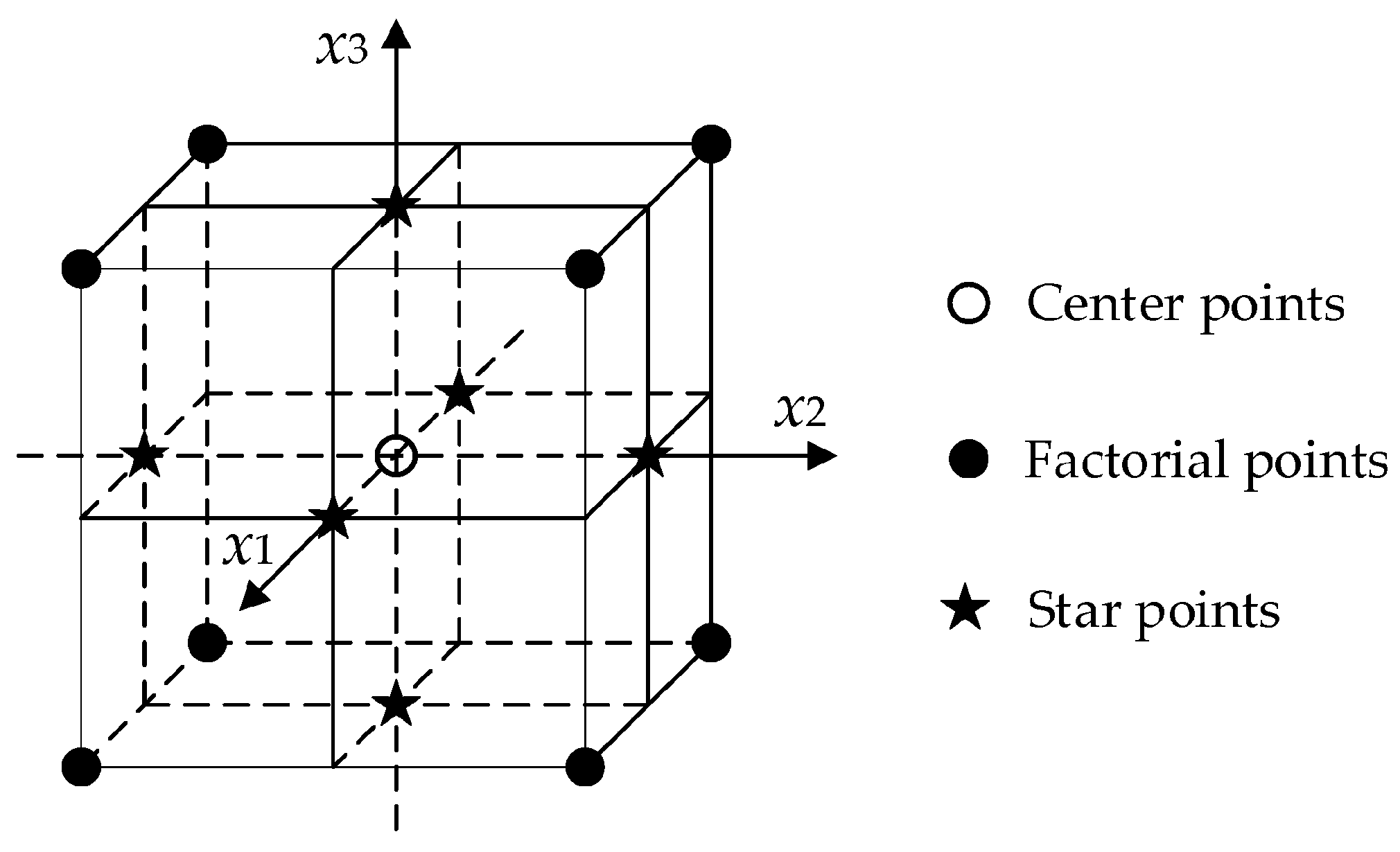
2.4. e-MIP and e-NIP Preparation: Optimization of the Experimental Condition by DoE
2.5. Bare, e-MIP and e-NIP Working Electrodes Surface Characterization
2.6. Study of the Electrochemical Behavior of Dopamine at the Bare and e-MIP-Modified Electrodes
2.7. Quantification of Dopamine by Differential Pulse Voltammetry (DPV)
3. Results and Discussion
3.1. Modification and Characterization of the Working Electrode of the Screen-Printed Cell
3.2. Characterization of the Working Electrode Surface before and after Modification
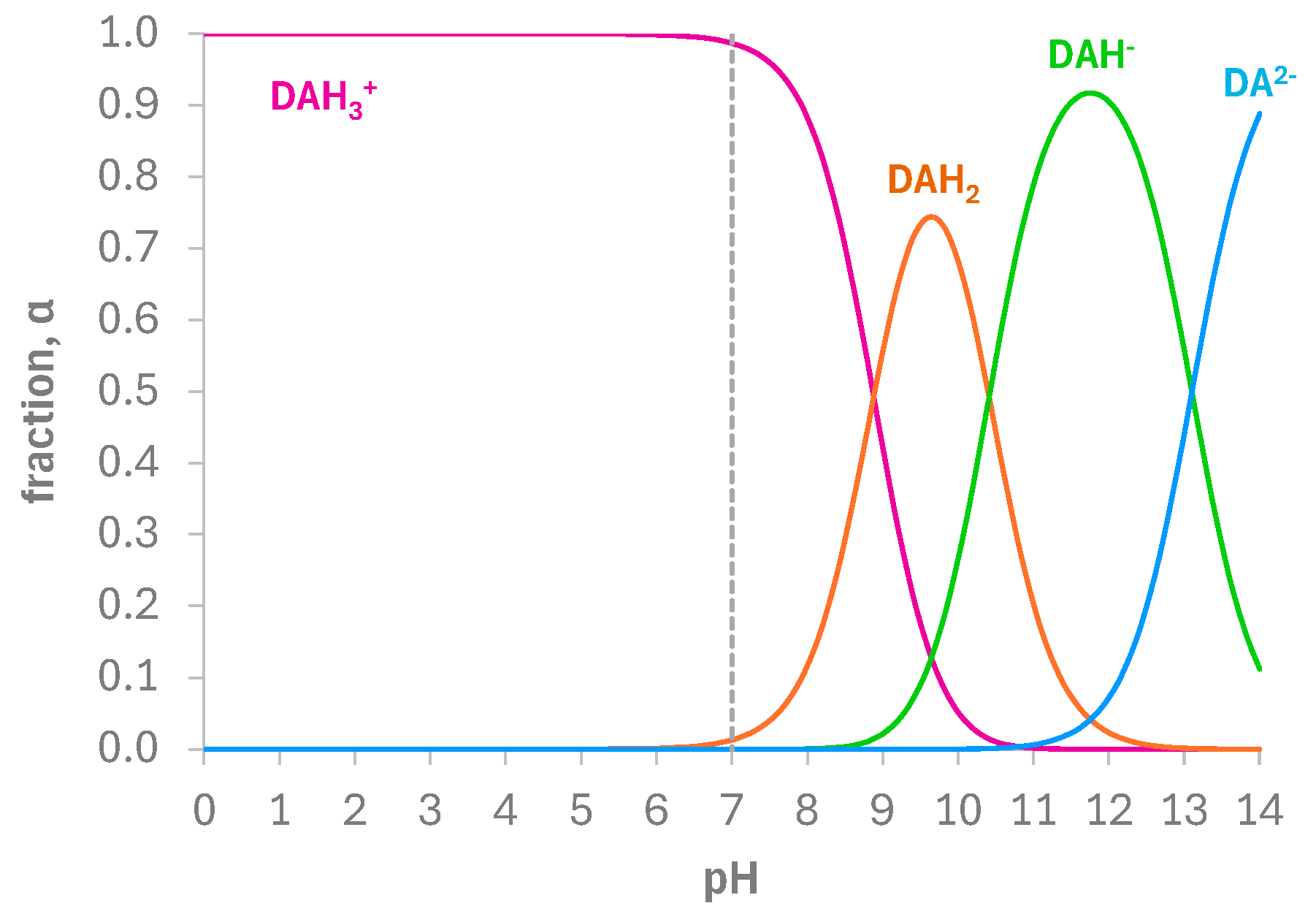
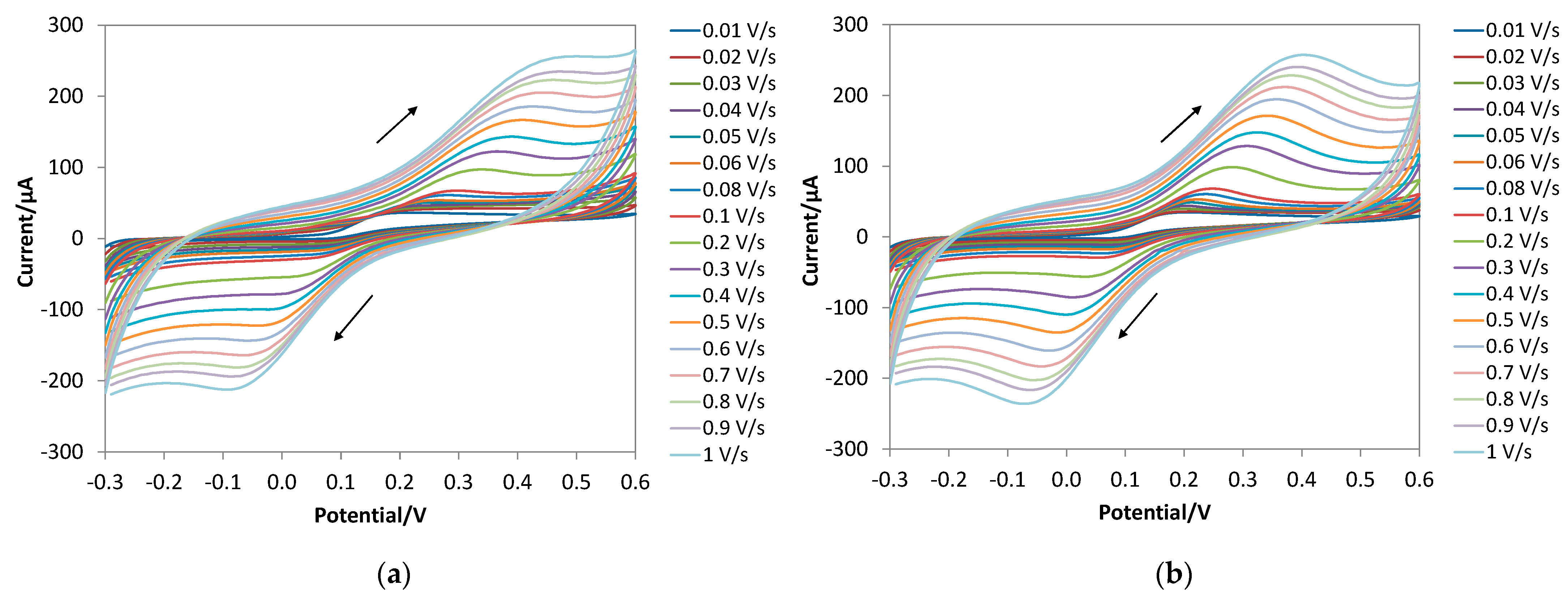
3.3. Electrochemical Behavior of DA at the Bare and e-MIP-Modified Electrode
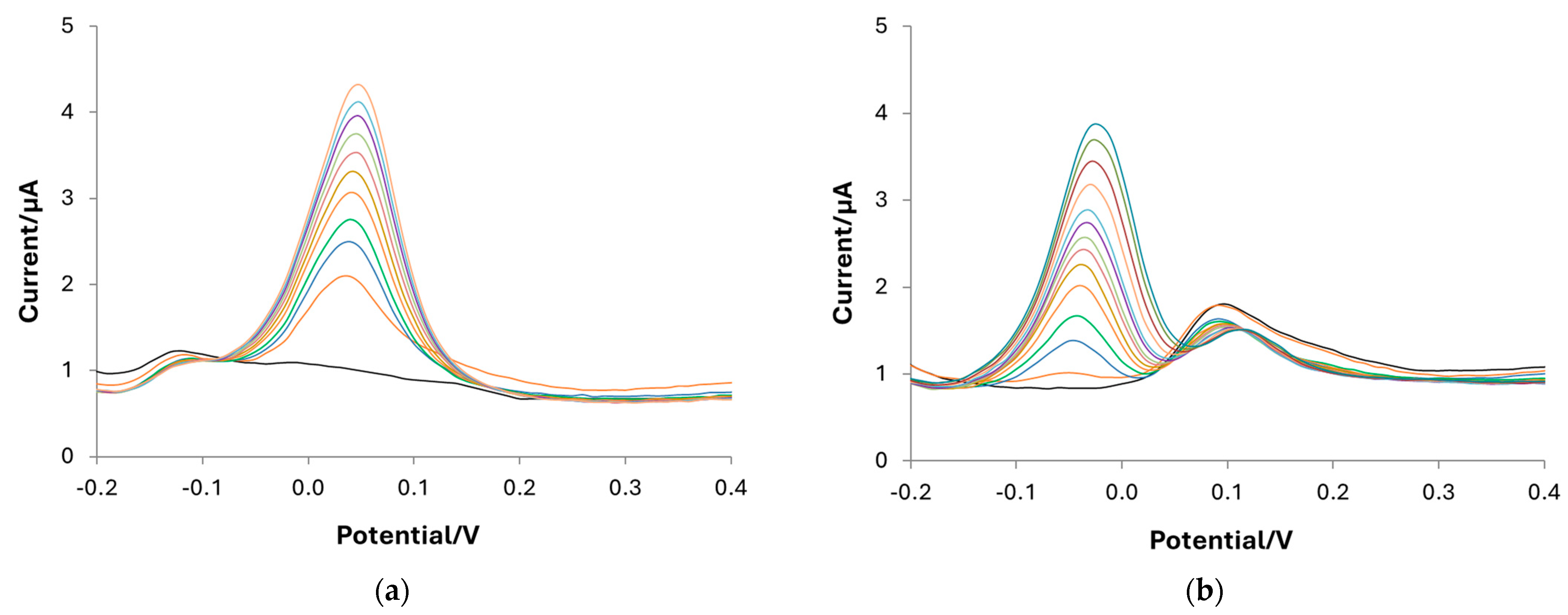
3.4. Quantification of DA by Differential Pulse Voltammetry (DPV): Calibrations and Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamal Eddin, F.B.; Wing Fen, Y. Recent Advances in Electrochemical and Optical Sensing of Dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef]
- Krishna, V.M.; Somanathan, T.; Manikandan, E.; Tadi, K.K.; Uvarajan, S. Neurotransmitter dopamine enhanced sensing detection using fiber-like carbon nanotubes by chemical vapor deposition technique. J. Nanosci. Nanotechnol. 2018, 18, 5380–5389. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Chen, W.; Wu, P. Electrocatalytic oxidation and determination of dopamine in the presence of ascorbic acid and uric acid at a poly (p-nitrobenzenazo resorcinol) modified glassy carbon electrode. Sens. Actuators B Chem. 2007, 122, 309–314. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, Y.; Lao, G.; Wang, Y.; Guan, L.; Wei, J.; Yang, Z.; Ni, P.; Li, X.; Jiang, Z.; et al. Association study of dopamine receptor genes polymorphism with cognitive functions in bipolar I disorder patients. J. Affect. Disord. 2015, 170, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Peciña, M.; Martínez-Jauand, M.; Love, T.; Heffernan, J.; Montoya, P.; Hodgkinson, C.; Stohler, C.S.; Goldman, D.; Zubieta, J.K. Valence-specific effects of BDNF Val66Met polymorphism on dopaminergic stress and reward processing in humans. J. Neurosci. 2014, 34, 5874–5881. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Polonsky, K.S.; Larsen, P.R.; Kronenberg, H.M. Chapter 16—Endocrine Hypertension. In Williams Textbook of Endocrinology, 12th ed.; Young, W.F., Ed.; Saunders Company: Philadelphia, PA, USA, 2011. [Google Scholar]
- Baines, A.D.; Drangova, R. Regulation of sodium transport by endogenous dopamine production in proximal tubular and OK cells. Clin. Exp. Hypertens. 1997, 19, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.; Dinan, T.G. Prolactin and dopamine: What is the connection? A review article. J. Psychopharmacol. 2008, 22, 12–19. [Google Scholar] [CrossRef]
- Harris, R.C.; Zhang, M.Z. Dopamine, the kidney, and hypertension. Curr. Hypertens. Rep. 2012, 14, 138–143. [Google Scholar] [CrossRef]
- Bove, C.; Anselmi, L.; Travagli, R.A. Altered gastric tone and motility response to brain-stem dopamine in a rat model of parkinsonism. Am. J. Physiol. Gastrointest. Liver. Physiol. 2019, 317, G1–G7. [Google Scholar] [CrossRef]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, immunity, and disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef]
- Liaudet, L.; Calderari, B.; Pacher, P. Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail. Rev. 2014, 19, 815–824. [Google Scholar] [CrossRef]
- Ferrer, D.G.; García, A.G.; Peris-Vicente, J.; Gimeno-Adelantado, J.V.; Esteve-Romero, J. Analysis of epinephrine, norepinephrine, and dopamine in urine samples of hospital patients by micellar liquid chromatography. Anal. Bioanal. Chem. 2015, 407, 9009–9018. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Nemeroff, C.B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; SÄkö, E.; PaljÄrvi, L.; Mölsä, P.K.; Rinne, U.K. Brain dopamine D-2 receptors in senile dementia. J. Neural Transm. 1986, 65, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Li, J.I.E.; Zhu, M.I.N.; Manning-Bog, A.B.; Di Monte, D.A.; Fink, A.L. Dopamine and L-dopa disaggregate amyloid fibrils: Implications for Parkinson’s and Alzheimer’s disease. FASEB J. 2004, 18, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Aydın, B.S.; Bulduk, İ. A validated HPLC-UV method for determination of dopamine HCl in injectable solutions. Eurasian J. Biol. Chem. Sci. 2020, 3, 116–120. [Google Scholar]
- Chen, F.; Fang, B.; Wang, S. A fast and validated HPLC method for simultaneous determination of dopamine, dobutamine, phentolamine, furosemide, and aminophylline in infusion samples and injection formulations. J. Anal. Methods Chem. 2021, 2021, 8821126. [Google Scholar] [CrossRef]
- Mirzaei, H.; Mogaddam, M.R.A.; Khandaghi, J. Simultaneous determination of four biogenic amines in whey samples using a new solid phase extraction method prior to their analysis by HPLC-MS/MS. Microchem. J. 2022, 177, 107313. [Google Scholar] [CrossRef]
- Boyang, C.; Oleskin, A.V.; Vlasova, T. Detecting biogenic amines in food and drug plants with HPLC: Medical and nutritional implications. J. Pharm. Nutr. Sci. 2020, 10, 88–91. [Google Scholar] [CrossRef]
- Bouri, M.; Lerma-García, M.J.; Salghi, R.; Zougagh, M.; Ríos, A. Selective extraction and determination of catecholamines in urine samples by using a dopamine magnetic molecularly imprinted polymer and capillary electrophoresis. Talanta 2012, 99, 897–903. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, S.; Huang, J.; Ye, F. Quantum dot-enhanced chemiluminescence detection for simultaneous determination of dopamine and epinephrine by capillary electrophoresis. Talanta 2011, 85, 2650–2654. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Francis, K.A.; Patel, J.; Jha, S.K.; Basu, S. A decoupler-free simple paper microchip capillary electrophoresis device for simultaneous detection of dopamine, epinephrine and serotonin. RSC Adv. 2020, 10, 25487–25495. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wu, L.; Zhong, H.; Wu, H.; Zhang, S.; Meng, J.; Li, F. Analysis of neurotransmitter catecholamines and related amines in human urine and serum by chromatography and capillary electrophoresis with 1, 3, 5, 7-tetramethyl-8-(N-hydroxysuccinimidyl propionic ester)-difluoro-boradiaza-s-indacene. Acta Chromatogr. 2022, 34, 276–286. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Z.; Wang, Z. A simple dopamine detection method based on fluorescence analysis and dopamine polymerization. Microchem. J. 2019, 145, 55–58. [Google Scholar] [CrossRef]
- Zhou, T.; Su, Z.; Tu, Y.; Yan, J. Determination of dopamine based on its enhancement of gold-silver nanocluster fluorescence. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119519. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bai, J.; Li, J.; Lei, L.; Zhang, W.; Yan, S.; Li, Y. Fluorescence detection of dopamine based on the polyphenol oxidase–mimicking enzyme. Anal. Bioanal. Chem. 2020, 412, 5291–5297. [Google Scholar] [CrossRef]
- Pan, J.; Miao, C.; Chen, Y.; Ye, J.; Wang, Z.; Han, W.; Huang, Z.; Zheng, Y.; Weng, S. Facile fluorescence dopamine detection strategy based on acid phosphatase (ACP) enzymatic oxidation dopamine to polydopamine. Chem. Pharm. Bull. 2020, 68, 628–634. [Google Scholar] [CrossRef]
- Li, R.; Zhang, D.; Li, X.; Qi, H. Sensitive and selective electrogenerated chemiluminescence aptasensing method for the determination of dopamine based on target-induced conformational displacement. Bioelectrochemistry 2022, 146, 108148. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, Y.; Lv, C.; Liu, W.; Zhang, Z.; Peng, X. Copper-based metal-organic xerogels on paper for chemiluminescence detection of dopamine. Anal. Methods 2020, 12, 4191–4198. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, M.; Yang, X.; Zhang, C.; Qi, H.; Qi, H. Label-Free Electrogenerated Chemiluminescence Aptasensing Method for Highly Sensitive Determination of Dopamine via Target-Induced DNA Conformational Change. Anal. Chem. 2023, 95, 5500–5506. [Google Scholar] [CrossRef]
- Liu, F.; Xia, S.; Alboull, A.A.M.A.; Dong, Z.; Liu, H.; Meng, C.; Wu, F.; Xu, G. Remarkably Enhanced Luminol/H2O2 Chemiluminescence with Excellent Peroxidase-like Activity of FeCoNi-based Metal-Organic Xerogels for the Sensitive Detection of Dopamine. Anal. Chem. 2023, 95, 9380–9387. [Google Scholar] [CrossRef]
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed]
- Bel Bruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.; Turner, A. Electrochemical Sensors Based on Molecularly Imprinted Polymers. Electroanalysis 2002, 14, 317–323. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly imprinted polymer-based electrochemical sensors for food contaminants determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.; Cordeiro, M.N.D.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Blanco-López, M.C.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tunon-Blanco, P. Electrochemical sensors based on molecularly imprinted polymers. TrAC Trends Anal. Chem. 2004, 23, 36–48. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Amine, A. Recent Advances in Electrochemical Sensors Based on Molecularly Imprinted Polymers and Nanomaterials. Electroanalysis 2019, 31, 188. [Google Scholar] [CrossRef]
- Gonçalves, L.M. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; Grinsven, B.V.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent Advances in Electrosynthesized Molecularly Imprinted Polymer Sensing Platforms for Bioanalyte Detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed]
- Unger, C.; Lieberzeit, P.A. Molecularly imprinted thin film surfaces in sensing: Chances and challenges. React. Funct. Polym. 2021, 161, 104855. [Google Scholar] [CrossRef]
- Sharma, P.S.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204. [Google Scholar] [CrossRef] [PubMed]
- Ramanavičius, S.; Morkvėnaitė-Vilkončienė, I.; Samukaitė-Bubnienė, U.; Ratautaitė, V.; Plikusienė, I.; Viter, R.; Ramanavičius, A. Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors. Sensors 2022, 22, 1282. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Ascorbic Acid Sensing by Molecularly Imprinted Electrosynthesized Polymer (e-MIP) on Screen-Printed Electrodes. Chemosensors 2023, 11, 348. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 12. [Google Scholar]
- Witkowski, A.; Freund, M.S.; Brajter-Toth, A. Effect of Electrode Substrate on the Morphology and Selectivity of Overoxidized Polypyrrole Films. Anal. Chem. 1991, 63, 622–626. [Google Scholar] [CrossRef]
- Hsueh, C.; Brajter-Toth, A. Electrochemical Preparation and Analytical Applications of Ultrathin Overoxidized Polypyrrole Films. Anal. Chem. 1994, 66, 2458–2464. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, F.; Kan, X. Voltammetric dopamine sensor based on three-dimensional electrosynthesized molecularly imprinted polymers and polypyrrole nanowires. Microchim. Acta 2017, 184, 2515–2522. [Google Scholar] [CrossRef]
- Kan, X.; Zhou, H.; Li, C.; Zhu, A.; Xing, Z.; Zhao, Z. Imprinted electrochemical sensor for dopamine recognition and determination based on a carbon nanotube/polypyrrole film. Electrochim. Acta 2012, 63, 69–75. [Google Scholar] [CrossRef]
- Maouche, N.; Guergouri, M.; Gam-Derouich, S.; Jouini, M.; Nessark, B.; Chehimi, M.M. Molecularly imprinted polypyrrole films: Some key parameters for electrochemical picomolar detection of dopamine. J. Electroanal. Chem. 2012, 685, 21–27. [Google Scholar] [CrossRef]
- Slimi, S.; Mabrouk, C.; Barhoumi, H.; Jaffrezic-Renault, N. A Simple Over-Oxidized Molecularly Imprinted Polypyrrole for the Sensitive Detection of Dopamine in Human Serum. J. Sens. Technol. 2022, 12, 33–44. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Zhou, X.; Ma, P.; Wu, S.; Xu, L.; Shen, J. Ultrasensitive dopamine sensor based on novel molecularly imprinted polypyrrole-coated carbon nanotubes. Biosens. Bioelectron. 2014, 58, 237–241. [Google Scholar] [CrossRef]
- Chelly, S.; Chelly, M.; Zribi, R.; Gdoura, R.; Bouaziz-Ketata, H.; Neri, G. Electrochemical Detection of Dopamine and Riboflavine on a Screen-Printed Carbon Electrode Modified by AuNPs Derived from Rhanterium suaveolens Plant Extract. ACS Omega 2021, 6, 23666–23675. [Google Scholar] [CrossRef]
- Pavličková, M.; Lorencová, L.; Hatala, M.; Kováč, M.; Tkáč, J.; Gemeiner, P. Facile fabrication of screen-printed MoS2 electrodes for electrochemical sensing of dopamine. Sci. Rep. 2022, 12, 11900. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ju, F.; Li, G.; Ma, L. Smartphone-Based Electrochemical Potentiostat Detection System Using PEDOT: PSS/Chitosan/Graphene Modified Screen-Printed Electrodes for Dopamine Detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). Available online: http://www.gruppochemiometria.it/index.php/software/19-download-the-rbased-chemometric-software (accessed on 4 July 2024).
- Burak, D.; Emregul, E.; Emregul, K.C. Copper–zinc alloy nanoparticle-based enzyme-free superoxide radical sensing on a screen-printed electrode. Talanta 2015, 134, 206–214. [Google Scholar]
- Pesavento, M.; Merli, D.; Biesuz, R.; Alberti, G.; Marchetti, S.; Milanese, C. A MIP-based low-cost electrochemical sensor for 2-furaldehyde detection in beverages. Anal. Chim. Acta 2021, 1142, 201–210. [Google Scholar] [CrossRef]
- Pesavento, M.; D’Agostino, G.; Alberti, G.; Biesuz, R.; Merli, D. Voltammetric platform for detection of 2,4,6-trinitrotoluene based on a molecularly imprinted polymer. Anal. Bioanal. Chem. 2013, 405, 3559–3570. [Google Scholar] [CrossRef]
- Akhoundian, M.; Alizadeh, T.; Ganjali, M.R.; Rafiei, F. A new carbon paste electrode modified with MWCNTs and nano-structured molecularly imprinted polymer for ultratrace determination of trimipramine: The crucial effect of electrode components mixing on its performance. Biosens. Bioelectron. 2018, 111, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Merli, D.; Lio, E.; Protti, S.; Coccia, R.; Profumo, A.; Alberti, G. Molecularly Imprinted Polymer-based voltammetric sensor for amino acids/indazole derivatives synthetic cannabinoids detection. Anal. Chim. Acta 2024, 1288, 342151. [Google Scholar] [CrossRef] [PubMed]
- Capucciati, A.; Cacciatore, L.; Protti, S.; Profumo, A.; Merli, D. Electrochemical analysis and characterization of psychoactive substances glaucine and tetrahydropalmatine. J. Electroanal. Chem. 2022, 907, 116032. [Google Scholar] [CrossRef]
- Capucciati, A.; Burato, A.; Bersani, C.; Protti, S.; Profumo, A.; Merli, D. Electrochemical Behavior and Voltammetric Determination of Two Synthetic Aroyl Amides Opioids. Chemosensors 2023, 11, 198. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment (IUPAC Technical Report). Pure App. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Calibration methods in instrumental analysis: Regression and correlation. In Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: Harlow Essex, UK, 2010; pp. 124–126. [Google Scholar]
- Desimoni, E.; Brunetti, B. About estimating the limit of detection by the signal to noise approach. Pharm. Anal. Acta 2015, 6, 1–4. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Oyejola, B.A.; Nwanya, J.C. Selecting the right central composite design. Int. J. Stat. Appl. 2015, 5, 21–30. [Google Scholar]
- Wang, W.; Cheng, Y.; Tan, G. Design Optimization of SBS-Modified Asphalt Mixture Reinforced with Eco-Friendly Basalt Fiber Based on Response Surface Methodology. Materials 2018, 11, 1311. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Wu, K.; Zhang, L.; Ge, S.; Yu, J. A molecularly imprinted polypyrrole for ultrasensitive voltammetric determination of glyphosate. Microchim. Acta 2017, 184, 1959–1967. [Google Scholar] [CrossRef]
- Zelano, V.; Zerbinati, O.; Ostacoli, G. Ternary Cu (II) complex-formation with L-dopa or dopamine and valine, leucine, phenylalanine and threonine in aqueous-solution. Annal. Chim. 1988, 78, 273–283. [Google Scholar]
- Kiss, T.; Gergely, A. Complexes of 3,4-dhydroxyphnyl derivatives, III. Equilibrium study of parent and some mixed ligand complexes of dopamine, alanine and pyrocatechol with nickel(II), copper(II) and zinc(II) ions. Inorg. Chim. Acta 1979, 36, 31–36. [Google Scholar] [CrossRef]
- Ismail, I.; Okajima, T.; Kawauchi, S.; Ohsaka, T. Studies on the early oxidation process of dopamine by electrochemical measurements and quantum chemical calculations. Electrochim. Acta 2016, 211, 777–786. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Rajabi, H.; Beitollahi, H.; Mirjalili, B.B.F.; Taghavinia, N.; Akbari, A. Voltammetric determination of dopamine at the surface of TiO2 nanoparticles modified carbon paste electrode. Int. J. Electrochem. Sci. 2010, 5, 147–157. [Google Scholar] [CrossRef]
- Zanello, P. Voltammetric Techniques. In Inorganic Electrochemistry. Theory, Practice and Application, 1st ed.; The Royal Society of Chemistry: Cambridge, UK, 2003; pp. 59–93. [Google Scholar]
- Corona-Avendaño, S.; Alarcón-Angeles, G.; Ramírez-Silva, M.T.; Rosquete-Pina, G.; Romero-Romo, M.; Palomar-Pardavé, M. On the electrochemistry of dopamine in aqueous solution. Part I: The role of [SDS] on the voltammetric behavior of dopamine on a carbon paste electrode. J. Electroanal. Chem. 2007, 609, 17–26. [Google Scholar] [CrossRef]


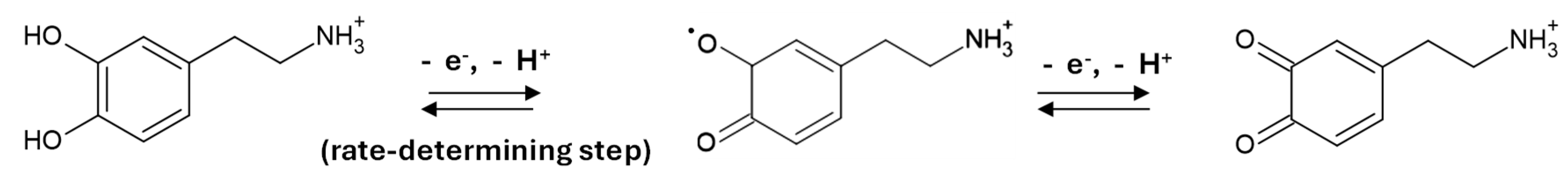
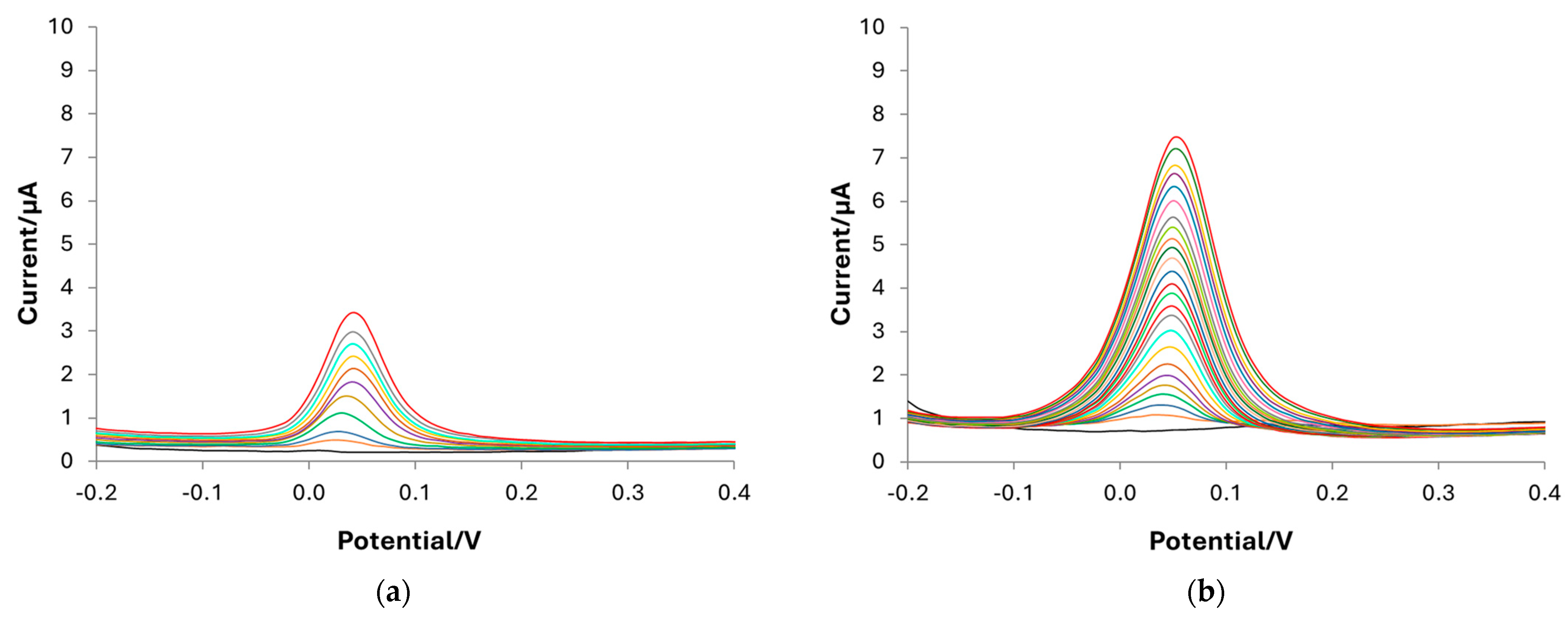
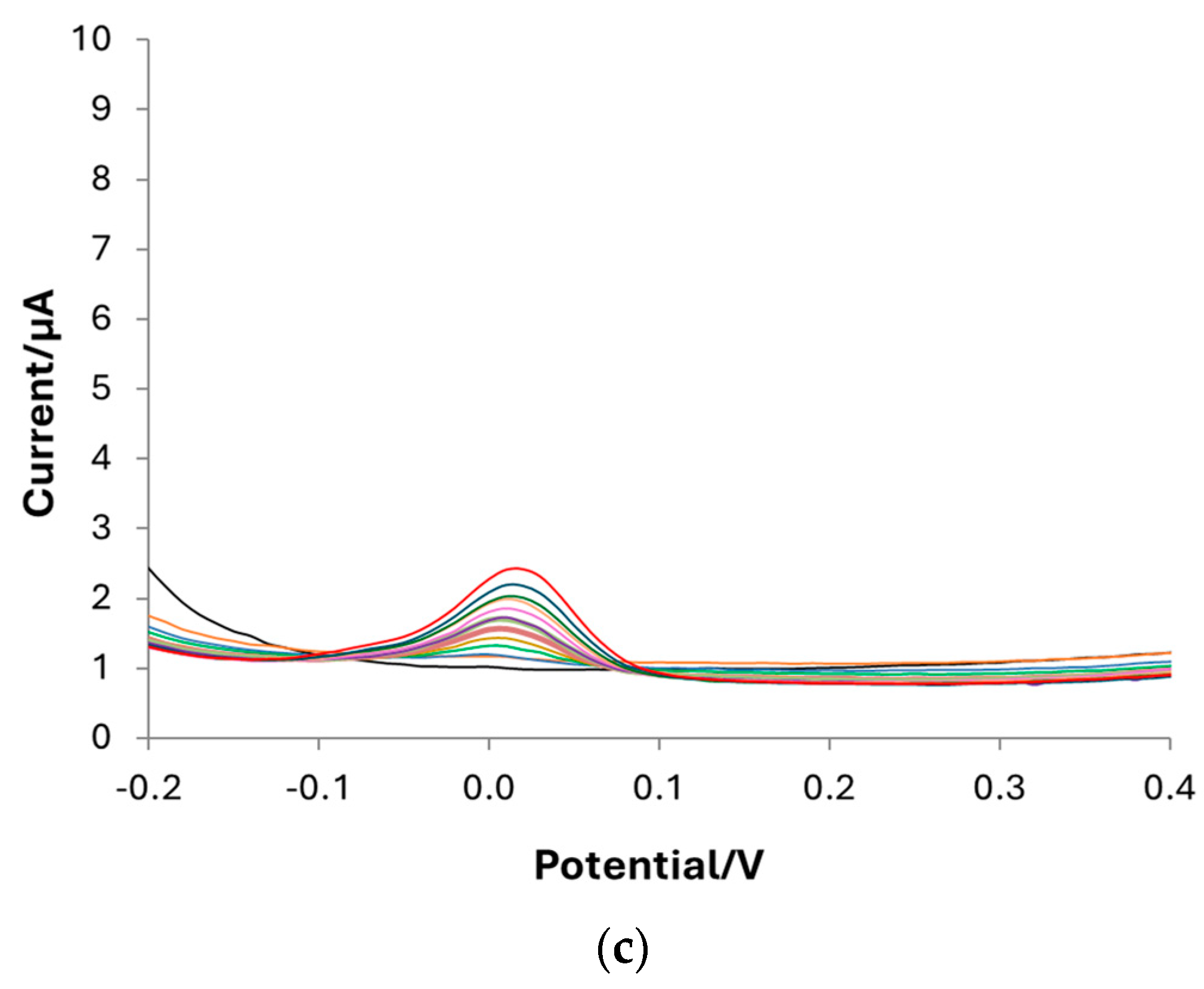
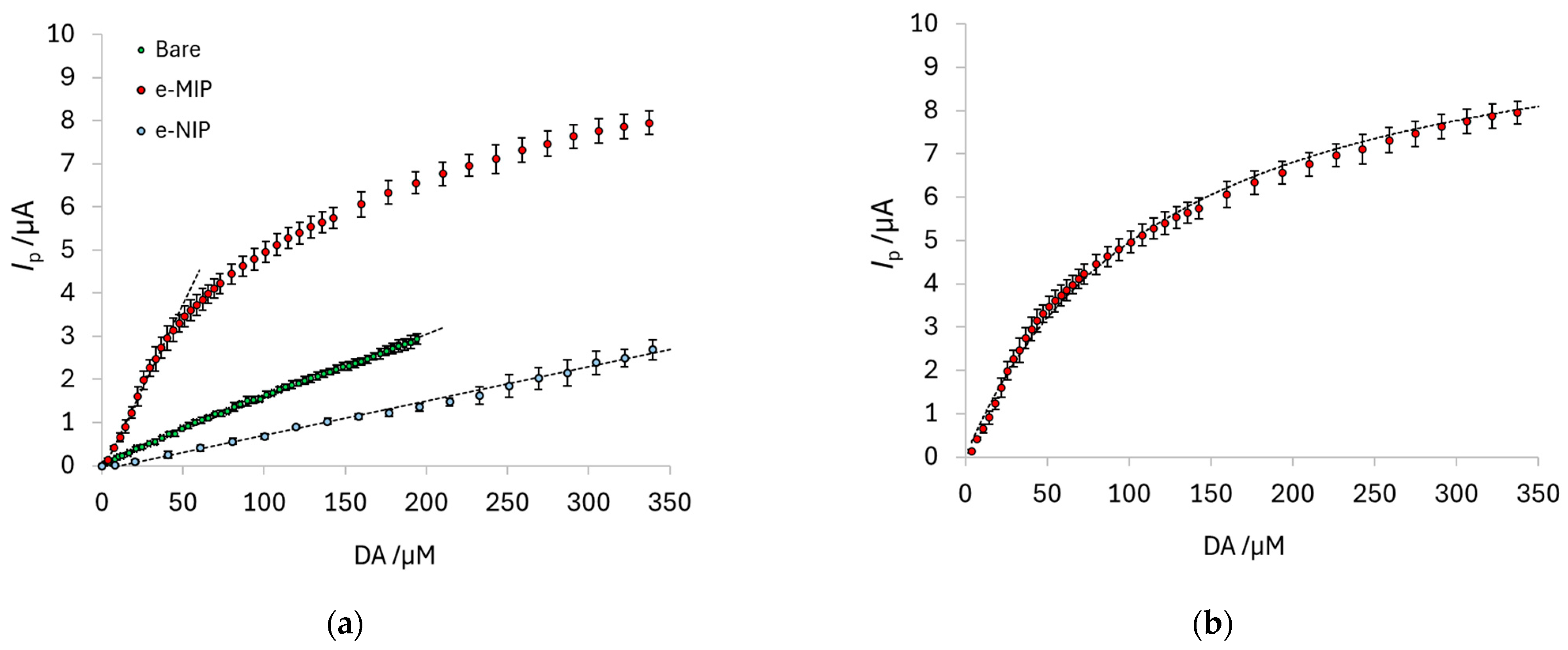
| Component | Amount (g L−1) |
|---|---|
| C5H4N4O3 | 0.25 |
| Na3C6H5O7·2H2O | 0.72 |
| C4H7N3O | 0.88 |
| CH4N2O | 1.50 |
| Na2SO4 | 1.70 |
| KCl | 2.30 |
| NaCl | 1.75 |
| CaCl2 | 0.18 |
| NH4Cl | 1.30 |
| K2C2O4·H2O | 0.04 |
| MgSO4·7H2O | 1.08 |
| NaH2PO4·2H2O | 0.29 |
| Na2HPO4·2H2O | 0.83 |
| Factor | −1 | 0 | +1 |
|---|---|---|---|
| n. CV scans (n. CV) | 3 | 5 | 7 |
| DA/Py (ratio) | 1/15 | 1/10 | 1/5 |
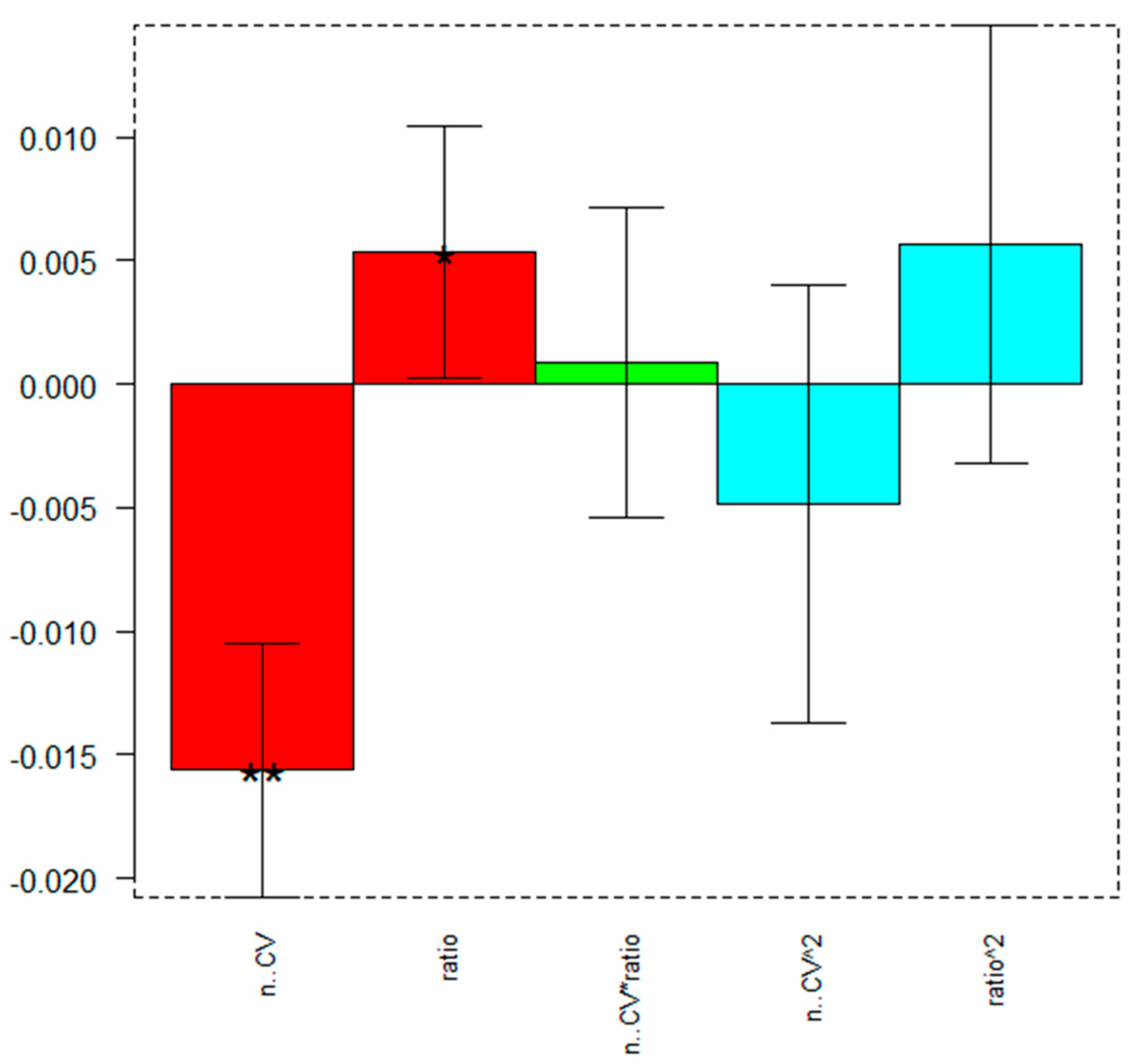
| Coefficient | Value | Significance |
|---|---|---|
| b0 | 0.024(3) | |
| b1 | −0.016(2) | ** |
| b2 | 0.005(2) | * |
| b12 | 0.001(2) | |
| b11 | −0.005(3) | |
| b22 | 0.006(3) |
| Slope (µA µM−1) | |
|---|---|
| Average | 0.028 |
| Standard deviation | 0.005 |
| Upper bound CI | 0.037 |
| Lower bound CI | 0.019 |
| Predicted response (b0) | 0.024 |
| Electrode | Active Area mm2 | Double-Layer Capacitance μF |
|---|---|---|
| bare | 4.8(1) | 2.8(1) |
| e-MIP-modified | 2.0(3) | 3.4(1) |
| e-NIP-modified | 1.7(2) | 3.2(2) |
| geometric area (circular-shaped working electrode of the screen-printed cell ø 1.2 mm) | 4.5 |
| Bare Electrode | e-MIP-Modified Electrode | |
|---|---|---|
| E0′/V | 0.17(2) | 0.16(1) |
| D/cm2 s−1 | 3.9(1)·10−6 | 4.5(3)·10−6 |
| αA | 0.35(2) | 0.38(2) |
| Sensitivity (µA µM−1) | Linear Range (µM) | Dynamic Range (µM) | LOD (µM) | LOQ (µM) | |
|---|---|---|---|---|---|
| Bare electrode | 0.0148(1) | 10–200 | - | 10 | 30 |
| e-MIP-modified electrode | 0.078(2) | 0.8–45 | 0.8–350 | 0.8 | 2 |
| e-NIP-modified electrode | 0.0080(1) | 30–350 | - | 30 | 92 |
| Sample | DA Content (µM) | DA Added (µM) | DA Found (µM) | Recovery (%) |
|---|---|---|---|---|
| PBS 0.1 M pH 7, AA 20 µM, UA 5 µM | - | 8 | 8.4(2) | 103 |
| PBS 0.1 M pH 7, AA 20 µM, UA 20 µM | - | 16 | 16.5(2) | 102 |
| Simulated urine (1) | - | 2 | 1.73(2) | 87 |
| Simulated urine (2) | - | 4 | 3.6(1) | 94 |
| Simulated urine (3) | - | 8 | 7.5(8) | 93 |
| Real human urine | - | 3.31(2) | ||
| Spiked real human urine (1) | 3.31(2) | 2 | 5.28(1) | 99 |
| Spiked real human urine (2) | 3.31(2) | 4 | 7.27(1) | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merli, D.; Cutaia, A.; Hallulli, I.; Bonanni, A.; Alberti, G. Molecularly Imprinted Polypyrrole-Modified Screen-Printed Electrode for Dopamine Determination. Polymers 2024, 16, 2528. https://doi.org/10.3390/polym16172528
Merli D, Cutaia A, Hallulli I, Bonanni A, Alberti G. Molecularly Imprinted Polypyrrole-Modified Screen-Printed Electrode for Dopamine Determination. Polymers. 2024; 16(17):2528. https://doi.org/10.3390/polym16172528
Chicago/Turabian StyleMerli, Daniele, Alessandra Cutaia, Ines Hallulli, Alessandra Bonanni, and Giancarla Alberti. 2024. "Molecularly Imprinted Polypyrrole-Modified Screen-Printed Electrode for Dopamine Determination" Polymers 16, no. 17: 2528. https://doi.org/10.3390/polym16172528










