Polyhydroxyalkanoate Production by Methanotrophs: Recent Updates and Perspectives
Abstract
:1. Introduction
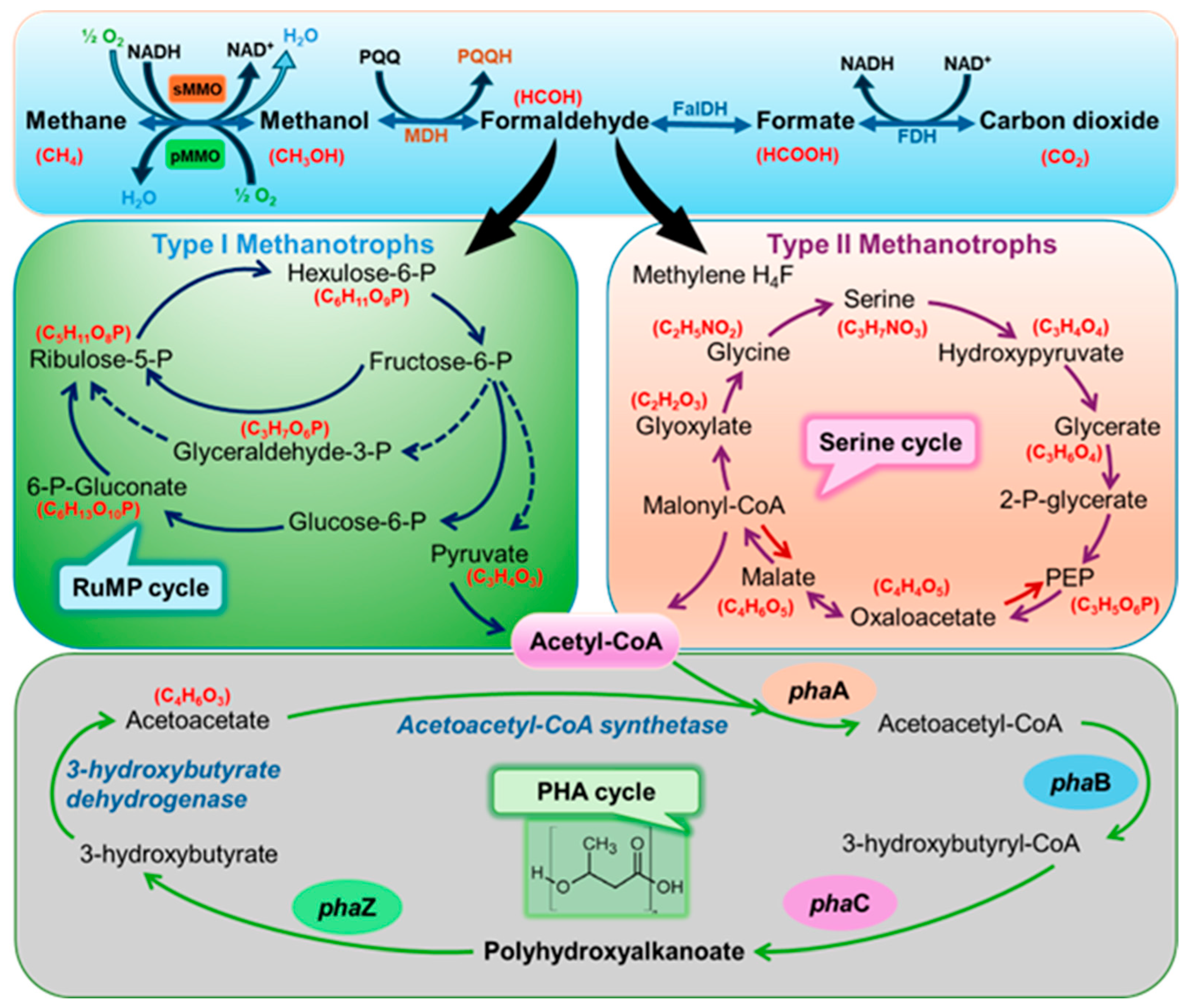
2. Methanotrophs and Their Metabolism
3. Production of Polyhydroxyalkanoates by Methanotrophs from Methane and Their Physical Characteristics
4. Genetic Engineering in Methanotrophs for Producing Polyhydroxyalkanoates (PHAs)
5. Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ge, X.; Yang, L.; Sheets, J.P.; Yu, Z.; Li, Y. Biological conversion of methane to liquid fuels: Status and opportunities. Biotechnol. Adv. 2014, 32, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.-K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energ. Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Singh, R.; Ryu, J.; Kim, S.W. An overview on methanotrophs and the role of Methylosinus trichosporium OB3b for biotechnological applications. Biotechnol. Bioprocess Eng. 2022, 27, 468–481. [Google Scholar] [CrossRef]
- Bachleitner, S.; Ata, O.; Mattanovich, D. The potential of CO2-based production cycles in biotechnology to fight the climate crisis. Nat. Commun. 2023, 14, 6978. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, U.M.; Mai, D.H.A.; Krishna, S.; Lee, E.Y. Methanotrophs as a reservoir for bioactive secondary metabolites: Pitfalls, insights and promises. Biotechnol. Adv. 2023, 63, 108097. [Google Scholar]
- Fei, Q.; Guarnieri, M.T.; Tao, L.; Laurens, L.M.L.; Dowe, N.; Pienkos, P.T. Bioconversion of natural gas to liquid fuel: Opportunities and challenges. Biotechnol. Adv. 2014, 32, 596–614. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Shanmugam, R.; Lee, J.-K.; Kalia, V.C.; Kim, I.-W. Biomolecules production from greenhouse gases by methanotrophs. Indian J. Microbiol. 2021, 61, 449–457. [Google Scholar] [CrossRef]
- Chen, R.; Weng, G.-M. Sustainable energy resources for driving methane conversion. Adv. Energy Mater. 2023, 13, 2301734. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Jeon, M.S.; Gupta, R.K.; Jeon, Y.; Kalia, V.C.; Kim, S.C.; Cho, B.K.; Kim, D.R.; Lee, J.-K. Hierarchical macroporous particles for efficient whole-cell immobilization: Application in bioconversion of greenhouse gases to methanol. ACS Appl. Mater. Interfaces 2019, 11, 18968–18977. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gupta, R.K.; Kalia, V.C.; Lee, J.-K. Integrating anaerobic digestion of potato peels to methanol production by methanotrophs immobilized on banana leaves. Bioresour. Technol. 2021, 323, 124550. [Google Scholar] [CrossRef]
- Samanta, D.; Sani, R.K. Methane oxidation via chemical and biological methods: Challenges and solutions. Methane 2023, 2, 279–303. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Mardina, P.; Kim, D.; Kim, S.-Y.; Kalia, V.C.; Kim, I.-W.; Lee, J.-K. Improvement in methanol production by regulating the composition of synthetic gas mixture and raw biogas. Bioresour. Technol. 2016, 218, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yu, W.; Zhang, C.; Ning, P.; Li, J.; Liu, Y.; Du, G.; Liu, L. C1-based biomanufacturing: Advances, challenges and perspectives. Bioresour. Technol. 2023, 367, 128259. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Kalia, V.C.; Lee, J.-K. Integration of biogas derived from dark fermentation and anaerobic digestion of biowaste to enhance methanol production by methanotrophs. Bioresour. Technol. 2023, 367, 128427. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gupta, R.K.; Kondaveeti, S.; Otari, S.V.; Kumar, A.; Kalia, V.C.; Lee, J.-K. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour. Technol. 2020, 315, 123791. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kalia, V.C.; Joo, J.B.; Kang, Y.C.; Lee, J.-K. Biotransformation of methane into methanol by methanotrophs immobilized on coconut coir. Bioresour. Technol. 2020, 297, 122433. [Google Scholar] [CrossRef]
- Priyadarsini, A.; Singh, R.; Barbora, L.; Maitra, S.S.; Moholkar, V.S. Methanotroph detection and bioconversion of methane to methanol by enriched microbial consortium from rice field soil. Bioresour. Biotechnol. Rep. 2023, 22, 101410. [Google Scholar] [CrossRef]
- Zheng, X.-C.; Li, H.-S.; Wang, Z.-H.; Sun, Z.-F.; Zhao, L. Intermediates production in methane oxidation coupled with denitrification: Current status, challenges, and future opportunities. Fermentation 2023, 9, 645. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances towards biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.-K. Exploiting polyhydroxyalkanoates for biomedical applications. Polymers 2023, 15, 1937. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.S.; Karthikeyan, K.K.; Jeya, M.; Kim, I.-W.; Lee, J.-K. Manipulating microbial cell morphology for the sustainable production of biopolymers. Polymers 2024, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gupta, R.K.; Kalia, V.C.; Lee, J.-K. Synthetic design of methanotroph co-cultures and their immobilization within polymers containing magnetic nanoparticles to enhance methanol production from wheat straw-based biogas. Bioresour. Technol. 2022, 364, 128032. [Google Scholar] [CrossRef] [PubMed]
- Pieja, A.J.; Morse, M.C.; Cal, A.J. Methane to bioproducts: The future of the bioeconomy? Curr. Opin. Chem. Biol. 2017, 41, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Fenibo, E.O.; Selvarajan, R.; Wang, H.; Wang, Y.; Abia, A.L.K. Untapped talents, insight into the ecological significance of methanotrophs and its prospects. Sci. Total Environ. 2023, 903, 166145. [Google Scholar] [CrossRef]
- Yoon, J.; Oh, M.-K. Strategies for biosynthesis of C1 gas-derived polyhydroxyalkanoates: A review. Bioresour. Technol. 2022, 344, 126307. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Lee, J.-K. Plastic eating enzymes: A step towards sustainability. Indian J. Microbiol. 2022, 62, 658–661. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Xie, G.-J.; Xing, D.-F.; Liu, B.-F.; Ding, J.; Ren, N.-Q. Biological conversion of methane to polyhydroxyalkanoates, Current advances, challenges, and perspectives. Environ. Sci. Ecotechnol. 2021, 2, 100029. [Google Scholar] [CrossRef]
- Bagi, Z.; Ács, N.; Böjti, T.; Kakuk, B.; Rakhely, G.; Strang, O.; Szuhal, M.; Wirth, R.; Kovacs, K.L. Biomethane: The energy storage, platform chemical and greenhouse gas mitigation target. Anaerobe 2017, 46, 13–22. [Google Scholar] [CrossRef]
- Amabile, C.; Abate, T.; De Crescenzo, C.; Muñoz, R.; Sabbaresse, S.; Chianese, S.; Musmarra, D. Sustainable process for the production of poly(3-hydroxybutyrate- co-3-hydroxyvalerate) from renewable resources: A simulation study. ACS Sustain. Chem. Eng. 2022, 10, 14230–14239. [Google Scholar] [CrossRef]
- Salem, R.; Eldyasti, A.; Audette, G.F. Biomedical applications of biomolecules isolated from methanotrophic bacteria in wastewater treatment systems. Biomolecules 2021, 11, 1217. [Google Scholar] [CrossRef]
- Amabile, C.; Abate, T.; Muñoz, R.; Chianese, S.; Musmarra, D. Production of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from methane and volatile fatty acids, properties, metabolic routes and current trend. Sci. Total Environ. 2024, 927, 172138. [Google Scholar] [CrossRef] [PubMed]
- Ashoor, S.; Jun, S.-H.; Ko, H.D.; Lee, J.; Hamelin, J.; Milferstedt, K.; Na, J.-G. Polyhydroxybutyrate production from methane and carbon dioxide by a syntrophic consortium of methanotrophs with oxygenic photogranules without an external oxygen supply. Microorganisms 2023, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gupta, R.K.; Kim, I.-W.; Lee, J.-K. Encapsulation of methanotrophs within a polymeric matrix containing copper-and iron-based nanoparticles to enhance methanol production from a simulated biogas. Polymers 2023, 15, 3667. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Kumar, V.; Mardina, P.; Li, J.; Lestari, R.; Kalia, V.C.; Lee, J.-K. Methanol production from simulated biogas mixtures by co-immobilized Methylomonas methanica and Methylocella tundrae. Bioresour. Technol. 2018, 263, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bordel, S.; Rodríguez, E.; Muñoz, R. Genome sequence of Methylocystis hirsuta CSC1, a polyhydroxyalkanoate producing methanotroph. MicrobiologyOpen 2019, 8, e00771. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Shanmugam, R.; Kalia, V.C.; Lee, J.-K. Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour. Technol. 2020, 304, 123022. [Google Scholar] [CrossRef]
- Xin, J.Y.; Zhang, Y.X.; Zhang, S.; Xia, C.G.; Li, S.B. Methanol production from CO2 by resting cells of the methanotrophic bacterium Methylosinus trichosporium IMV3011. J. Basic Microbiol. 2007, 47, 426–435. [Google Scholar] [CrossRef]
- Mai, D.H.A.; Nguyen, T.T.; Lee, E.Y. The ethylmalonyl-CoA pathway for methane-based biorefineries: A case study of using Methylosinus trichosporium OB3b, an alpha-proteobacterial methanotroph, for producing 2-hydroxyisobutyric acid and 1,3-butanediol from methane. Green Chem. 2021, 23, 7712–7723. [Google Scholar] [CrossRef]
- Kim, Y.; Flinkstrom, Z.; Candry, P.; Winkler, M.-K.H.; Myung, J. Resource availability governs polyhydroxyalkanoate (PHA) accumulation and diversity of methanotrophic enrichments from wetlands. Front. Bioeng. Biotechnol. 2023, 11, 1210392. [Google Scholar] [CrossRef]
- Chau, T.H.T.; Duc Nguyen, A.; Lee, E.Y. Engineering type I methanotrophic bacteria as novel platform for sustainable production of 3-hydroxybutyrate and biodegradable polyhydroxybutyrate from methane and xylose. Bioresour. Technol. 2022, 363, 127898. [Google Scholar]
- Ray, S.; Jin, J.-O.; Choi, I.; Kim, M. Recent trends of biotechnological production of polyhydroxyalkanoates from C1 carbon sources. Front. Bioeng. Biotechnol. 2023, 10, 907500. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Galega, W.M.; Van Nostrand, J.D.; Yuan, T.; Zhou, J.; Criddle, C.S. Long-term cultivation of a stable Methylocystis-dominated methanotrophic enrichment enabling tailored production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioresour. Technol. 2015, 198, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Kim, M.; Pan, M.; Criddle, C.S.; Tang, S. Low energy emulsion-based fermentation enabling accelerated methane mass transfer and growth of poly(3-hydroxybutyrate)-accumulating methanotrophs. Bioresour. Technol. 2016, 207, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Flanagan, J.C.A.; Waymouth, R.M.; Criddle, C.S. Methane or methanol-oxidation dependent synthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by obligate type II methanotrophs. Process Biochem. 2016, 51, 561–567. [Google Scholar] [CrossRef]
- López, J.C.; Quijano, G.; Pérez, R.; Muñoz, R. Assessing the influence of CH4 concentration during culture enrichment on the biodegradation kinetics and population structure. J. Environ. Manag. 2014, 146, 116–123. [Google Scholar] [CrossRef] [PubMed]
- López, J.C.; Quijano, G.; Pérez, R.; Muñoz, R. Effect of pollutant concentration during isolation on the CH4 biodegradation kinetics, population structure and PHB accumulation. Chem. Eng. Transact. 2014, 40, 211–216. [Google Scholar]
- Helm, J.; Wendlandt, K.-D.; Rogge, G.; Kappelmeyer, U. Characterizing a stable methane-utilizing mixed culture used in the synthesis of a high-quality biopolymer in an open system. J. Appl. Microbiol. 2006, 101, 387–395. [Google Scholar] [CrossRef]
- Helm, J.; Wendlandt, K.D.; Jechorek, M.; Stottmeister, U. Potassium deficiency results in accumulation of ultra-high molecular weight poly-beta-hydroxybutyrate in a methaneutilizing mixed culture. J. Appl. Microbiol. 2008, 105, 1054–1061. [Google Scholar] [CrossRef]
- Cal, A.J.; Sikkema, W.D.; Ponce, M.I.; Franqui-Villanueva, D.; Riiff, T.J.; Orts, W.J.; Pieja, A.J.; Lee, C.C. Methanotrophic production of polyhydroxybutyrate-co-hydroxyvalerate with high hydroxyvalerate content. Int. J. Biol. Macromol. 2016, 87, 302–307. [Google Scholar] [CrossRef]
- García-Pérez, T.; Lópeza, J.C.; Passosc, F.; Lebreroa, R.; Revah, S.; Muñoza, R. Simultaneous methane abatement and PHB production by Methylocystis hirsuta in a novel gas-recycling bubble column bioreactor. Chem. Eng. J. 2018, 334, 691–697. [Google Scholar] [CrossRef]
- Cantera, S.; Sánchez-Andrea, I.; Lebrero, R.; García-Encina, P.A.; Stams, A.J.M.; Muñoz, R. Multi-production of high added market value metabolites from diluted methane emissions via methanotrophic extremophiles. Bioresour. Technol. 2018, 267, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, K.D.; Jechorek, M.; Helm, J.; Stottmeister, U. Producing poly-3-hydroxybutyrate with a high molecular mass from methane. J. Biotechnol. 2001, 86, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Rostkowski, K.H.; Pfluger, A.R.; Criddle, C.S. Stoichiometry and kinetics of the PHB-producing Type II methanotrophs Methylosinus trichosporium OB3b and Methylocystis parvus OBBP. Bioresour. Technol. 2013, 132, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Levett, I.; Birkett, G.; Davies, N.; Bell, A.; Langford, A.; Laycock, B.; Lant, P.; Pratt, S. Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—The case for thermophilic bioprocessing. J. Environ. Chem. Eng. 2016, 4, 3724–3733. [Google Scholar] [CrossRef]
- López, J.C.; Arnáiz, E.; Merchán, L.; Lebrero, R.; Muñoz, R. Biogas-based polyhydroxyalkanoates production by Methylocystis hirsuta: A step further in anaerobic digestion biorefineries. Chem. Eng. J. 2018, 333, 529–536. [Google Scholar] [CrossRef]
- Pérez, R.; Cantera, S.; Bordel, S.; Garcia-Encina, P.A.; Muñoz, R. The effect of temperature during culture enrichment on methanotrophic polyhydroxyalkanoate production. Int. Biodeter. Biodegrad. 2019, 140, 144–151. [Google Scholar] [CrossRef]
- Luangthongkam, P.; Laycock, B.; Evans, P.; Lant, P.; Pratt, S. Thermophilic production of poly(3-hydroxybutyrate-co-3-hydrovalerate) by a mixed methane-utilizing culture. New Biotechnol. 2019, 53, 49–56. [Google Scholar] [CrossRef]
- Pérez, R.; Casal, J.; Muñoz, R.; Lebrero, R. Polyhydroxyalkanoates production from methane emissions in Sphagnum mosses: Assessing the effect of temperature and phosphorus limitation. Sci. Total Environ. 2019, 688, 684–690. [Google Scholar] [CrossRef]
- Luangthongkam, P.; Strong, P.J.; Syed Mahamud, S.N.; Evans, P.; Jensen, P.; Tyson, G.; Laycock, B.; Lant, P.A.; Pratt, S. The effect of methane and odd-chain fatty acids on 3-hydroxybutyrate (3HB) and 3-hydroxyvalerate (3HV) synthesis by a Methylosinus-dominated mixed culture. Bioresour. Bioproces. 2019, 6, 50. [Google Scholar] [CrossRef]
- Eam, H.; Ko, D.; Lee, C.; Myung, J. Methylosinus trichosporium OB3b bioaugmentation unleashes polyhydroxybutyrate-accumulating potential in waste-activated sludge. Microb. Cell Fact. 2024, 23, 160. [Google Scholar] [CrossRef]
- Pham, D.N.; Mai, D.H.A.; Lee, E.Y. Biosynthesis of polyhydroxybutyrate from methane and carbon dioxide using type II methanotrophs. Bioresour. Technol. 2024, 405, 130931. [Google Scholar] [CrossRef] [PubMed]
- Delherbe, N.A.; Pearce, D.; But, S.Y.; Murrell, J.C.; Khmelenina, V.N.; Kalyuzhnaya, M.G. Genomic insights into moderately thermophilic methanotrophs of the genus Methylocaldum. Microorganisms 2024, 12, 469. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Flanagan, J.C.A.; Waymouth, R.M.; Criddle, C.S. Ethane-dependent synthesis of polyhydroxyalkanoates by the obligate methanotroph Methylocystis parvus OBBP. Chem. Eng. J. 2024, 483, 149210. [Google Scholar] [CrossRef]
- Gęsicka, A.; Gutowska, N.; Palaniappan, S.; Oleskowicz-Popiel, P.; Łężyk, M. Enrichment of mixed methanotrophic cultures producing polyhydroxyalkanoates (PHAs) from various environmental sources. Sci. Total Environ. 2024, 912, 168844. [Google Scholar] [CrossRef] [PubMed]
- Pieja, A.J.; Sundstrom, E.R.; Criddle, C.S. Poly-3-hydroxybutyrate metabolism in the type II methanotroph Methylocystis parvus OBBP. Appl. Environ. Microbiol. 2011, 77, 6012–6019. [Google Scholar] [CrossRef] [PubMed]
- Pieja, A.J.; Sundstrom, E.R.; Criddle, C.S. Cyclic, alternating methane and nitrogen limitation increases PHB production in a methanotrophic community. Bioresour. Technol. 2012, 107, 385–392. [Google Scholar] [CrossRef]
- Pieja, A.J.; Rostkowski, K.H.; Criddle, C.S. Distribution and selection of poly-3-hydroxybutyrate production capacity in methanotrophic proteobacteria. Microb. Ecol. 2011, 62, 564–573. [Google Scholar] [CrossRef]
- Tran, M.H.; Choi, T.-R.; Yang, Y.-H.; Lee, O.K.; Lee, E.Y. An efficient and eco-friendly approach for the sustainable recovery and properties characterization of polyhydroxyalkanoates produced by methanotrophs. Int. J. Biol. Macromol. 2023, 257, 128687. [Google Scholar] [CrossRef]
- de Sousa Junior, R.R.; Cezario, F.E.M.; Antonino, L.D.; dos Santos, D.J.; Lackner, M. Characterization of poly(3-hydroxybutyrate) (P3HB) from alternative, scalable (Waste) feedstocks. Bioengineering 2023, 10, 1382. [Google Scholar] [CrossRef]
- Lee, O.K.; Kang, S.G.; Choi, T.-R.; Yang, Y.-H.; Lee, E.Y. Production and characterization of a biodegradable polymer, poly(3-hydroxybutyrate-co-3-hydroxyvalerate), using the type II methanotroph, Methylocystis sp. MJC1. Bioresour. Technol. 2023, 389, 129853. [Google Scholar] [CrossRef]
- Safaeian, P.; Yazdian, F.; Khosravi-Darani, K.; Rashedi, H.; Lackner, M. P3HB from CH4 using methanotrophs: Aspects of bioreactor, fermentation process and modelling for cost-effective biopolymer production. Front. Bioeng. Biotechnol. 2023, 11, 1137749. [Google Scholar] [CrossRef] [PubMed]
- Amabile, C.; Abate, T.; De Crescenzo, C.; Sabbaresse, S.; Migliaccio, A.; Chianese, S.; Musmarra, D. Poly(3-hydroxybutyrate) production from methane in bubble column bioreactors: Process simulation and design optimization. New Biotechnol. 2022, 70, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.P.; Chavan, S.B.; Deshpande, M.S.; Sagotra, D.; Kumbhar, P.S.; Ghosalkar, A.R. Enrichment of Methylocystis dominant mixed culture from rice field for PHB production. J. Biotechnol. 2022, 343, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Vecherskaya, M.; Dijkema, C.; Stams, A.J. Intracellular PHB conversion in a type II methanotroph studied by 13C NMR. J. Ind. Microbiol. Biotechnol. 2001, 26, 15–21. [Google Scholar] [CrossRef]
- Wendlandt, K.D.; Geyer, W.; Mirschel, G.; Hemidi, F.A. Possibilities for controlling a PHB accumulation process using various analytical methods. J. Biotechnol. 2005, 117, 119–129. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, Y.; Dong, J.; Song, H.; Xia, C. An experimental study on molecular weight of poly-3-hydroxybutyrate (PHB) accumulated in Methylosinus trichosporium IMV 3011. Afr. J. Biotechnol. 2011, 10, 7078–7087. [Google Scholar]
- Zhang, Y.; Xin, J.; Chen, L.; Song, H.; Xia, C. Biosynthesis of poly-3-hydroxybutyrate with a high molecular weight by methanotroph from methane and methanol. J. Nat. Gas Chem. 2008, 17, 103–109. [Google Scholar] [CrossRef]
- Zúniga, C.; Morales, M.; Le Borgne, S.; Revah, S. Production of poly-β-hydroxybutyrate (PHB) by Methylobacterium organophilum isolated from a methanotrophic consortium in a two-phase partition bioreactor. J. Hazard. Mater. 2011, 190, 876–882. [Google Scholar] [CrossRef]
- Zúniga, C.; Morales, M.; Revah, S. Polyhydroxyalkanoates accumulation by Methylobacterium organophilum CZ-2 during methane degradation using citrate or propionate as cosubstrates. Bioresour. Technol. 2013, 129, 686–689. [Google Scholar] [CrossRef]
- Malvar, S.; Cardoso, L.O.B.; Karolski, B.; Perpetuo, E.A.; Carmo, B.S.; Meneghini, J.R. A rheological approach to identify efficient biopolymer producing bacteria. Biotechnol. Bioeng. 2021, 118, 622–632. [Google Scholar] [CrossRef]
- Chen, X.; Rodríguez, Y.; López, J.C.; Muñoz, R.; Ni, B.-J.; Sin, G. Modeling of polyhydroxyalkanoate synthesis from biogas by Methylocystis hirsuta. ACS Sustain. Chem. Eng. 2020, 8, 3906–3912. [Google Scholar] [CrossRef]
- Carere, C.R.; McDonald, B.; Peach, H.A.; Greening, C.; Gapes, D.J.; Collet, C.; Stott, M.B. Hydrogen oxidation influences glycogen accumulation in a verrucomicrobial methanotroph. Front. Microbiol. 2019, 10, 1873. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Flanagan, J.C.A.; Waymouth, R.M.; Criddle, C.S. Expanding the range of polyhydroxyalkanoates synthesized by methanotrophic bacteria through the utilization of omega-hydroxyalkanoate co-substrates. AMB Express 2017, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Chidambarampadmavathy, K.; Karthikeyan, O.P.; Heimann, K. Sustainable bio-plastic production through landfill methane recycling. Renew. Sustain. Energy Rev. 2017, 71, 555–562. [Google Scholar] [CrossRef]
- Flanagan, J.C.A.; Myung, J.; Criddle, C.S.; Waymouth, R.M. Poly(hydroxyalkanoate)s from waste biomass: A combined chemical–biological approach. ChemistrySelect 2016, 1, 2327–2331. [Google Scholar] [CrossRef]
- Hyun, S.W.; Krishna, S.; Chau, T.H.T.; Lee, E.Y. Methanotrophs mediated biogas valorization: Sustainable route to polyhydroxybutyrate production. Bioresour. Technol. 2024, 402, 130759. [Google Scholar] [CrossRef]
- Pérez, V.; Lebrero, R.; Muñoz, R.; Pérez, R. The fundamental role of pH in CH4 bioconversion into polyhydroxybutyrate in mixed methanotrophic cultures. Chemosphere 2024, 355, 141832. [Google Scholar] [CrossRef]
- Hong, H.J.; Hyung, J.S.; Lee, J.; Na, J.-G. Effects of methane to oxygen ratio on cell growth and polyhydroxybutyrate synthesis in high cell density cultivation of Methylocystis sp. MJC1. Environ. Sci. Pollut. Res. 2024, in press. [Google Scholar] [CrossRef]
- Naizabekov, S.; Hyun, S.W.; Na, J.-G.; Yoon, S.; Lee, O.K.; Lee, E.Y. Comparative genomic analysis of Methylocystis sp. MJC1 as a platform strain for polyhydroxybutyrate biosynthesis. PLoS ONE 2023, 18, e0284846. [Google Scholar] [CrossRef]
- Meraz, J.L.; Abel, A.J.; Clark, D.S.; Criddle, C.S. Biological conversion of methane to bioplastics: Kinetics, stoichiometry, and thermodynamic considerations for process optimization. Chem. Eng. J. 2023, 454, 140166. [Google Scholar] [CrossRef]
- Cardoso, L.O.B.; Karolski, B.; Gracioso, L.H.; Borrego, B.B.; do Nascimento, C.A.O.; Perpetuo, E.A. Enrichment of Methylosinus-dominant consortia from mangroves for polyhydroxybutyrate (PHB) production. J. Environ. Chem. Eng. 2022, 10, 108490. [Google Scholar] [CrossRef]
- Fergala, A.; Alsayed, A.; Eldyasti, A. Utilization of polyhydroxybutyrate (PHB) as intracellular reducing power for methanol production to alleviate the reliance on external energy sources by Methylocystis hirsuta. J. Environ. Chem. Eng. 2021, 9, 105314. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Firmino, P.I.M.; Arnáiz, E.; Lebrero, R.; Muñoz, R. Elucidating the influence of environmental factors on biogas-based polyhydroxybutyrate production by Methylocystis hirsuta CSC1. Sci. Total Environ. 2020, 706, 135136. [Google Scholar] [CrossRef]
- Bordel, S.; Rojas, A.; Muñoz, R. Reconstruction of a genome scale metabolic model of the polyhydroxybutyrate producing methanotroph Methylocystis parvus OBBP. Microb. Cell Fact. 2019, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Fergala, A.; Alsayed, A.; Khattab, S.; Ramirez, M.; Eldyasti, A. Development of methane-utilizing mixed cultures for the production of polyhydroxyalkanoates (PHAs) from anaerobic digester sludge. Environ. Sci. Technol. 2018, 52, 12376–12387. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.Z.; Stein, L.Y.; Sauvageau, D. Defining nutrient combinations for optimal growth and polyhydroxybutyrate production by Methylosinus trichosporium OB3b using response surface methodology. Front. Microbiol. 2018, 9, 1513. [Google Scholar]
- Chidambarampadmavathy, K.; Karthikeyan, O.P.; Huerlimann, R.; Maes, G.E.; Heimann, K. Response of mixed methanotrophic consortia to different methane to oxygen ratios. Waste Manag. 2017, 61, 220–228. [Google Scholar] [CrossRef]
- Bordel, S.; Rodríguez, Y.; Hakobyan, A.; Rodriguez, E.; Lebrero, R.; Muñoz, R. Genome scale metabolic modeling reveals the metabolic potential of three Type II methanotrophs of the genus Methylocystis. Metab. Eng. 2019, 54, 191–199. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lee, E.Y. Methane-based biosynthesis of 4-hydroxybutyrate and P(3-hydroxybutyrate-co-4-hydroxybutyrate) using engineered Methylosinus trichosporium OB3b. Bioresour. Technol. 2021, 335, 125263. [Google Scholar] [CrossRef]
- Kulkarni, P.P.; Khonde, V.K.; Ghosalkar, A.R. Simultaneous production of lipase and PHB using methanotrophic organism Methylosinus trichosporium OB3b. Bioresour. Technol. Rep. 2024, 25, 101751. [Google Scholar] [CrossRef]
- Lazic, M.; Gudneppanavar, R.; Whiddon, K.; Sauvageau, D.; Stein, L.Y.; Konopka, M. In vivo quantification of polyhydroxybutyrate (PHB) in the alphaproteobacterial methanotroph, Methylocystis sp. Rockwell. Appl. Microbiol. Biotechnol. 2022, 106, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Rashedi, H.; Mofradnia, S.R.; Khosravi-Darani, K.; Ashouri, R.; Yazdian, F. Polyhydroxybutyrate production from natural gas in a bubble column bioreactor: Simulation using COMSOL. Bioengineering 2019, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Darani, K.K.; Yazdian, F.; Rashedi, H.; Bozorg, N.M.; Moradi, M.; Mofradnia, S.R.; Koller, M. Simulation of bioreactors for PHB production from natural gas. Iran. J. Chem. Chem. Eng. 2020, 39, 1. [Google Scholar]
| Methanotrophs | CH4 (%) | Fermentation Conditions (Mode/Working Capacity (L)//Incubation Period (h)) | PHAs | Reference | |
|---|---|---|---|---|---|
| % in cdw | Mw (×106) | ||||
| Enriched methanotrophs/consortia | 25 | Batch/70.0/24 | 46.2 | 2.41 | [47] |
| 25 | Batch/70.0/24 | 10.4–33.6 | 1.81–3.10 | [48] | |
| 50 | Batch/0.05/24 | 46.0 | - | [67] | |
| 80 a | Continuous/2.00/384 | 34.0 | - | [78] | |
| 150 b | Continuous/4.00/24 | 25.0 | - | [66] | |
| 5 | Continuous/0.40/432 | 12.6 | - | [45] | |
| 5 | Continuous/0.40/310 | 1.0–12.6 | - | [46] | |
| 50 | Batch/0.05/48 | 39.0–45.0 | 0.93–1.20 | [42] | |
| 40 | Batch/0.20/480 | 25.0 d | - | [97] | |
| 46 a | Continuous/2.00/150 | 0.01 e | - | [51] | |
| 50 | Batch/0.05/48 | 51.0 | - | [95] | |
| 50 | Semi-continuous/0.24/72 | 8.6–14.1 | - | [59] | |
| 50 | Batch/0.03/48 | 8.0–10.0 | - | [57] | |
| 177 b | Batch/0.20/- | 34.1–35.1 | - | [56] | |
| 161 b | Batch/0.20/384 | 13.6 | - | [58] | |
| - c | Batch/0.05/192 | 0.19 e | - | [80] | |
| 12.3 | Batch/-/268 | 0.18 e | - | [91] | |
| 50 | Continuous/8.00/120 | 22.2 | 2.20 | [72] | |
| 20 | Batch/0.05/144 | 12.6 | - | [39] | |
| 30 | Batch/0.04/168 | 12.9 | - | [64] | |
| 9 | Batch/2.50/192 | 43.7 | - | [87] | |
| Activated sludge and Methylosinus trichosporium OB3b | 50 | Batch/0.04/72 | 37.1 | - | [60] |
| Methanotrophic bacterium MTS | 25 | Batch/0.30/- | 3.00 | - | [74] |
| Methylobacterium organophilus CZ-2 | 80 a | Continuous/2.00/384 | 38.0–39.0 | - | [78] |
| 42 a | Continuous/2.00/240 | 88.0 | - | [79] | |
| Methylococcus capsulatus HD6T | 50 | Batch/0.10/120 | - | 0.95 | [76] |
| Methylocystis | 4 | Semi-continuous/400/- | 37.5 | - | [72] |
| Methylocystis 42/22 | 50 | Batch/0.05/24 | 25.0 | - | [67] |
| Methylocystis SC2 | 50 | Batch/0.05/24 | 30.0 | - | [67] |
| Methylocystis hirsuta CSC1 | 50 | Batch/0.05/24 | 7.0 | - | [67] |
| 29.2 | Batch/0.40/504 | 45.0 | - | [93] | |
| M. hirsuta DSMZ 18500 | 4 | Batch/2.50/1632 | 28–34.6 | - | [50] |
| 35 | Batch/0.05/168 | 45.0–54.0 | - | [55] | |
| M. hirsuta | 50 | Continuous/10.0/120 | 51.6 | - | [71] |
| Methylocystis parvus OBBP | 50 | Batch/0.05/66 | 30.5–50.3 | - | [65] |
| 50 | Batch/0.05/24 | 36.0 | - | [67] | |
| 30 | Batch/0.05/22 | 60.0 | - | [53] | |
| 40 | Batch/0.05/24 | 48.0–64.0 d | 1.18–1.47 | [85] | |
| 40 | Batch/-/168 | 32.0 | - | [43] | |
| 40 | Batch/0.05/48 | 32.0–60.0 | - | [44] | |
| 40 | Batch/0.05/48 | 59.0 | 1.22–1.33 | [83] | |
| 40 | Batch/0.05/24 | 35.0–48.0 | - | [63] | |
| M. parvus | 74 b | Batch/0.02/- | 50.0 | - | [94] |
| Methylocystis rosea SV99 | 50 | Batch/0.05/24 | 9.00 | - | [67] |
| Methylocystis sp. MJC1 | 30 | Batch/0.05/96 | 41.9 | - | [70] |
| 30 | Batch/3.00/96 | 44.5 | - | [89] | |
| 30 | Batch/2.50/208 | 61.7 | - | [88] | |
| 30 | Batch/1.20/140 | 2.90 e | - | [86] | |
| 20 | Batch/0.10/24 | 38.0 | - | [61] | |
| Methylocystis sp. GB25 | 20 | Batch/70.0/24 | 28.3–51.3 | - | [52] |
| 15 | Batch/70.0/24 | 45.0–51.0 | 2.50 | [75] | |
| - | Batch/30.0/504 | - | 1.08 | [69] | |
| Methylocystis strain M | 50 | Batch/0.05/24 | 14.0 | - | [67] |
| Methylocystis sp. WRRC1 | 50 | Batch/0.02/72 | 0.20–0.57 e | - | [49] |
| Methylomonas sp. GYJB | 50 | Batch/0.10/120 | - | 0.30 | [76] |
| Methylosinus sp. LW4 | 50 | Batch/0.05/24 | 100 | - | [67] |
| Methylosinus sporium | 50 | Batch/0.05/24 | 9.00 | - | [67] |
| M. trichosporium IMV3011 | 50 | Batch/0.10/120 | - | 1.20 | [76] |
| 50 | Batch/0.05/168 | 41.0 | [37] | ||
| 50 | Batch/0.10/144 | 38.1 | 1.50 | [77] | |
| M. trichosporium OB3b | 50 | Batch/0.05/24 | 38.0 | [67] | |
| 50 | Batch/0.10/120 | - | 0.95 | [76] | |
| 80 a | Continuous/2.00/384 | 57.0 | - | [78] | |
| 30 | Batch/0.05/28 | 29.0–60.0 | - | [53] | |
| 50 | Batch/0.10/120 | 52.5 | - | [95] | |
| Methylotuvimicrobium alcaliphilum 20Z | - | Batch/0.10/168 | 1.30 | - | [40] |
| Methylomonas sp. DH-1 and M. trichosporium OB3b | 30 | Batch/0.10/168 | 0.08 e | - | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.K.S.; Singh, D.; Pant, D.; Gupta, R.K.; Busi, S.; Singh, R.V.; Lee, J.-K. Polyhydroxyalkanoate Production by Methanotrophs: Recent Updates and Perspectives. Polymers 2024, 16, 2570. https://doi.org/10.3390/polym16182570
Patel SKS, Singh D, Pant D, Gupta RK, Busi S, Singh RV, Lee J-K. Polyhydroxyalkanoate Production by Methanotrophs: Recent Updates and Perspectives. Polymers. 2024; 16(18):2570. https://doi.org/10.3390/polym16182570
Chicago/Turabian StylePatel, Sanjay K. S., Deepshikha Singh, Diksha Pant, Rahul K. Gupta, Siddhardha Busi, Rahul V. Singh, and Jung-Kul Lee. 2024. "Polyhydroxyalkanoate Production by Methanotrophs: Recent Updates and Perspectives" Polymers 16, no. 18: 2570. https://doi.org/10.3390/polym16182570








