Carboxyalkylated Lignin as a Sustainable Dispersant for Coal Water Slurry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coal Water Slurry
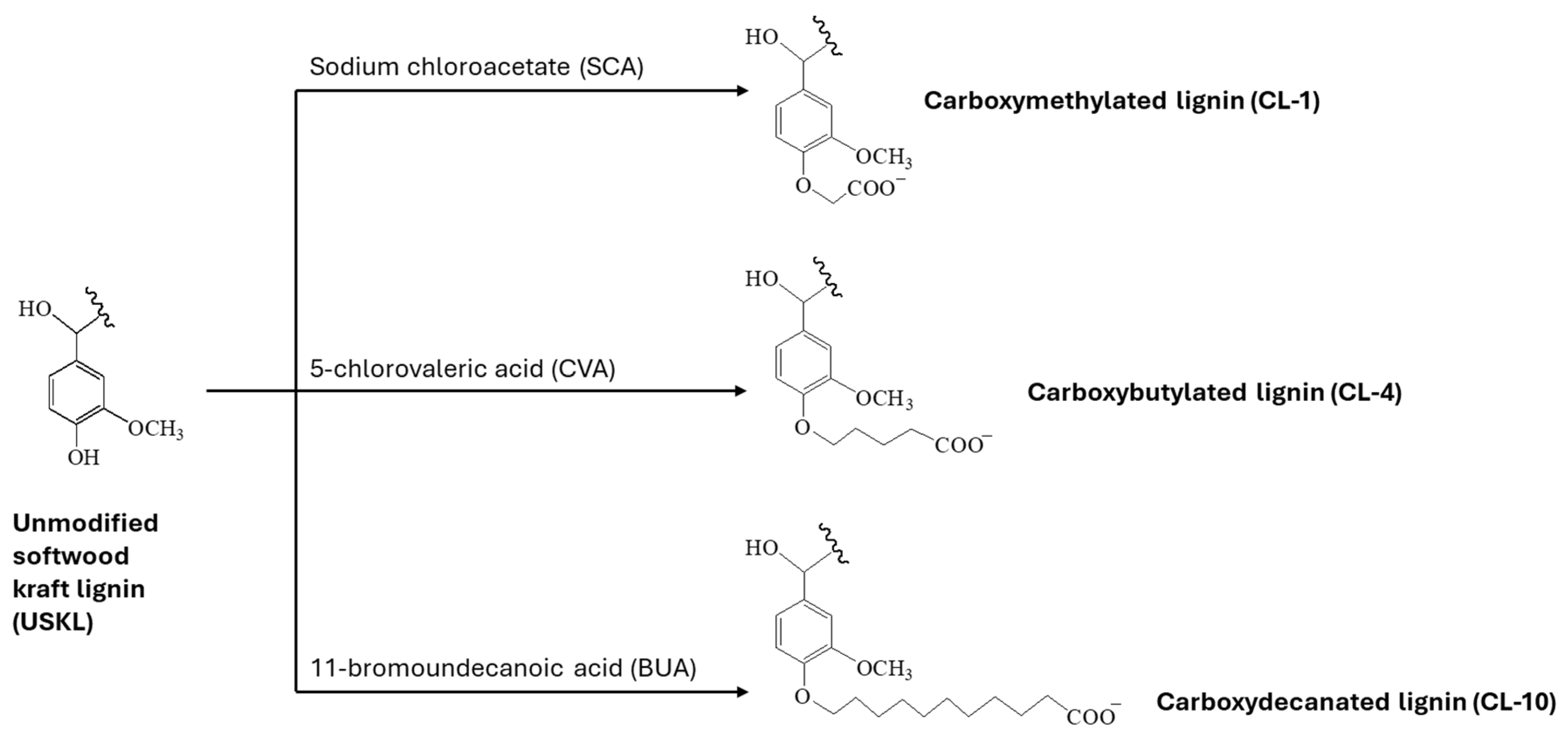
2.3. Synthesis of CL Samples
2.4. Solubility Measurement
2.5. Charge Density and Carboxyl Group Analysis
2.6. NMR Analysis
2.7. Molecular Weight Analysis
2.8. Zeta Potential Analysis
2.9. Contact Angle Analysis
2.10. Viscosity Analysis
2.11. Stability Analysis
3. Results and Discussion
3.1. Properties of Coal and Lignin Samples
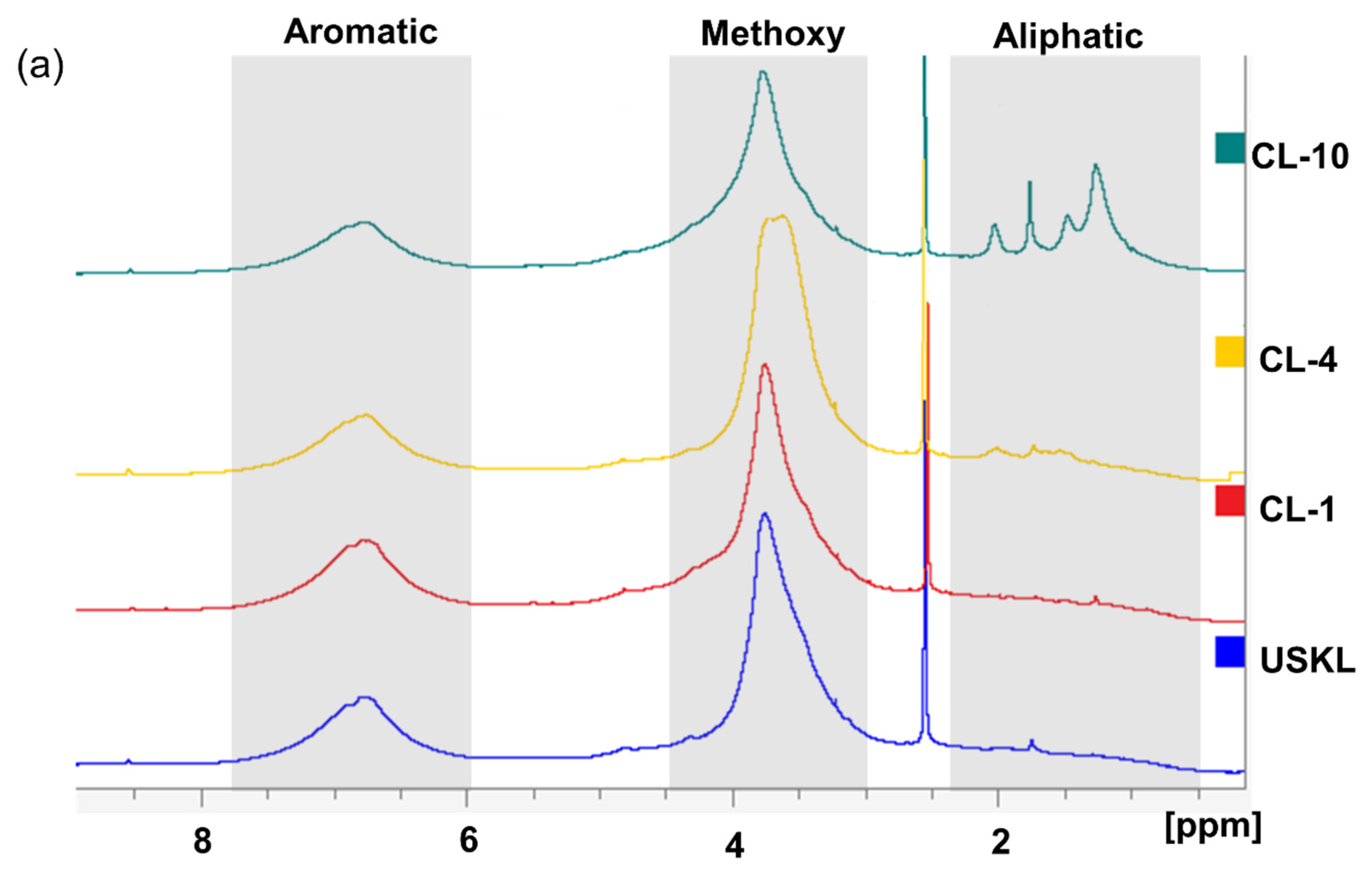
3.2. NMR Spectroscopy
3.3. Stability Studies
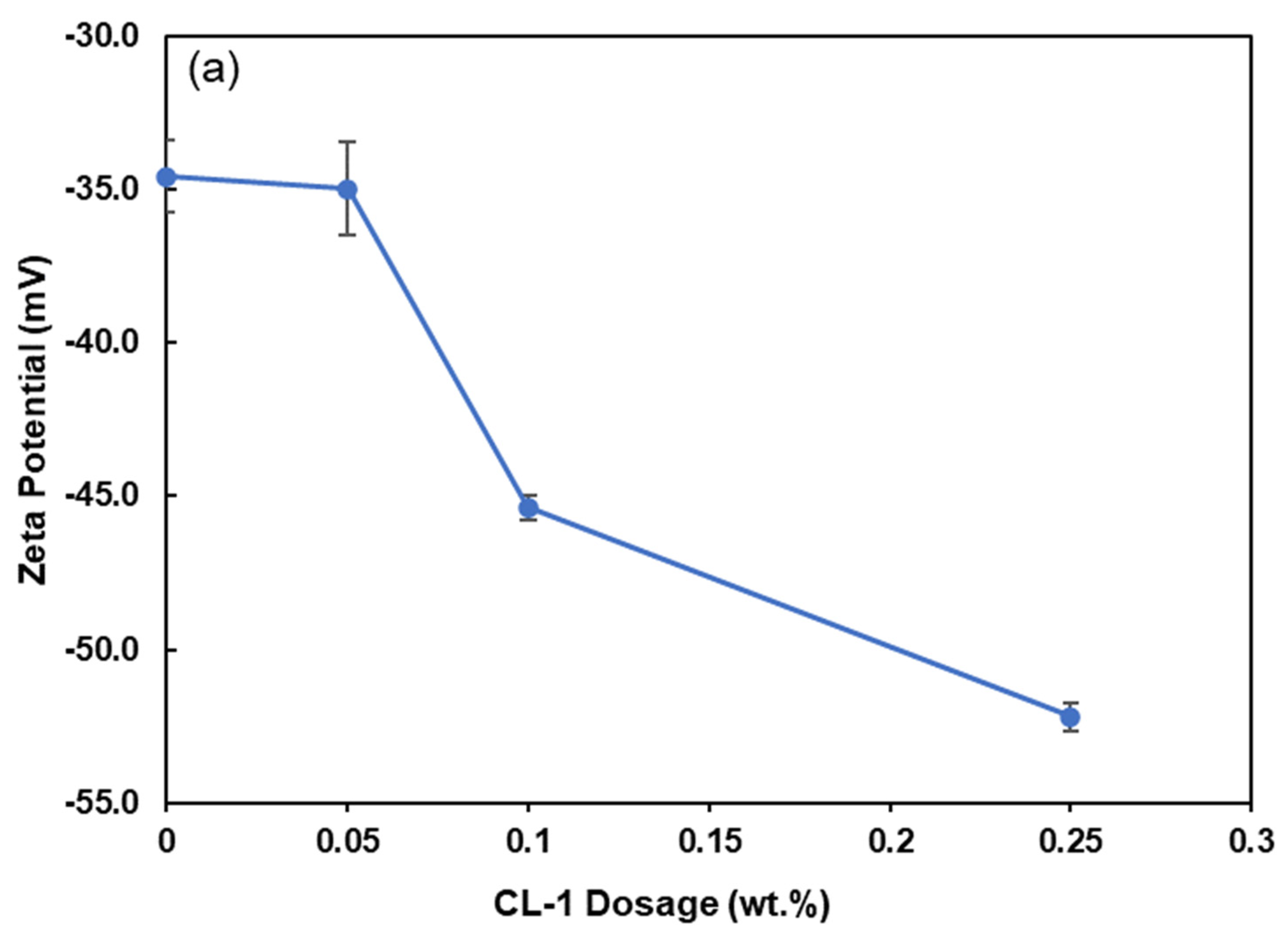
3.4. Zeta Potential of Coal Particles
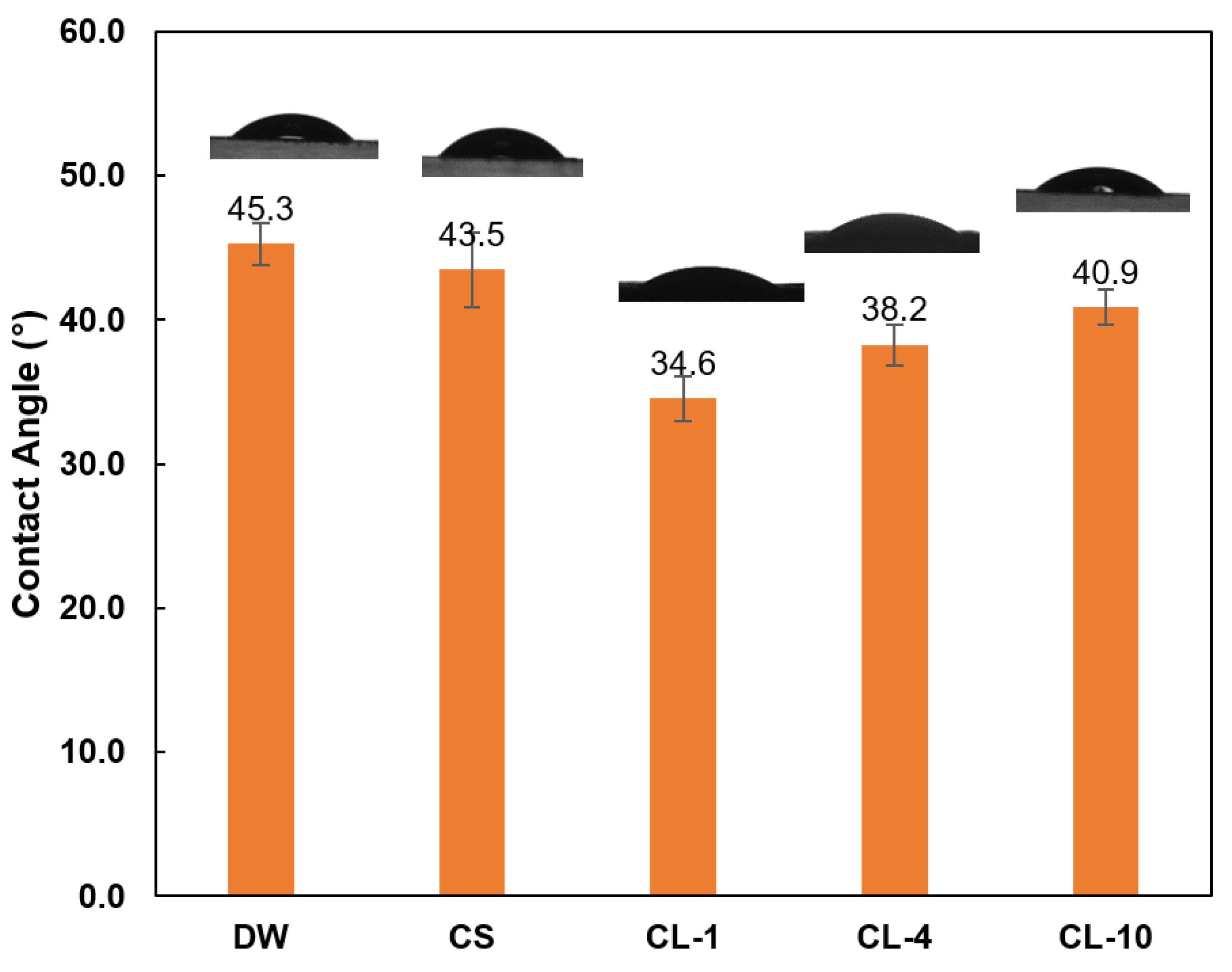
3.5. Contact Angle Studies
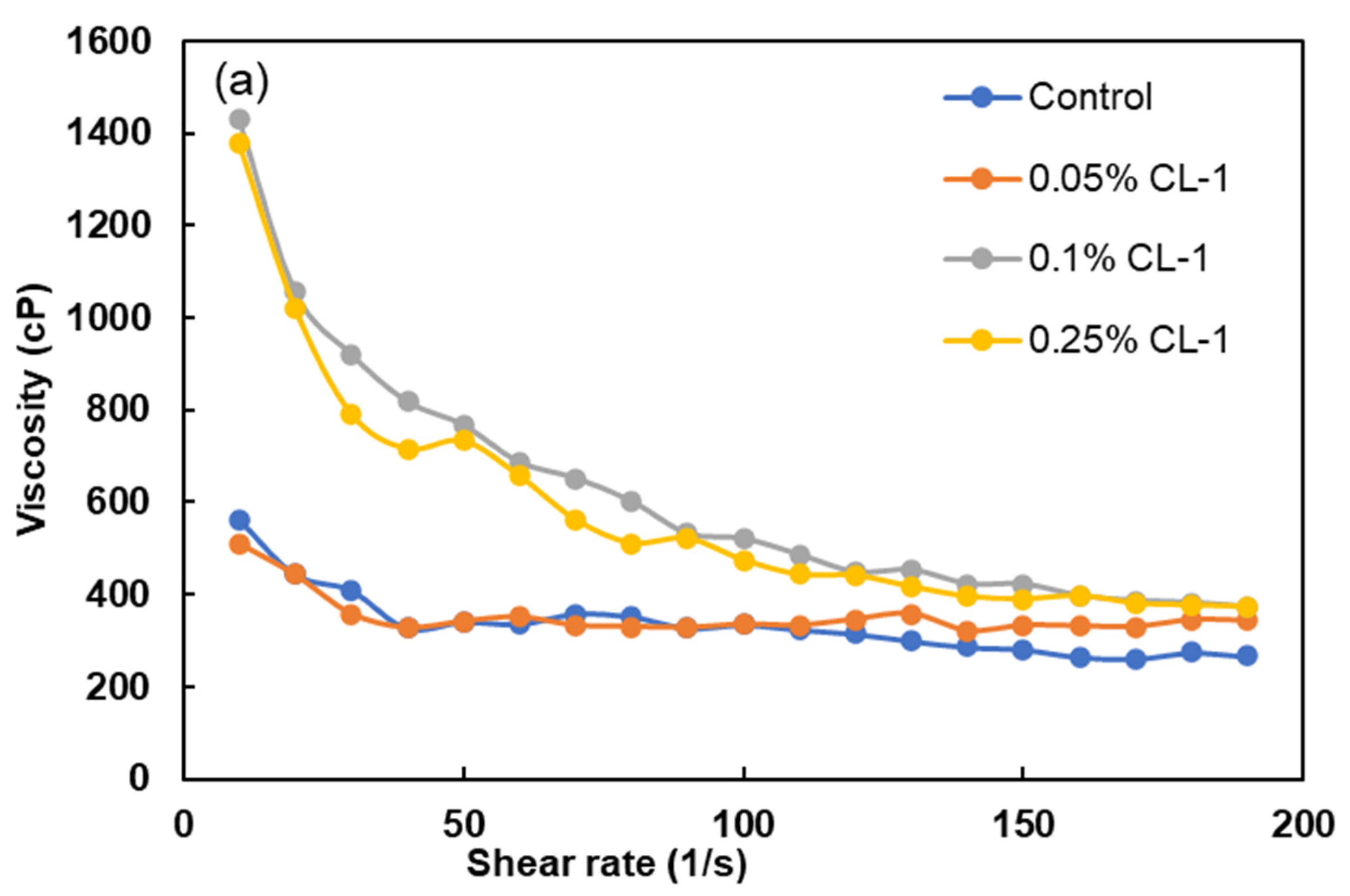
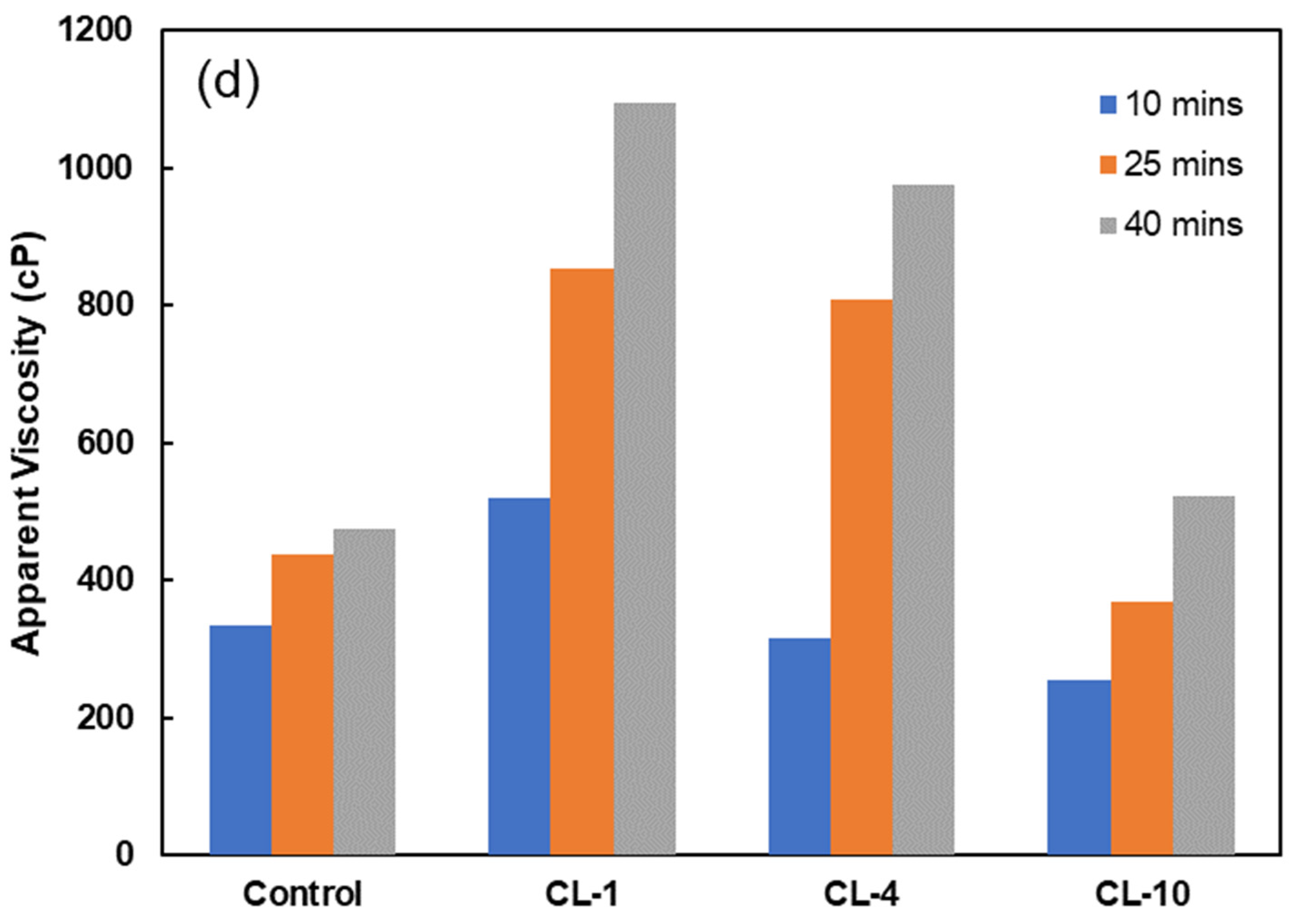
3.6. Viscosity Studies
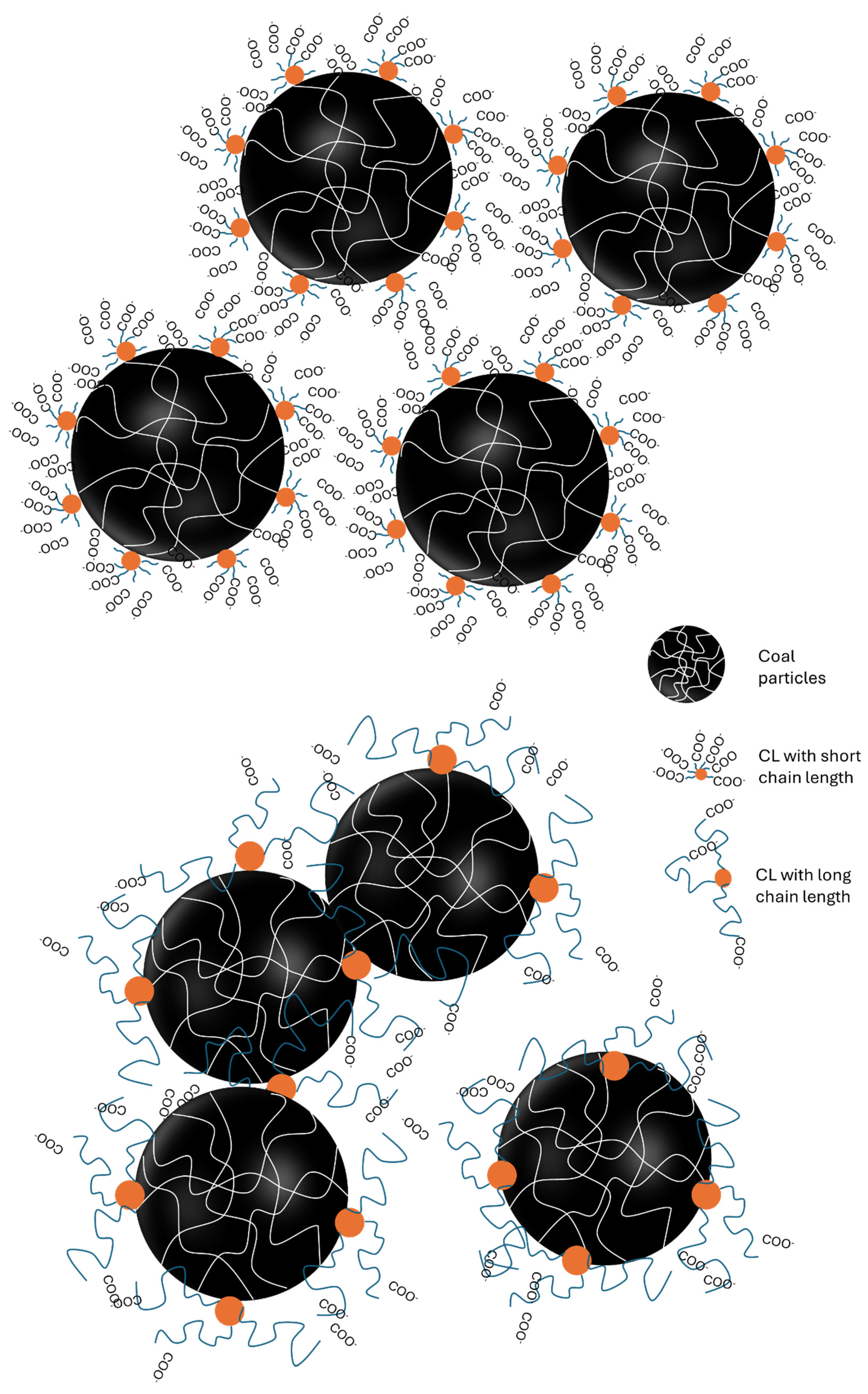
3.7. Mechanism of Dispersion
3.8. Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Chen, S.; Cui, L.; Xu, E.; Chen, H.; Meng, X.; Wu, G. Eco-friendly utilization of microplastics for preparing coal water slurry: Rheological behavior and dispersion mechanism. J. Clean. Prod. 2022, 330, 129881. [Google Scholar] [CrossRef]
- Nyashina, G.S.; Vershinina, K.Y.; Dmitrienko, M.A.; Strizhak, P.A. Environmental benefits and drawbacks of composite fuels based on industrial wastes and different ranks of coal. J. Hazard. Mater. 2018, 347, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Feng, Y.; He, Q.; Yan, W.; Yuan, M.; Hu, B. Review and Perspectives of Anionic Dispersants for Coal–Water Slurry. Energ. Fuel. 2023, 37, 4816–4834. [Google Scholar] [CrossRef]
- Pawlik, M. Polymeric dispersants for coal–water slurries. Colloids Surf. A: Physicochem. Eng. Asp. 2005, 266, 82–90. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, X.; Ma, X.; Yang, H. Synthesis and Performance of a Novel Cotton Linter Based Cellulose Derivatives Dispersant for Coal–Water Slurries. Polymers 2022, 14, 1103. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Potential of Coal–Water Slurries as an Alternative Fuel Source during the Transition Period for the Decarbonization of Energy Production: A Review. Appl. Sci. 2020, 10, 2470. [Google Scholar] [CrossRef]
- D’Orsi, R.; Irimia, C.V.; Lucejko, J.J.; Kahraman, B.; Kanbur, Y.; Yumusak, C.; Bednorz, M.; Babudri, F.; Irimia-Vladu, M.; Operamolla, A. Kraft Lignin: From Pulping Waste to Bio-Based Dielectric Polymer for Organic Field-Effect Transistors. Adv. Sustain. Syst. 2022, 6, 2200285. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to materials: A focused review on recent novel lignin applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Wang, T.; Li, H.; Diao, X.; Lu, X.; Ma, D.; Ji, N. Lignin to dispersants, adsorbents, flocculants and adhesives: A critical review on industrial applications of lignin. Ind. Crop. Prod. 2023, 199, 116715. [Google Scholar] [CrossRef]
- Konduri, M.K.R.; Fatehi, P. Adsorption and dispersion performance of oxidized sulfomethylated kraft lignin in coal water slurry. Fuel Process. Technol. 2018, 176, 267–275. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, D.; Gu, F.; Li, X.; Xiong, W.; Zhu, J.Y. Biorefinery lignosulfonates as a dispersant for coal water slurry. Sustain. Chem. Process. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Yu, X.; Yang, B.; Zhu, W.; Deng, T.; Pu, Y.; Ragauskas, A.; Wang, H. Towards functionalized lignin and its derivatives for high-value material applications. Ind. Crop. Prod. 2023, 200, 116824. [Google Scholar] [CrossRef]
- Konduri, M.K.; Kong, F.; Fatehi, P. Production of carboxymethylated lignin and its application as a dispersant. Eur. Polym. J. 2015, 70, 371–383. [Google Scholar] [CrossRef]
- Hu, S.; Liu, L.; Yang, X.; Li, J.; Zhou, B.; Wu, C.; Weng, L.; Liu, K. Influence of different dispersants on rheological behaviors of coal water slurry prepared from a low quality coal. RSC adv. 2019, 9, 32911–32921. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhuang, W.; He, Q.; Cai, J.; Hu, B.; Shen, J. Effects of chemical structure on the properties of carboxylate-type copolymer dispersant for coal-water slurry. AIChE J. 2009, 55, 2461–2467. [Google Scholar] [CrossRef]
- Shomali, Z.; Fatehi, P. Carboxyalkylated Lignin Nanoparticles with Enhanced Functionality for Oil–Water Pickering Emulsion Systems. ACS Sustain. Chem. Eng. 2022, 10, 16563–16577. [Google Scholar] [CrossRef]
- Keboletse, K.P.; Ntuli, F.; Oladijo, O.P. Influence of coal properties on coal conversion processes-coal carbonization, carbon fiber production, gasification and liquefaction technologies: A review. Int. J. Coal Sci. Technol. 2021, 8, 817–843. [Google Scholar] [CrossRef]
- Kouisni, L.; Holt-Hindle, P.; Maki, K.; Paleologou, M. The Lignoforce system: A new process for the production of high-quality lignin from black liquor. J. Sci. Technol. For. Prod. Processes 2012, 2, 6–10. Available online: https://www.academia.edu/download/78836264/J-FOR_Vol2-issue4-ART1-The_20Lignoforce_20System.pdf (accessed on 1 August 2024).
- ASTM International. ASTM D197-19 Standard Test Method for Sampling and Fineness Test of Pulverized Coal. ASTM: West Conshohocken, PA, USA, 2019. Available online: https://www.astm.org/d0197-19.html (accessed on 1 August 2024).
- Cong, K.; Zhang, Y.; Han, F.; Li, Q. Influence of particle sizes on combustion characteristics of coal particles in oxygen-deficient atmosphere. Energy 2019, 170, 840–848. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Nyashina, G.S.; Strizhak, P.A. Major gas emissions from combustion of slurry fuels based on coal, coal waste, and coal derivatives. J. Clean. Prod. 2018, 177, 284–301. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, Z.; He, Q.; Gong, Y.; Huang, Y.; Yu, G. Characteristics of High-Carbon-Content Slag and Utilization for Coal-Water Slurry Preparation. Energ. Fuel. 2020, 34, 14058–14064. [Google Scholar] [CrossRef]
- Bahrpaima, K.; Fatehi, P. Synthesis and Characterization of Carboxyethylated Lignosulfonate. ChemSusChem 2018, 11, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Bahrpaima, K.; Fatehi, P. Preparation and Coagulation Performance of Carboxypropylated and Carboxypentylated Lignosulfonates for Dye Removal. Biomolecules 2019, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Gellerstedt, G.; Sjöholm, E.; Brodin, I. The Wood-Based Biorefinery: A Source of Carbon Fiber? Open Agric. J. 2010, 4, 119–124. [Google Scholar] [CrossRef]
- Keller, D.V. The contact angle of water on coal. Colloid. Surface. 1987, 22, 21–35. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Hosseinpour Feizi, Z.; Fatehi, P. Grafting strategies for hydroxy groups of lignin for producing materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef]
- Kalliola, A.; Kangas, P.; Winberg, I.; Vehmas, T.; Kyllönen, H.; Heikkinen, J.; Poukka, O.; Kemppainen, K.; Sjögård, P.; Pehu-Lehtonen, L.; et al. Oxidation process concept to produce lignin dispersants at a kraft pulp mill. Nord. Pulp Pap. Res. J. 2022, 37, 394–404. [Google Scholar] [CrossRef]
- Schieppati, D.; Dreux, A.; Gao, W.; Fatehi, P.; Boffito, D.C. Ultrasound-assisted carboxymethylation of LignoForce Kraft lignin to produce biodispersants. J. Clean. Prod. 2022, 366, 132776. [Google Scholar] [CrossRef]
- Alekhina, M.; Mikkonen, K.S.; Alén, R.; Tenkanen, M.; Sixta, H. Carboxymethylation of alkali extracted xylan for preparation of bio-based packaging films. Carbohydr. Polym. 2014, 100, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Feng, X.; Zhou, M.; Qian, Y.; Yu, H.; Qiu, X. Investigation of Aggregation and Assembly of Alkali Lignin Using Iodine as a Probe. Biomacromolecules 2011, 12, 1116–1125. [Google Scholar] [CrossRef]
- Gan, L.H.; Zhou, M.S.; Qiu, X.Q. Preparation of Water-Soluble Carboxymethylated Lignin from Wheat Straw Alkali Lignin. Adv. Mater. Res. 2012, 550-553, 1293–1298. [Google Scholar] [CrossRef]
- Zeng, J.; Helms, G.L.; Gao, X.; Chen, S. Quantification of wheat straw lignin structure by comprehensive NMR analysis. J. Agric. Food Chem. 2013, 61, 10848–10857. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lundquist, K. A new method for the analysis of phenolic groups in lignins by ′H NMR spectrometry. Nord. Pulp Pap. Res. J. 1994, 9, 191–195. [Google Scholar] [CrossRef]
- Antonino, L.D.; Gouveia, J.R.; de Sousa Júnior, R.R.; Garcia, G.E.S.; Gobbo, L.C.; Tavares, L.B.; dos Santos, D.J. Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI. Molecules 2021, 26, 2131. [Google Scholar] [CrossRef]
- Guadix-Montero, S.; Sainna, M.A.; Jin, J.; Reynolds, J.; Forsythe, W.G.; Sheldrake, G.N.; Willock, D.; Sankar, M. Ruthenium ion catalysed C–C bond activation in lignin model compounds—Towards lignin depolymerisation. Catal. Sci. Technol. 2023, 13, 5912–5923. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Beery, S.R.; Kong, F.; Fatehi, P. Impact of Molecular Weight of Oxidized Lignin on its Coagulation Performance in Aluminum Oxide Suspension. Waste Biomass Valori. 2023, 14, 2349–2365. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Hernández-García, H.; Zamudio-Aguilar, M.A.M.; Oropeza-Guzmán, M.T.; Ochoa-Terán, A.; López-Martínez, L.M.; Martinez-Quiroz, M.; Valdez, R.; Olivas, A. Chemical issues of coffee and Tule lignins as ecofriendly materials for the effective removal of hazardous metal ions contained in metal finishing wastewater. Chem. Eng. J. 2020, 397, 125384. [Google Scholar] [CrossRef]
- Zhou, M.; Qiu, X.; Yang, D.; Lou, H.; Ouyang, X. High-performance dispersant of coal–water slurry synthesized from wheat straw alkali lignin. Fuel Process. Technol. 2007, 88, 375–382. [Google Scholar] [CrossRef]
- Mori, T.; Hori, Y.; Fei, H.; Inamine, I.; Asai, K.; Kiguchi, T.; Tsubaki, J. Experimental study about the agglomeration behavior in slurry prepared by adding excess polyelectrolyte dispersant. Adv. Powder Technol. 2012, 23, 661–666. [Google Scholar] [CrossRef]
- Klose, W.; Lent, M. Agglomeration kinetics of coking coal particles during the softening phase. Fuel 1985, 64, 193–199. [Google Scholar] [CrossRef]
- Ogura, T.; Tanoura, M.; Hiraki, A. Behavior of Surfactants in a Highly Loaded Coal–Water Slurry. I. Effects of Surfactant Concentration on Its Properties. Bull. Chem. Soc. JPN 1993, 66, 1343–1349. [Google Scholar] [CrossRef]
- Yang, D.; Qiu, X.; Zhou, M.; Lou, H. Properties of sodium lignosulfonate as dispersant of coal water slurry. Energ. Convers. Manag. 2007, 48, 2433–2438. [Google Scholar] [CrossRef]
- Zhang, Z. A new method for estimating zeta potential of carboxylic acids’ functionalised particles. Mol. Phys. 2024, 122, e2260014. [Google Scholar] [CrossRef]
- Cao, R.; Liu, X.; Guo, J.; Xu, Y. Comparison of various organic acids for xylo-oligosaccharide productions in terms of pKa values and combined severity. Biotechnol. Biofuels 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Stevens, S.; Hofmeyr, J.H.S. Effects of ethanol, octanoic and decanoic acids on fermentation and the passive influx of protons through the plasma membrane of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 38, 656–663. [Google Scholar] [CrossRef]
- Žilnik, L.F.; Likozar, B. Back-extraction process operation and modeling through thermodynamic equilibrium solubility of valeric acid in aqueous and organic phase mixtures. Sep. Purif. Technol. 2019, 222, 125–135. [Google Scholar] [CrossRef]
- Mishra, S.K.; Senapati, P.K.; Panda, D. Rheological behavior of coal-water slurry. Energy Sources 2002, 24, 159–167. [Google Scholar] [CrossRef]
- Huang, J.; Xu, J.; Wang, D.; Li, L.; Guo, X. Effects of amphiphilic copolymer dispersants on rheology and stability of coal water slurry. Ind. Eng. Chem. Res. 2013, 52, 8427–8435. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, S.; Mohapatra, S.K.; Prasad, S.B.; Singh, J. Slurryability and flowability of coal water slurry: Effect of particle size distribution. J. Clean. Prod. 2021, 323, 129183. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, H.; Dai, Z.; Li, W.; Liu, H. Influence of alkaline additive on viscosity of coal water slurry. Fuel 2019, 235, 639–646. [Google Scholar] [CrossRef]
- Hassanabadi, H.M.; Wilhelm, M.; Rodrigue, D. A rheological criterion to determine the percolation threshold in polymer nano-composites. Rheol. Acta 2014, 53, 869–882. [Google Scholar] [CrossRef]
- Amin, N.; Tahir, M.S.; Saleem, M.; Khan, Z.; Aslam, M.; Bazmi, A.A.; Ghauri, M.; Sagir, M. Rheological improvement in performance of low-rank coal–water slurries using novel cost-effective additives. Asia-Pac. J. Chem. Eng. 2020, 15, e2400. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Pei, Y.; Chen, M.; Liu, H.; Li, X. Influence of flocculation effect on the apparent viscosity of cement slurry and analysis of different influencing factors. Constr. Build. Mater. 2021, 281, 122602. [Google Scholar] [CrossRef]
- Atesok, G.; Boylu, F.; Sirkeci, A.A.; Dincer, H. The effect of coal properties on the viscosity of coal–water slurries. Fuel 2002, 81, 1855–1858. [Google Scholar] [CrossRef]
- Zürcher, S.; Graule, T. Influence of dispersant structure on the rheological properties of highly-concentrated zirconia dispersions. J. Eur. Ceram. Soc. 2005, 25, 863–873. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Yang, X.; Jiang, F.; Wu, C.; Li, J.; Liu, K. Performance and mechanism of polyacrylamide stabilizers in coal water slurry. Colloid. Surface. A Physicochem. Eng. Asp. 2021, 630, 127544. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Basu, S.K.; Bit, K.C.; Banerjee, S.; Mishra, K.K. High-concentration coal–water slurry from Indian coals using newly developed additives. Fuel Process. Technol. 2004, 85, 31–42. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Wu, P.; Lv, Y.; Bai, Y.; Li, Z.; Xie, Q. Preparation of semicokes by medium–low temperature pyrolysis of long flame coal and their adsorption performance on sodium lignin sulfonate dispersant for high concentration coal water slurry. J. Disper. Sci. Technol. 2021, 42, 1610–1622. [Google Scholar] [CrossRef]
- Hong, N.; Li, Y.; Zeng, W.; Zhang, M.; Peng, X.; Qiu, X. Ultrahigh molecular weight, lignosulfonate-based polymers: Preparation, self-assembly behaviours and dispersion property in coal–water slurry. RSC Adv. 2015, 5, 21588–21595. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, D.; Guo, W.; Qiu, X. Investigation of grafted sulfonated alkali lignin polymer as dispersant in coal-water slurry. J. Ind. Eng. Chem. 2015, 27, 192–200. [Google Scholar] [CrossRef]
- Lu, H.Y.; Li, X.F.; Zhang, C.Q.; Li, W.H.; Xu, D.P. β-Cyclodextrin grafted on alkali lignin as a dispersant for coal water slurry. Energ. Source Part A 2019, 41, 1716–1724. [Google Scholar] [CrossRef]





| Sample ID | Charge Density (mmol/g) | Solubility (g/L) | MW (kg/mol) | PDI | Carboxylic Acid Content (mmol/g) | ||
|---|---|---|---|---|---|---|---|
| RI | UV | RI | UV | ||||
| USKL | −0.35 ± 0.02 | 2.7 | - | - | - | - | 0.63 |
| CS | −1.34 ± 0.02 | 9.9 | 1.50 | 1.79 | 1.95 | 2.14 | 0.93 |
| CL-1 | −1.95 ± 0.03 | 9.9 | 1.61 | 2.12 | 2.15 | 2.21 | 1.17 |
| CL-4 | −2.04 ± 0.01 | 9.6 | 2.09 | 2.20 | 2.04 | 2.22 | 1.22 |
| CL-10 | −1.98 ± 0.02 | 9.9 | 2.31 | 2.58 | 1.69 | 1.87 | 1.20 |
| Sample | C5 Substituted | Guaiacyl | p-Hydroxyphenyl | Total Phenolic OH | Carboxylic Acid OH | Aliphatic OH |
|---|---|---|---|---|---|---|
| USKL | 2.27 | 1.95 | 0.26 | 4.48 | 0.76 | 1.90 |
| CL-1 | 1.42 | 1.33 | 0.15 | 2.90 | 1.06 | 2.04 |
| CL-4 | 1.08 | 1.20 | 0.15 | 2.42 | 1.01 | 1.42 |
| CL-10 | 1.44 | 1.29 | 0.16 | 2.89 | 1.15 | 1.88 |
| Mw, kg/mol | Charge Density, mmol/g | Dosage, % | CWS Concentration, % | Contact Angle, ° | Zeta Potential, mV | Viscosity at 100 s−1, cP | Reference | |
|---|---|---|---|---|---|---|---|---|
| CL-1 | 1.6 | −1.95 | 0.1 | 50 | 34.6 | −45.4 | 520 | This study |
| Sodium lignosulfonate | 10 | 2.34 * | 1 | 65 | 74.4 | - | 632 | [59] |
| Sodium lignosulfonate | 9 | 1.12 * | 0.4 | 67 | - | −52 | 600 | [43] |
| Crosslinked lignosulfonate | 42.8–115 | 1.16–1.31 * | 0.6 | 60 | - | - | 536–639 | [60] |
| Lignosulfonate | 9 | - | 1 | 60 | - | −28 | 624–857 | [61] |
| Grafted sulfonated alkali lignin | 31.5 | 2.52 * | 1 | 61.5 | 72 | −65 | 650 | [11] |
| β-Cyclodextrin grafted alkali lignin | - | - | 0.5 | 55–59 | - | −50~−62 | 1000 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qulatein, H.A.; Gao, W.; Fatehi, P. Carboxyalkylated Lignin as a Sustainable Dispersant for Coal Water Slurry. Polymers 2024, 16, 2586. https://doi.org/10.3390/polym16182586
Qulatein HA, Gao W, Fatehi P. Carboxyalkylated Lignin as a Sustainable Dispersant for Coal Water Slurry. Polymers. 2024; 16(18):2586. https://doi.org/10.3390/polym16182586
Chicago/Turabian StyleQulatein, Hussein Ahmad, Weijue Gao, and Pedram Fatehi. 2024. "Carboxyalkylated Lignin as a Sustainable Dispersant for Coal Water Slurry" Polymers 16, no. 18: 2586. https://doi.org/10.3390/polym16182586
APA StyleQulatein, H. A., Gao, W., & Fatehi, P. (2024). Carboxyalkylated Lignin as a Sustainable Dispersant for Coal Water Slurry. Polymers, 16(18), 2586. https://doi.org/10.3390/polym16182586









