Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
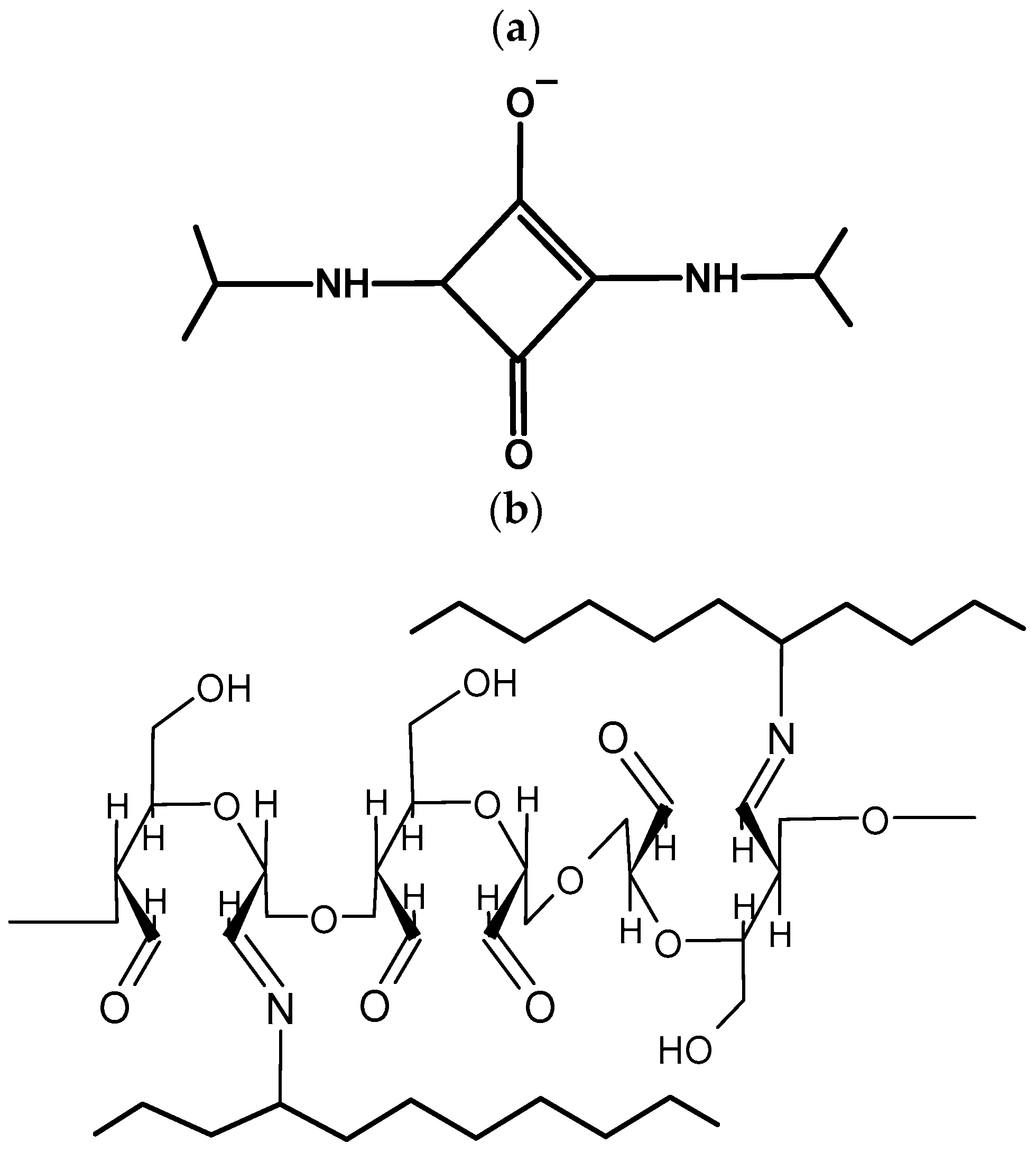
2.2. Hydrogel Preparation
2.3. Infrared Spectroscopy
2.4. Thermal Analysis
2.5. Mechanical Properties
2.6. Swelling Ability
2.7. SEM Images
2.8. Biological Research—Cytotoxicity Test
2.9. Viscosity Measurements
2.10. Printability—Preliminary Test
3. Results
3.1. Preliminary Results
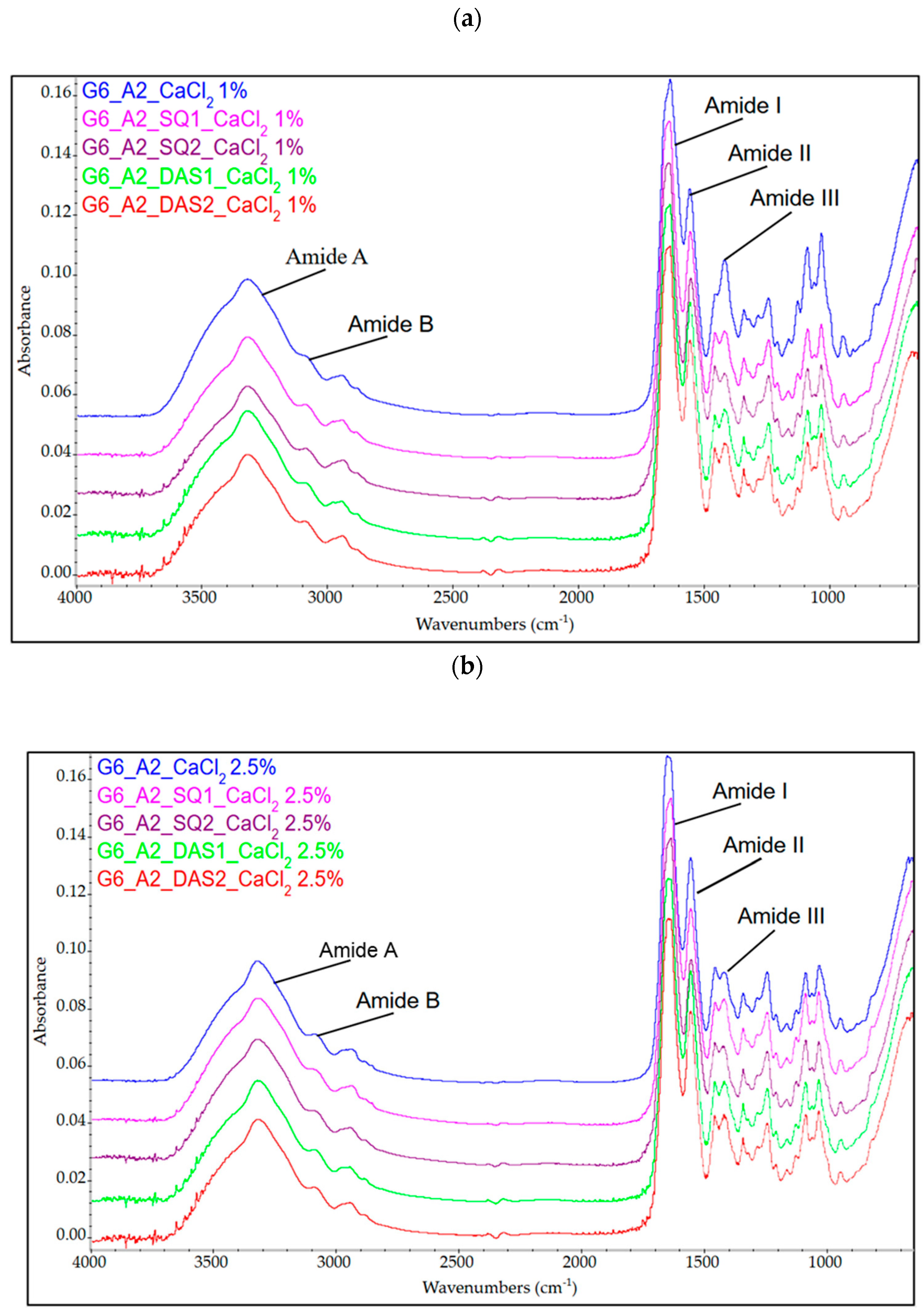
3.2. Infrared Spectroscopy FTIR Analysis
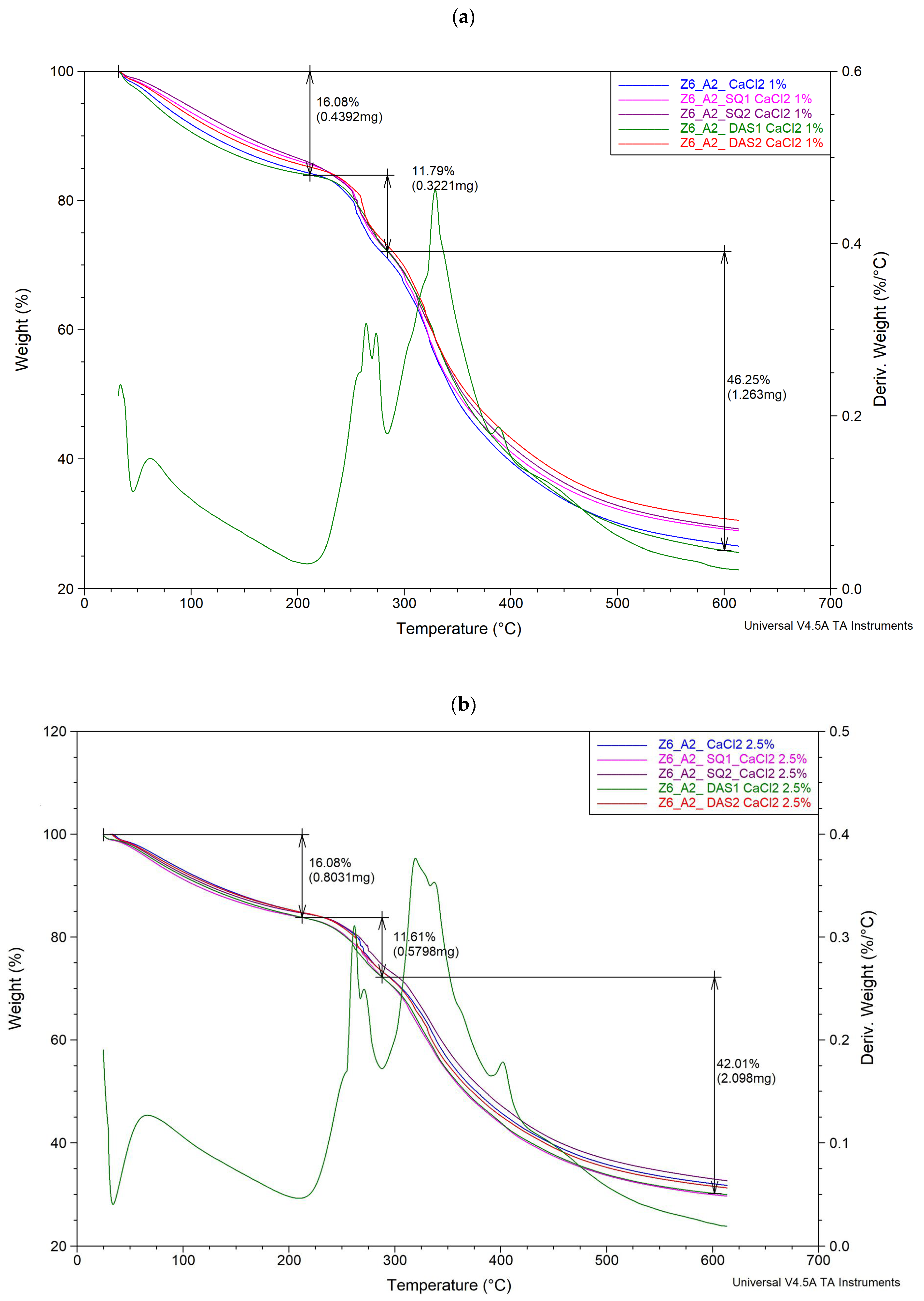
3.3. Thermal Analysis Results
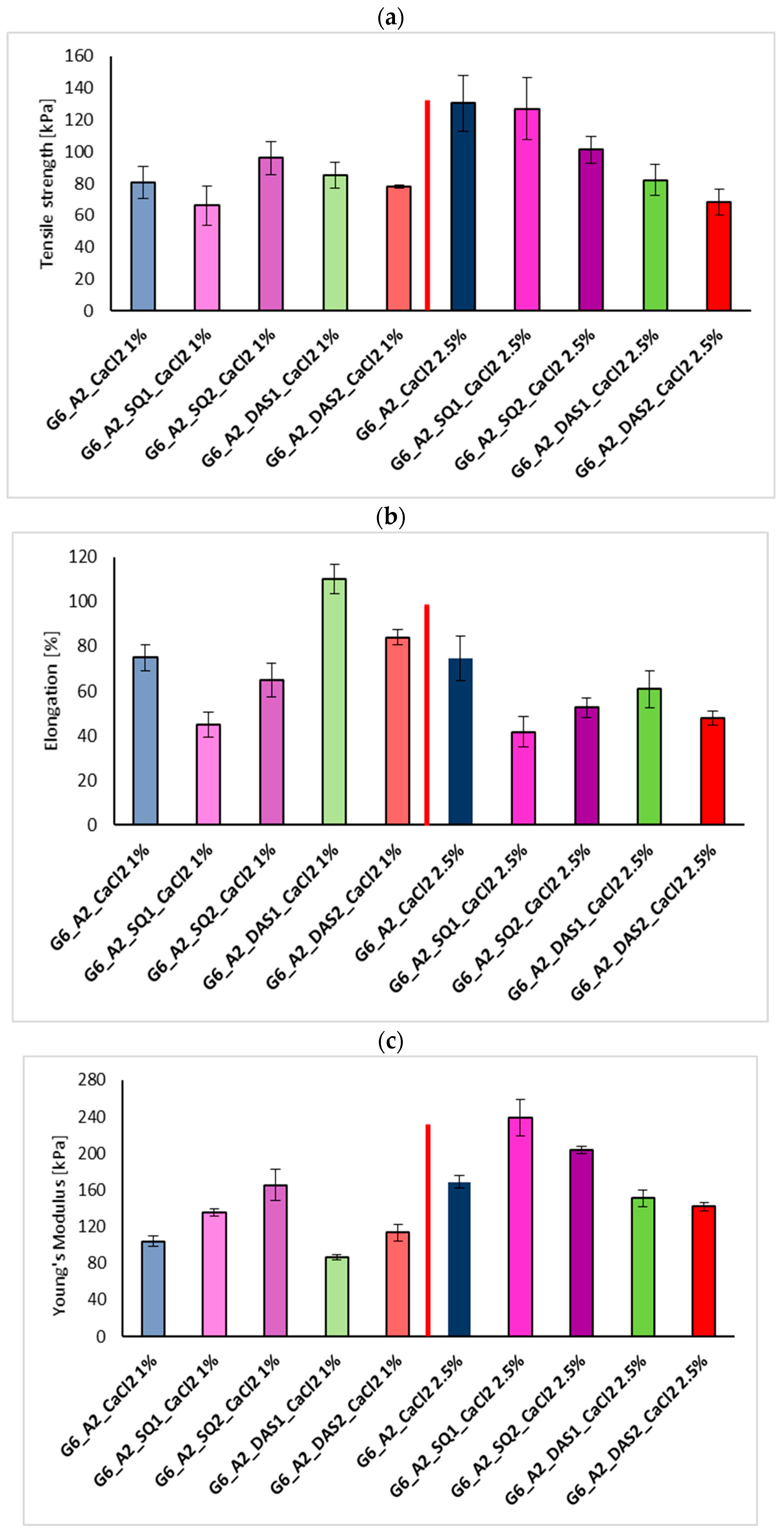
3.4. Mechanical Properties Test
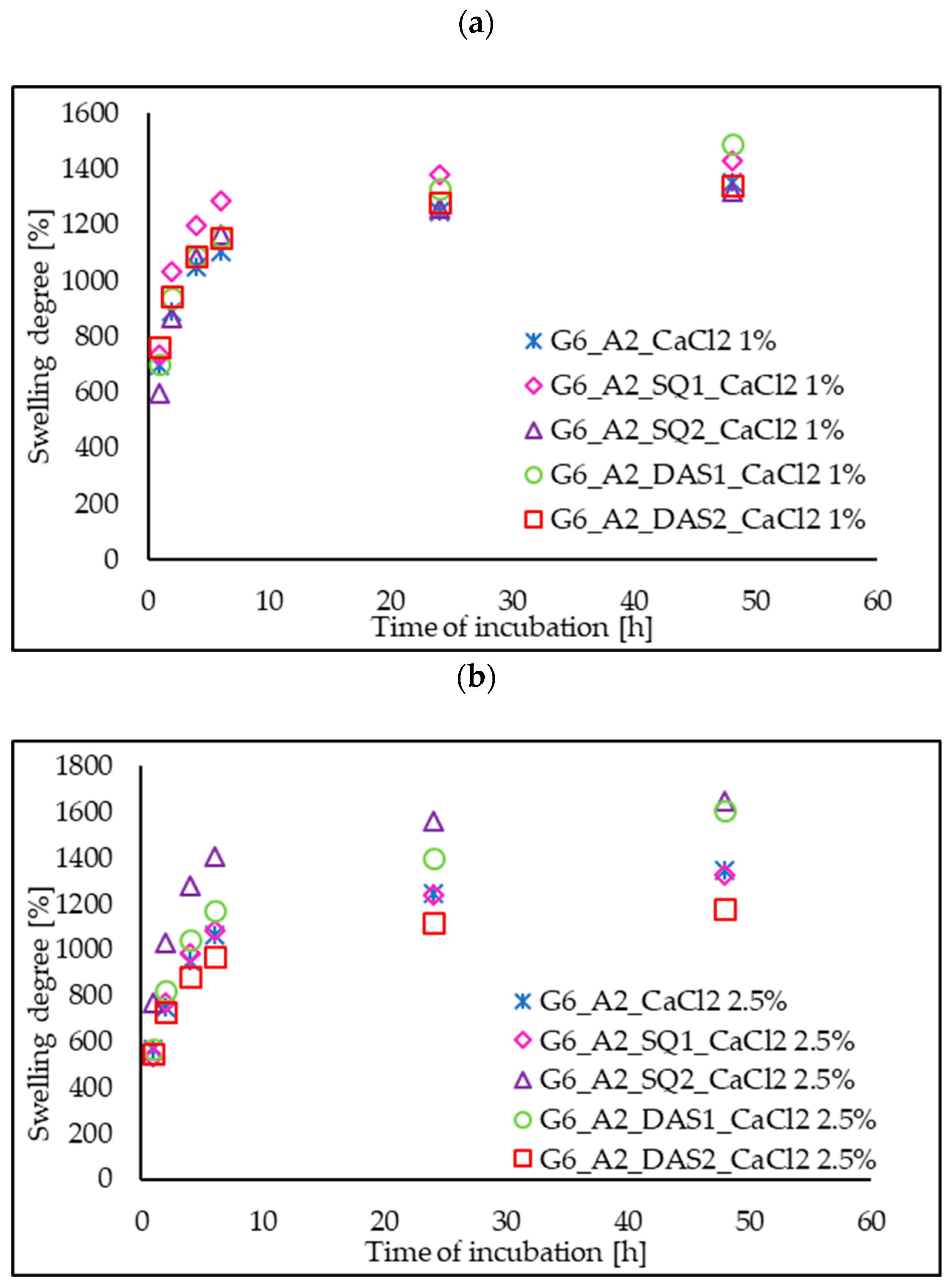
3.5. Swelling Ability
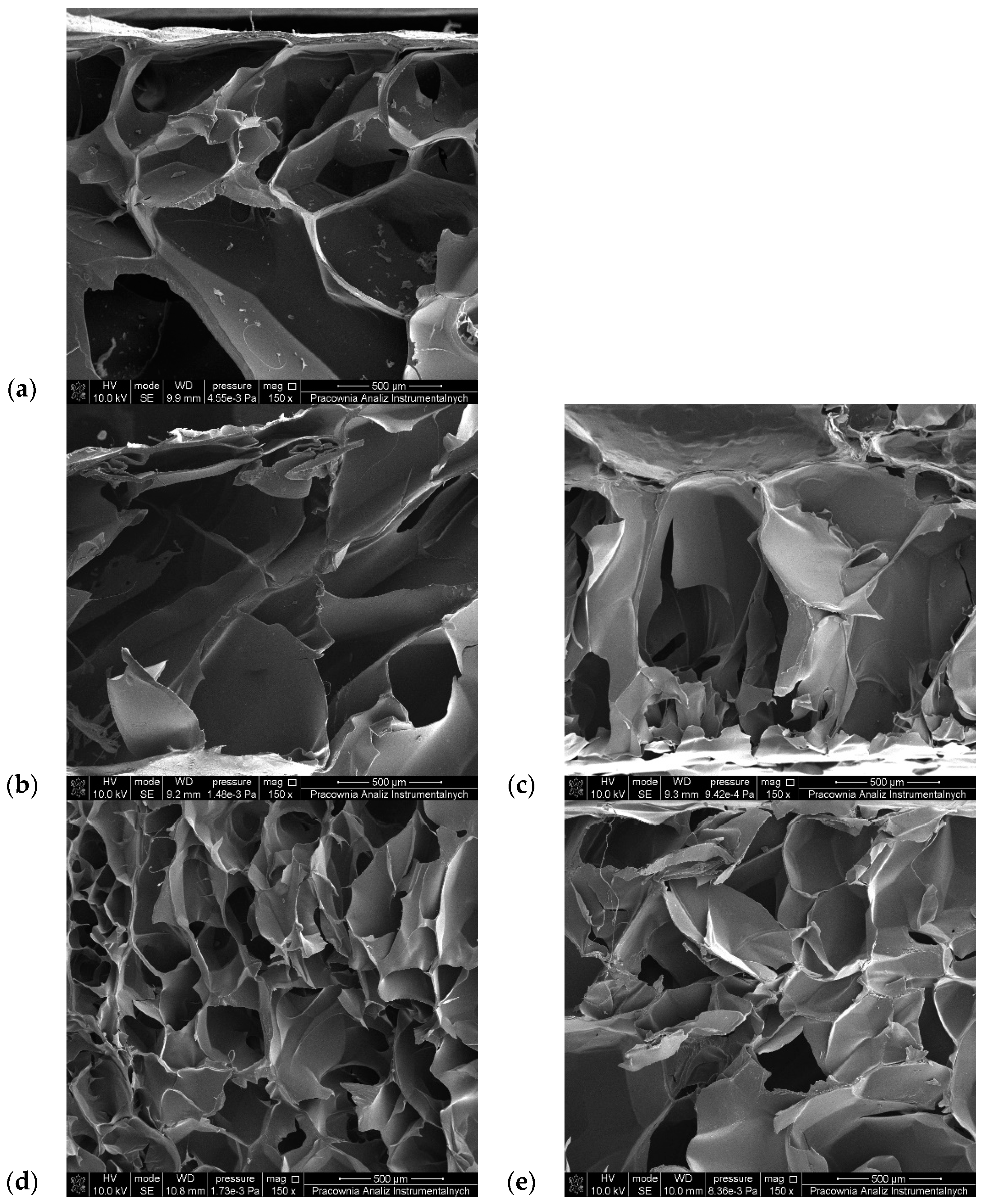
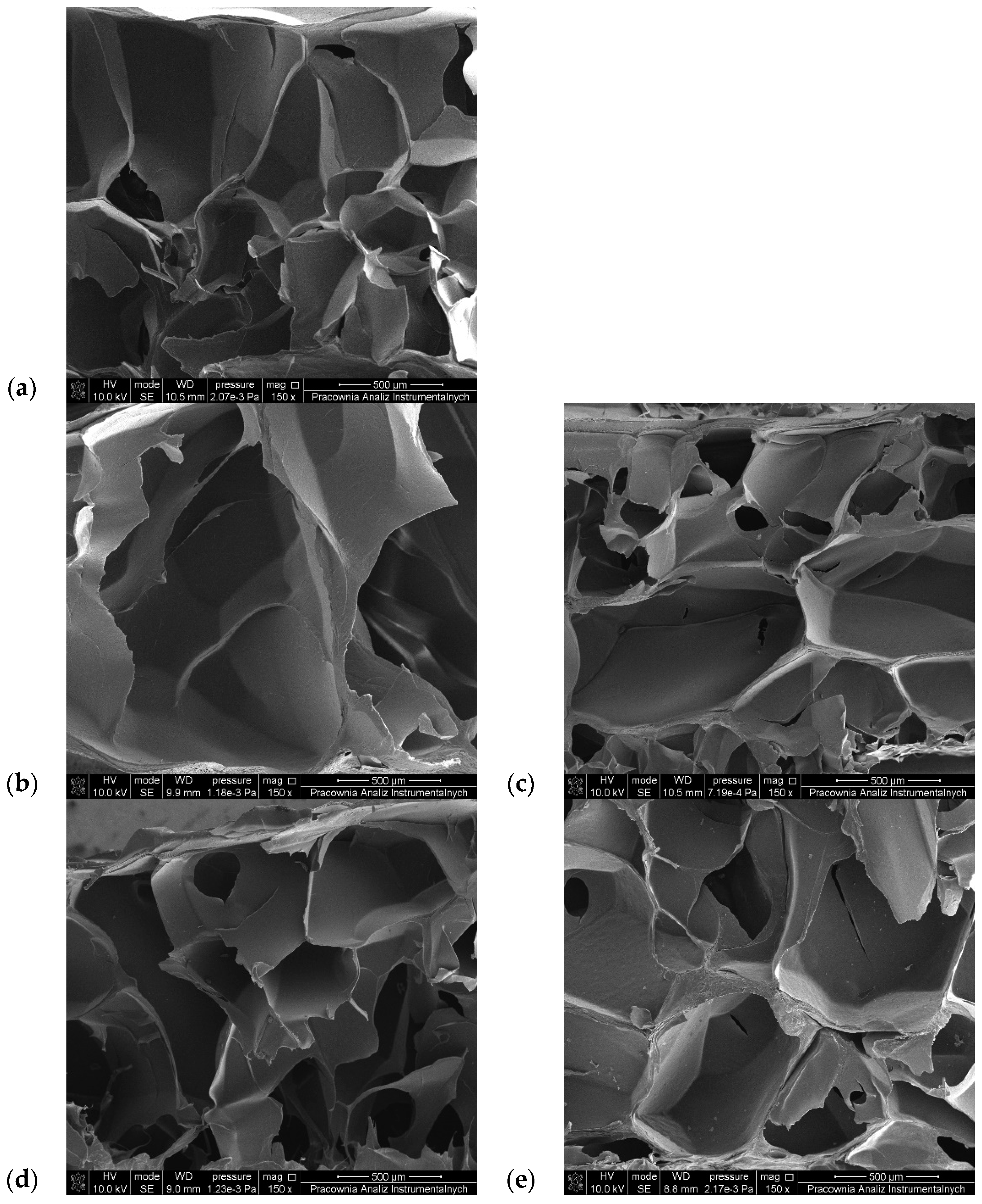
3.6. SEM Observations
3.7. Biological Research—Cytotoxicity Test Results
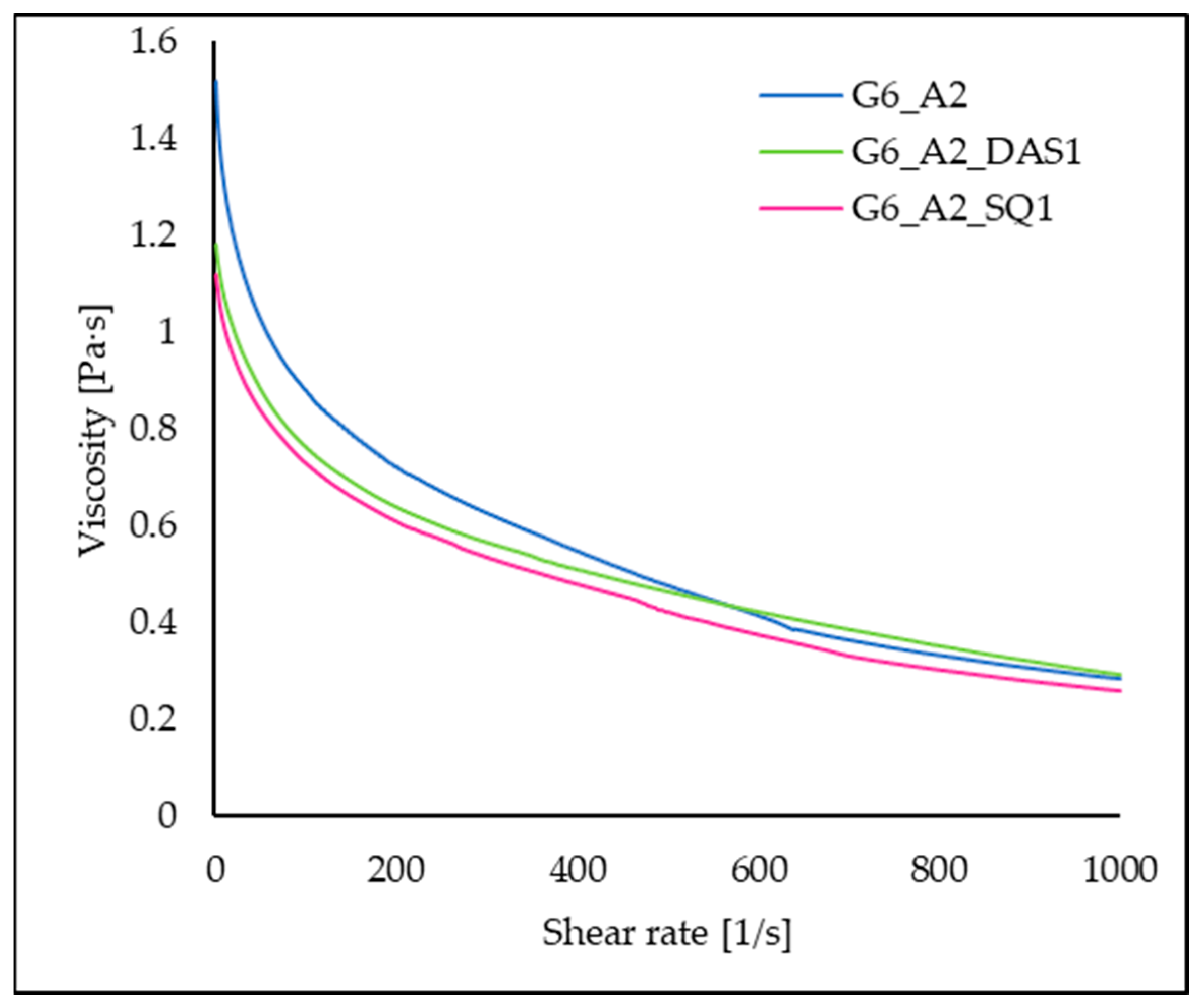
3.8. Viscosity
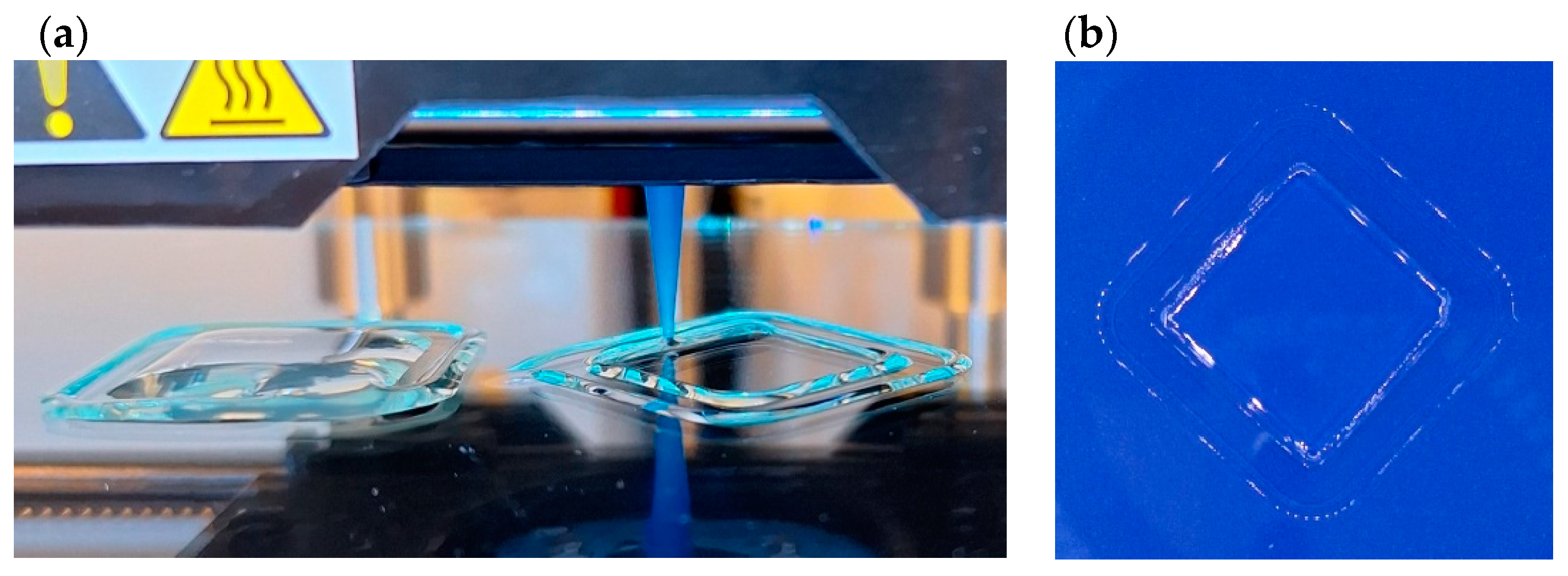
3.9. Printability—Preliminary Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, E.; Cristallini, C.; Barbani, N.; Vozzi, G.; Giusti, P. Preparation and Characterization of Alginate/Gelatin Blend Films for Cardiac Tissue Engineering. J. Biomed. Mater. Res. A 2009, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent Advances in Smart Hydrogels for Biomedical Applications: From Self-Assembly to Functional Approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.W. Emerging Properties of Hydrogels in Tissue Engineering. J. Tissue Eng. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- Ferris, C.J.; Gilmore, K.J.; Beirne, S.; McCallum, D.; Wallace, G.G.; In Het Panhuis, M. Bio-Ink for on-Demand Printing of Living Cells. Biomater. Sci. 2013, 1, 224–230. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-Ink Properties and Printability for Extrusion Printing Living Cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef]
- Badhe, R.V.; Chatterjee, A.; Bijukumar, D.; Mathew, M.T. Current Advancements in Bio-Ink Technology for Cartilage and Bone Tissue Engineering. Bone 2023, 171, 116746. [Google Scholar] [CrossRef]
- Barron, J.A.; Spargo, B.J.; Ringeisen, B.R. Biological Laser Printing of Three Dimensional Cellular Structures. Appl. Phys. A Mater. Sci. Process. 2004, 79, 1027–1030. [Google Scholar] [CrossRef]
- Gao, Q.; Kim, B.S.; Gao, G. Advanced Strategies for 3D Bioprinting of Tissue and Organs Analogs Using Alginate Hydrogel Bioinks. Mar. Drugs 2021, 19, 708. [Google Scholar] [CrossRef] [PubMed]
- Semba, J.A.; Mieloch, A.A.; Tomaszewska, E.; Cywoniuk, P.; Rybka, J.D. Formulation and Evaluation of a Bioink Composed of Alginate, Gelatin, and Nanocellulose for Meniscal Tissue Engineering. Int. J. Bioprint 2023, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.D.; Onofrillo, C.; Duchi, S.; Li, X.; Zhang, Y.; Tian, P.; Lu, L.; Trengove, A.; Quigley, A.; Gambhir, S.; et al. Evaluation of Sterilisation Methods for Bio-Ink Components: Gelatin, Gelatin Methacryloyl, Hyaluronic Acid and Hyaluronic Acid Methacryloyl. Biofabrication 2019, 11, 035003. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Cao, N.; Chen, X.B.; Schreyer, D.J. Influence of Calcium Ions on Cell Survival and Proliferation in the Context of an Alginate Hydrogel. ISRN Chem. Eng. 2012, 2012, 516461. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, Source, Extraction and Industrial Applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Tosh, S.M.; Marangoni, A.G. Determination of the Maximum Gelation Temperature in Gelatin Gels. Appl. Phys. Lett. 2004, 84, 4242–4244. [Google Scholar] [CrossRef]
- Petros, S.; Tesfaye, T.; Ayele, M. A Review on Gelatin Based Hydrogels for Medical Textile Applications. J. Eng. 2020, 2020, 8866582. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Bartniak, M.; Rosińska, K.; Bociąga, D. Optimization of the preparation process stages of the bioink compositions based on sodium alginate and gelatin to improve the viability of biological material contained in hydrogel 3d printouts. Eng. Biomater. 2022, 25, 7–16. [Google Scholar] [CrossRef]
- Asim, S.; Tabish, T.A.; Liaqat, U.; Ozbolat, I.T.; Rizwan, M. Advances in Gelatin Bioinks to Optimize Bioprinted Cell Functions. Adv. Healthc. Mater. 2023, 12, 2203148. [Google Scholar] [CrossRef]
- Gaglio, C.G.; Baruffaldi, D.; Pirri, C.F.; Napione, L.; Frascella, F. GelMA Synthesis and Sources Comparison for 3D Multimaterial Bioprinting. Front. Bioeng. Biotechnol. 2024, 12, 1383010. [Google Scholar] [CrossRef]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium Alginate/Gelatine Hydrogels for Direct Bioprinting-the Effect of Composition Selection and Applied Solvents on the Bioink Properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef]
- Koch, F.; Tröndle, K.; Finkenzeller, G.; Zengerle, R.; Zimmermann, S.; Koltay, P. Generic Method of Printing Window Adjustment for Extrusion-Based 3D-Bioprinting to Maintain High Viability of Mesenchymal Stem Cells in an Alginate-Gelatin Hydrogel. Bioprinting 2020, 20, e00094. [Google Scholar] [CrossRef]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical Behaviour of Alginate-Gelatin Hydrogels for 3D Bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef]
- Amr, M.; Dykes, I.; Counts, M.; Kernan, J.; Mallah, A.; Mendenhall, J.; Van Wie, B.; Abu-Lail, N.; Gozen, B.A. 3D Printed, Mechanically Tunable, Composite Sodium Alginate, Gelatin and Gum Arabic (SA-GEL-GA) Scaffolds. Bioprinting 2021, 22, e00133. [Google Scholar] [CrossRef]
- Kim, J.; Choi, Y.J.; Gal, C.W.; Sung, A.; Park, H.; Yun, H.S. Development of an Alginate–Gelatin Bioink Enhancing Osteogenic Differentiation by Gelatin Release. Int. J. Bioprint 2022, 9, 142–157. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Qin, X.; Wa, Q. 3D Printing of Concentrated Alginate/Gelatin Scaffolds with Homogeneous Nano Apatite Coating for Bone Tissue Engineering. Mater. Des. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning Alginate-Gelatin Bioink Properties by Varying Solvent and Their Impact on Stem Cell Behavior. Sci. Rep. 2018, 8, 8020. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts. Tissue Eng. Part. A 2021, 27, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Sonaye, S.Y.; Ertugral, E.G.; Kothapalli, C.R.; Sikder, P. Extrusion 3D (Bio)Printing of Alginate-Gelatin-Based Composite Scaffolds for Skeletal Muscle Tissue Engineering. Materials 2022, 15, 7945. [Google Scholar] [CrossRef] [PubMed]
- Mirek, A.; Belaid, H.; Barranger, F.; Grzeczkowicz, M.; Bouden, Y.; Cavaillès, V.; Lewińska, D.; Bechelany, M. Development of a New 3D Bioprinted Antibiotic Delivery System Based on a Cross-Linked Gelatin-Alginate Hydrogel. J. Mater. Chem. B 2022, 10, 8862–8874. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhao, S.; Hao, S.; He, Y.; Feng, M.; Zhou, K.; He, Y.; Yang, J.; Mao, H.; Gu, Z. Functionalized Gelatin-Alginate Based Bioink with Enhanced Manufacturability and Biomimicry for Accelerating Wound Healing. Int. J. Biol. Macromol. 2023, 240, 124364. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Liu, Y.; Zhang, C.J.; Zhang, C.; Zhu, P. Alginate/Gelatin Blended Hydrogel Fibers Cross-Linked by Ca 2+ and Oxidized Starch: Preparation and Properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Fayyazbakhsh, F.; Khayat, M.J.; Leu, M.C. 3D-Printed Gelatin-Alginate Hydrogel Dressings for Burn Wound Healing: A Comprehensive Study. Int. J. Bioprint 2022, 8, 274–291. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wisniewska, J.; Kaczmarek, H.; Marszałł, M.P. Chitosan–Collagen Coated Magnetic Nanoparticles for Lipase Immobilization—New Type of “Enzyme Friendly” Polymer Shell Crosslinking with Squaric Acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef]
- Chasák, J.; Šlachtová, V.; Urban, M.; Brulíková, L. Squaric Acid Analogues in Medicinal Chemistry. Eur. J. Med. Chem. 2021, 209, 112872. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Kuderko, J.; Bajek, A.; Maj, M.; Sionkowska, A.; Ziegler-Borowska, M. Collagen/Elastin Hydrogels Cross-Linked by Squaric Acid. Mater. Sci. Eng. C 2016, 60, 100–108. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Wegrzynowska-Drzymalska, K.; Bajek, A.; Maj, M.; Sionkowska, A. Is Dialdehyde Starch a Valuable Cross-Linking Agent for Collagen/Elastin Based Materials? J. Mater. Sci. Mater. Med. 2016, 27, 67. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, M.; Kapuśniak, J.; Karolczyk-Kostuch, S.; Khachatryan, G.; Kowalski, S.; Para, A.; Sikora, M.; Staroszczyk, H.; Szymońska, J.; Tomasik, P. Selected Novel Materials from Polysaccharides. Polimery 2006, 51, 517–523. [Google Scholar] [CrossRef]
- International Organization for Standardization 10993 Biological Evaluation of Medical Devices-Part 5:2009 Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 7 May 2024).
- International Organization for Standardization 10993 Biological Evaluation of Medical Devices-Part 12:2021 Sample Preparation and Reference Materials. Available online: https://www.iso.org/standard/75769.html (accessed on 7 May 2024).
- Luo, Y.; Lode, A.; Akkineni, A.R.; Gelinsky, M. Concentrated Gelatin/Alginate Composites for Fabrication of Predesigned Scaffolds with a Favorable Cell Response by 3D Plotting. RSC Adv. 2015, 5, 43480–43488. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of Alginate-Gelatin Crosslinked Hydrogel Microcapsules and Evaluation of the Microstructure and Physico-Chemical Properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Saha, P. On the Development and Characterisation of Crosslinked Sodium Alginate/Gelatine Hydrogels. J. Mech. Behav. Biomed. Mater. 2013, 18, 152–166. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and Its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Grabska-Zielinska, S.; Sionkowska, A.; Olewnik-Kruszkowska, E.; Reczynska, K.; Reczynska, R.; Elzbieta Pamula, E. Is Dialdehyde Chitosan a Good Substance to Modify Physicochemical Properties of Biopolymeric Materials? Int. J. Mol. Sci. 2021, 22, 3391. [Google Scholar] [CrossRef]
- Dos Santos Araújo, P.; Belini, G.B.; Mambrini, G.P.; Yamaji, F.M.; Waldman, W.R. Thermal Degradation of Calcium and Sodium Alginate: A Greener Synthesis towards Calcium Oxide Micro/Nanoparticles. Int. J. Biol. Macromol. 2019, 140, 749–760. [Google Scholar] [CrossRef]
- Correia, D.M.; Padrão, J.; Rodrigues, L.R.; Dourado, F.; Lanceros-Méndez, S.; Sencadas, V. Thermal and Hydrolytic Degradation of Electrospun Fish Gelatin Membranes. Polym. Test. 2013, 32, 995–1000. [Google Scholar] [CrossRef]
- Pieróg, M.; Ostrowska-Czubenko, J.; Gierszewska-Druzyńska, M. Thermal Degradation of Double Crosslinked Hydrogel Chitosan Membranes. Prog. Chem. Appl. Chitin Deriv. 2012, 2012, 67–74. [Google Scholar]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of Chitosan-Sodium Alginate Films through Layer-by-Layer Assembly and Ferulic Acid Crosslinking: Film Properties, Characterization, and Formation Mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Sobczak-Kupiec, A. In Vitro Cytotoxicity of Hydrogels Based on Chitosan and Modified with Gold Nanoparticles. J. Polym. Res. 2017, 24, 153. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Velasquillo-Martínez, C.; Knauth, P.; López, Z.; Moreno-Valtierra, M.; Bravo-Madrigal, J.; Jiménez-Palomar, I.; Luna-Bárcenas, G.; Espinosa-Andrews, H.; García-Carvajal, Z.Y. Sterilized Chitosan-Based Composite Hydrogels: Physicochemical Characterization and In Vitro Cytotoxicity. J. Biomed. Mater. Res. A 2020, 108, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.; Baldy-Chudzik, K.; Mazurek-Popczyk, J.; Zaręba, Ł.; Klekiel, T.; Będziński, R. Changes in the Mechanical Properties of Alginate-Gelatin Hydrogels with the Addition of Pygeum Africanum with Potential Application in Urology. Int. J. Mol. Sci. 2022, 23, 10324. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Palmer, G.; Knowles, J.C.; Salih, V.; Young, A.M. Chemical, Modulus and Cell Attachment Studies of Reactive Calcium Phosphate Filler-Containing Fast Photo-Curing, Surface-Degrading, Polymeric Bone Adhesives. Acta Biomater. 2010, 6, 2695–2703. [Google Scholar] [CrossRef]
- Rimessi, A.; Giorgi, C.; Pinton, P.; Rizzuto, R. The Versatility of Mitochondrial Calcium Signals: From Stimulation of Cell Metabolism to Induction of Cell Death. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 808–816. [Google Scholar] [CrossRef]
- Li, Y.; Jia, H.; Cheng, Q.; Pan, F.; Jiang, Z. Sodium Alginate-Gelatin Polyelectrolyte Complex Membranes with Both High Water Vapor Permeance and High Permselectivity. J. Memb. Sci. 2011, 375, 304–312. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical Properties of Alginate-Based Films: Effect of Ionic Crosslinking and Mannuronic and Guluronic Acid Ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Martucci, J.; Vázquez, A.; Ruseckaite, R. Nanocomposites Based on Gelatin and Montmorillonite and Thermal Studies. J. Ofthermal Anal. Calorim. 2007, 89, 117–122. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between Gelatin and Sodium Alginate: UV and FTIR Studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.; Lau, H.C.; Kim, A. Gelatin-Alginate Complexes for EGF Encapsulation: Effects of H-Bonding and Electrostatic Interactions. Pharmaceutics 2019, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Grabska-Zielińska, S.; Skopińska-Wiśniewska, J.; Jakubowska, E. Examining the Impact of Squaric Acid as a Crosslinking Agent on the Properties of Chitosan-Based Films. Int. J. Mol. Sci. 2021, 22, 3329. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Rubini, K.; Panzavolta, S.; Bigi, A. Chemico-Physical Characterization of Gelatin Films Modified with Oxidized Alginate. Acta Biomater. 2010, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of Fibroblasts Adhesion and Proliferation on Alginate-Gelatin Crosslinked Hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef]



| Sample Full Name | Shortcut |
|---|---|
| Gelatin 6% + Sodium Alginate 1.5% + Calcium Chloride 10% | G6_A1.5_CaCl2 10% |
| Gelatin 6% + Sodium Alginate 2% + SQ 1% + Calcium Chloride 5% | G6_A2_SQ1_CaCl2 5% |
| Gelatin 6% + Sodium Alginate 2% + DAS 2% + Calcium Chloride 2.5% | G6_A2_DAS2_CaCl2 2.5% |
| Gelatin 6% + Sodium Alginate 2% + Calcium Chloride 1% | G6_A2_CaCl2 1% |
| Sample | Tensile Strength [kPa] | Elongation at the Breaking Point [%] | Young’s Modulus [kPa] |
|---|---|---|---|
| G6_A1.5_CaCl2 10% | 53.79 ± 8.03 | 40.71 ± 3.78 | 139.40 ± 12.76 |
| G6_A2_CaCl2 10% | 61.49 ± 5.12 | 28.42 ± 3.82 | 274.80 ± 23.98 |
| G6_A2_CaCl2 5% | 56.17 ± 11.52 | 91.98 ± 10.33 | 199.12 ± 11.05 |
| G6_A2_CaCl2 2.5% | 130.44 ± 17.62 | 74.68 ± 9.95 | 168.95 ± 7.02 |
| G6_A2_CaCl2 1% | 80.64 ± 10.02 | 74.99 ± 5.78 | 103.93 ± 5.99 |
| Sample | Amide A | Amide B | CH3 | Amide I | Amide II | Amide III | C=O Sym. | C-O C-C | C-C C-O-C | C-O |
|---|---|---|---|---|---|---|---|---|---|---|
| G6_A2_CaCl2 1% | 3316 | 3090 | 2940 | 1632 | 1553 | 1240 | 1416 | 1084 | 1030 | 945 |
| G6_A2_SQ1_CaCl2 1% | 3316 | 3089 | 2940 | 1635 | 1551 | 1240 | 1416 | 1084 | 1030 | 941 |
| G6_A2_SQ2_CaCl2 1% | 3317 | 3083 | 2934 | 1636 | 1550 | 1239 | 1417 | 1084 | 1030 | 938 |
| G6_A2_DAS1_CaCl2 1% | 3316 | 3090 | 2942 | 1633 | 1552 | 1240 | 1417 | 1083 | 1030 | 938 |
| G6_A2_DAS2_CaCl2 1% | 3316 | 3090 | 2941 | 1633 | 1552 | 1240 | 1417 | 1084 | 1030 | 938 |
| G6_A2_CaCl2 2.5% | 3316 | 3090 | 2940 | 1632 | 1552 | 1240 | 1417 | 1083 | 1029 | 942 |
| G6_A2_SQ1_CaCl2 2.5% | 3317 | 3090 | 2935 | 1633 | 1552 | 1240 | 1417 | 1084 | 1030 | 941 |
| G6_A2_SQ2_CaCl2 2.5% | 3317 | 3090 | 2941 | 1633 | 1551 | 1241 | 1417 | 1083 | 1030 | 941 |
| G6_A2_DAS1_CaCl2 2.5% | 3317 | 3089 | 2941 | 1644 | 1552 | 1240 | 1417 | 1083 | 1030 | 938 |
| G6_A2_DAS2_CaCl2 2.5% | 3317 | 3089 | 2940 | 1644 | 1552 | 1240 | 1417 | 1084 | 1030 | 938 |
| Gelatin | 3308 | 3080 | 2940 | 1644 | 1551 | 1239 | 1454 | 1082 | 1032 | 973 |
| Sodium alginate | 3358 | - | 2934 | 1600 | - | 1124 | 1412 | 1087 | 1029 | 948 |
| Sample | I Stage | II Stage | III Stage | ||
|---|---|---|---|---|---|
| Δm [%] | T [°C] | Δm [%] | T [°C] | Δm [%] | |
| G6_A2_CaCl2 1% | 15.77 | 256 | 12.09 | 323 | 45.31 |
| G6_A2_SQ1_CaCl2 1% | 14.40 | 256 | 12.99 | 320 | 43.45 |
| G6_A2_SQ2_CaCl2 1% | 14.09 | 255 | 13.57 | 330 | 42.79 |
| G6_A2_DAS1_CaCl2 1% | 16.08 | 264 | 11.79 | 329 | 46.25 |
| G6_A2_DAS2_CaCl2 1% | 14.83 | 261 | 11.52 | 320 | 42.86 |
| G6_A2_CaCl2 2.5% | 15.22 | 268 | 11.94 | 336 | 40.74 |
| G6_A2_SQ1_CaCl2 2.5% | 16.11 | 271 | 12.20 | 323 | 41.69 |
| G6_A2_SQ2_CaCl2 2.5% | 15.33 | 274 | 11.59 | 333 | 40.04 |
| G6_A2_DAS1_CaCl2 2.5% | 16.08 | 274 | 11.61 | 229 | 42.01 |
| G6_A2_DAS2_CaCl2 2.5% | 15.20 | 264 | 11.31 | 332 | 41.88 |
| Sample | Pore Size (µm) | Sample | Pore Size (µm) |
|---|---|---|---|
| G6_A2_CaCl2 1% | 347.21 ± 49.71 | G6_A2_CaCl2 2.5% | 379.79 ± 38.66 |
| G6_A2_SQ1_CaCl2 1% | 428.05 ± 102.28 | G6_A2_SQ1_CaCl2 2.5% | 504.31 ± 218.23 |
| G6_A2_SQ2_CaCl2 1% | 477.44 ± 91.20 | G6_A2_SQ2_CaCl2 2.5% | 445.31 ± 120.81 |
| G6_A2_DAS1_CaCl2 1% | 235.94 ± 42.76 | G6_A2_DAS1_CaCl2 2.5% | 425.03 ± 58.43 |
| G6_A2_DAS2_CaCl2 1% | 330.41 ± 22.68 | G6_A2_DAS2_CaCl2 2.5% | 561.16 ± 109.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skopinska-Wisniewska, J.; Tuszynska, M.; Kaźmierski, Ł.; Bartniak, M.; Bajek, A. Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink. Polymers 2024, 16, 2560. https://doi.org/10.3390/polym16182560
Skopinska-Wisniewska J, Tuszynska M, Kaźmierski Ł, Bartniak M, Bajek A. Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink. Polymers. 2024; 16(18):2560. https://doi.org/10.3390/polym16182560
Chicago/Turabian StyleSkopinska-Wisniewska, Joanna, Marta Tuszynska, Łukasz Kaźmierski, Mateusz Bartniak, and Anna Bajek. 2024. "Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink" Polymers 16, no. 18: 2560. https://doi.org/10.3390/polym16182560
APA StyleSkopinska-Wisniewska, J., Tuszynska, M., Kaźmierski, Ł., Bartniak, M., & Bajek, A. (2024). Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink. Polymers, 16(18), 2560. https://doi.org/10.3390/polym16182560







