Entrapment of Cyanase from Thermomyces lanuginosus Using Biomimetic Silica and Its Application for Cyanate Bioremediation
Abstract
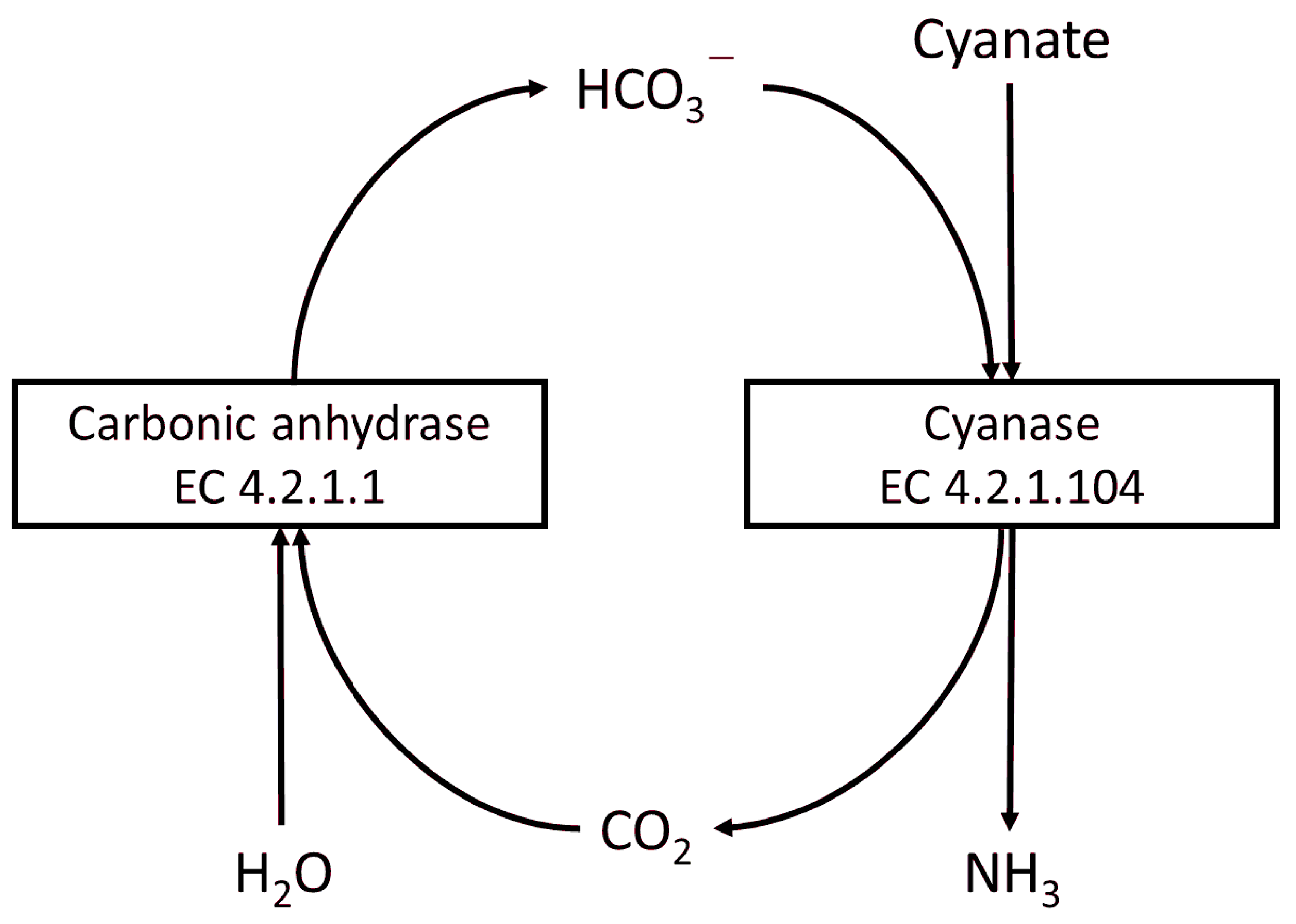
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Vector Construction
2.2. Expression and Purification of TlCyn
2.3. Activity Assays
2.4. Entrapment of TlCyn
2.5. Cyanate Degradation by TlCyn-SP
3. Results and Discussion
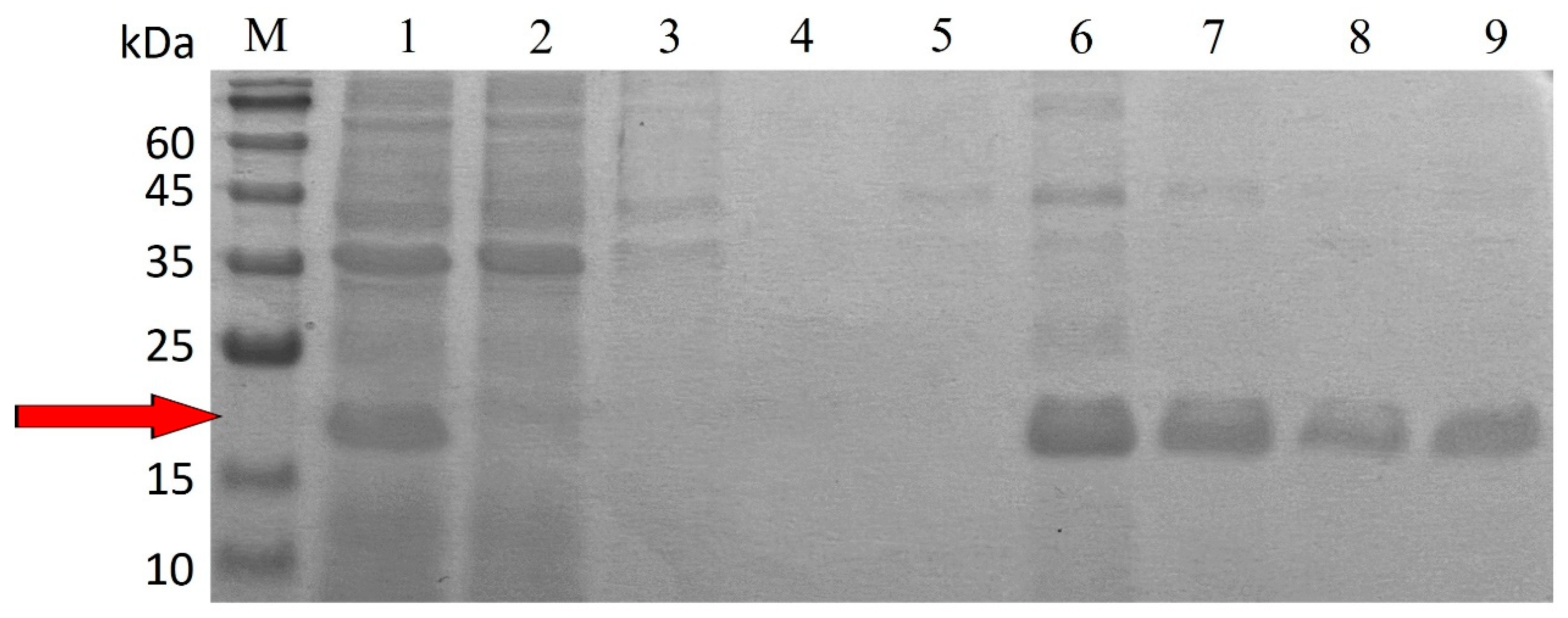
3.1. Expression and Purification of TlCyn
3.2. Entrapment of TlCyn
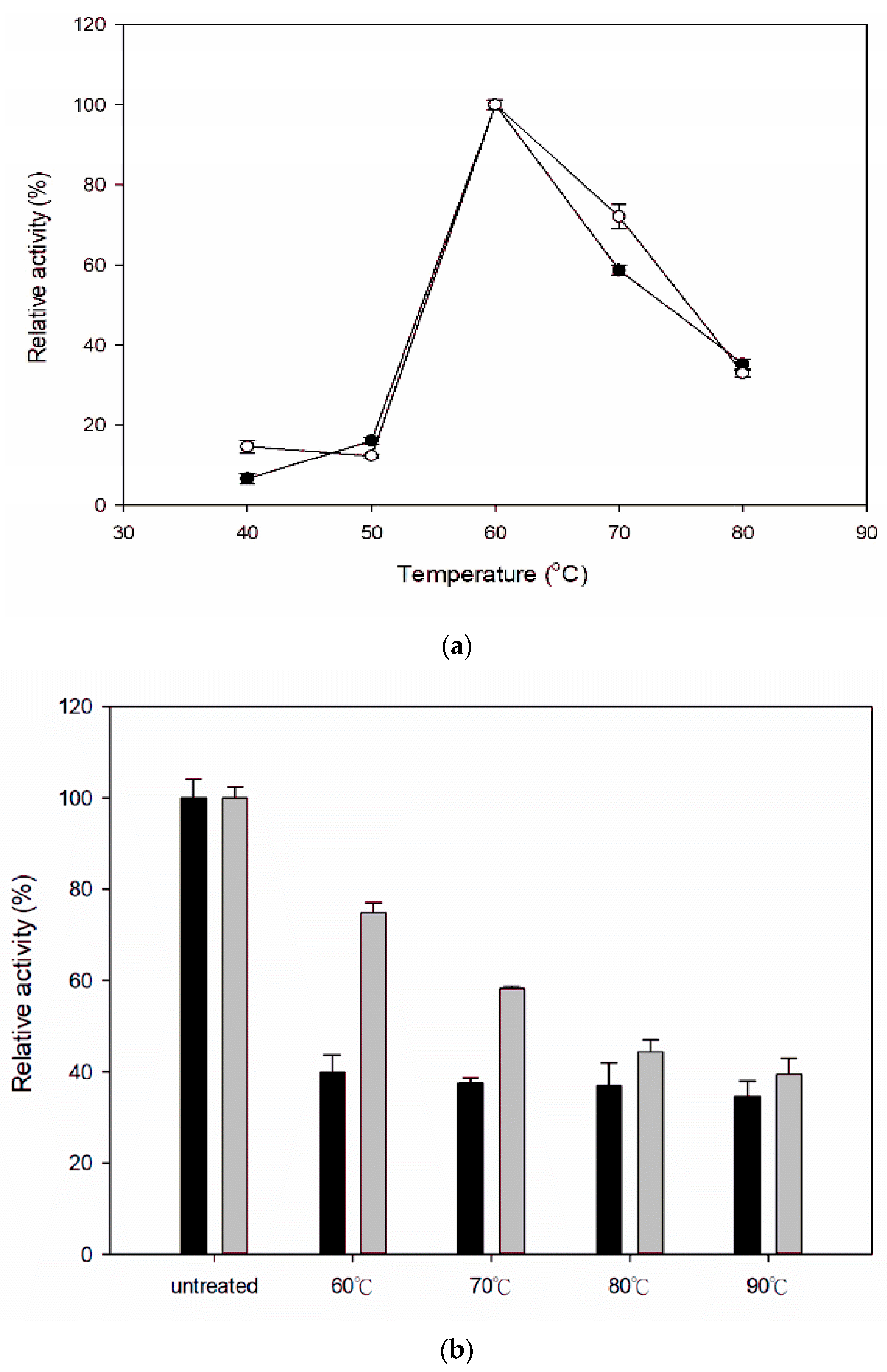
3.3. Effect of Temperature on Activity and Stability
3.4. Effect of pH on Activity and Stability
3.5. Cation Tolerance
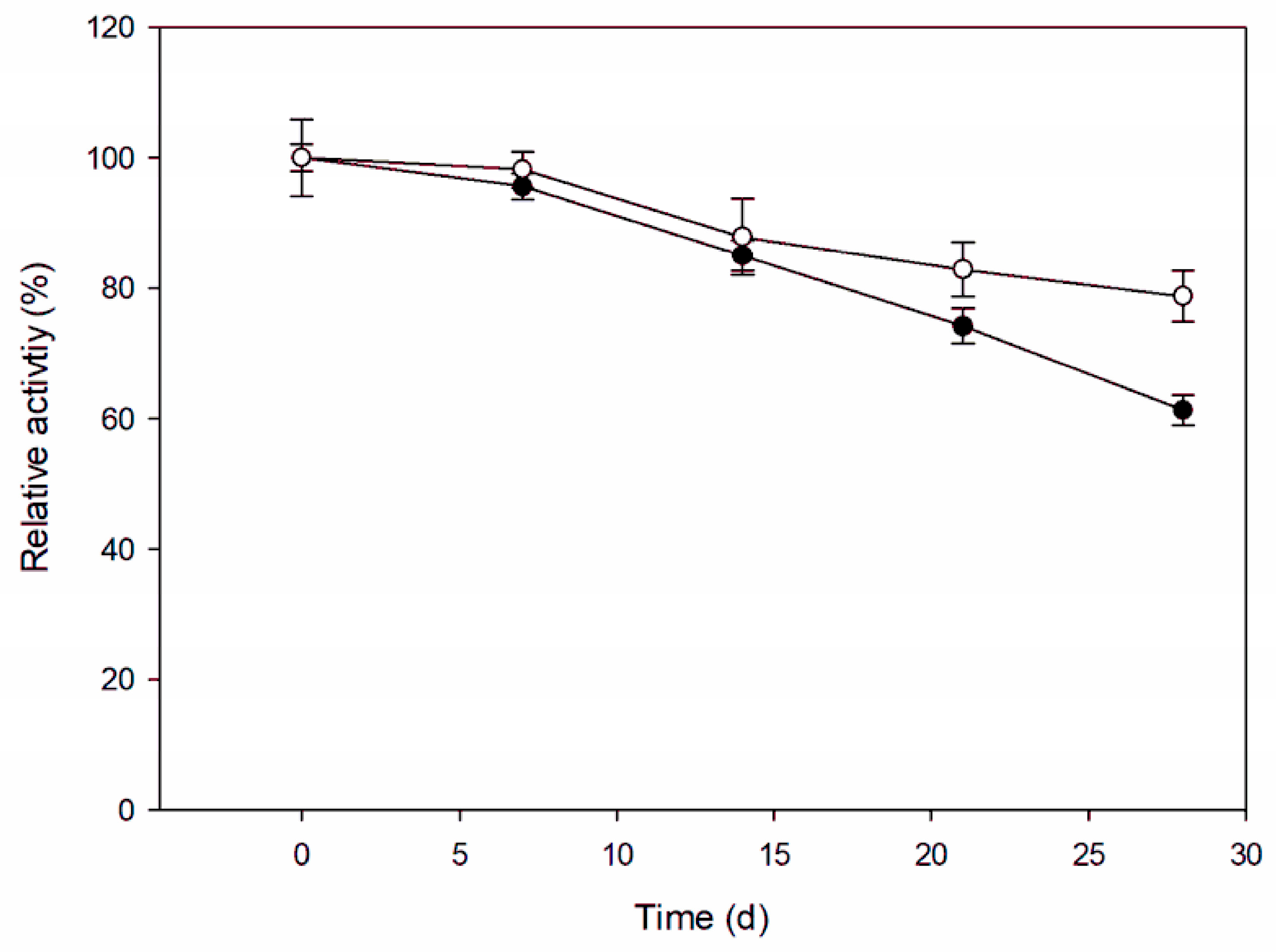
3.6. Storage Stability
3.7. The Enzyme Kinetic Constants
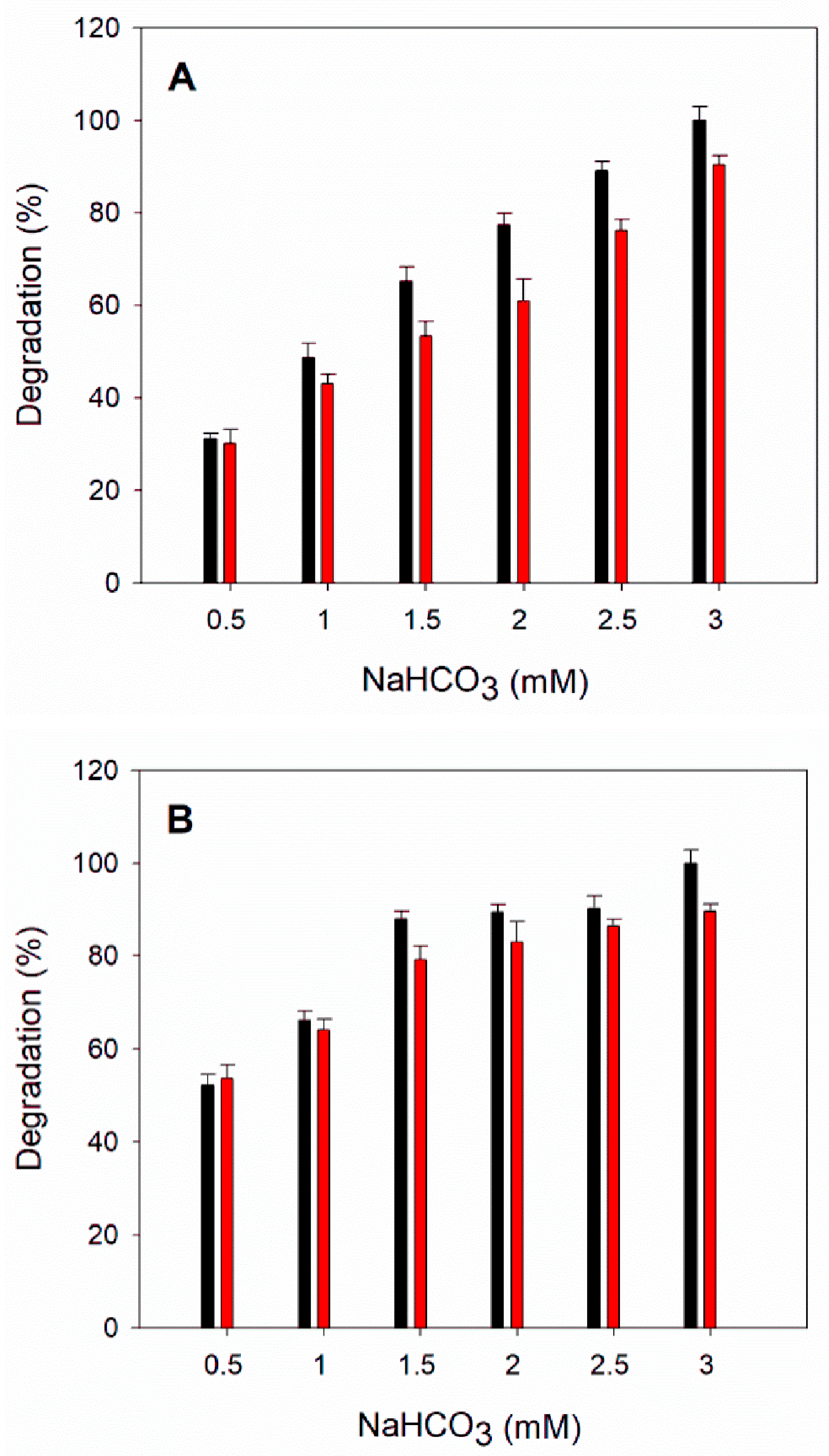
3.8. Cyanate Degradation Using TlCyn-SP and TlCyn-SazCA-SP
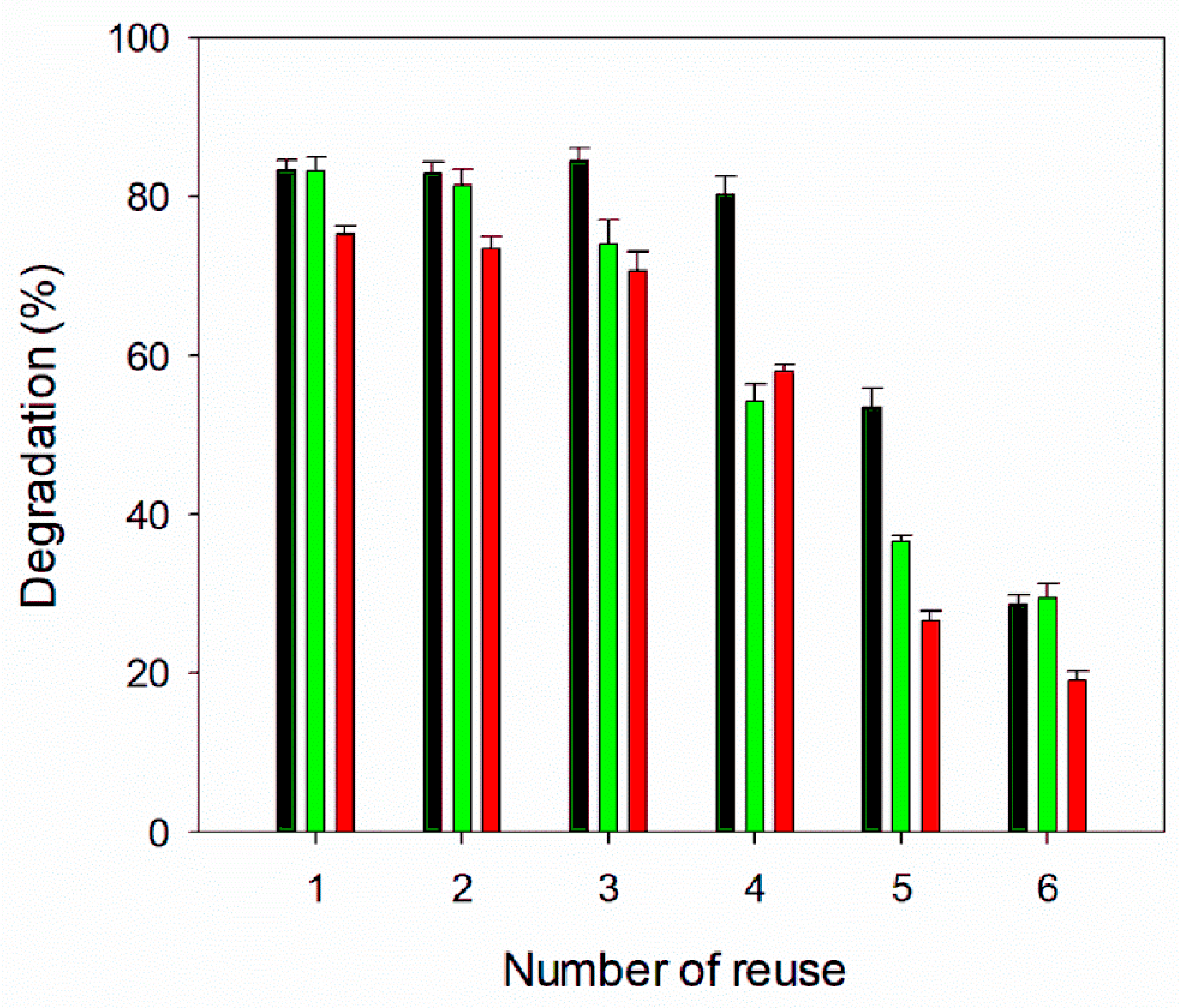
3.9. Reusability of TlCyn-SP and TlCyn-SazCA-SP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Palatinszky, M.; Herbold, C.; Jehmlich, N.; Pogoda, M.; Han, P.; von Bergen, M.; Lagkouvardos, I.; Karst, S.M.; Galushko, A.; Koch, H.; et al. Cyanate as an Energy Source for Nitrifiers. Nature 2015, 524, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-J.; Hu, C.-J.; Yu, C.-Y. Biomimetic Carbon Sequestration and Cyanate Detoxification Using Heat-Purified Carbonic Anhydrase from Sulfurihydrogenibium Yellowstonense. Biomimetics 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lee, Y.; Han, S.O.; Hyeon, J.E. Detoxifying Cyanides Using Cyanase Enzyme Complexes Composed of Carbonic Anhydrase via Irreversible Covalent Bonds. J. Agric. Food Chem. 2024, 72, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Trevor Sewell, B.; Benedik, M.J. Cyanide Bioremediation: The Potential of Engineered Nitrilases. Appl. Microbiol. Biotechnol. 2017, 101, 3029–3042. [Google Scholar] [CrossRef]
- Sharma, M.; Akhter, Y.; Chatterjee, S. A review on Remediation of Cyanide Containing Industrial Wastes Using Biological Systems with Special Reference to Enzymatic Degradation. World J. Microbiol. Biotechnol. 2019, 35, 70. [Google Scholar] [CrossRef]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. Expression of a Novel Recombinant Cyanate Hydratase (rTl-Cyn) in Pichia Pastoris, Characteristics and Applicability in the Detoxification of Cyanate. Bioresour. Technol. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Huertas, M.-J.; Saez, L.P.; Luque-Romero, M.M.; Moreno-Vivian, C.; Castillo, F.; Roldan, M.D.; Blasco, R. Characterization of the Pseudomonas pseudoalcaligenes CECT5344 cyanase, an enzyme that is not essential for cyanide assimilation. Appl. Environ. Microbiol. 2008, 74, 6280–6288. [Google Scholar] [CrossRef]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. A Novel Strategy for the Efficient Removal of Toxic Cyanate by the Combinatorial Use of Recombinant Enzymes Immobilized on Aminosilane Modified Magnetic Nanoparticles. Bioresour. Technol. 2018, 253, 105–111. [Google Scholar] [CrossRef]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. Simultaneous Removal of Heavy Metals and Cyanate in a Wastewater Sample Using Immobilized Cyanate Hydratase on Magnetic-Multiwall Carbon Nanotubes. J. Hazard. Mater. 2019, 363, 73–80. [Google Scholar] [CrossRef]
- Feng, L.; Han, Y.; Niu, J.; Guo, J.; Zhang, J.; Li, H.; Hou, Y.; Song, Y. Synergistic removal performance and mechanism in denitrification system under phenol stress. J. Environ. Chem. Eng. 2023, 11, 110767. [Google Scholar] [CrossRef]
- Caresani, J.R.F.; Dallegrave, A.; Santos, J.H.Z.D. Amylases encapsulated in organosilane-modified silicas prepared by sol–gel: Evaluation of starch saccharification. J. Sol-Gel Sci. Technol. 2021, 97, 340–350. [Google Scholar] [CrossRef]
- Tikhonov, S.L.; Tikhonova, N.V.; Kudryashov, L.S.; Kudryashova, O.A.; Moskovenko, N.V.; Tretyakova, I.N. Efficiency of microencapsulation of proteolytic enzymes. Catalysts 2021, 11, 1270. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Zhou, Y.; Zhu, L.; Zhang, C.; Yan, X.; You, S.; Qi, W.; Su, R. A reusable and leakage-proof immobilized laccase@ UiO-66-NH2 (30) for the efficient biodegradation of rifampicin and lincomycin. Biochem. Eng. J. 2023, 194, 108897. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Montazeri, E. Nano co-immobilization of α-amylase and maltogenic amylase by nanomagnetic combi-cross-linked enzyme aggregates method for maltose production from corn starch. Carbohydr. Res. 2020, 488, 107904. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Spain, J.C.; Naik, R.R.; Stone, M.O. Enzyme Immobilization in a Biomimetic Silica Support. Nat. Biotechnol. 2004, 22, 211–213. [Google Scholar] [CrossRef]
- Bialas, F.; Reichinger, D.; Becker, C.F.W. Biomimetic and Biopolymer-Based Enzyme Encapsulation. Enzym. Microb. Technol. 2021, 150, 109864. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and Bioinspired Silicifications: Recent Advances for Biomaterial Design and Applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef]
- Kröger, N.; Lorenz, S.; Brunner, E.; Sumper, M. Self-Assembly of Highly Phosphorylated Silaffins and Their Function in Biosilica Morphogenesis. Science 2002, 298, 584–586. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Clarson, S.J.; Perry, C.C. On the Role(s) of Additives in Bioinspired Silicification. Chem. Commun. 2005, 7, 1113–1121. [Google Scholar] [CrossRef]
- Hsieh, C.-J.; Yu, C.-Y. Cyanate Degradation in Different Matrices Using Heat-Purified Enzymes. Catalysts 2023, 13, 76. [Google Scholar] [CrossRef]
- Fu, W.-D.; Hsieh, C.-J.; Hu, C.-J.; Yu, C.-Y. Entrapment of Carbonic Anhydrase from Sulfurihydrogenibium Azorense with Polyallylamine-Mediated Biomimetic Silica. Bioresour. Technol. Rep. 2022, 20, 101217. [Google Scholar] [CrossRef]
- Hsieh, C.-J.; Cheng, J.-C.; Hu, C.-J.; Yu, C.-Y. Entrapment of the Fastest Known Carbonic Anhydrase with Biomimetic Silica and its Application for CO2 Sequestration. Polymers 2021, 13, 2452. [Google Scholar] [CrossRef]
- Ranjan, B.; Choi, P.H.; Pillai, S.; Permaul, K.; Tong, L.; Singh, S. Crystal Structure of a Thermophilic Fungal Cyanase and its Implications on the Catalytic Mechanism for Bioremediation. Sci. Rep. 2021, 11, 277. [Google Scholar] [CrossRef]
- Huertas, M.J.; Sáez, L.P.; Roldán, M.D.; Luque-Almagro, V.M.; Martínez-Luque, M.; Blasco, R.; Castillo, F.; Moreno-Vivián, C.; García-García, I. Alkaline Cyanide Degradation by Pseudomonas pseudoalcaligenes CECT5344 in a Batch Reactor. Influence of pH. J. Hazard. Mater. 2010, 179, 72–78. [Google Scholar] [CrossRef]
- Di Fiore, A.; Alterio, V.; Monti, S.M.; De Simone, G.; D’Ambrosio, K. Thermostable Carbonic Anhydrases in Biotechnological Applications. Int. J. Mol. Sci. 2015, 16, 15456–15480. [Google Scholar] [CrossRef]
- Luca, V.D.; Vullo, D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. An α-Carbonic Anhydrase from the Thermophilic Bacterium Sulphurihydrogenibium azorense is the Fastest Enzyme Known for the CO2 Hydration Reaction. Bioorg. Med. Chem. 2013, 21, 1465–1469. [Google Scholar] [CrossRef]


| Samples | KM (mM) | kcat (s−1) | kcat/KM (s−1·mM−1) |
|---|---|---|---|
| TlCyn | 0.44 | 18,417 | 41,857 |
| TlCyn-SP | 0.21 | 45,881 | 218,481 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
How, S.-C.; Hsieh, C.-J.; Yu, C.-Y. Entrapment of Cyanase from Thermomyces lanuginosus Using Biomimetic Silica and Its Application for Cyanate Bioremediation. Polymers 2024, 16, 2594. https://doi.org/10.3390/polym16182594
How S-C, Hsieh C-J, Yu C-Y. Entrapment of Cyanase from Thermomyces lanuginosus Using Biomimetic Silica and Its Application for Cyanate Bioremediation. Polymers. 2024; 16(18):2594. https://doi.org/10.3390/polym16182594
Chicago/Turabian StyleHow, Su-Chun, Chia-Jung Hsieh, and Chi-Yang Yu. 2024. "Entrapment of Cyanase from Thermomyces lanuginosus Using Biomimetic Silica and Its Application for Cyanate Bioremediation" Polymers 16, no. 18: 2594. https://doi.org/10.3390/polym16182594
APA StyleHow, S.-C., Hsieh, C.-J., & Yu, C.-Y. (2024). Entrapment of Cyanase from Thermomyces lanuginosus Using Biomimetic Silica and Its Application for Cyanate Bioremediation. Polymers, 16(18), 2594. https://doi.org/10.3390/polym16182594






