Partially Bio-Based and Biodegradable Poly(Propylene Terephthalate-Co-Adipate) Copolymers: Synthesis, Thermal Properties, and Enzymatic Degradation Behavior
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Poly(Propylene Terephthalate-Co-Adipate) Copolymers
2.3. Enzymatic Degradation Experiment
2.4. Characterization
2.4.1. Nuclear Magnetic Resonance (NMR)
2.4.2. Intrinsic Viscosity [η]
2.4.3. Gel Permeation Chromatography (GPC)
2.4.4. Wide Angle X-ray Diffraction Patterns (WAXD)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Thermogravimetric Analysis (TGA)
2.4.7. Water Contact Angles (WCA)
2.4.8. Scanning Electron Microscopy (SEM)
3. Results and Discussion
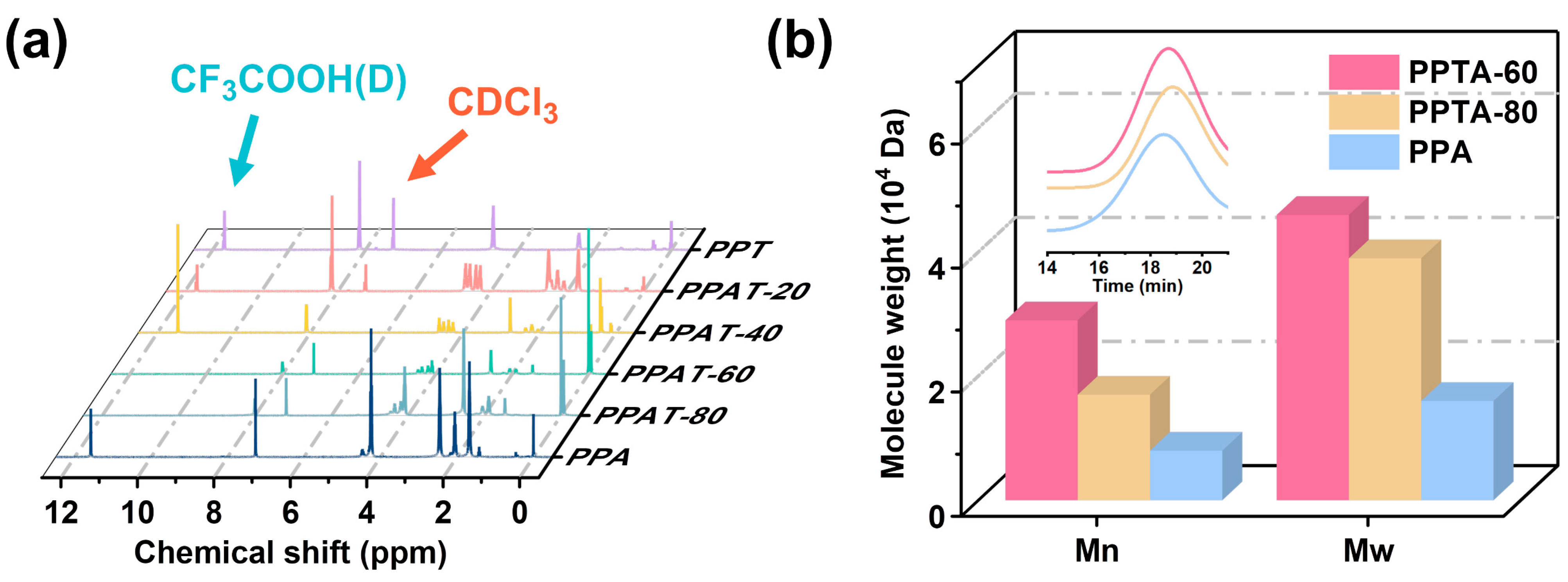
3.1. Synthesis and Structure Characterization of the PPTA Copolymers
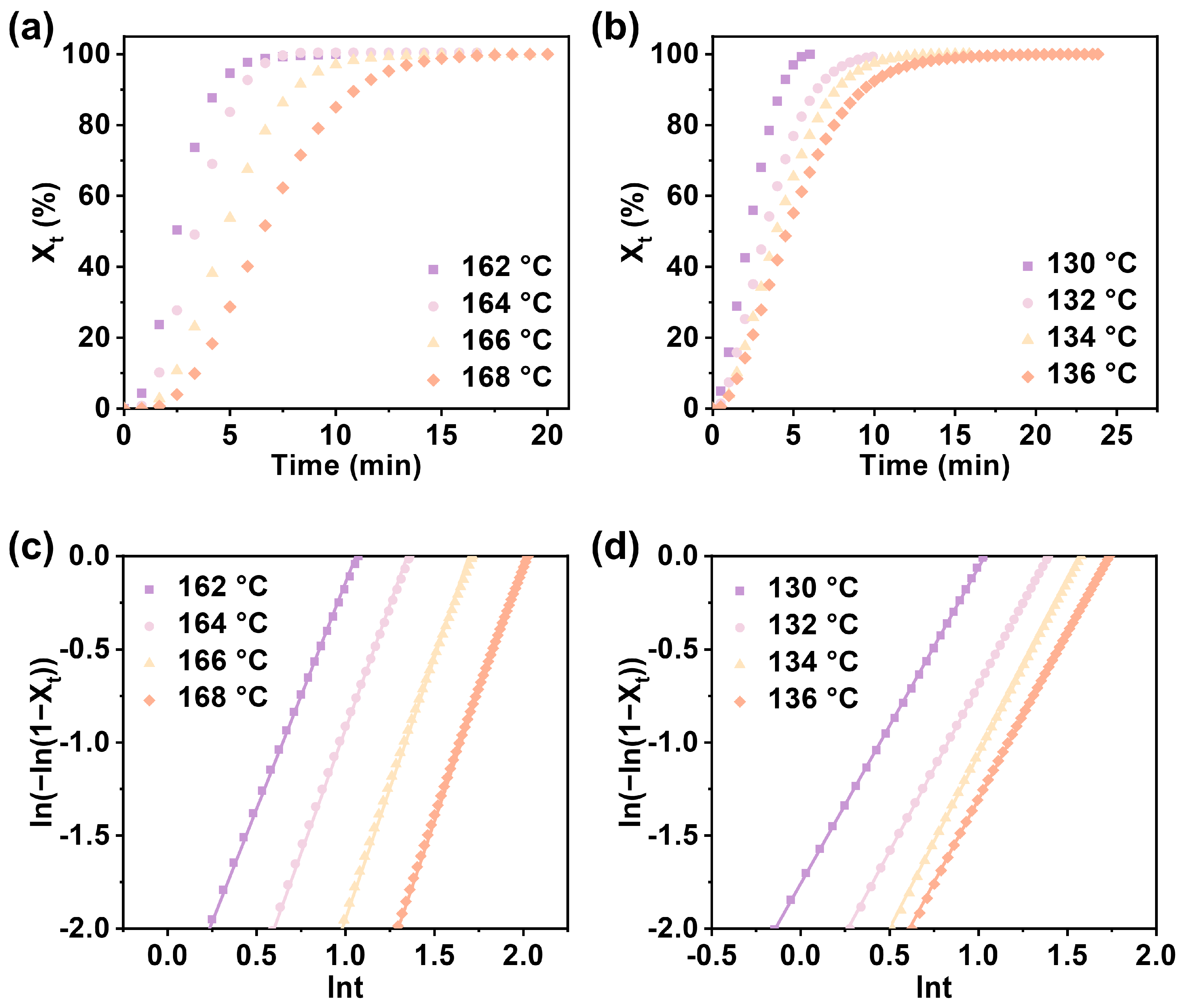
3.2. Non-Isothermal and Isothermal Crystallization
3.3. Crystal Structure of PPTA Copolymers
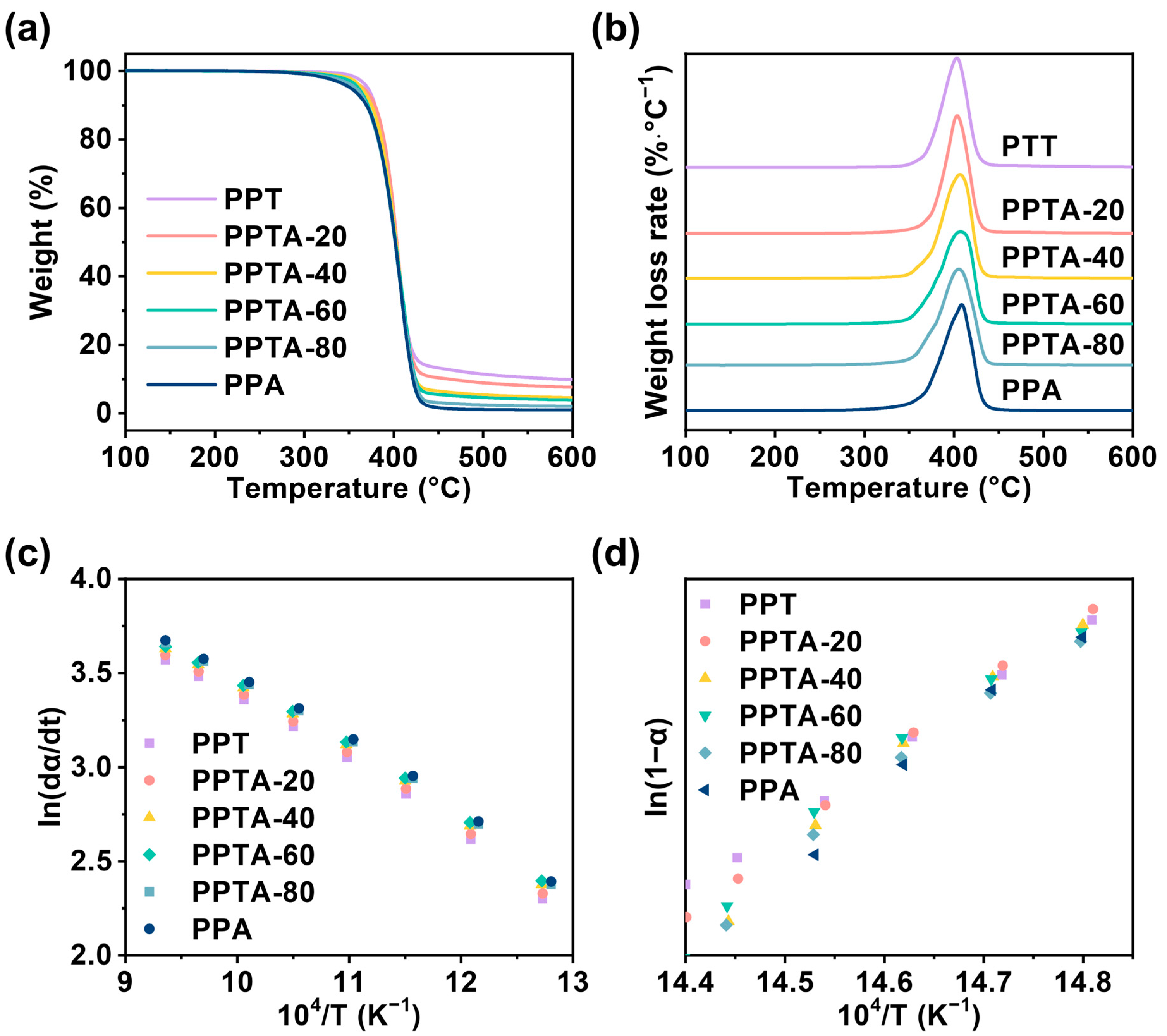
3.4. Thermal Stability and Thermal Degradation Kinetics of PPTA Copolymers
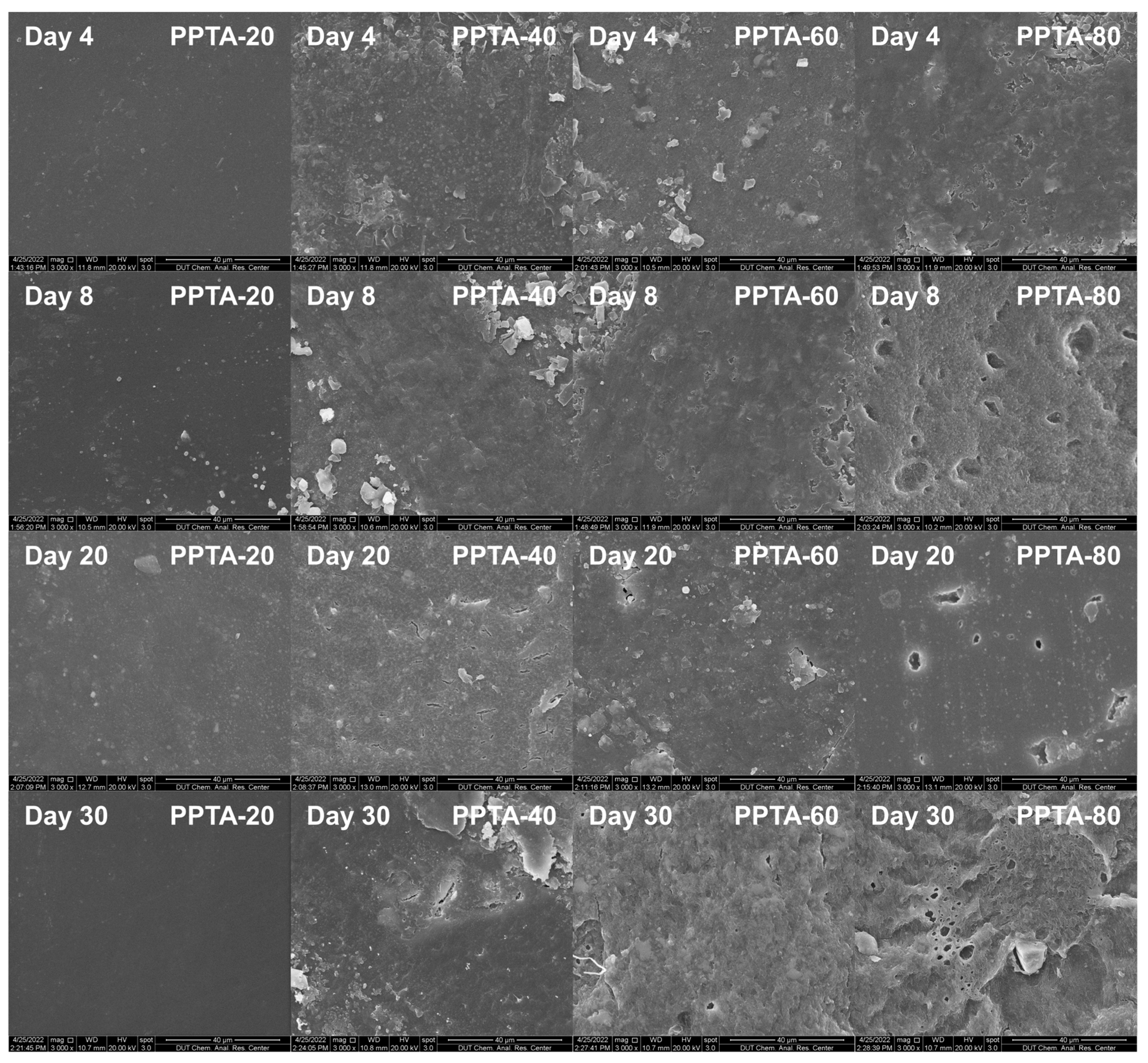
3.5. Enzymatic Degradation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Long, Z.; Wang, W.; Zhou, Y.; Yu, L.; Shen, L.; Dong, Y. Effect of Polybutylene Adipate Terephthalate on the Properties of Starch/Polybutylene Adipate Terephthalate Shape Memory Composites. Int. J. Biol. Macromol. 2023, 240, 124452. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Wang, S.; Li, J.; Liu, P.; Wu, L.; Wu, H.; Xu, J.; Severtson, S.J.; Wang, W.-J. Stiffening, Strengthening, and Toughening of Biodegradable Poly(butylene Adipate-Co-Terephthalate) with a Low Nanoinclusion Usage. Carbohydr. Polym. 2020, 247, 116687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, C.; Wang, Y.; Xie, L.; Li, W.; Li, B.; Guo, R.; Yan, H. Magnesium Oxide/Silver Nanoparticles Reinforced Poly(butylene Succinate-Co-Terephthalate) Biofilms for Food Packaging Applications. Food Packag. Shelf. 2021, 30, 100748. [Google Scholar] [CrossRef]
- Lin, S.; Wang, J.; Wang, H.; Wu, T. Synthesis, Mechanical Properties and Biodegradation of Various Acrylic Acid-Grafted Poly(Butylene Succinate-Co-Terephthalate)/Organically Modified Layered Zinc Phenylphosphonate Nanocomposites. Eur. Polym. J. 2019, 116, 1–8. [Google Scholar] [CrossRef]
- Chen, M.; Cai, C.; Bao, J.; Du, Y.; Gao, H.; Liu, X. Effect of Aliphatic Segment Length and Content on Crystallization and Biodegradation Properties of Aliphatic-Aromatic Co-Polyesters. Polym. Degrad. Stabil. 2022, 203, 110080. [Google Scholar] [CrossRef]
- Berti, C.; Celli, A.; Marchese, P.; Barbiroli, G.; Di Credico, F.; Verney, V.; Commereuc, S. Novel Copolyesters Based on Poly(Alkylene Dicarboxylate)S: 1. Thermal Behavior and Biodegradation of Aliphatic–Aromatic Random Copolymers. Eur. Polym. J. 2008, 44, 3650–3661. [Google Scholar] [CrossRef]
- Manker, L.P.; Dick, G.R.; Demongeot, A.; Hedou, M.A.; Rayroud, C.; Rambert, T.; Jones, M.J.; Sulaeva, I.; Vieli, M.; Leterrier, Y.; et al. Sustainable Polyesters via Direct Functionalization of Lignocellulosic Sugars. Nat. Chem. 2022, 14, 976–984. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, J.; Yang, X.; Ke, Y.; Ou, R.; Wang, Y.; Madbouly, S.A.; Wang, Q. From Plant Phenols to Novel Bio-Based Polymers. Prog. Polym. Sci. 2022, 125, 101473. [Google Scholar] [CrossRef]
- Bonjour, O.; Liblikas, I.; Pehk, T.; Khai-Nghi, T.; Rissanen, K.; Vares, L.; Jannasch, P. Rigid Biobased Polycarbonates with Good Processability Based on a Spirocyclic Diol Derived from Citric Acid. Green Chem. 2020, 22, 3940–3951. [Google Scholar] [CrossRef]
- Mankar, S.V.; Garcia Gonzalez, M.N.; Warlin, N.; Valsange, N.G.; Rehnberg, N.; Lundmark, S.; Jannasch, P.; Zhang, B. Synthesis, Life Cycle Assessment, and Polymerization of a Vanillin-Based Spirocyclic Diol toward Polyesters with Increased Glass-Transition Temperature. ACS Sustain. Chem. Eng. 2019, 7, 19090–19103. [Google Scholar] [CrossRef]
- Cheng, Y.; Jiao, Z.; Li, M.; Xia, M.; Zhou, Z.; Song, P.; Xu, Q.; Wei, Z. A New Class of Nucleating Agents for Poly(L-lactic Acid): Environmentally-Friendly Metal Salts with Biomass-Derived Ligands and Advanced Nucleation Ability. Int. J. Biol. Macromol. 2023, 225, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Ou, C. Study on Poly(Oxybenzoate-P-Trimethylene Terephthalate) Copolymer. Eur. Polym. J. 2002, 38, 2405–2411. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Liang, X.; Wei, M.; Liang, F.; Feng, D.; Xu, C.; Xian, M.; Zou, H. Production and Waste Treatment of Polyesters: Application of Bioresources and Biotechniques. Crit. Rev. Biotechnol. 2023, 43, 503–520. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, Z.; Li, X.; Chen, P.; Hou, Z. Selective Synthesis of 1,3-Propanediol from Glycidol Over a Carbon Film Encapsulated Co-Catalyst. Catal. Sci. Technol. 2019, 9, 5022–5030. [Google Scholar] [CrossRef]
- Özkan, M.; Duru Baykal, P.; Özkan, İ. Investigation on the Performance Properties of Polytrimethylene Terephthalate (PTT) Based Staple Fibers and Cotton Blended OE-Rotor Yarns. J. Text. Inst. 2021, 113, 449–459. [Google Scholar] [CrossRef]
- Palm, G.J.; Reisky, L.; Bottcher, D.; Muller, H.; Michels, E.A.; Walczak, M.C.; Berndt, L.; Weiss, M.S.; Bornscheuer, U.T.; Weber, G. Structure of the Plastic-Degrading Ideonella Sakaiensis Mhetase Bound to a Substrate. Nat. Commun. 2019, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B.; Wei, B.; Wei, Y.; Yao, J.; Zhang, H.; Chen, X.; Shao, Z. Enhanced Compatibility between Poly(lactic Acid) and Poly (butylene Adipate-Co-Terephthalate) by Incorporation of N-Halamine Epoxy Precursor. Int. J. Biol. Macromol. 2020, 165, 460–471. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, C.; Wang, L.; Ren, J. Preparation and Properties of Biodegradable Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blend with Epoxy-Functional Styrene Acrylic Copolymer as Reactive Agent. J. Polym. Environ. 2012, 21, 286–292. [Google Scholar] [CrossRef]
- Rajan, A.; Shankar, V. Purification and Characterization of Esterase Enzyme from Aspergillus Versicolor. Iran. J. Chem. Chem. Eng. 2023, 42, 1691–1700. [Google Scholar]
- Soccio, M.; Finelli, L.; Lotti, N.; Gazzano, M.; Munari, A. Poly(propylene Isophthalate), Poly(propylene Succinate), and Their Random Copolymers: Synthesis and Thermal Properties. J. Polym. Sci. Pol. Phys. 2007, 45, 310–321. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- De Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: New York, NY, USA, 1979. [Google Scholar]
- Bulkin, B.J.; Lewin, M.; Kim, J. Crystallization Kinetics of Poly(propylene Terephthalate) Studied by Rapid-Scanning Raman Spectroscopy and FT-IR Spectroscopy. Macromolecules 1987, 20, 830–835. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Miller, R.L. Kinetics of Crystallization from the Melt and Chain Folding in Polyethylene Fractions Revisited: Theory and Experiment. Polymer 1997, 38, 3151–3212. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, B. Crystallization Kinetics in Mixtures of Poly(epsilon-Caprolactone) and Poly(styrene-Co-Acrylonitrile). Macromolecules 1997, 30, 6223–6229. [Google Scholar] [CrossRef]
- Hong, P.; Chung, W.; Hsu, C. Crystallization Kinetics and Morphology of Poly(trimethylene Terephthalate). Polymer 2002, 43, 3335–3343. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Li, S. Hydrolytic Degradation Characteristics of Aliphatic Polyesters Derived from Lactic and Glycolic Acids. J. Biomed. Mater. Res. 1999, 48, 342–353. [Google Scholar] [CrossRef]
- Jimenez, A.; Berenguer, V.; Lopez, J.; Sanchez, A. Thermal Degradation Study of Poly(vinyl Chloride): Kinetic Analysis of Thermogravimetric Data. J. Appl. Polym. Sci. 1993, 50, 1565–1573. [Google Scholar] [CrossRef]
- Good, R.J. A Thermodynamic Derivation of Wenzel’s Modification of Young’s Equation for Contact Angles; Together with a Theory of Hysteresis. J. Am. Chem. Soc. 1952, 74, 5041–5042. [Google Scholar] [CrossRef]
- Neumann, A.W.; Good, R.J. Thermodynamics of contact angles. I. Heterogeneous solid surfaces. J. Colloid Interf. Sci. 1972, 38, 341–358. [Google Scholar] [CrossRef]
- Li, X.; Huang, M.; Guan, G.; Sun, T. Thermal Decomposition Kinetics of Liquid Crystalline p-Oxybenzoate/Ethylene Terephthalate/Third Monomer Terpolymers. Polym. Degrad. Stabil. 1999, 65, 463–472. [Google Scholar] [CrossRef]
- Zen, A.; Saphiannikova, M.; Neher, D.; Grenzer, J.; Grigorian, S.; Pietsch, U.; Asawapirom, U.; Janietz, S.; Scherf, U.; Lieberwirth, I.; et al. Effect of Molecular Weight on the Structure and Crystallinity of Poly(3-hexylthiophene). Macromolecules 2006, 39, 2162–2171. [Google Scholar] [CrossRef]
- Fernández, J.; Etxeberria, A.; Sarasua, J.R. In Vitro Degradation Studies and Mechanical Behavior of Poly(Ε-caprolactone-co-δ-Valerolactone) and Poly(Ε-caprolactone-co-L-Lactide) with Random and Semi-Alternating Chain Microstructures. Eur. Polym. J. 2015, 71, 585–595. [Google Scholar] [CrossRef]
- Frantziskonis, G.; Desai, C.S. Surface Degradation Mechanisms in Brittle Material Structural Systems. Int. J. Fract. 1991, 48, 231–244. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]



| Samples | n(PTA):n(AA) | Esterification Temperature (°C) | Esterification Time (h) | Polycondensation Temperature (°C) | Polycondensation Time (h) |
|---|---|---|---|---|---|
| PPT | 100:0 | 230 | 4 | 250 | 5 |
| PPTA-20 | 80:20 | 230 | 4 | 250 | 5 |
| PPTA-40 | 60:40 | 230 | 4 | 250 | 5 |
| PPTA-60 | 40:60 | 230 | 4 | 240 | 5 |
| PPTA-80 | 20:80 | 230 | 4 | 230 | 5 |
| PPA | 0:100 | 230 | 4 | 230 | 5 |
| Samples | n(PTA):n(AA) | a Composition (%) | [η] (dL/g) | b Mn (104) | Mw/Mn | LPT | LPA | R | |
|---|---|---|---|---|---|---|---|---|---|
| PT | PA | ||||||||
| PPT | 100:0 | 100 | 0 | 1.06 | — | — | — | — | — |
| PPTA-20 | 80:20 | 77.24 | 23.76 | 0.58 | — | — | 4.57 | 1.26 | 1.01 |
| PPTA-40 | 60:40 | 54.64 | 45.36 | 0.60 | — | — | 2.17 | 1.81 | 1.01 |
| PPTA-60 | 40:60 | 42.50 | 57.50 | 0.81 | 2.9 | 1.6 | 1.63 | 2.65 | 0.99 |
| PPTA-80 | 20:80 | 20.20 | 79.80 | 0.71 | 1.7 | 2.3 | 1.25 | 5.54 | 0.98 |
| PPA | 0:100 | 0 | 100 | 0.30 | 0.8 | 2.0 | — | — | — |
| Samples | Time (Days) | Mass Loss (%) | a PA Content in Copolymer (%) | b ΔHm (J/g) | Tg (°C) | Tm (°C) |
|---|---|---|---|---|---|---|
| PPTA-20 | 0 | 0 | 23.76 | 32.72 | 61.9 | 203.8 |
| 16 | 0 | 24.68 | 31.65 | 62.1 | 202.1 | |
| 20 | 0 | 24.77 | 32.51 | 63.5 | 203.6 | |
| 30 | 0 | 25.18 | 32.08 | 64.8 | 202.8 | |
| PPTA-40 | 0 | 0 | 45.36 | 26.25 | −4.6 | 152.4 |
| 16 | 0.50 ± 0.10 | 44.86 | 27.86 | −3.2 | 151.5 | |
| 20 | 0.78 ± 0.15 | 45.12 | 25.16 | −3.8 | 152.4 | |
| 30 | 1.23 ± 0.11 | 44.64 | 28.15 | −4.1 | 152.8 | |
| PPTA-60 | 0 | 0 | 57.50 | 0.14 | −11.0 | 107.6 |
| 16 | 4.64 ± 0.21 | 58.12 | 0.58 | −11.8 | 106.8 | |
| 20 | 5.32 ± 0.19 | 60.23 | 1.17 | −12.0 | 105.3 | |
| 30 | 6.70 ± 0.15 | 61.66 | 0.52 | −12.0 | 106.9 | |
| PPTA-80 | 0 | 0 | 79.80 | — | −22.4 | — |
| 16 | 26.62 ± 0.19 | 81.12 | — | −23.3 | — | |
| 20 | 36.53 ± 2.15 | 80.62 | — | −22.9 | — | |
| 30 | 59.10 ± 1.10 | 82.73 | — | −22.3 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, P.; Li, M.; Wang, H.; Cheng, Y.; Wei, Z. Partially Bio-Based and Biodegradable Poly(Propylene Terephthalate-Co-Adipate) Copolymers: Synthesis, Thermal Properties, and Enzymatic Degradation Behavior. Polymers 2024, 16, 2588. https://doi.org/10.3390/polym16182588
Song P, Li M, Wang H, Cheng Y, Wei Z. Partially Bio-Based and Biodegradable Poly(Propylene Terephthalate-Co-Adipate) Copolymers: Synthesis, Thermal Properties, and Enzymatic Degradation Behavior. Polymers. 2024; 16(18):2588. https://doi.org/10.3390/polym16182588
Chicago/Turabian StyleSong, Ping, Mingjun Li, Haonan Wang, Yi Cheng, and Zhiyong Wei. 2024. "Partially Bio-Based and Biodegradable Poly(Propylene Terephthalate-Co-Adipate) Copolymers: Synthesis, Thermal Properties, and Enzymatic Degradation Behavior" Polymers 16, no. 18: 2588. https://doi.org/10.3390/polym16182588
APA StyleSong, P., Li, M., Wang, H., Cheng, Y., & Wei, Z. (2024). Partially Bio-Based and Biodegradable Poly(Propylene Terephthalate-Co-Adipate) Copolymers: Synthesis, Thermal Properties, and Enzymatic Degradation Behavior. Polymers, 16(18), 2588. https://doi.org/10.3390/polym16182588






