Preparation and Stability Study of an Injectable Hydrogel for Artificial Intraocular Lenses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
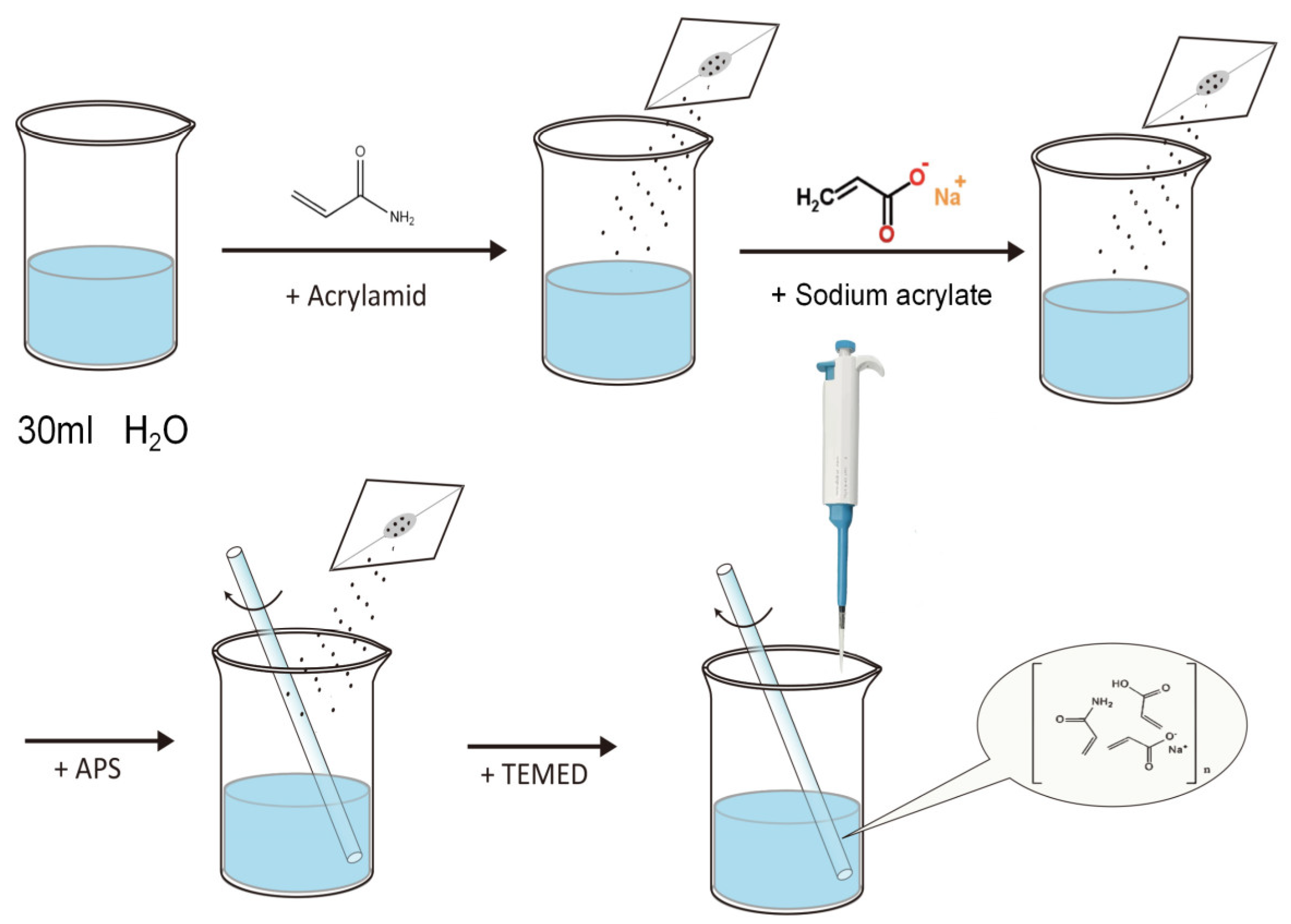
2.2. Preparation of Polyacrylamide–Sodium Acrylate Hydrogels
2.3. Characterization
2.4. Fourier Transform Infrared Spectroscopy Analysis
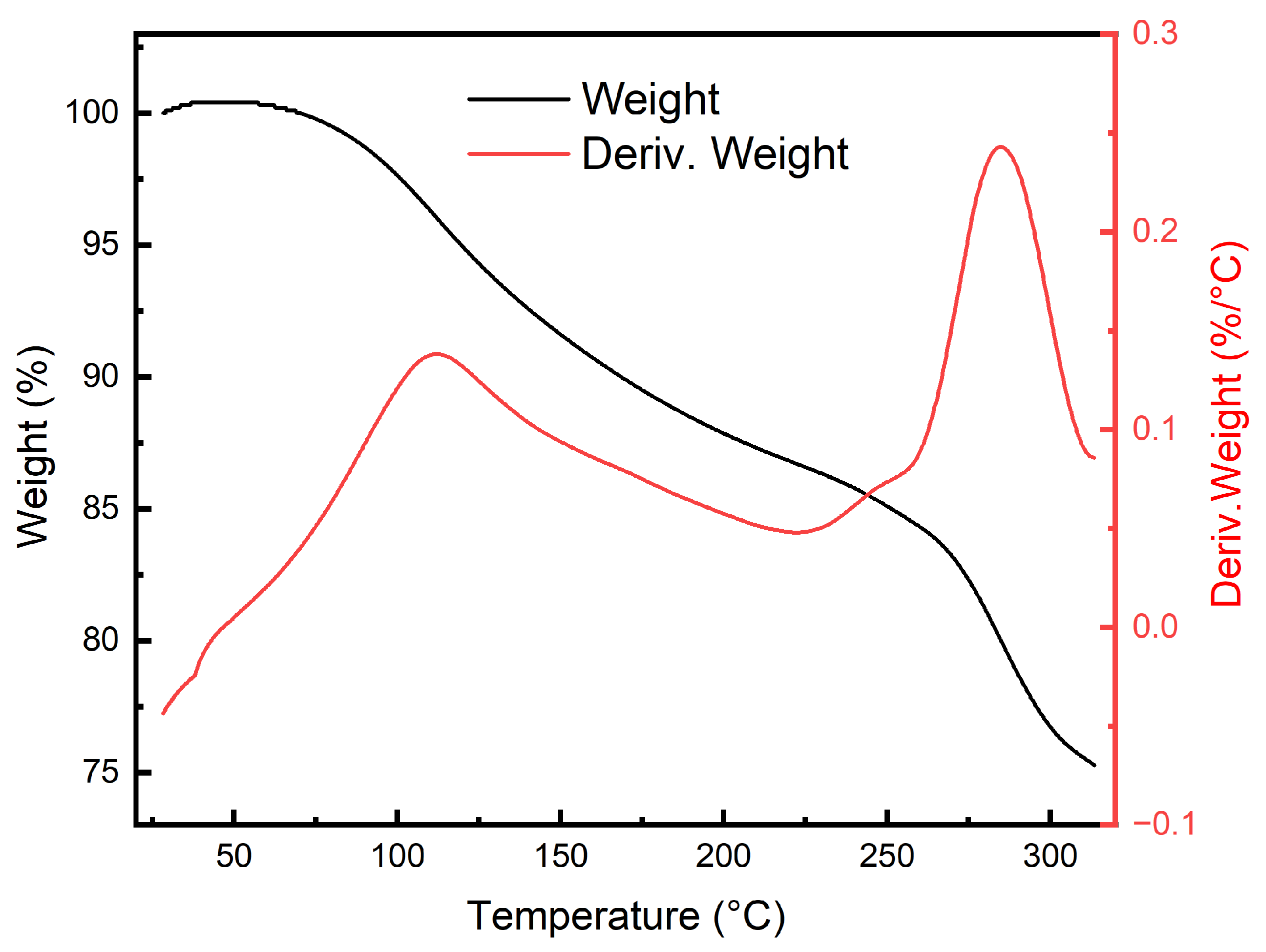
2.5. Thermal Stability Testing
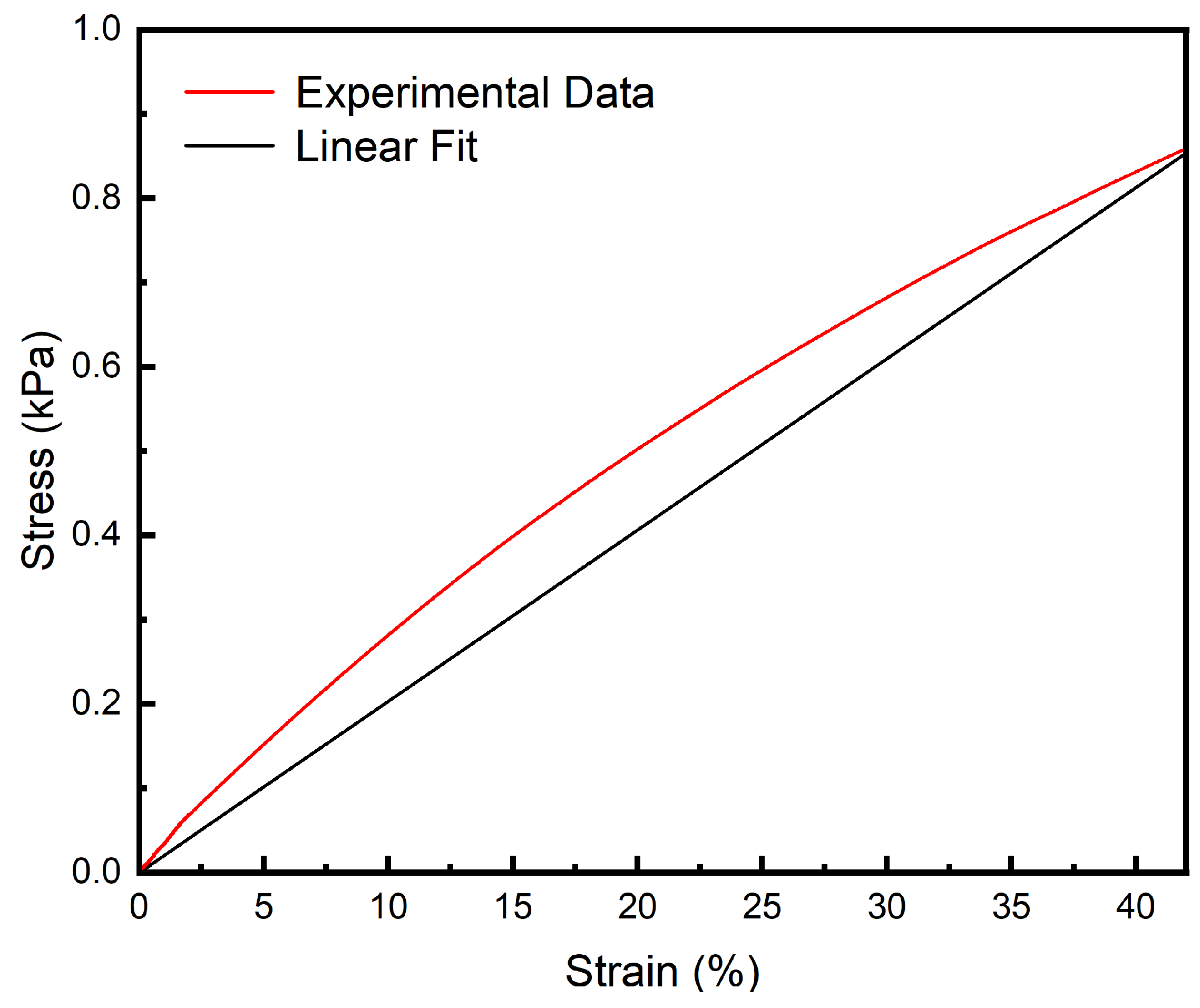
2.6. Tensile Performance Testing
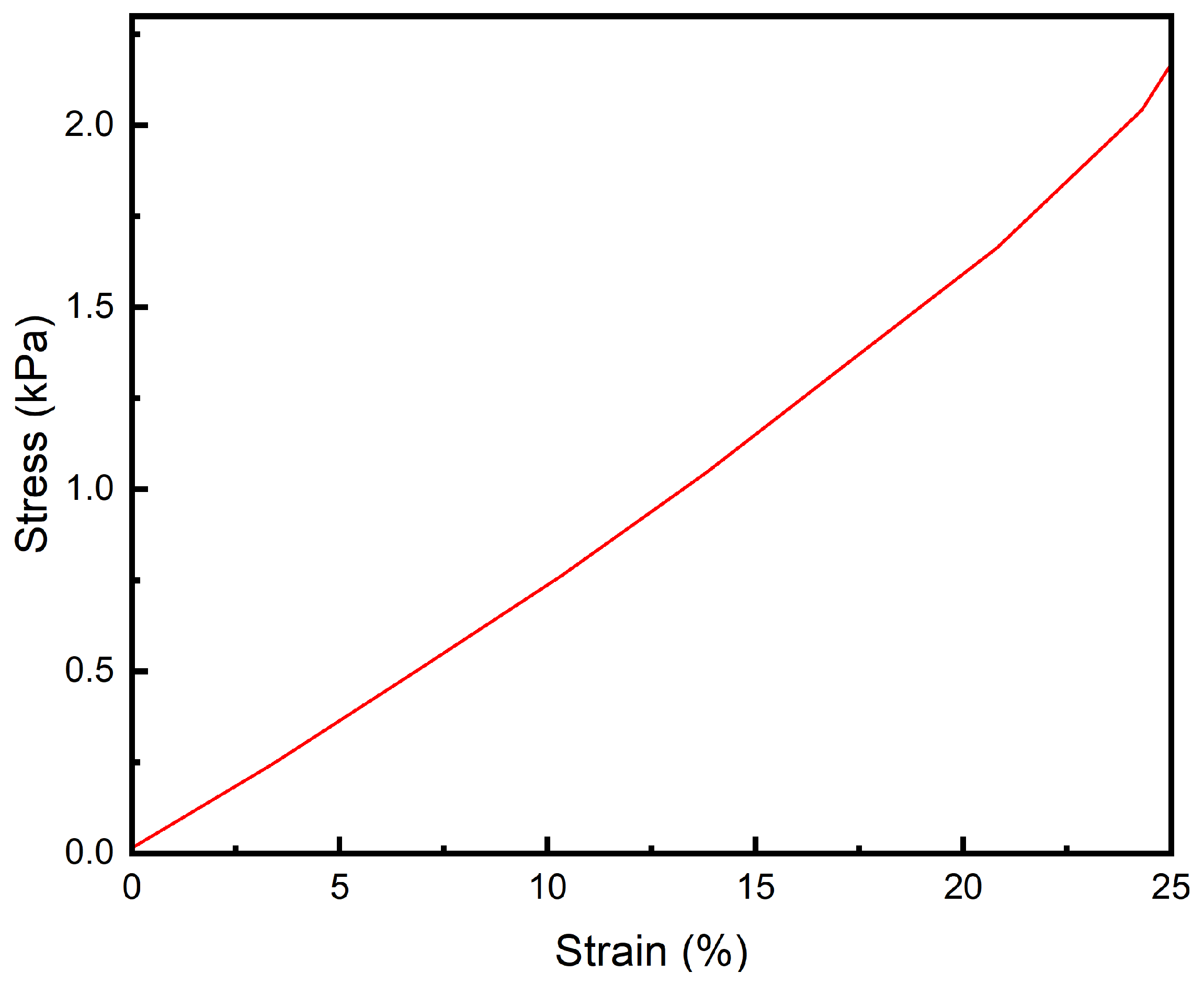
2.7. Compression Performance Testing
2.8. Fatigue Resistance Test
2.9. Transparency Testing
2.10. Refractive Index Testing
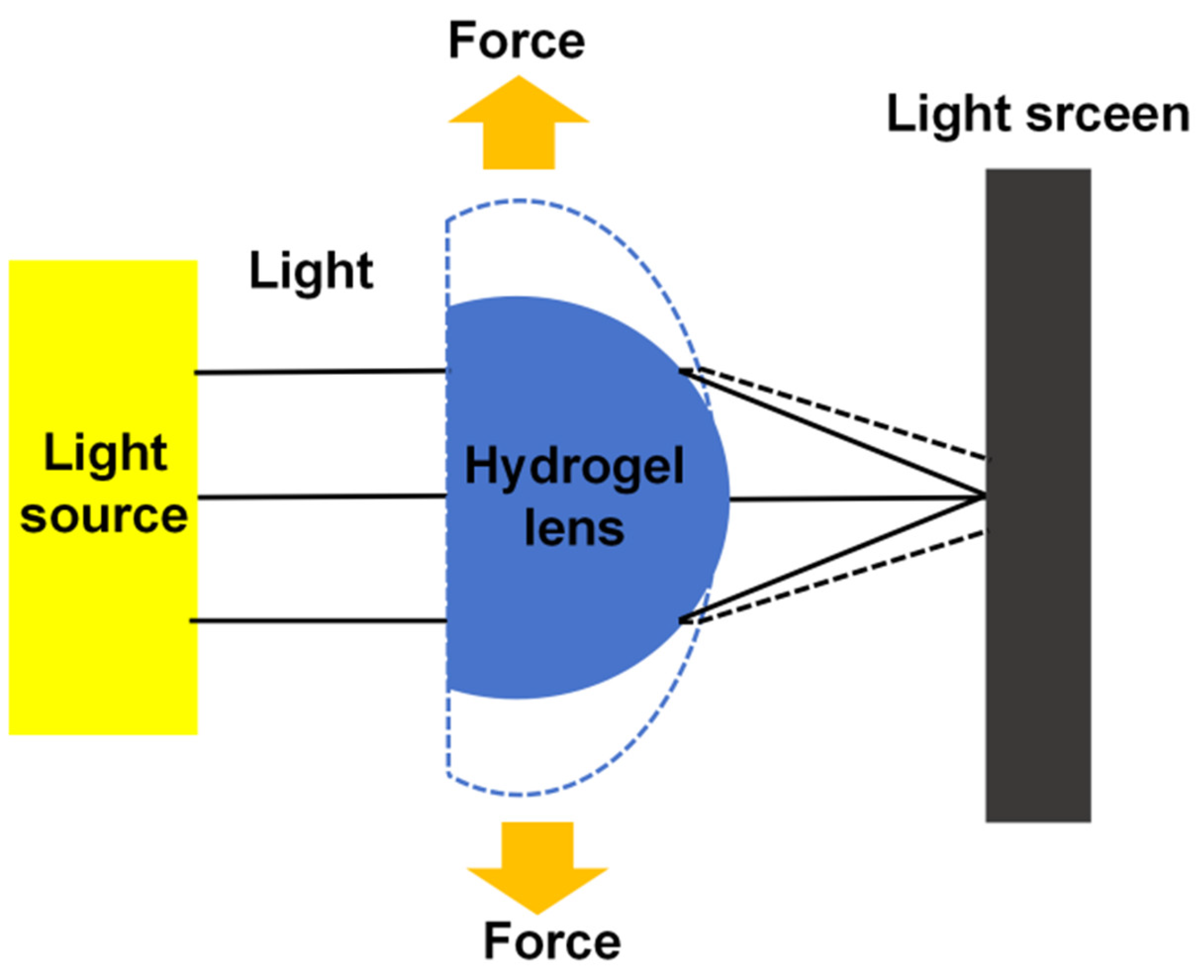
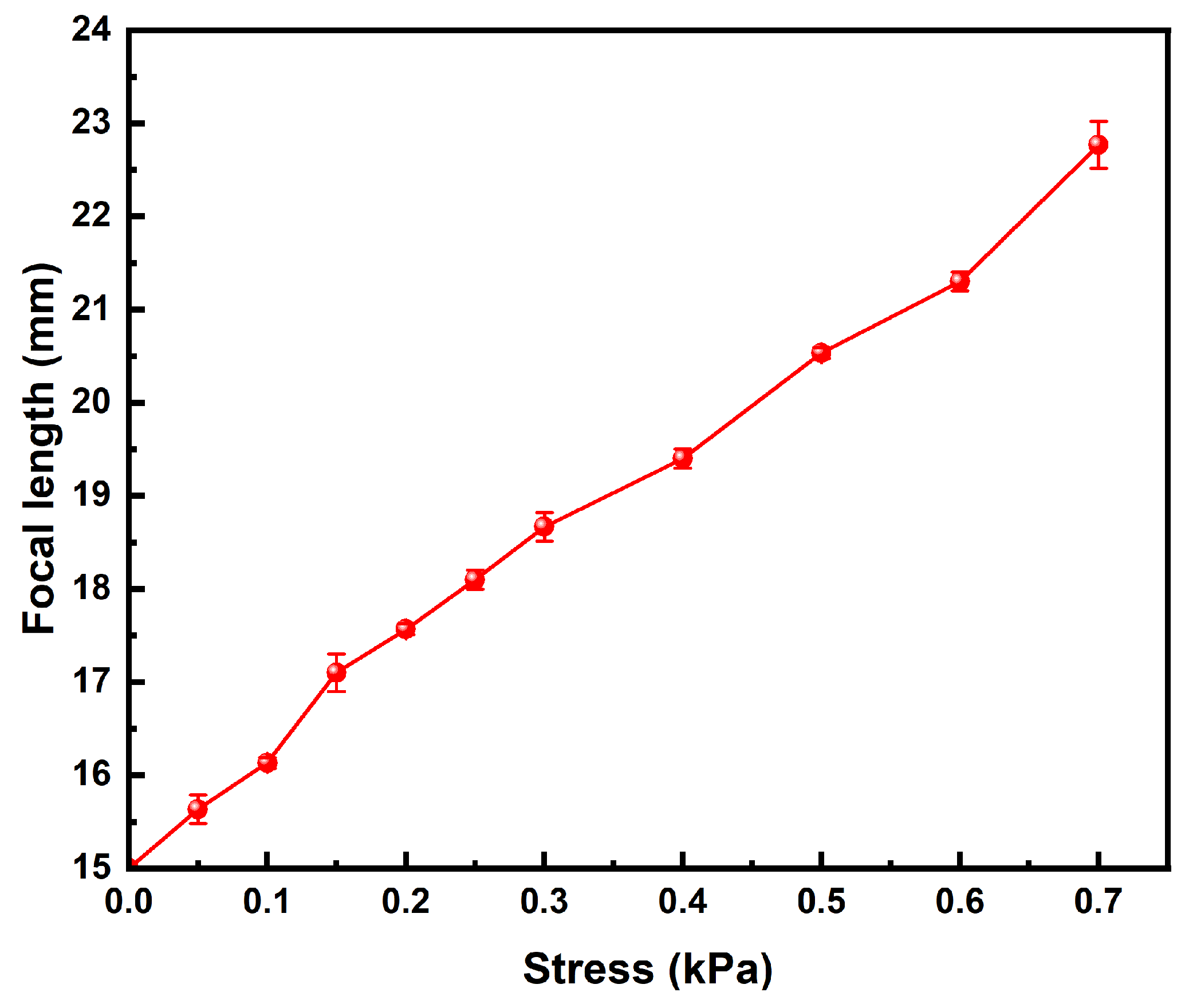
2.11. Focal Length Measurement
2.12. Zoom Performance Testing
2.13. Swelling Performance Testing
2.14. Rheological Performance Testing
3. Results and Discussion
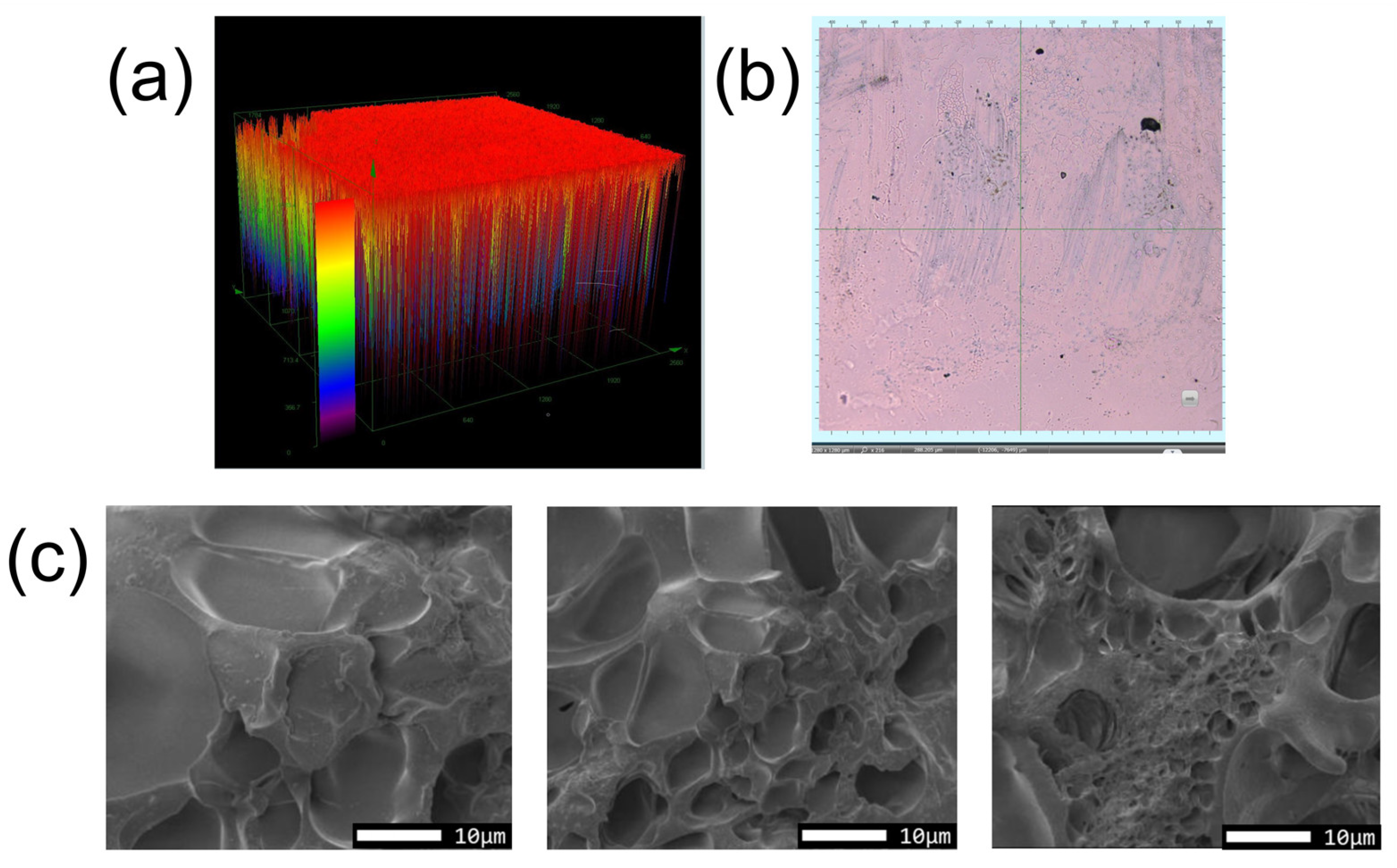
3.1. Hydrogel Characterization
3.2. FTIR Analysis Results
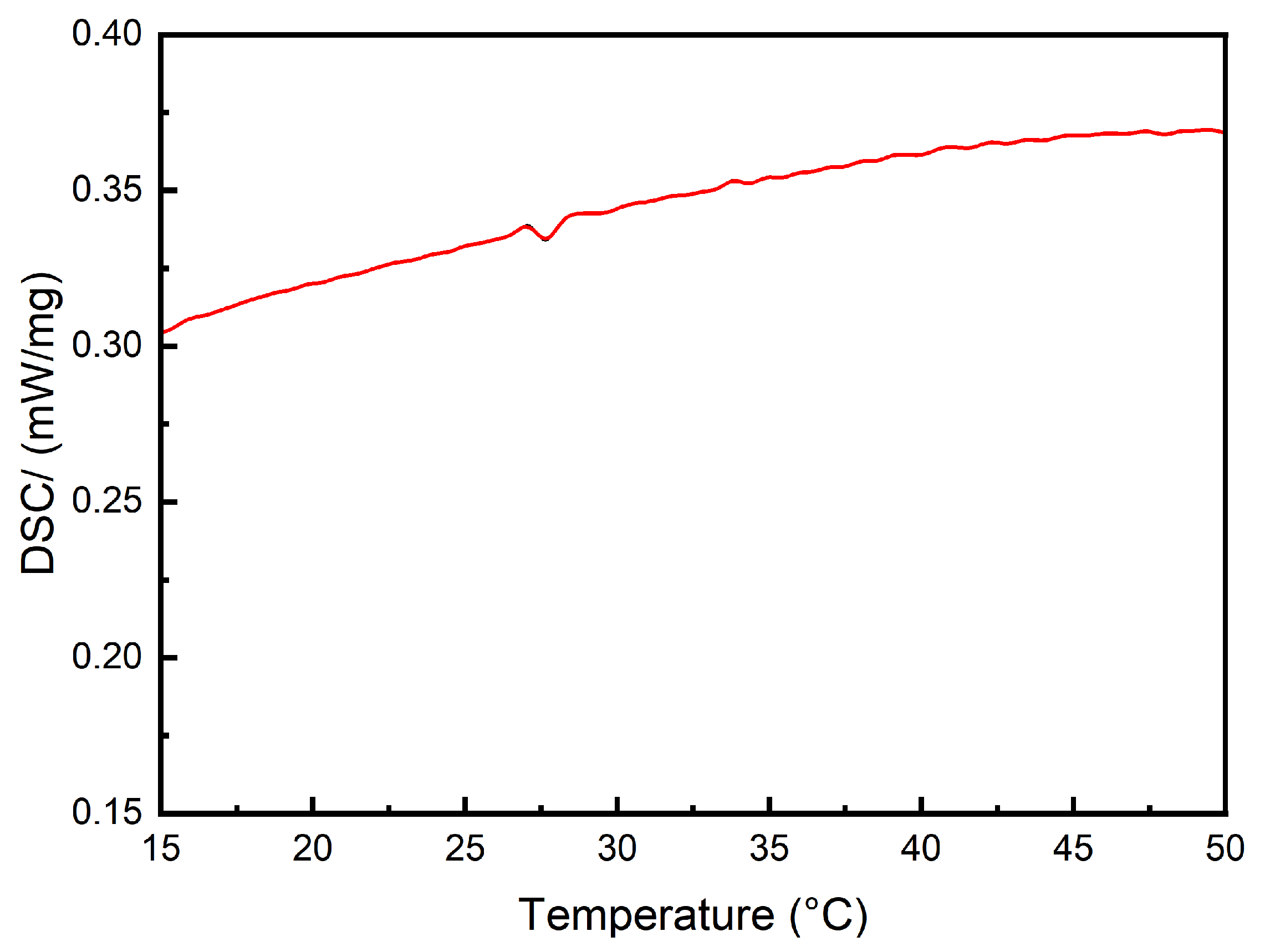
3.3. Thermal Stability Analysis
3.4. Tensile Performance
3.5. Compression Performance
3.6. Fatigue Resistance Analysis
3.7. Transparency Analysis
3.8. Refractive Index Analysis
3.9. Focal Length Measurement
3.10. Zoom Performance Analysis
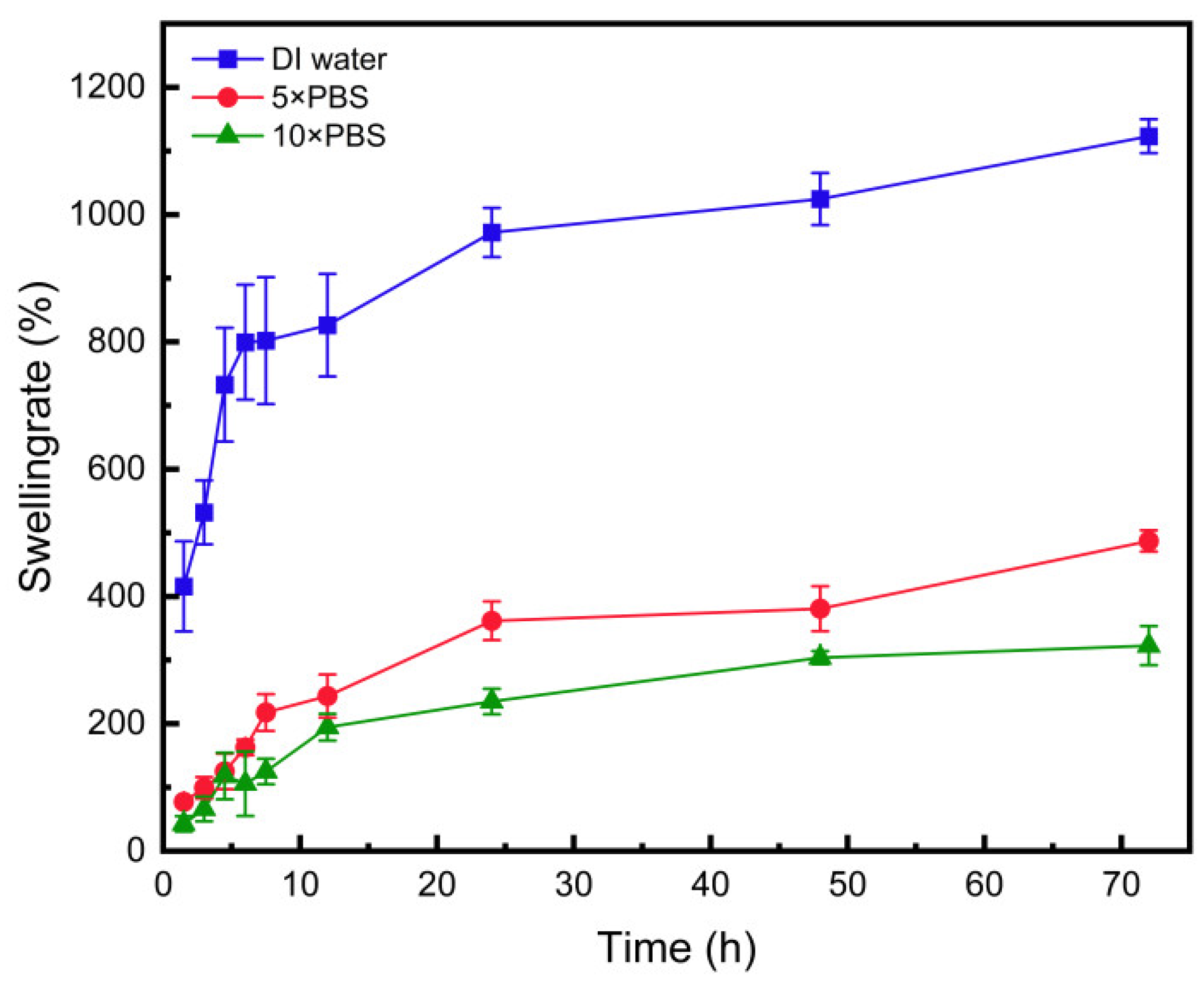
3.11. Swelling Performance
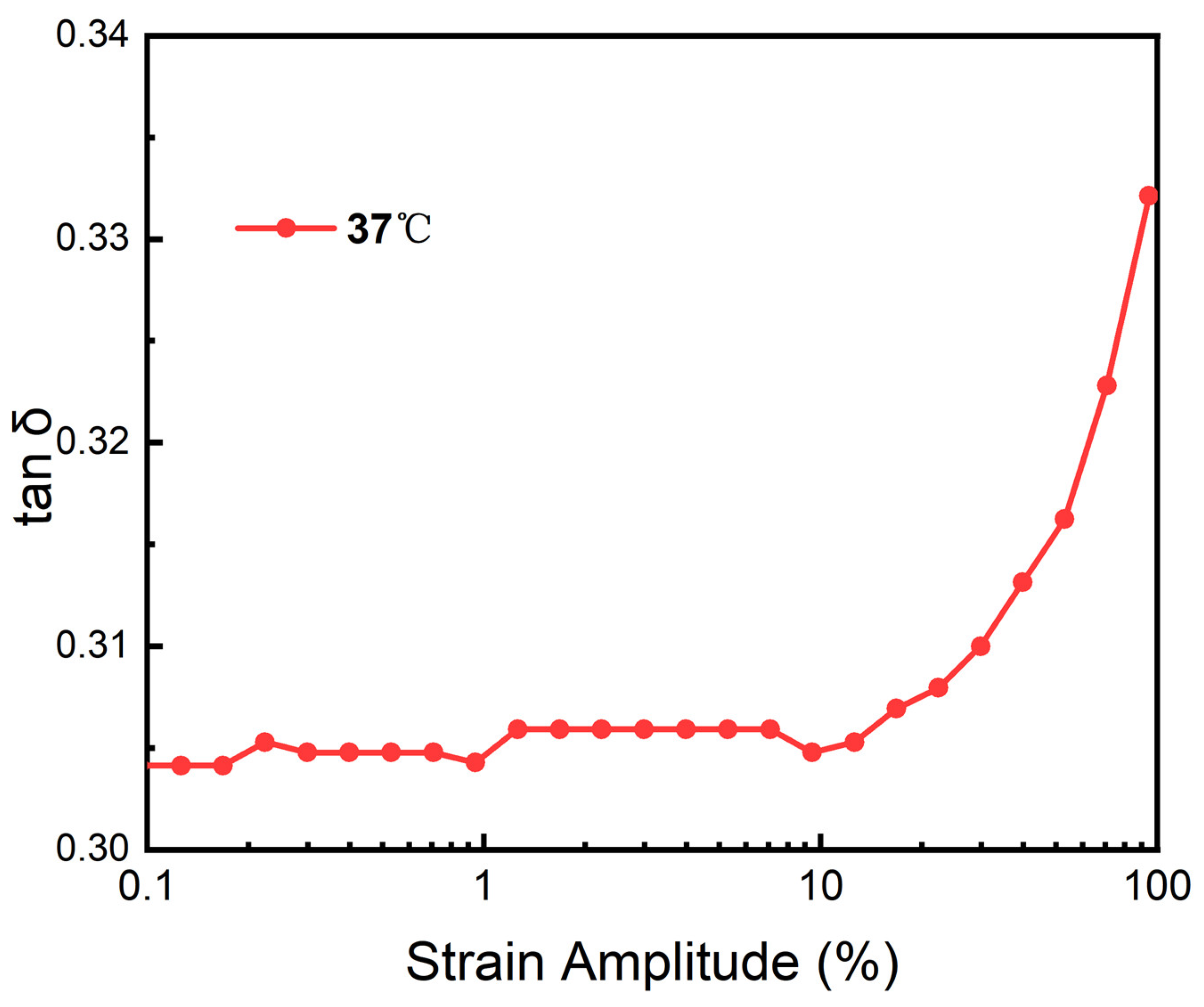
3.12. Heological Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-W.; Wei, R.-L.; Cai, J.-P.; Xi, G.-L.; Zhu, H.; Li, Y.; Ma, X.-Y. Efficacy of different intraocular lens materials and optic edge designs in preventing posterior capsular opacification: A meta-analysis. Arch. Ophthalmol. 2007, 143, 428–436.e3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dunn, A.C. Soft hydrated sliding interfaces as complex fluids. Soft Matter 2016, 12, 6536–6546. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Özyol, P.; Özyol, E.; Karel, F. Biocompatibility of intraocular lenses. Turk. J. Ophthalmol. 2017, 47, 221–225. [Google Scholar] [CrossRef]
- Tong, Z.; Jin, L.; Oliveira, J.M.; Reis, R.L.; Zhong, Q.; Mao, Z.; Gao, C. Adaptable hydrogel with reversible linkages for regenerative medicine: Dynamic mechanical microenvironment for cells. Bioact. Mater. 2020, 6, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. Phema: An overview for biomedical applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef]
- Hu, X.; Grinstaff, M.W. Advances in hydrogel adhesives for gastrointestinal wound closure and repair. Gels 2023, 9, 282. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, H.; Shi, S.; Chen, X.; Jiao, S. Preparation and properties of poly(acrylic ester) hydrogel as a potential intraocular lens for cataract treatment. Pharm. Nanotechnol. 2020, 8, 302–312. [Google Scholar]
- Li, X.; Zhao, Y.; Wang, K.; Wang, L.; Yang, X.; Zhu, S. Cyclodextrin-containing hydrogels as an intraocular lens for sustained drug release. PLoS ONE 2017, 12, e0189778. [Google Scholar] [CrossRef]
- Han, F.; Wang, T.; Liu, G.; Liu, H.; Xie, X.; Wei, Z.; Li, J.; Jiang, C.; He, Y.; Xu, F. Materials with tunable optical properties for wearable epidermal sensing in health monitoring. Adv. Mater. 2022, 34, e2109055. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D printing in ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Oh, H.J.; Yoon, K.C.; Tae, G.; Kim, Y.H. Fast in situ enzymatic gelation of PPO-PEO block copolymer for injectable intraocular lens in vivo. J. Biomater. Appl. 2013, 28, 1247–1263. [Google Scholar] [CrossRef]
- Singh, B.; Dhiman, A.; Rajneesh, K.A. Slow release of ciprofloxacin from β-cyclodextrin containing drug delivery system through network formation and supramolecular interactions. Int. J. Biol. Macromol. 2016, 92, 390–400. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.H.; Spaans, C.J.; van Calck, R.V.; van Beijma, F.J.; Norrby, S.; Pennings, A.J. Hydrogels for an accommodating intraocular lens. An explorative study. Biomacromolecules 2003, 4, 608–616. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, K.; He, Z.; Li, H.; Chen, T.; Lei, Y.; Xia, S.; He, G.; Xie, Y.; Zheng, Y.; et al. Development of inclusion complex of brinzolamide with hydroxypropyl β-cyclodextrin. Carbohydr. Polym. 2013, 98, 638–643. [Google Scholar] [CrossRef]
- Bozukova, D.; Pagnoulle, C.; De Pauw-Gillet, M.C.; Desbief, S.; Lazzaroni, R.; Ruth, N.; Jérôme, R.; Jérôme, C. Improved performances of intraocular lenses by poly (ethylene glycol) chemical coatings. Biomacromolecules 2007, 8, 2379–2387. [Google Scholar] [CrossRef]
- Wielders, L.H.; Lambermont, V.A.; Schouten, J.S.; Biggelaar, F.J.v.D.; Worthy, G.; Simons, R.W.; Winkens, B.; Nuijts, R.M. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: A systematic review and meta-analysis. Arch. Ophthalmol. 2015, 160, 968–981. [Google Scholar] [CrossRef]
- Shin, M.K.; Ji, Y.W.; Moon, C.E.; Lee, H.; Kang, B.; Jinn, W.S.; Ki, J.; Mun, B.; Kim, M.-H.; Lee, H.K.; et al. Matrix metalloproteinase 9-activatable peptide-conjugated hydrogel-based fluorogenic intraocular-lens sensor. Biosens. Bioelectron. 2020, 162, 112254. [Google Scholar] [CrossRef]
- Schwiegerling, J. Recent developments in pseudophakic dysphotopsia. Curr. Opin. Ophthalmol. 2006, 17, 27–30. [Google Scholar] [CrossRef]
- Masket, S.; Fram, N.R. Pseudophakic dysphotopsia: Review of incidence, cause, and treatment of positive and negative dysphotopsia. Ophthalmology 2021, 128, e195–e205. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sella, R.; Afshari, N.A. Dysphotopsia: A multifaceted optic phenomenon. Curr. Opin. Ophthalmol. 2018, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bonsemeyer, M.K.M.; Becker, E.; Liekfeld, A. Dysphotopsia and functional quality of vision after implantation of an intraocular lens with a 7.0 mm optic and plate haptic design. J. Cataract. Refract. Surg. 2021, 48, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.A.; Hollister, S.; Nelson, M.E.; Ohye, R.G.; Green, G.E. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 2013, 368, 2043–2045. [Google Scholar] [CrossRef]
- Shiblee, N.I.; Ahmed, K.; Khosla, A.; Kawakami, M.; Furukawa, H. 3D printing of shape memory hydrogels with tunable mechanical properties. Soft Matter 2018, 14, 7809–7817. [Google Scholar] [CrossRef]
- Hinze, U.; El-Tamer, A.; Reiß, S.; Stolz, H.; Guthoff, R.; Stachs, O.; Chichkov, B.N. Implantatdesign mittels multiphotonen–polymerisation. Klin. Monatsbl. Augenheilkd. 2015, 232, 1381–1385. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Li, Z.; Zhao, C.; Zeng, Y.; Zou, T.; Fu, C.; Liu, X.; Xu, H.; Yin, Z.Q. Grafted c-kit+/SSEA1− eye-wall progenitor cells delay retinal degeneration in mice by regulating neural plasticity and forming new graft-to-host synapses. Stem Cell Res. Ther. 2016, 7, 191. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in PAHrmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Abdelkader, H.; Ismail, S.; Hussein, A.; Wu, Z.; Al-Kassas, R.; Alany, R.G. Conjunctival and corneal tolerability assessment of ocular naltrexone niosomes and their ingredients on the hen’s egg chorioallantoic membrane and excised bovine cornea models. Int. J. Pharm. 2012, 432, 1–10. [Google Scholar] [CrossRef]
- Li, J.-W.; Li, Y.-J.; Hu, X.-S.; Gong, Y.; Xu, B.-B.; Xu, H.-W.; Yin, Z.-Q. Biosafety of a 3D-printed intraocular lens made of a poly(acrylamide-co-sodium acrylate) hydrogel in vitro and in vivo. Int. J. Ophthalmol. 2020, 13, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Shajari, M.; Kolb, C.M.; Petermann, K.; Böhm, M.; Herzog, M.; De’lorenzo, N.; Schönbrunn, S.; Kohnen, T. Comparison of 9 modern intraocular lens power calculation formulas for a quadrifocal intraocular lens. J. Cataract. Refract. Surg. 2018, 44, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Song, J.S.; Kim, H.M. Spectacle plane add power of multifocal intraocular lenses according to effective lens position. Can. J. Ophthalmol. 2016, 52, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.; Seyfoddin, A.; Fatihhi, S. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wong, T.T.; Mehta, J.S. Intraocular lens as a drug delivery reservoir. Curr. Opin. Ophthalmol. 2013, 24, 53–59. [Google Scholar] [CrossRef]
- Rim, S.-B.; Catrysse, P.B.; Dinyari, R.; Huang, K.; Peumans, P. The optical advantages of curved focal plane arrays. Opt. Express 2008, 16, 4965–4971. [Google Scholar] [CrossRef]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.-A.; Kaur, C. Retinal microglia—A key player in healthy and diseased retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Hosseinzadeh, H. Synthesis and properties of partially hydrolyzed acrylonitrile-co-acrylamide superabsorbent hydrogel. Bull. Korean Chem. Soc. 2010, 31, 3163–3172. [Google Scholar] [CrossRef]
- Siepser, S.B.; Wieland, M. Animal model experimentation using the expansile hydrogel intraocular lens. J. Cataract. Refract. Surg. 1991, 17, 491–494. [Google Scholar] [CrossRef]
- Ahmed, M.; Amirat, M. FTIR, 1H, and 13C NMR Characterization and Antibacterial Activity of the Combination of Euphorbia Honey and Potato Starch. Comb. Chem. High Throughput Screen. 2024, 27, 1913–1918. [Google Scholar] [CrossRef]
- Kumar, R.; Chaurasia, A.; Tewari, A.; Parashar, A.; Kumar, R.; Chaurasia, A.; Tewari, A.; Parashar, A. Atomistic modelling and experimental study of tensile strength of nanocomposite hydrogel. Int. J. Mech. Sci. 2024, 277, 109397. [Google Scholar] [CrossRef]
- Ma, H.; Zou, Y.; Liu, L.; Zhang, X.; Yu, J.; Fan, Y. Mussel-inspired chitin nanofiber adherable hydrogel sensor with interpenetrating network and great fatigue resistance for motion and acoustics monitoring. Int. J. Biol. Macromol. 2024, 263, 130059. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.-T.; Cheng, D.-Y.; Wu, I.-F.; Liao, Y.-C. 3D printed anti-swelling hydrogel scaffold with dialdehyde cellulose nanocrystals. Cellulose 2024, 31, 2975–2988. [Google Scholar] [CrossRef]
- GB/T-528-2009; Rubber, Vulcanized or Thermoplastic-Determination of Tensile Stress-Strain Properties. Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- GB/T 9341-2000; Plastics-Determination of Flexural Properties. Standardization Administration of the People’s Republic of China: Beijing, China, 2000.
- HG/T 2645-2011; Technical Specifications for Rubber Dies. Chemical Industry Press: Beijing, China, 2011.
- GB/T 1041-92; Standard Test Method for ‘PLASTICS-Determination of Compressive Peoperties’. Standardization Administration of the People’s Republic of China: Beijing, China, 1992.
- Madhi, A.; Shirkavand Hadavand, B.; Madhi, A.H. Bio-friendly fluorescent polyvinyl alcohol/gelatin/chitosan hydrogel membranes strengthened by g-C3N4/CQDs nanocomposite: Preparation, investigation of UV-absorption, mechanical and rheological properties. Fuller. Nanotub. Carbon Nanostruct. 2024, 32, 611–620. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, Y.; Wang, X.; Han, C.; Chen, Z. A thermal management strategy for electronic devices based on copper double skin inspired hydrogel. Int. J. Heat Mass Transf. 2023, 206, 123946. [Google Scholar] [CrossRef]





| Number | Material | Molar Mass (g/mol) | Molar Ratio (%) | Concentration (%) | Volume (μL) |
|---|---|---|---|---|---|
| 1 | Acrylamide | 71.08 | 87 | 30 | 55.61 |
| 2 | Sodium Acrylate | 94.04 | 12.5 | 20 | 15.86 |
| 3 | MBA | 154.2 | 0.5 | 2 | 10.4 |
| 4 | TMEDA | 116.2 | - | - | 0.3 |
| 5 | APS | 228.2 | - | 10 | 1 |
| 6 | Water | - | - | - | 118.13 |
| Number | Angle of Incidence (°) | Angle of Reflection (°) | Refractive Index |
|---|---|---|---|
| 1 | 23.446 | 102.538 | 1.416 |
| 2 | 19.576 | 86.186 | 1.413 |
| 3 | 25.307 | 110.320 | 1.417 |
| 4 | 26.835 | 117.150 | 1.412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Li, P.; Su, Z.; Guan, S.; Dong, H.; Dong, X. Preparation and Stability Study of an Injectable Hydrogel for Artificial Intraocular Lenses. Polymers 2024, 16, 2562. https://doi.org/10.3390/polym16182562
Cui H, Li P, Su Z, Guan S, Dong H, Dong X. Preparation and Stability Study of an Injectable Hydrogel for Artificial Intraocular Lenses. Polymers. 2024; 16(18):2562. https://doi.org/10.3390/polym16182562
Chicago/Turabian StyleCui, Haifeng, Pengfei Li, Zekun Su, Shiqiang Guan, He Dong, and Xufeng Dong. 2024. "Preparation and Stability Study of an Injectable Hydrogel for Artificial Intraocular Lenses" Polymers 16, no. 18: 2562. https://doi.org/10.3390/polym16182562







