Cellulose-Based Hydrogels for Wastewater Treatment: A Focus on Metal Ions Removal
Abstract
:1. Introduction
2. Synthesis of Cellulose- and Cellulose Derivative-Based Hydrogels
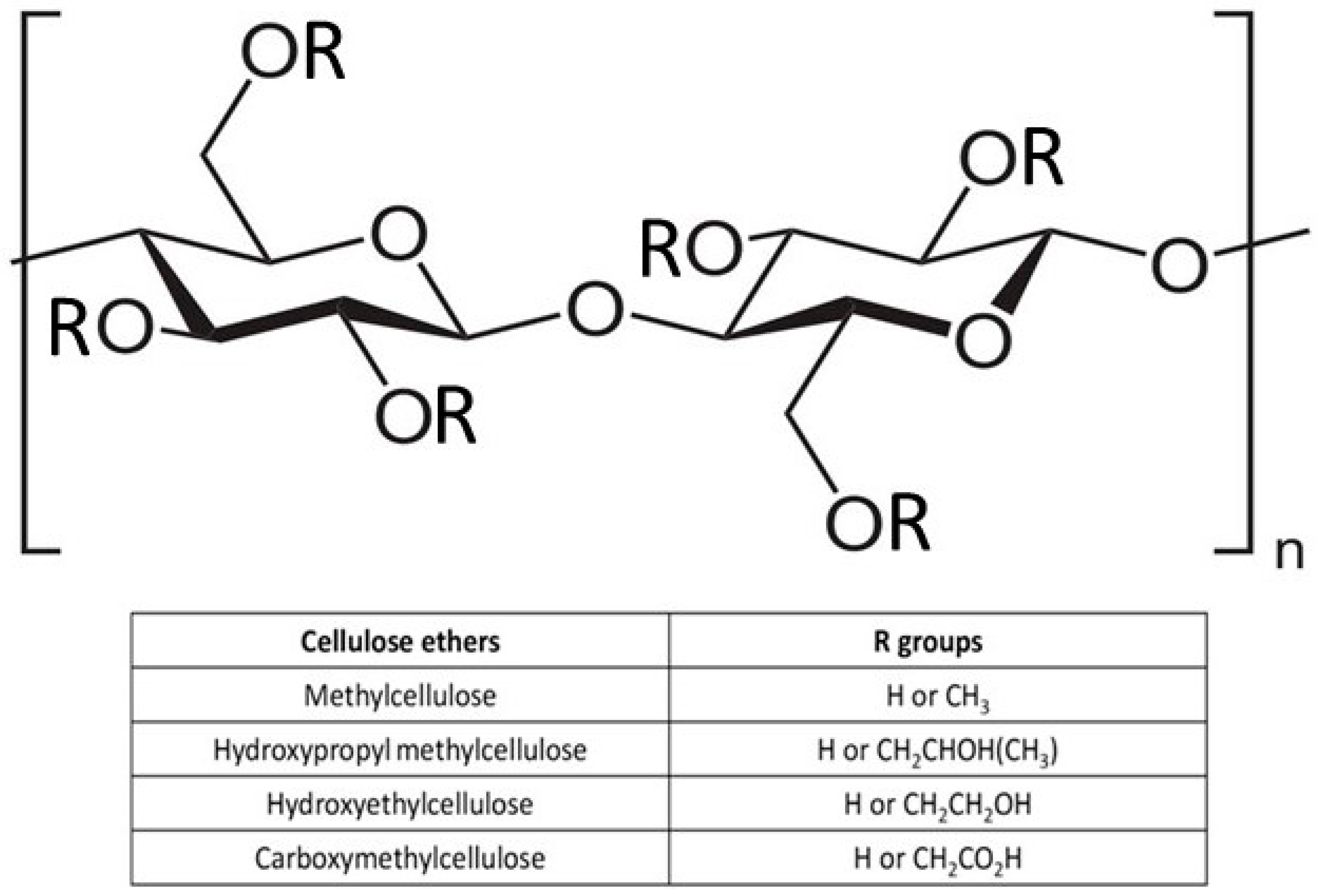
2.1. Structure of Cellulose and Its Derivatives
2.2. Physically Cross-Linked Hydrogels
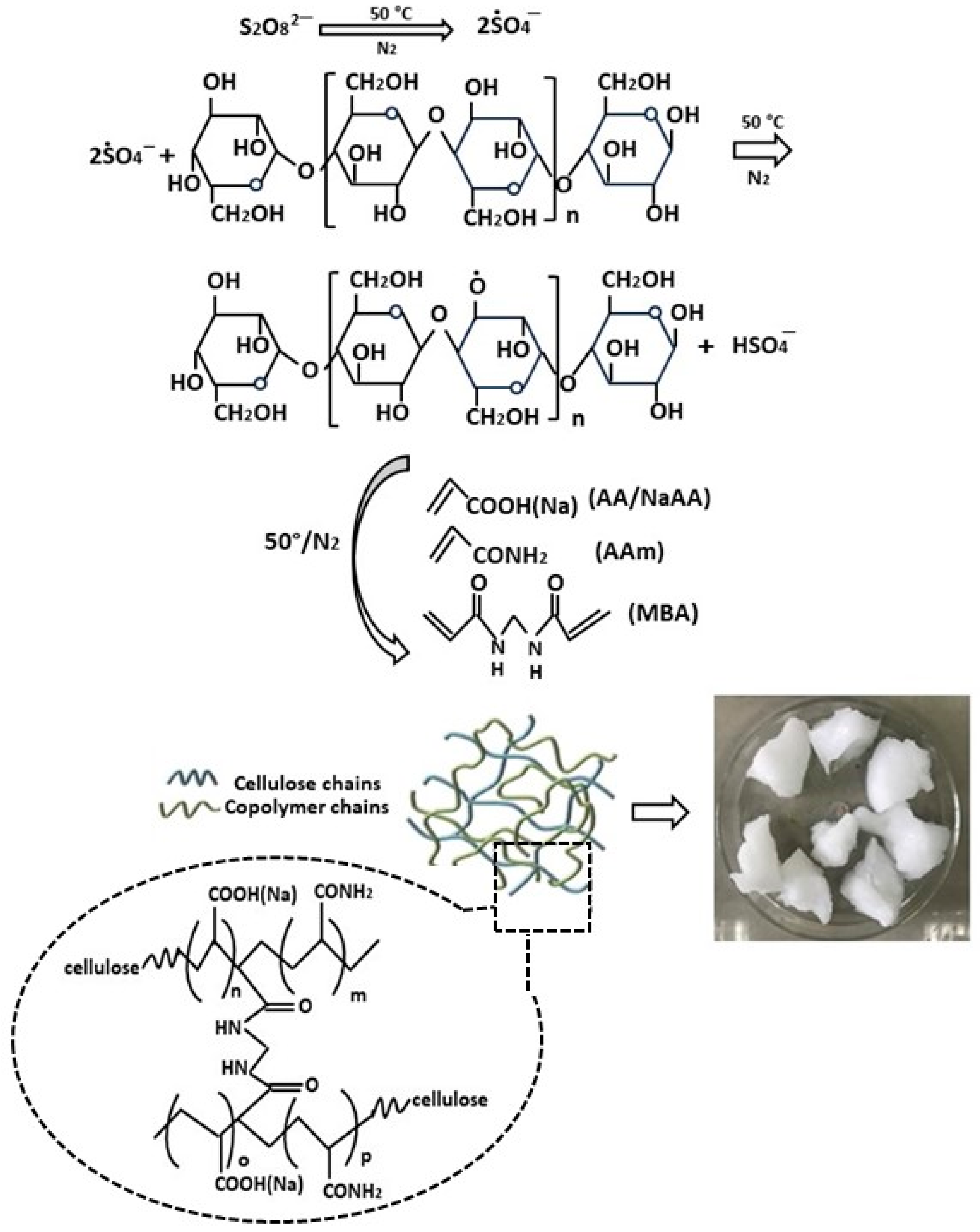
2.3. Chemically Cross-Linked Hydrogels
3. Hydrogels Based on Cellulose and Cellulose Derivatives for Water Decontamination of Heavy Metals
3.1. Kinetics and Adsorption Isotherms
3.2. Non-Composite Hydrogels
3.3. Composite Hydrogels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CMC | carboxy methyl cellulose |
| –COOH | carboxyl groups |
| CCNF | carboxylated cellulose nanofiber |
| CM | carboxymethyl group |
| CH | chitosan |
| CA | citric acid |
| EDTA | ethylenediaminetetraacetic acid group |
| EGDE | glutaraldehyde |
| HEC | hydroxyethyl cellulose |
| –OH | hydroxyl groups |
| MC | methylcellulose |
| MCC | microcrystalline cellulose |
| CNF | nanofibrillated cellulose |
| NOCNF | nitro-oxidized carboxycellulose nanofibers |
| PVA | poly(vinyl alcohol) |
| TOCNF | TEMPO-oxidized negatively charged (unidimensional) cellulose nanofiber |
| XRD | X-ray diffraction |
| TEMED | N,N,N′,N′-tetramethylethylenediamine |
| MBA | N,N′-methylenebisacrylamide |
References
- Soliman, N.K.; Moustafa, A.F. Industrial solid waste for heavy metals adsorption features and challenges; A review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Diaconu, L.I.; Covaliu-Mierlă, C.I.; Păunescu, O.; Covaliu, L.D.; Iovu, H.; Paraschiv, G. Phytoremediation of Wastewater Containing Lead and Manganese Ions Using Algae. Biology 2023, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chen, J.; Zhu, F.; Chai, L.; Lin, Z.; Zhang, K.; Shi, Y. Biological Toxicity of Heavy Metal (loid) s in Natural Environments: From Microbes to Humans. Front. Environ. Sci. 2022, 681, 920957. [Google Scholar] [CrossRef]
- Malitesta, C.; Di Masi, S.; Mazzotta, E. From electrochemical biosensors to biomimetic sensors based on molecularly imprinted polymers in environmental determination of heavy metals. Front. Chem. 2017, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Guascito, M.R.; Malitesta, C.; Mazzotta, E.; Turco, A. Inhibitive determination of metal ions by an amperometric glucose oxidase biosensor: Study of the effect of hydrogen peroxide decomposition. Sens. Actuators B Chem. 2008, 131, 394–402. [Google Scholar] [CrossRef]
- Guascito, M.R.; Malitesta, C.; Mazzotta, E.; Turco, A. Screen-printed glucose oxidase-based biosensor for inhibitive detection of heavy metal ions in a flow injection system. Sens. Lett. 2009, 7, 153–159. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Vinci, G.; Maddaloni, L.; Mancini, L.; Prencipe, S.A.; Ruggeri, M.; Tiradritti, M. The Health of the Water Planet: Challenges and Opportunities in the Mediterranean Area. An Overview. Earth 2021, 2, 894–919. [Google Scholar] [CrossRef]
- Turco, A.; Monteduro, A.G.; Mazzotta, E.; Maruccio, G.; Malitesta, C. An innovative porous nanocomposite material for the removal of phenolic compounds from aqueous solutions. Nanomaterials 2018, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Turco, A.; Pennetta, A.; Caroli, A.; Mazzotta, E.; Monteduro, A.G.; Primiceri, E.; de Benedetto, G.; Malitesta, C. Easy fabrication of mussel inspired coated foam and its optimization for the facile removal of copper from aqueous solutions. J. Colloid Interface Sci. 2019, 552, 401–411. [Google Scholar] [CrossRef]
- Turco, A.; Malitesta, C.; Barillaro, G.; Greco, A.; Maffezzoli, A.; Mazzotta, E. A magnetic and highly reusable macroporous superhydrophobic/superoleophilic PDMS/MWNT nanocomposite for oil sorption from water. J. Mater. Chem. A 2015, 3, 17685–17696. [Google Scholar] [CrossRef]
- Turco, A.; Malitesta, C. Removal of phenolic compounds from olive mill wastewater by a polydimethylsiloxane/oxMWCNTs porous nanocomposite. Water 2020, 12, 3471. [Google Scholar] [CrossRef]
- López, Y.C.; Ortega, G.A.; Reguera, E. Hazardous ions decontamination: From the element to the material. Chem. Eng. J. Adv. 2022, 11, 100297. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Gala, O.; Helińska, K.; Kołodzińska, K.; Konczak, H.; Mroczyński, Ł.; Siarka, E. Membrane Separation in the Nickel-Contaminated Wastewater Treatment. Waste 2023, 1, 482–496. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Su, X.; Suss, M.E.; Tian, H.; Guyes, E.N.; Shocron, A.N.; Conforti, K.M.; de Souza, J.P.; Kim, N.; Tedesco, M.; et al. Electrochemical methods for water purification, ion separations, and energy conversion. Chem. Rev. 2022, 122, 13547–13635. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and opportunities of biocoagulant/bioflocculant application for drinking water and wastewater treatment and its potential for sludge recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, V. Recovery and recycle of wastewater contaminated with heavy metals using adsorbents incorporated from waste resources and nanomaterials-A review. Chemosphere 2021, 273, 129677. [Google Scholar]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A review of adsorbents for heavy metal decontamination: Growing approach to wastewater treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C. Zeolite application in wastewater treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Du, J.; Qi, F.; Donne, S.W.; Islam, M.M. Mesoporous biopolymer architecture enhanced the adsorption and selectivity of aqueous heavy-metal ions. ACS Omega 2021, 6, 15316–15331. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Magalhães, M.I.; Almeida, A.P. Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D. Appl. Biosci. 2023, 2, 94–114. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent advances in cellulose-based hydrogels for tissue engineering applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef] [PubMed]
- Topare, N.S.; Wadgaonkar, V.S. A review on application of low-cost adsorbents for heavy metals removal from wastewater. Mater. Today Proc. 2023, 77, 8–18. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, B.; Sarkar, K. Nanocellulose as sustainable biomaterials for drug delivery. Sens. Int. 2022, 3, 100135. [Google Scholar] [CrossRef]
- Badsha, M.A.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhu, Y.; Jiang, G.; Cao, K.; Zeng, S.; Chen, W.; Zhao, D.; Yu, H. Sustainable cellulose and its derivatives for promising biomedical applications. Prog. Mater. Sci. 2023, 138, 101152. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and opportunities in the characterization of cellulose—An important regulator of cell wall growth and mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant-vs. Bacterial-derived cellulose for wound healing: A review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- See, W.Q.; Jamaluddin, J.; Basar, N.; Bakar, N.F.A.; Azizan, A.; Alam, M.N.H.Z.; Lai, J.C.; Asmadi, M.; Adrus, N. Green Chemistry Approaches to Cellulose Dissolution and Regeneration. In Regenerated Cellulose and Composites: Morphology-Property Relationship; Springer Nature: Singapore, 2023; pp. 9–36. [Google Scholar]
- Morais, E.S.; Lopes, A.M.D.C.; Freire, M.G.; Freire, C.S.; Coutinho, J.A.; Silvestre, A.J. Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef] [PubMed]
- Kayra, N.; Aytekin, A.Ö. Synthesis of cellulose-based hydrogels: Preparation, formation, mixture, and modification. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2018; pp. 1–28. [Google Scholar]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A review on the modification of cellulose and its applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Kamide, K. Characterization of molecular structure of cellulose derivatives. In Cellulose and Cellulose Derivatives; Elsevier: Amsterdam, The Netherlands, 2005; pp. 25–188. [Google Scholar]
- McMullen, R.L.; Ozkan, S.; Gillece, T. Physicochemical properties of cellulose ethers. Cosmetics 2022, 9, 52. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.; Aruan, H.K.; Herfananda, A.L. Hydrogel and effects of crosslinking agent on cellulose-based hydrogels: A review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Muhamad, I.I.; Pa’e, N.; Hashim, Z. Strategies in improving properties of cellulose-based hydrogels for smart applications. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2019; pp. 887–908. [Google Scholar]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.F. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Zannagui, C.; Amhamdi, H.; El Barkany, S.; Jilal, I.; Sundman, O.; Salhi, A.; Chaouf, S.; Abou-Salama, M.; El Idrissi, A.; Zaghrioui, M. Design, characterization and investigation of heavy metal ions removal by new cellulose-ether based adsorbent. Moroc. J. Chem. 2020, 8, 332–346. [Google Scholar]
- Durpekova, S.; Di Martino, A.; Dusankova, M.; Drohsler, P.; Sedlarik, V. Biopolymer hydrogel based on acid whey and cellulose derivatives for enhancement water retention capacity of soil and slow release of fertilizers. Polymers 2021, 13, 3274. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.I.H.; Haque, M.O. Cellulosic hydrogels: A greener solution of sustainability. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2019; pp. 3–35. [Google Scholar]
- Morales, A.; Labidi, J.; Gullón, P. Assessment of green approaches for the synthesis of physically crosslinked lignin hydrogels. J. Ind. Eng. Chem. 2020, 81, 475–487. [Google Scholar] [CrossRef]
- Mah, E.; Ghosh, R. Thermo-responsive hydrogels for stimuli-responsive membranes. Processes 2013, 1, 238–262. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Deng, X.; Wang, T.; Yang, Z.; Zhou, W.; Wang, C. Dual physically cross-linked hydrogels with high stretchability, toughness, and good self-recoverability. Macromolecules 2016, 49, 5660–5668. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Das, D. Cellulose: A fascinating biopolymer for hydrogel synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Wang, M.J. Removal of heavy metal ions by poly (vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze–thaw method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Guan, Y.; Bian, J.; Peng, F.; Zhang, X.M.; Sun, R.C. High strength of hemicelluloses based hydrogels by freeze/thaw technique. Carbohydr. Polym. 2014, 101, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of hydrogels: From synthetic strategies, classification, and properties to biomedical applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Yao, M.; He, S.; Dong, X.; Yao, F.; et al. Dual physically cross-linked carboxymethyl cellulose-based hydrogel with high stretchability and toughness as sensitive strain sensors. Cellulose 2020, 27, 9975–9989. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z. Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate 2021, 2, e21. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Lei, H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels 2022, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef] [PubMed]
- Coma, V.; Sebti, I.; Pardon, P.; Pichavant, F.H.; Deschamps, A. Film properties from crosslinking of cellulosic derivatives with a polyfunctional carboxylic acid. Carbohydr. Polym. 2003, 51, 265–271. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, C.C. Physical properties of the crosslinked cellulose catalyzed with nanotitanium dioxide under UV irradiation and electronic field. Appl. Catal. A Gen. 2005, 293, 171–179. [Google Scholar] [CrossRef]
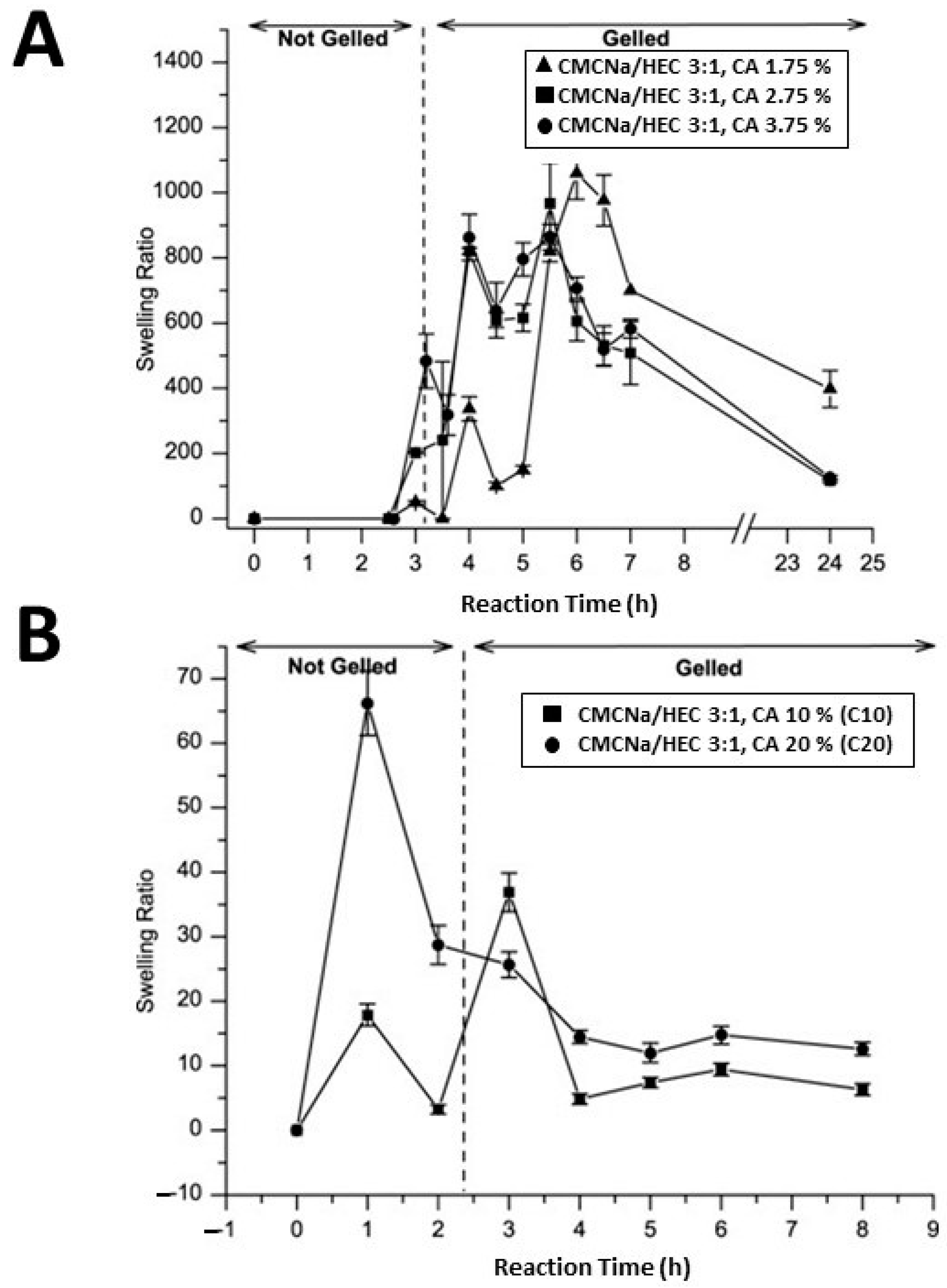
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Raucci, M.G.; Alvarez-Perez, M.A.; Demitri, C.; Giugliano, D.; De Benedictis, V.; Sannino, A.; Ambrosio, L. Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. J. Biomed. Mater. Res. Part A 2015, 103, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Shuai, Q.; Gong, X.; Gu, Q.; Yu, H. Synthesis of microporous cationic hydrogel of hydroxypropyl cellulose (HPC) and its application on anionic dye removal. CLEAN Soil Air Water 2009, 37, 392–398. [Google Scholar] [CrossRef]
- Peng, N.; Wang, Y.; Ye, Q.; Liang, L.; An, Y.; Li, Q.; Chang, C. Biocompatible cellulose-based superabsorbent hydrogels with antimicrobial activity. Carbohydr. Polym. 2016, 137, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Phoothong, F.; Boonmahitthisud, A. Superabsorbent cellulose-based hydrogels cross-liked with borax. Sci. Rep. 2022, 12, 8920. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’—Proposal of a comprehensive definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Seth, A.; Singh, A.K.; Rajput, M.S.; Sikandar, M. Remediation strategies for heavy metals contaminated ecosystem: A review. Environ. Sustain. Indic. 2021, 12, 100155. [Google Scholar] [CrossRef]
- Qiao, A.; Cui, M.; Huang, R.; Ding, G.; Qi, W.; He, Z.; Klemes, J.J.; Su, R. Advances in nanocellulose-based materials as adsorbents of heavy metals and dyes. Carbohydr. Polym. 2021, 272, 118471. [Google Scholar] [CrossRef] [PubMed]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-based adsorbent material for the effective removal of heavy metals from wastewater: A comprehensive review. Gels 2022, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent advances in cellulose-based hydrogels: Food applications. Foods 2023, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, L.; Guo, S. Mechanisms of lead biosorption on cellulose/chitin beads. Water Res. 2005, 39, 3755–3762. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, J.; He, J.; Kan, D.; Chen, K.; Lu, J. Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application. Gels 2023, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef] [PubMed]
- Hossain, L.; Raghuwanshi, V.S.; Tanner, J.; Wu, C.M.; Kleinerman, O.; Cohen, Y.; Garnier, G. Structure and swelling of cross-linked nanocellulose foams. J. Colloid Interface Sci. 2020, 568, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Garg, A.; Vishwavidyalaya, R.D. Hydrogel: Classification, properties, preparation and technical features. Asian J. Biomater. Res. 2016, 2, 163–170. [Google Scholar]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Kaliaraj, G.S.; Shanmugam, D.K.; Dasan, A.; Mosas, K.K.A. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Batys, P.; Kivistö, S.; Lalwani, S.M.; Lutkenhaus, J.L.; Sammalkorpi, M. Comparing water-mediated hydrogen-bonding in different polyelectrolyte complexes. Soft Matter 2019, 15, 7823–7831. [Google Scholar] [CrossRef] [PubMed]
- Kopač, T.; Abrami, M.; Grassi, M.; Ručigaj, A.; Krajnc, M. Polysaccharide-based hydrogels crosslink density equation: A rheological and LF-NMR study of polymer-polymer interactions. Carbohydr. Polym. 2022, 277, 118895. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Z.; Luo, S.Y.; Zong, M.H.; Lou, W.Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Niamnuy, C.; Seubsai, A. Removal of heavy metal ions using modified celluloses prepared from pineapple leaf fiber. ACS Omega 2020, 5, 5285–5296. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Song, Y.L.; Yang, H.R.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R. Carboxymethyl cellulose-based cryogels for efficient heavy metal capture: Aluminum-mediated assembly process and sorption mechanism. Int. J. Biol. Macromol. 2020, 164, 3275–3286. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fu, S.; Liu, H.; Zhou, Y.; Li, X. Hydrogel beads based on carboxymethyl cellulose for removal heavy metal ions. J. Appl. Polym. Sci. 2011, 119, 1204–1210. [Google Scholar] [CrossRef]
- Rong, L.; Zhu, Z.; Wang, B.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Sui, X. Facile fabrication of thiol-modified cellulose sponges for adsorption of Hg2+ from aqueous solutions. Cellulose 2018, 25, 3025–3035. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Zhang, M.; Zhuo, X.; Niu, J. Preparation and characterization of modified cellulose for adsorption of Cd(II), Hg(II), and acid fuchsin from aqueous solutions. Ind. Eng. Chem. Res. 2013, 52, 876–884. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, L.; Jin, C.; Sun, Q. Cellulose hydrogel functionalized titanate microspheres with self-cleaning for efficient purification of heavy metals in oily wastewater. Cellulose 2020, 27, 7751–7763. [Google Scholar] [CrossRef]
- Peng, H.; Fan, G.; Zheng, X.; Luo, J.; Zhou, J. Impact of structure and morphology of titanate nanomaterials on Pb2+ adsorption in aqueous solution. Desalination Water Treat. 2018, 136, 306–319. [Google Scholar] [CrossRef]
- Hara, K.; Iida, M.; Yano, K.; Nishida, T. Metal ion absorption of carboxymethylcellulose gel formed by γ-ray irradiation: For the environmental purification. Colloids Surf. B Biointerfaces 2004, 38, 227–230. [Google Scholar] [PubMed]
- Kamel, S.; Hassan, E.M.; El-Sakhawy, M. Preparation and application of acrylonitrile-grafted cyanoethyl cellulose for the removal of copper (II) ions. J. Appl. Polym. Sci. 2006, 100, 329–334. [Google Scholar] [CrossRef]
- Wang, D.; Yu, H.; Fan, X.; Gu, J.; Ye, S.; Yao, J.; Ni, Q. High aspect ratio carboxylated cellulose nanofibers cross-linked to robust aerogels for superabsorption–flocculants: Paving way from nanoscale to macroscale. ACS Appl. Mater. Interfaces 2018, 10, 20755–20766. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- De Nooy, A.E.; Besemer, A.C.; van Bekkum, H. Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydr. Res. 1995, 269, 89–98. [Google Scholar] [CrossRef]
- Li, L.; Guo, J.; Kang, C.; Song, H. Reinforcement of Nanocomposite Hydrogel with Dialdehyde Cellulose Nanofibrils via Physical and Double Network Crosslinking Synergies. Polymers 2023, 15, 1765. [Google Scholar] [CrossRef] [PubMed]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Dax, D.; Tapiero, Y.; Xu, C.; Willför, S. Bio-based hydrogels with ion exchange properties applied to remove Cu(II), Cr(VI), and As(V) ions from water. Front. Bioeng. Biotechnol. 2021, 9, 656472. [Google Scholar] [CrossRef] [PubMed]
- Baiya, C.; Nannuan, L.; Tassanapukdee, Y.; Chailapakul, O.; Songsrirote, K. The synthesis of carboxymethyl cellulose-based hydrogel from sugarcane bagasse using microwave-assisted irradiation for selective adsorption of copper(II) ions. Environ. Prog. Sustain. Energy 2019, 38, S157–S165. [Google Scholar] [CrossRef]
- Song, L.; Liu, F.; Zhu, C.; Li, A. Facile one-step fabrication of carboxymethyl cellulose based hydrogel for highly efficient removal of Cr(VI) under mild acidic condition. Chem. Eng. J. 2019, 369, 641–651. [Google Scholar] [CrossRef]
- Kundu, D.; Mondal, S.K.; Banerjee, T. Development of β-cyclodextrin-cellulose/hemicellulose-based hydrogels for the removal of Cd(II) and Ni(II): Synthesis, kinetics, and adsorption aspects. J. Chem. Eng. Data 2019, 64, 2601–2617. [Google Scholar] [CrossRef]
- Astrini, N.; Anah, L.; Haryadi, H.R. Adsorption of heavy metal ion from aqueous solution by using cellulose based hydrogel composite. In Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2015; Volume 353, pp. 191–197. [Google Scholar]
- Fan, X.; Wang, X.; Cai, Y.; Xie, H.; Han, S.; Hao, C. Functionalized cotton charcoal/chitosan biomass-based hydrogel for capturing Pb2+, Cu2+ and MB. J. Hazard. Mater. 2022, 423, 127191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, L.; Zeng, H.; Yan, P.; Nie, J.; Sharma, V.K.; Wang, C. A three-dimensional macroporous network structured chitosan/cellulose biocomposite sponge for rapid and selective removal of mercury(II) ions from aqueous solution. Chem. Eng. J. 2019, 363, 192–202. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.; Yu, J.; Fan, Y.; Yang, Y.; Ma, J.; Wang, Z. Synthesis of lignocellulose-based composite hydrogel as a novel biosorbent for Cu2+ removal. Cellulose 2018, 25, 7315–7328. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Navarathna, C.; Schueneman, G.T.; Hassan, E.B. Biohybrid hydrogel and aerogel from self-assembled nanocellulose and nanochitin as a high-efficiency adsorbent for water purification. ACS Appl. Mater. Interfaces 2019, 11, 46714–46725. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Hu, D.; Yang, R.; Zhang, J.; Guan, Y.; Lv, F.; Gao, H. A mesoporous nanocellulose/sodium alginate/carboxymethyl-chitosan gel beads for efficient adsorption of Cu2+ and Pb2+. Int. J. Biol. Macromol. 2021, 187, 922–993. [Google Scholar] [CrossRef] [PubMed]
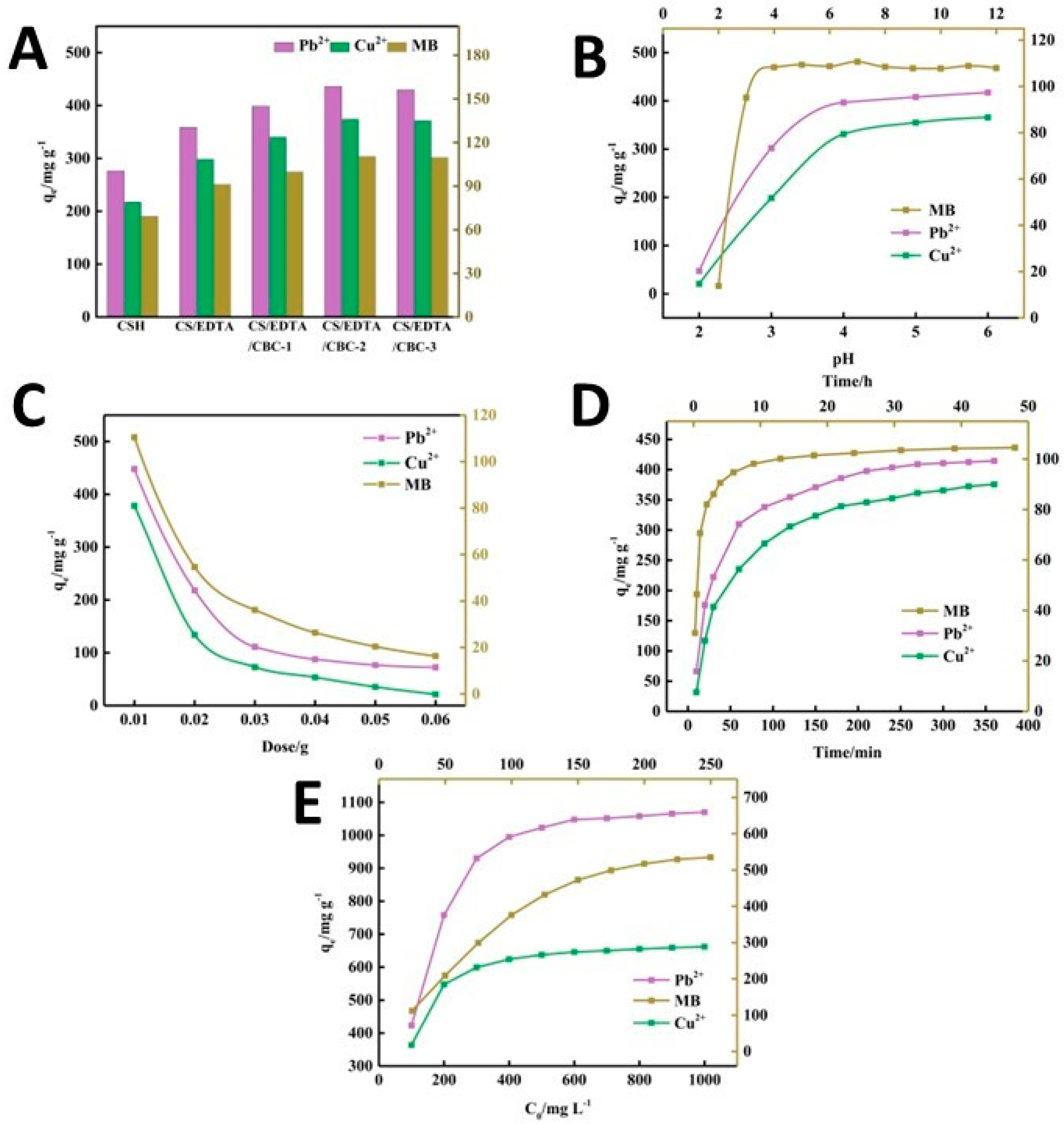
- Godiya, C.B.; Revadekar, C.; Kim, J.; Park, B.J. Amine-bilayer-functionalized cellulose-chitosan composite hydrogel for the efficient uptake of hazardous metal cations and catalysis in polluted water. J. Hazard. Mater. 2022, 436, 129112. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Ahmmed, M.R.; Ul Hoque, M.I.; Ebna Mohsen, T.; Mondol, A.; Rahaman, M.H.; Yesmin, M.; Rahman, M.S.; Nur Alam, S.M. Biodegradable Composite of Gelatin Blend Microcrystalline Cellulose for Cd2+, Pb2+, and Cr3+ Adsorption from an Aqueous Solution. Adv. Polym. Technol. 2023, 2023, 1893660. [Google Scholar] [CrossRef]
- Rodrigues, F.H.; de, C. Magalhães, C.E.; Medina, A.L.; Fajardo, A.R. Hydrogel composites containing nanocellulose as adsorbents for aqueous removal of heavy metals: Design, optimization, and application. Cellulose 2019, 26, 9119–9133. [Google Scholar] [CrossRef]
- Zhao, H.; Ouyang, X.K.; Yang, L.Y. Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber–sodium alginate hydrogel beads. J. Mol. Liq. 2021, 324, 115122. [Google Scholar] [CrossRef]
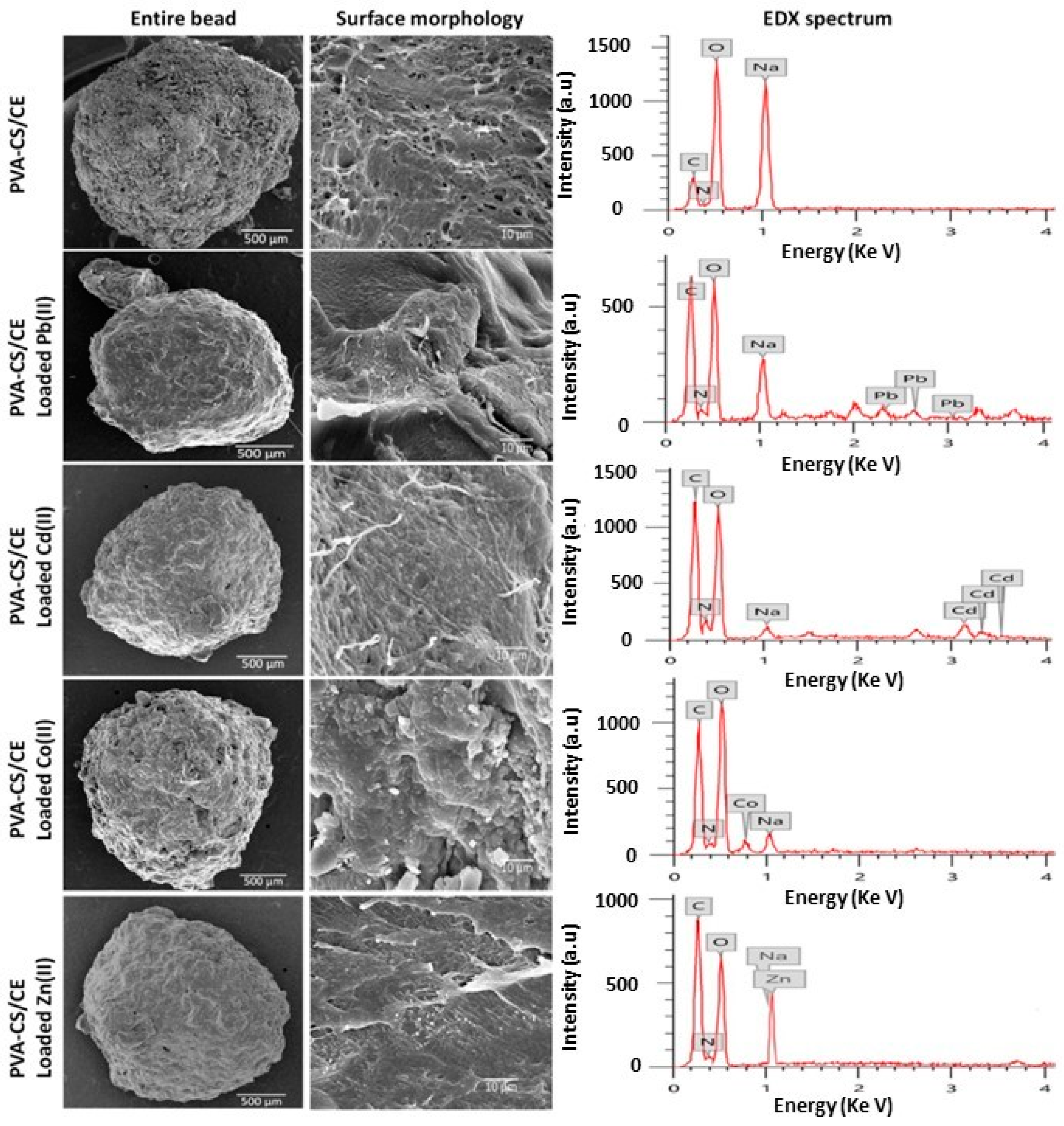
- Aljar, M.A.A.; Rashdan, S.; Almutawah, A.; El-Fattah, A.A. Synthesis and Characterization of Biodegradable Poly (vinyl alcohol)-Chitosan/Cellulose Hydrogel Beads for Efficient Removal of Pb(II), Cd(II), Zn(II), and Co(II) from Water. Gels 2023, 9, 328. [Google Scholar] [CrossRef] [PubMed]




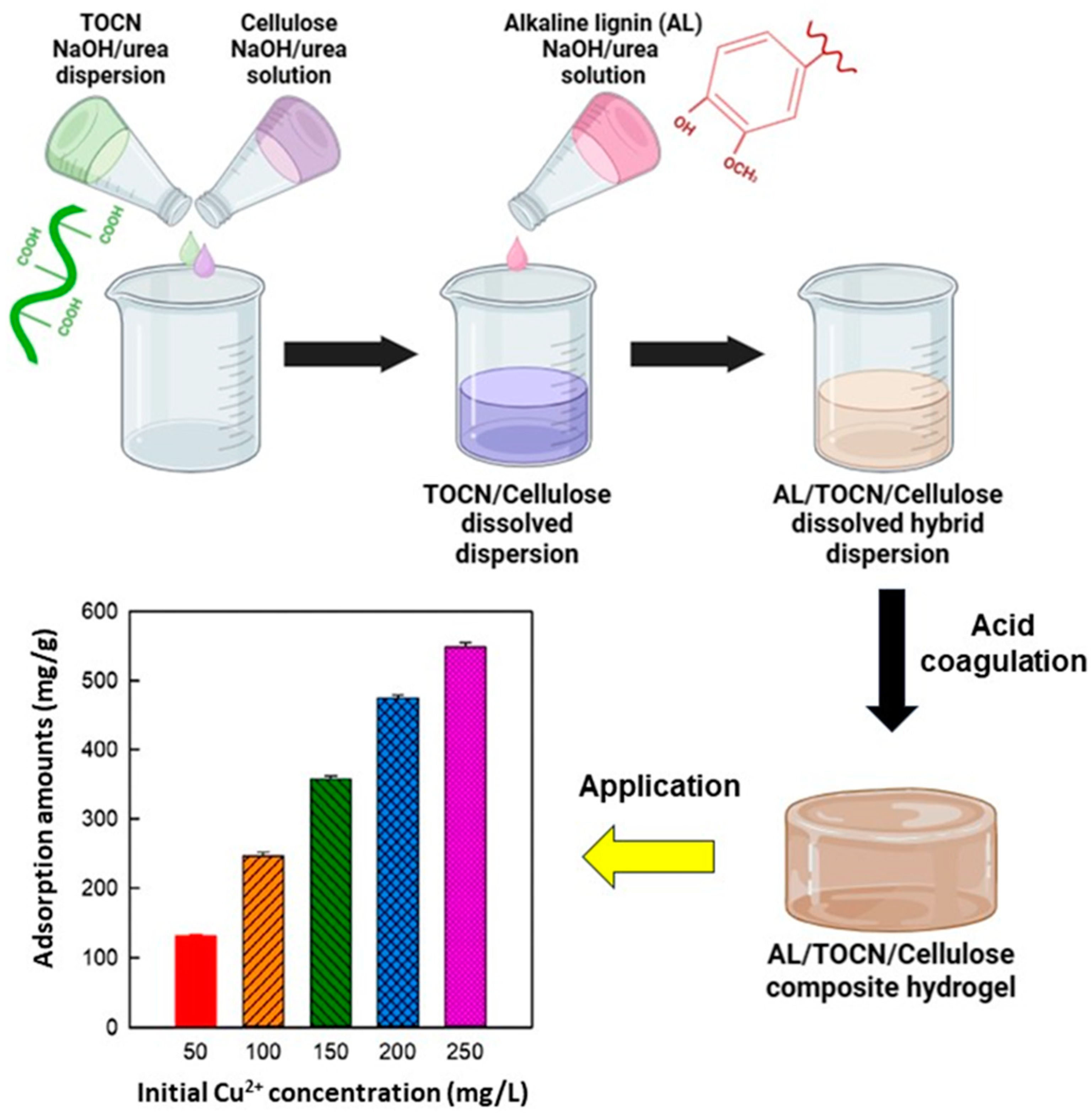
| Model | Equation | Significance | Adsorbent | Reference |
|---|---|---|---|---|
| Pseudo-First-Order | dqt/dt = k1 (qe − qt) (dqt/dt) represents the rate of change in the quantity of adsorbate with respect to time. (k1) is the pseudo-first-order rate constant. (qe) is the equilibrium adsorption capacity (the maximum amount of ions that can be adsorbed by the adsorbent). (qt) represents the amount of ions adsorbed at a specific time (t). | It is applicable in the initial phase before reaching the equilibrium absorption capacity. | M. speciose cellulose (MSCC)/chitosan cellulose fiber was extracted from pineapple leaves and modified with CM. | [92] [93] |
| Pseudo-Second-Order | dqt/dt = k2 (qe − qt)2 (dqt/dt) represents the rate of change in the quantity of adsorbate with respect to time. (k2) is the pseudo-second-order rate constant. (qe) is the equilibrium adsorption capacity (the maximum amount of ions that can be adsorbed by the adsorbent). (qt) represents the amount of ions adsorbed at a specific time (t). | Chemical adsorption represents the rate-limiting step, involving valence forces for chemical coordination through the sharing or exchange of electrons between the adsorbent and heavy metals (ion exchange). The occupancy rate of adsorption sites is proportional to the square of the number of unoccupied sites. | cellulose fiber was extracted from pineapple leaves and modified with EDTA. CMC/PAM CMC-based bead-like | [93] [94] [95] |
| Model | Equation | Significance | Adsorbent | Reference |
|---|---|---|---|---|
| Langmuir | qe = (qmax KL Ce)/1 + (KL Ce) (qe): amount of adsorbed metal ions per unit mass of adsorbent at equilibrium. (Ce): metal ion concentration in solution at equilibrium. (KL): Langmuir binding constant. (qmax): maximum amount of metal adsorbed per unit weight of adsorbent. | Describes monolayer chemical adsorption on a homogeneous surface. | CMC Thiol-modified cellulose sponges. | [96] [97] |
| Freundlich | qe = KF (Ce)1/n (qe): amount of adsorbed metal ions per unit mass of adsorbent at equilibrium. (Ce): metal ion concentration in solution at equilibrium. (KF): Freundlich isotherm constant related to adsorption capacity. (n): constant related to adsorption intensity. | Describes multilayer physical adsorption on a non-uniform surface. | CMC-based bead-like | [95] |
| qe = (RT/bT) ln(KTCe) | ||||
| Temkin | (qe): amount of adsorbed metal ions per unit weignt of adsorbent at equilibrium. (Ce): metal ion concentration in solution at equilibrium. (KT): Temkin isotherm equilibrium binding constant corresponding to the maximum binding energy. (bT): Temkin isotherm constant. (R): universal gas constant. (T): absolute temperature (°K). | Describes the indirect interaction between the adsorbent and the adsorbate and shows a linear decrease in heat of adsorption with increasing surface coverage of the adsorbent. | Cellulose grafted with the vinyl glycidyl methacrylate monomer. | [98] |
| Cellulose or Cellulose Derivative | Additional Polymer | Other Components or Modifications | Adsorption Capacity | Ref. | |
|---|---|---|---|---|---|
| Non-composite hydrogels | CMC | ― | epichlorohydrin as cross-linking agent | Cu2+: 6.49 mmol/g Pb2+: 5.15 mmol/g Ni2+: 4.06 mmol/g | [96] |
| CMC | ― | aluminum nitrate as cross-linking agent | Pb2+: 550 mg/g Ni2+: 620 mg/g Co2+: 760 mg/g | [95] | |
| Cellulose from bamboo fibers | ― | titanate nanotubes | Cu2+: 176.4 mg/g Pb2+: 613.5 mg/g Sr2+: 113.3 mg/g Fe2+: 158.2 mg/g Cd2+: 294.6 mg/g Zn2+: 187.8 mg/g Cr3+: 97.8 mg/g | [99] | |
| Cellulose fiber from pineapple leaves | ― | modification with carboxymethyl group (CM) and ethylenediaminetetraacetic acid (EDTA) group to produce Cell-CM and Cell-EDTA, respectively | For Cell-CM Pb2+: 63.4mg/g Cd2+: 23 mg/g For Cell-EDTA Pb2+: 41.2mg/g Cd2+: 33.2 mg/g | [93] | |
| HEC | ― | EDTA dianhydride (EDTAD) as cross-linking agent | Pb2+: 405.37 mg/g Cu2+: 265.47 mg/g Cd2+: 203.36 mg/g Zn2+: 108.36 mg/g | [49] | |
| Ginger fibers | ― | citric acid (C6H8O7) and hydrochloric acid (HCl) | Cu2+: 45.053 mg/g | [103] | |
| Jute | ― | sodium nitrite and nitric acid | Pb2+: 2270 mg/g | [104] | |
| Composite hydrogels | CMC and microcrystalline cellulose | Xilan | ethylene glycol diglycidyl ether as a crosslinking agent | Cd2+: 61.44 mg/g Ni2+: 55.85 mg/g | [111] |
| CMC | Polyacrylic acid | montmorillonite | |||
| Pb2+: 146.19 mg/g Zn2+: 286.67 mg/g | [112] | ||||
| CMC | PVA | ― | Ag+: 8.4 mg/g | [57] | |
| Degreasing cotton | Chitosan | ethylenediaminetetraacetic acid (EDTA) | Pb2+: 1105.78 mg/g Cu2+: 678.04 mg/g | [113] | |
| Cellulose | Chitosan | glutaraldehyde (EGDE) as crosslinking agent | Hg2+: 495 mg/g | [114] | |
| TEMPO-oxidized negatively charged (unidimensional) cellulose nanofibers (TOCNFs) | Partially deacetylated chitin nanofibers | ― | As(III) ion: 217 mg/g | [116] | |
| TEMPO-oxidized nanocellulose | Carboxymethyl chitosan and sodium alginate | Ca2+ as cross-linking agent | Pb2+: 472.59 mg/g Cu2+: 169.94 mg/g | [117] | |
| Microcrystalline cellulose | Chitosan, polydopamine (PDA), and polyethyleneimine (PEI) | ― | Cu2+: 434.8 mg/g Zn2+: 277.7 mg/g Ni2+ 261.8 mg/g | [118] | |
| Cellulose nanowhiskers (CNWs) | Chitosan-g-poly (acrylic acid) | MBA as cross-linking agent and TEMED as catalyst | Pb2+: 818.4 mg/g Cu2+: 325.5 mg/g | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persano, F.; Malitesta, C.; Mazzotta, E. Cellulose-Based Hydrogels for Wastewater Treatment: A Focus on Metal Ions Removal. Polymers 2024, 16, 1292. https://doi.org/10.3390/polym16091292
Persano F, Malitesta C, Mazzotta E. Cellulose-Based Hydrogels for Wastewater Treatment: A Focus on Metal Ions Removal. Polymers. 2024; 16(9):1292. https://doi.org/10.3390/polym16091292
Chicago/Turabian StylePersano, Francesca, Cosimino Malitesta, and Elisabetta Mazzotta. 2024. "Cellulose-Based Hydrogels for Wastewater Treatment: A Focus on Metal Ions Removal" Polymers 16, no. 9: 1292. https://doi.org/10.3390/polym16091292
APA StylePersano, F., Malitesta, C., & Mazzotta, E. (2024). Cellulose-Based Hydrogels for Wastewater Treatment: A Focus on Metal Ions Removal. Polymers, 16(9), 1292. https://doi.org/10.3390/polym16091292









