Exploiting the Properties of Non-Wood Feedstocks to Produce Tailorable Lignin-Containing Cellulose Nanofibers
Abstract
:1. Introduction
2. Experimentation
2.1. Materials
2.2. Methods
Characterization
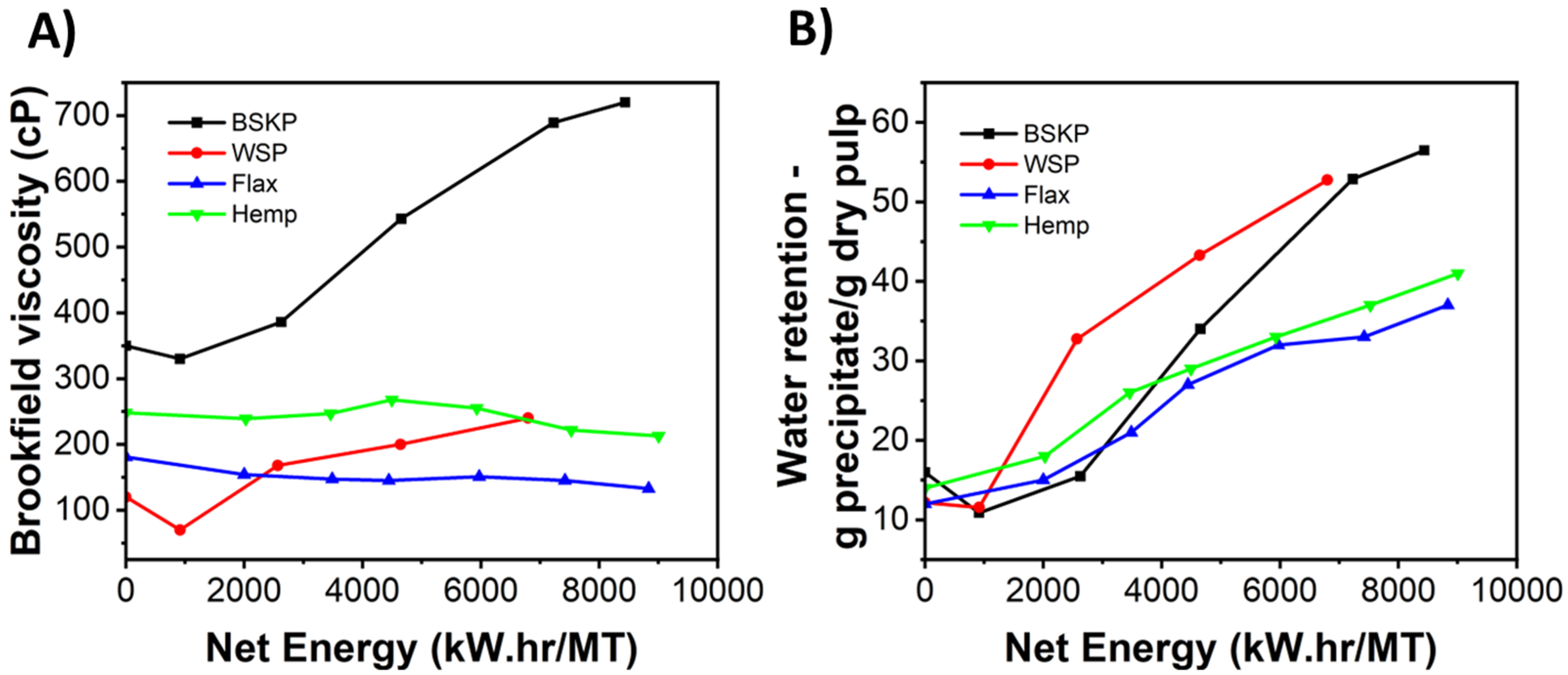
3. Results
3.1. Feedstock Analysis
3.2. CNF/LCNF Production and Analysis
3.3. CNF/LCNF Materials in Paper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Groschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, e1703779. [Google Scholar] [CrossRef] [PubMed]
- Lamm, M.E.; Li, K.; Qian, J.; Wang, L.; Lavoine, N.; Newman, R.; Gardner, D.J.; Li, T.; Hu, L.; Ragauskas, A.J.; et al. Recent Advances in Functional Materials through Cellulose Nanofiber Templating. Adv. Mater. 2021, 33, e2005538. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Clarkson, C.M.; Wang, L.; Liu, Y.; Lamm, M.; Pang, Z.; Zhou, Y.; Qian, J.; Tajvidi, M.; Gardner, D.J.; et al. Alignment of Cellulose Nanofibers: Harnessing Nanoscale Properties to Macroscale Benefits. ACS Nano 2021, 15, 3646–3673. [Google Scholar] [CrossRef]
- Lamm, M.E.; Wang, L.; Kishore, V.; Tekinalp, H.; Kunc, V.; Wang, J.; Gardner, D.J.; Ozcan, S. Material Extrusion Additive Manufacturing of Wood and Lignocellulosic Filled Composites. Polymers 2020, 12, 2115. [Google Scholar] [CrossRef]
- Kelly, P.V.; Shams Es-haghi, S.; Lamm, M.E.; Copenhaver, K.; Ozcan, S.; Gardner, D.J.; Gramlich, W.M. Polymer-Grafted Cellulose Nanofibrils with Enhanced Interfacial Compatibility for Stronger Poly(lactic acid) Composites. ACS Appl. Polym. Mater. 2023, 5, 3661–3676. [Google Scholar] [CrossRef]
- Marcuello, C.; Chabbert, B.; Berzin, F.; Bercu, N.B.; Molinari, M.; Aguié-Béghin, V. Influence of Surface Chemistry of Fiber and Lignocellulosic Materials on Adhesion Properties with Polybutylene Succinate at Nanoscale. Materials 2023, 16, 2440. [Google Scholar] [CrossRef]
- Mu, W.; Chen, X.; Li, S.; Sun, Y.; Wang, Q.; Na, J. Mechanical Performances Analysis and Prediction of Short Plant Fiber-Reinforced PLA Composites. Polymers 2023, 15, 3222. [Google Scholar] [CrossRef]
- Wang, L.; Li, K.; Copenhaver, K.; Mackay, S.; Lamm, M.E.; Zhao, X.; Dixon, B.; Wang, J.; Han, Y.; Neivandt, D.; et al. Review on Nonconventional Fibrillation Methods of Producing Cellulose Nanofibrils and Their Applications. Biomacromolecules 2021, 22, 4037–4059. [Google Scholar] [CrossRef]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2019, 27, 575–593. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production—Current knowledge and future prospects. Ind. Crops Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Lu, H.L.; Zhang, L.L.; Liu, C.C.; He, Z.B.; Zhou, X.F.; Ni, Y.H. A novel method to prepare lignocellulose nanofibrils directly from bamboo chips. Cellulose 2018, 25, 7043–7051. [Google Scholar] [CrossRef]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of Nanocellulose from Pineapple Leaf Fibers via High-Shear Homogenization and Ultrasonication. Fibers 2018, 6, 28. [Google Scholar] [CrossRef]
- Muna, N.; Fauzi, A.A.N.; Setyaningsih, D.; Yuliani, S. Isolation of Microfibrilated Cellulose from Oil Palm Empty Fruit Bunches (EFB) Through Peracetic Acid Delignification and Enzyme Hydrolysis. IOP Conf. Ser. Earth Environ. Sci. 2019, 309, 012063. [Google Scholar] [CrossRef]
- Liu, X.Y.; Jiang, Y.; Wang, L.J.; Song, X.P.; Qin, C.R.; Wang, S.F. Tuning of size and properties of cellulose nanofibers isolated from sugarcane bagasse by endoglucanase-assisted mechanical grinding. Ind. Crops Prod. 2020, 146, 112201. [Google Scholar] [CrossRef]
- Naeem, M.A.; Siddiqui, Q.; Khan, M.R.; Mushtaq, M.; Wasim, M.; Farooq, A.; Naveed, T.; Wei, Q.F. Bacterial cellulose-natural fiber composites produced by fibers extracted from banana peel waste. J. Ind. Text. 2020, 51, 990S–1006S. [Google Scholar] [CrossRef]
- Narkpiban, K.; Sakdaronnarong, C.; Nimchua, T.; Pinmanee, P.; Thongkred, P.; Poonsawat, T. The Effect of Mechano-enzymatic Treatment on the Characteristics of Cellulose Nanofiber Obtained from Kenaf (Hibiscus cannabinus L.) Bark. BioResources 2019, 14, 99–119. [Google Scholar] [CrossRef]
- Beluns, S.; Gaidukovs, S.; Platnieks, O.; Gaidukova, G.; Mierina, I.; Grase, L.; Starkova, O.; Brazdausks, P.; Thakur, V.K. From Wood and Hemp Biomass Wastes to Sustainable Nanocellulose Foams. Ind. Crops Prod. 2021, 170, 113780. [Google Scholar] [CrossRef]
- Espinosa, E.; Arrebola, R.I.; Bascon-Villegas, I.; Sanchez-Gutierrez, M.; Dominguez-Robles, J.; Rodriguez, A. Industrial application of orange tree nanocellulose as papermaking reinforcement agent. Cellulose 2020, 27, 10781–10797. [Google Scholar] [CrossRef]
- Prado, K.S.; Jacinto, A.A.; Spinacé, M.A.S. Cellulose Nanostructures Extracted from Pineapple Fibres. In Pineapple Leaf Fibers; Jawaid, M., Asim, M., Tahir, P., Nasir, M., Eds.; Green Energy and Technology; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Johnson, D.A.; Paradis, M.A. Surface application of cellulose nanofibrils to fine paper using different base sheet freeness levels. In Proceedings of the Tappi PaperCon, Minneapolis, MN, USA, 23–26 April 2017. [Google Scholar]
- Johnson, D.A.; Paradis, M.A.; Tajvidi, M.; Al-Gharrawi, M.; Bousfield, D. Surface application of cellulose microfibrils on paper—Effects of basis weight and surface coverage levels. In Proceedings of the Tappi PaperCon, Indianapolis, IN, USA, 5–8 May 2019. [Google Scholar]
- Johnson, D.A.; Paradis, M.A.; Bilodeau, M.; Crossley, B.; Foulger, M.; Gelinas, P. Effects of cellulosic nanofibrils on papermaking properties of fine papers. Tappi J. 2016, 15, 395–402. [Google Scholar] [CrossRef]
- Ashby, M.F. Chapter 15—Material profiles. In Materials and the Environment, 2nd ed.; Ashby, M.F., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2013; pp. 459–595. [Google Scholar]
- Bhagia, S.; Nunez, A.; Wyman, C.E.; Kumar, R. Robustness of two-step acid hydrolysis procedure for composition analysis of poplar. Bioresour. Technol. 2016, 216, 1077–1082. [Google Scholar] [CrossRef]
- Suzuki, M. Method and Apparatus for Manufacturing Microfibrillated Cellulose Fiber. U.S. Patent No. 7,381,294, 3 June 2008. [Google Scholar]
- Kumar, R.; Hu, F.; Hubbell, C.A.; Ragauskas, A.J.; Wyman, C.E. Comparison of laboratory delignification methods, their selectivity, and impacts on physiochemical characteristics of cellulosic biomass. Bioresour. Technol. 2013, 130, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, M.R.; Siengchin, S.; Parameswaranpillai, J.; Jawaid, M.; Pruncu, C.I.; Khan, A. A comprehensive review of techniques for natural fibers as reinforcement in composites: Preparation, processing and characterization. Carbohydr. Polym. 2019, 207, 108–121. [Google Scholar] [CrossRef]
- Njoku, C.E. Natural Fibers As Viable Sources For The Development Of Structural, Semi-Structural, And Technological Materials—A Review. Adv. Mater. Lett. 2019, 10, 682–694. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Copenhaver, K.; Li, K.; Lamm, M.E.; Walker, C.; Johnson, D.; Han, Y.; Wang, L.; Zhao, X.; Pu, Y.; Hinton, H.; et al. Recycled Cardboard Containers as a Low Energy Source for Cellulose Nanofibrils and Their Use in Poly(l-lactide) Nanocomposites. ACS Sustain. Chem. Eng. 2021, 9, 13460–13470. [Google Scholar] [CrossRef]
- Rana, S.S.; Gupta, M.K. Isolation of nanocellulose from hemp (Cannabis sativa) fibers by chemo-mechanical method and its characterization. Polym. Compos. 2020, 41, 5257–5268. [Google Scholar] [CrossRef]
- Espinosa, E.; Rol, F.; Bras, J.; Rodriguez, A. Production of lignocellulose nanofibers from wheat straw by different fibrillation methods. Comparison of its viability in cardboard recycling process. J. Clean. Prod. 2019, 239, 118083. [Google Scholar] [CrossRef]
- Shanks, R.A. 2—Chemistry and structure of cellulosic fibres as reinforcements in natural fibre composites. In Natural Fibre Composites; Hodzic, A., Shanks, R., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 66–83. [Google Scholar]
- Bhagia, S.; Dunlap, J.R.; Khuraishi, M.Z.A.; Lowden, R.R.; Muchero, W.; Vaidya, U.K.; Pu, Y.; Ragauskas, A.J. Fabrication of lignocellulosic biomass paper containing nanofibrillated biomass. BioResources 2021, 16, 209–222. [Google Scholar] [CrossRef]
- Koubaa, A.; Koran, Z. Measure of the internal bond strength of paper/board. Tappi J. 1995, 78, 103–111. [Google Scholar]
- Hasan, I.; Wang, J.; Bousfield, D.; Tajvidi, M. Turning Recycled Cardboard Container-Derived Lignin-Containing Cellulose Nanofibrils into a Robust Gas Barrier UV-Shielding Film. ACS Sustain. Chem. Eng. 2023, 11, 3720–3731. [Google Scholar] [CrossRef]
- Tyagi, P.; Gutierrez, J.N.; Nathani, V.; Lucia, L.A.; Rojas, O.J.; Hubbe, M.A.; Pal, L. Hydrothermal and mechanically generated hemp hurd nanofibers for sustainable barrier coatings/films. Ind. Crops Prod. 2021, 168, 113582. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Tajvidi, M.; Bousfield, D. Paper-Based Oil Barrier Packaging using Lignin-Containing Cellulose Nanofibrils. Molecules 2020, 25, 1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Wang, Q.; Ji, X.; Yang, G.; Chen, J.; Fatehi, P. Strong, ductile and biodegradable polylactic acid/lignin-containiThng cellulose nanofibril composites with improved thermal and barrier properties. Ind. Crops Prod. 2021, 171, 113898. [Google Scholar] [CrossRef]
- Yook, S.; Park, H.; Park, H.; Lee, S.-Y.; Kwon, J.; Youn, H.J. Barrier coatings with various types of cellulose nanofibrils and their barrier properties. Cellulose 2020, 27, 4509–4523. [Google Scholar] [CrossRef]
- Linares, M.G.; Delgado Aguilar, M.; Tarrés, J.; Aguado, R.; Pereira, M.; Valerio, O. Impact of lignocellulosic nanofiber source on the performance of polylactic acid. J. Appl. Polym. Sci. 2024, e56088. [Google Scholar] [CrossRef]




| Feedstock | Embodied Energy (MJ/kg) |
|---|---|
| BSKP | 37.3 |
| Wheat straw | 18.8 |
| Flax | 10–12 |
| Hemp | 9.5–10.5 |
| Material | Cellulose % | Hemicellulose % | Lignin % | Extractives % |
|---|---|---|---|---|
| Bleached softwood kraft pulp (BSKP) | 79.3 (±1.7) | 18.4 (±0.7) | 0 | 2.3 (±1.2) |
| Wheat straw pulp (WS) | 49.9 (±0.9) | 21.8 (±0.5) | 24.8 (±2.2) | 3.5 (±1.2) |
| Flax (F) | 73.5 (±0.7) | 7.0 (±1.7) | 15.3 (±0.3) | 5.9 (±0.8) |
| Hemp (H) | 77.4 (±3.8) | 5.4 (±0.8) | 11.2 (±0.3) | 6.1 (±1.6) |
| Material | Cellulose Crystallinity % | Td (5%, °C) | Char Yield (%) |
|---|---|---|---|
| BSKP | 42.9 | 304 | 12.3 |
| BSKP CNFs low E | 45.0 | 293 | 15.9 |
| BSKP CNFs high E | 41.8 | 292 | 16.5 |
| WS pulp | 40.0 | 271 | 20.5 |
| WS LCNFs low E | 44.3 | 236 | 30.8 |
| WS LCNFs high E | 47.9 | 230 | 32.6 |
| Flax | 70.0 | 273 | 18.8 |
| Flax LCNFs low E | 73.8 | 242 | 25.8 |
| Flax LCNFs high E | 68.2 | 269 | 27.4 |
| Hemp | 67.9 | 278 | 20.1 |
| Hemp LCNFs low E | 61.8 | 265 | 24.2 |
| Hemp LCNFs high E | 63.0 | 250 | 28.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamm, M.E.; Johnson, D.A.; Copenhaver, K.; Bhagia, S.; Hubbard, A.M.; Walker, C.C.; Doyle, K.; Ozcan, S. Exploiting the Properties of Non-Wood Feedstocks to Produce Tailorable Lignin-Containing Cellulose Nanofibers. Polymers 2024, 16, 2598. https://doi.org/10.3390/polym16182598
Lamm ME, Johnson DA, Copenhaver K, Bhagia S, Hubbard AM, Walker CC, Doyle K, Ozcan S. Exploiting the Properties of Non-Wood Feedstocks to Produce Tailorable Lignin-Containing Cellulose Nanofibers. Polymers. 2024; 16(18):2598. https://doi.org/10.3390/polym16182598
Chicago/Turabian StyleLamm, Meghan E., Donna A. Johnson, Katie Copenhaver, Samarthya Bhagia, Amber M. Hubbard, Colleen C. Walker, Kevin Doyle, and Soydan Ozcan. 2024. "Exploiting the Properties of Non-Wood Feedstocks to Produce Tailorable Lignin-Containing Cellulose Nanofibers" Polymers 16, no. 18: 2598. https://doi.org/10.3390/polym16182598







