Crystal Structure, Photophysical Properties and Antibacterial Activity of a Cd(II) Complex with Trans-2,3,4-Trimethoxycinnamic Acid and 4,4′-Bipyridine Ligands
Abstract
1. Introduction
2. Experimental
2.1. Materials and Physicochemical Measurements
2.2. Single-Crystal Structure Determination
2.3. Synthesis of Complex Cd-Tmca-Bpy
2.4. Computational Details
2.5. Agar Well Diffusion Method
3. Results and Discussion
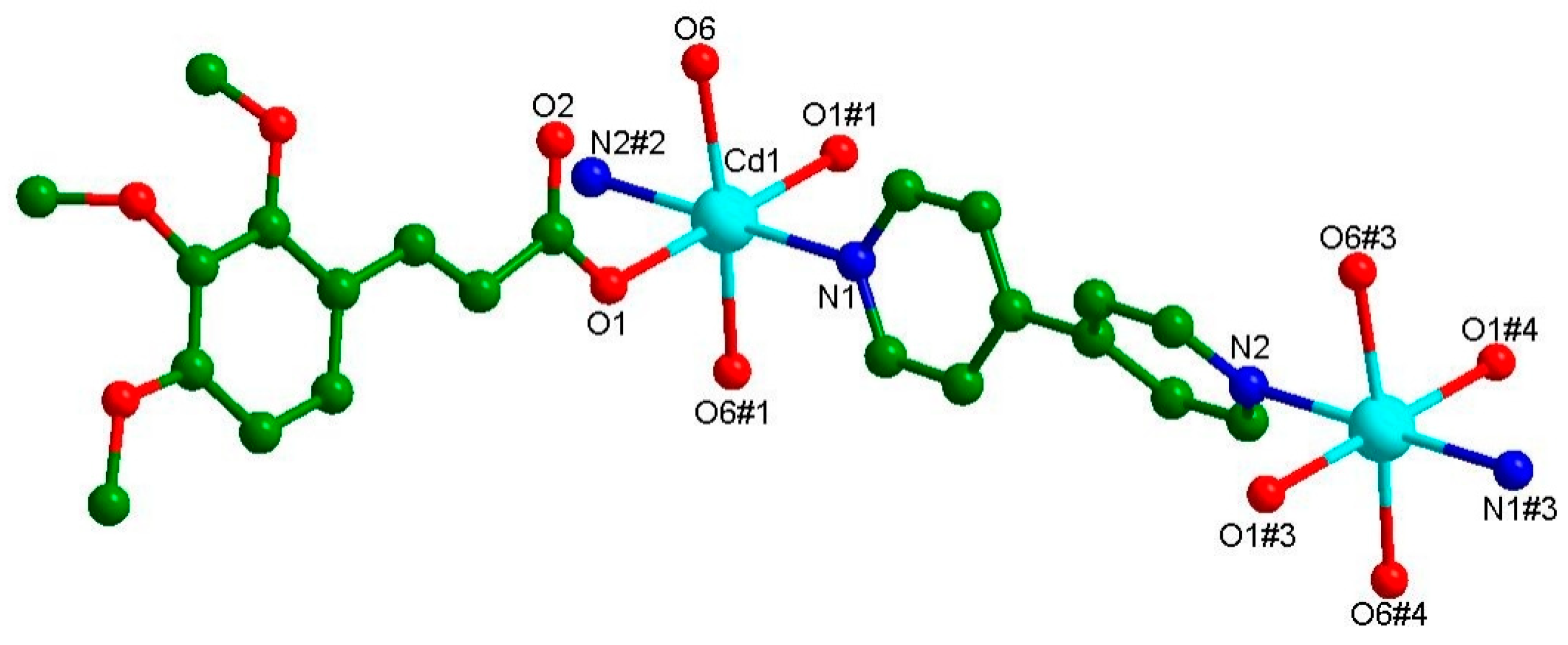
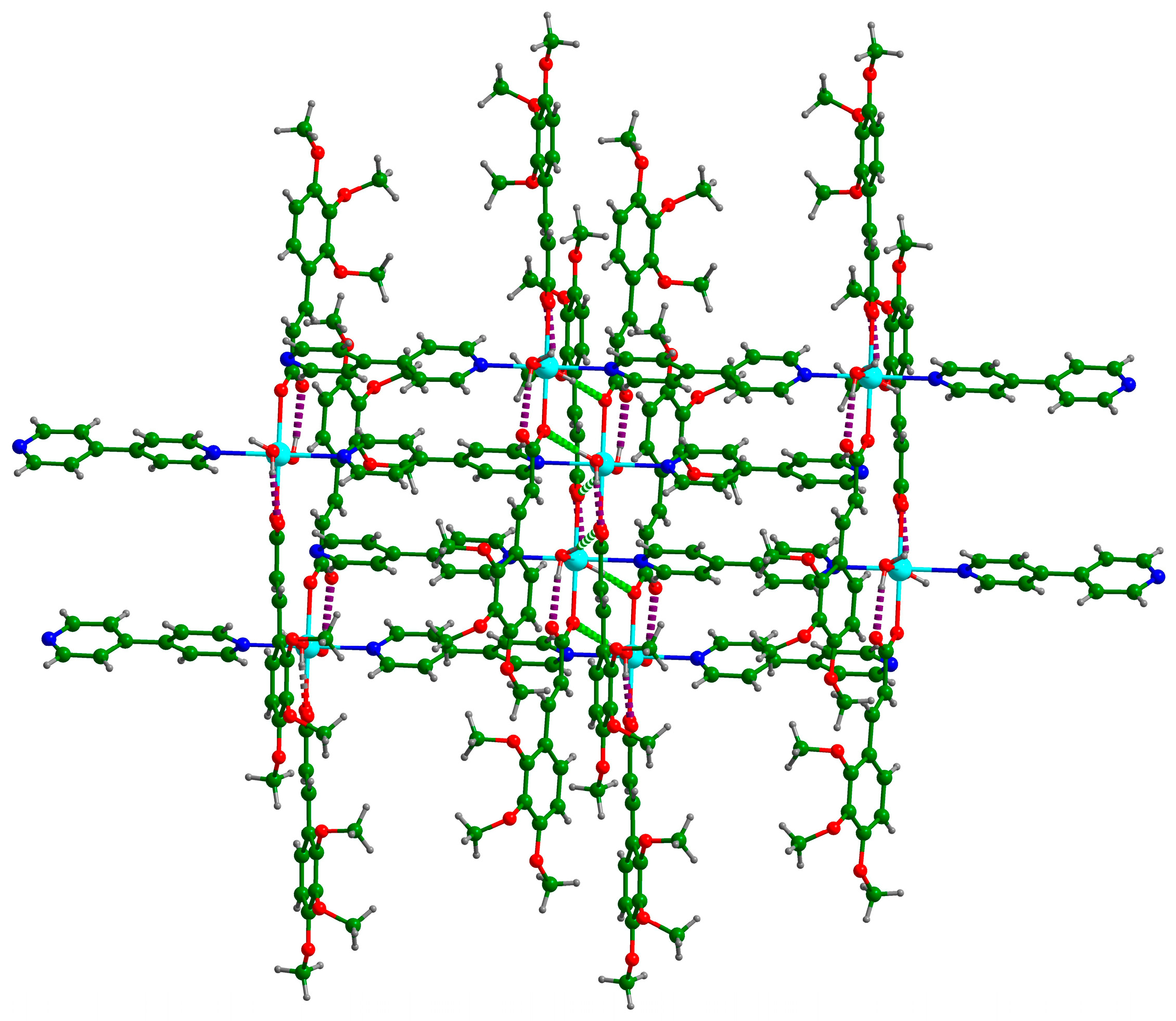
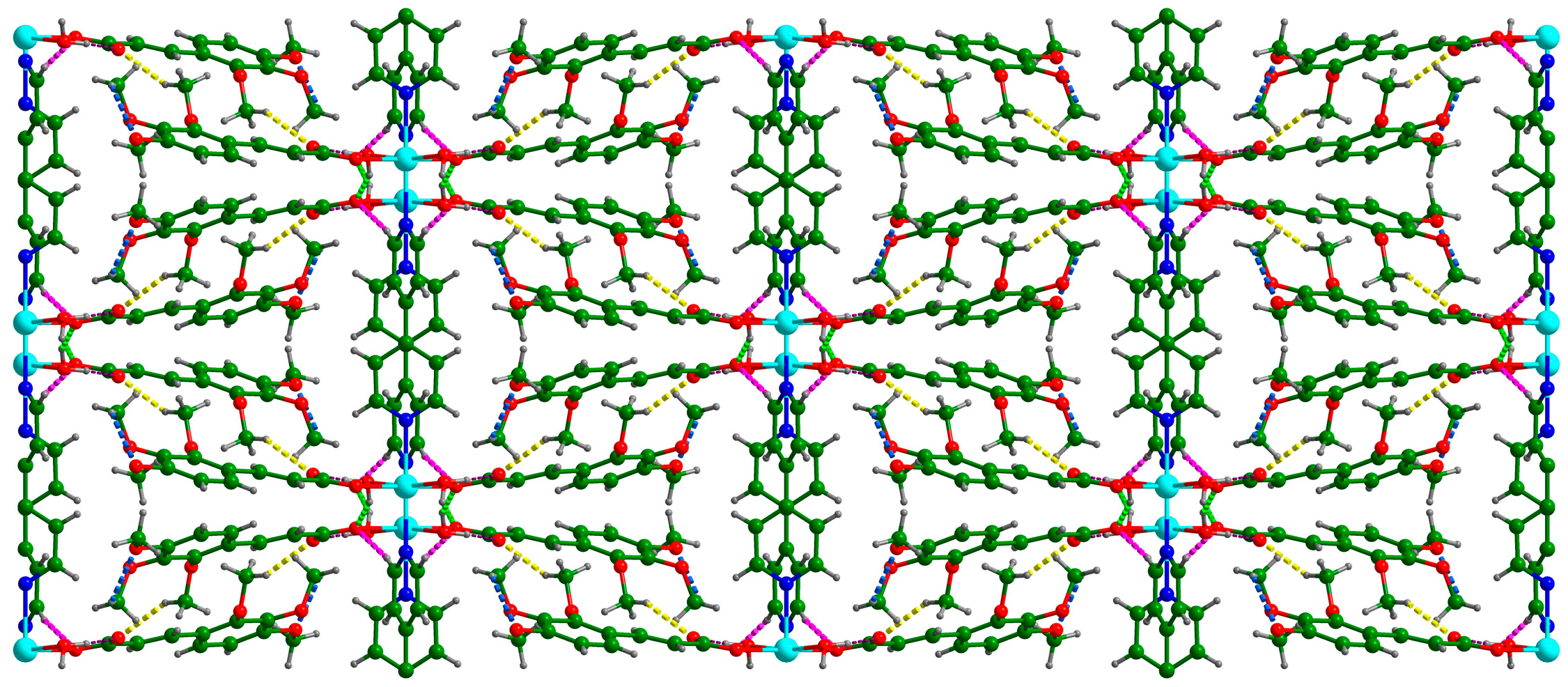
3.1. Crystal Structure of {[Cd(C12H13O5)2(4,4′-bpy)(H2O)2]}n (Cd-Tmca-bpy)
3.2. PXRD
3.3. FT-IR Spectra
3.4. Thermogravimetric Analyses
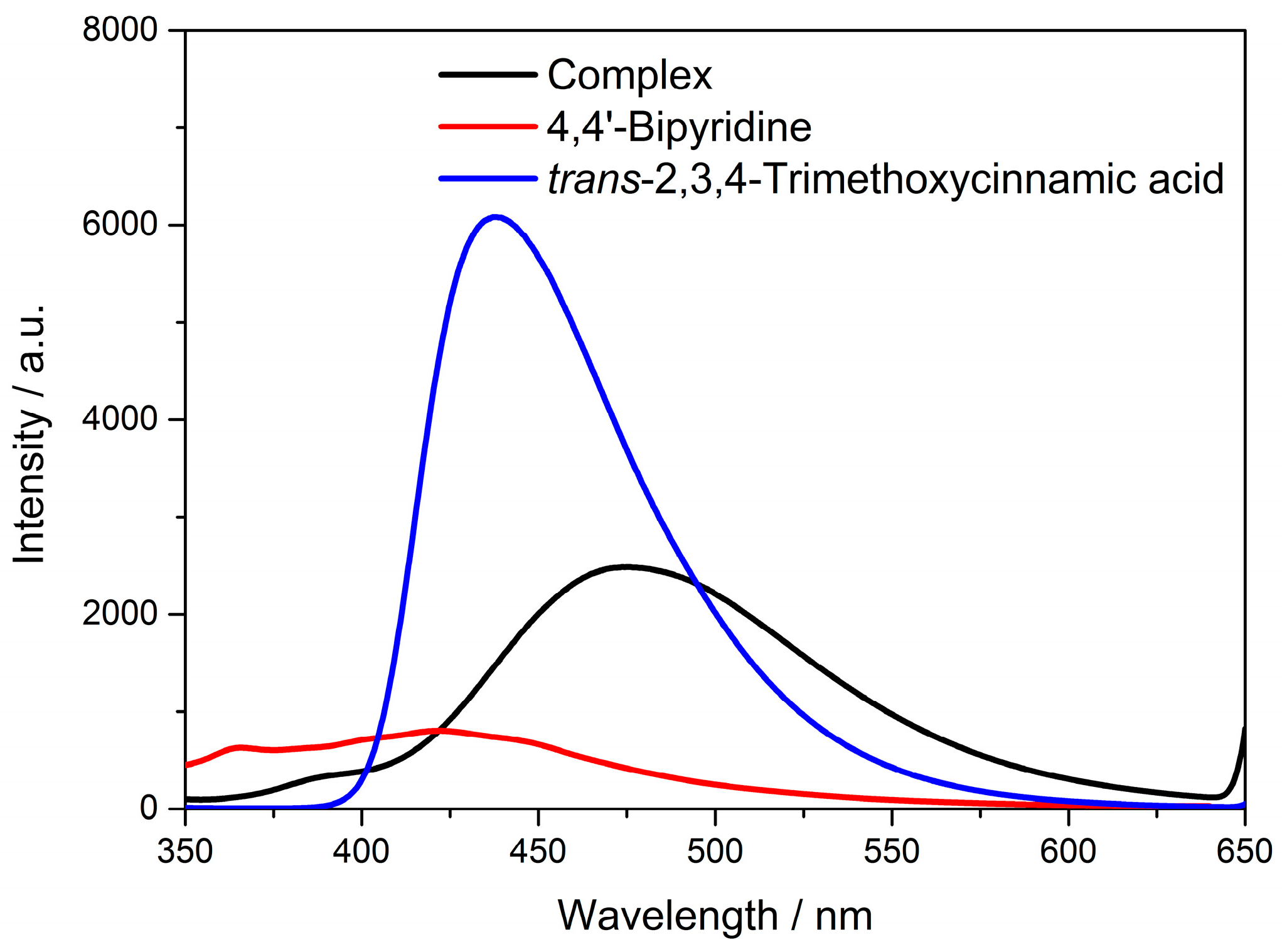
3.5. Solid-State Luminescence Emission
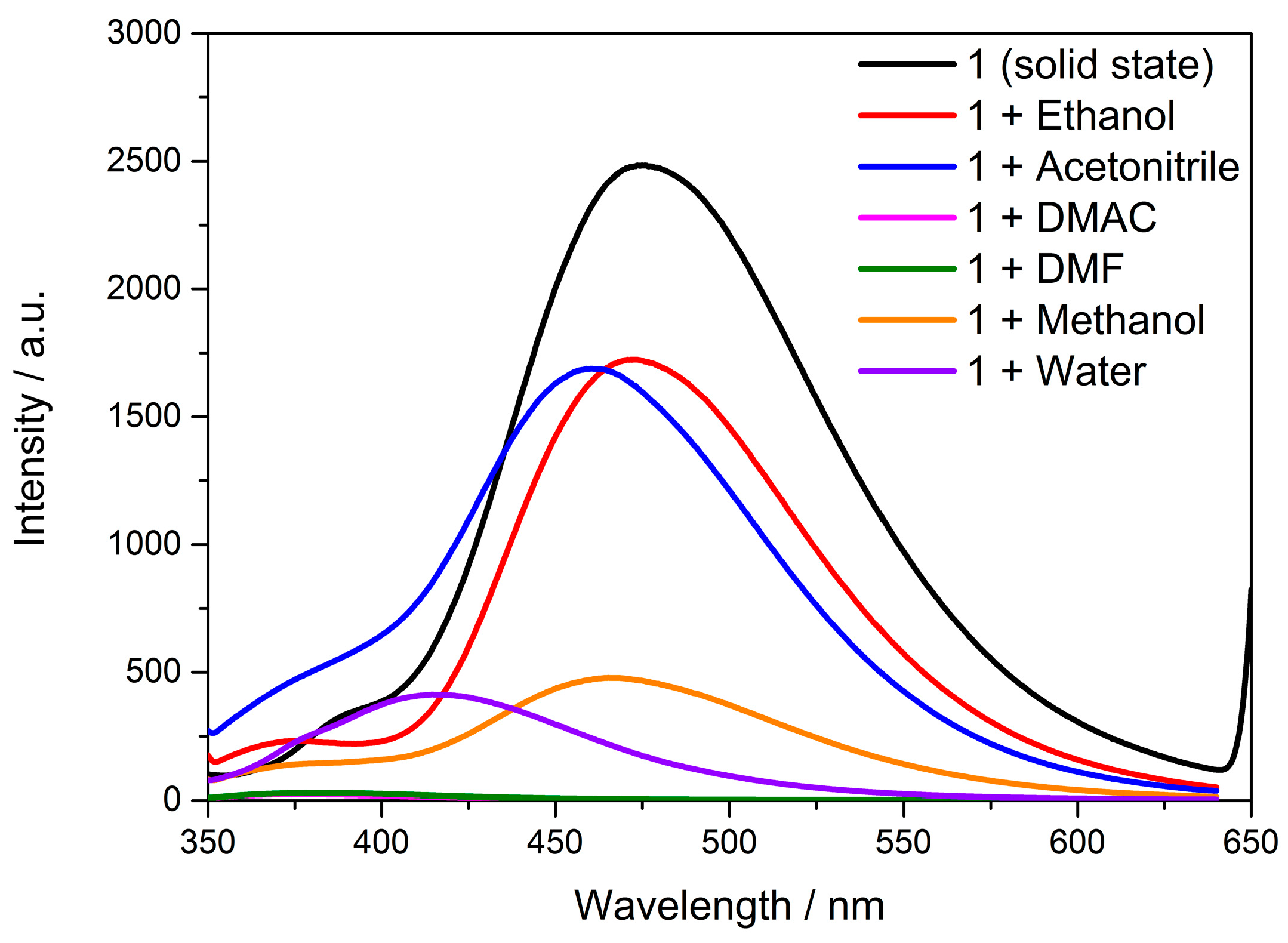
3.6. Solvent Effect
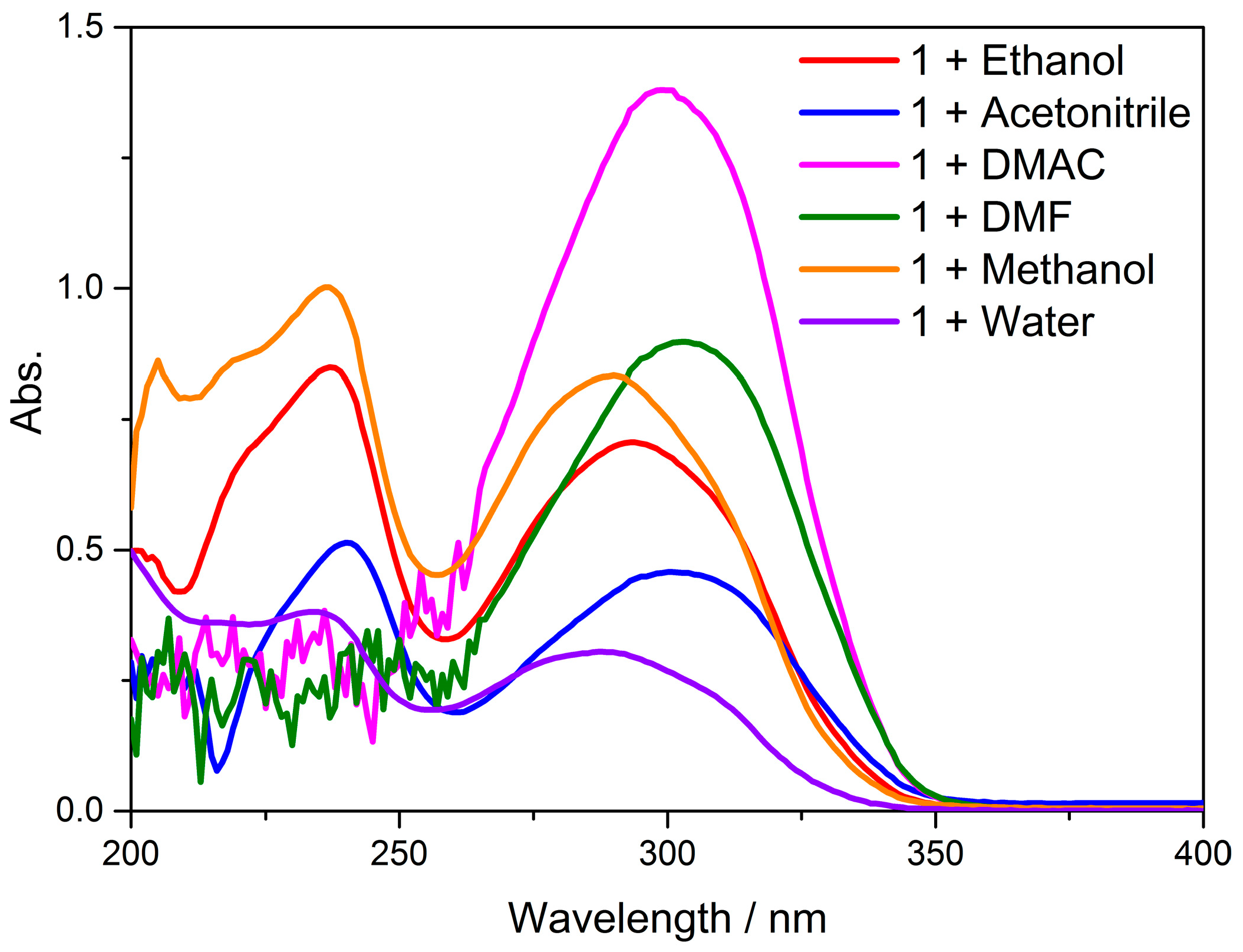
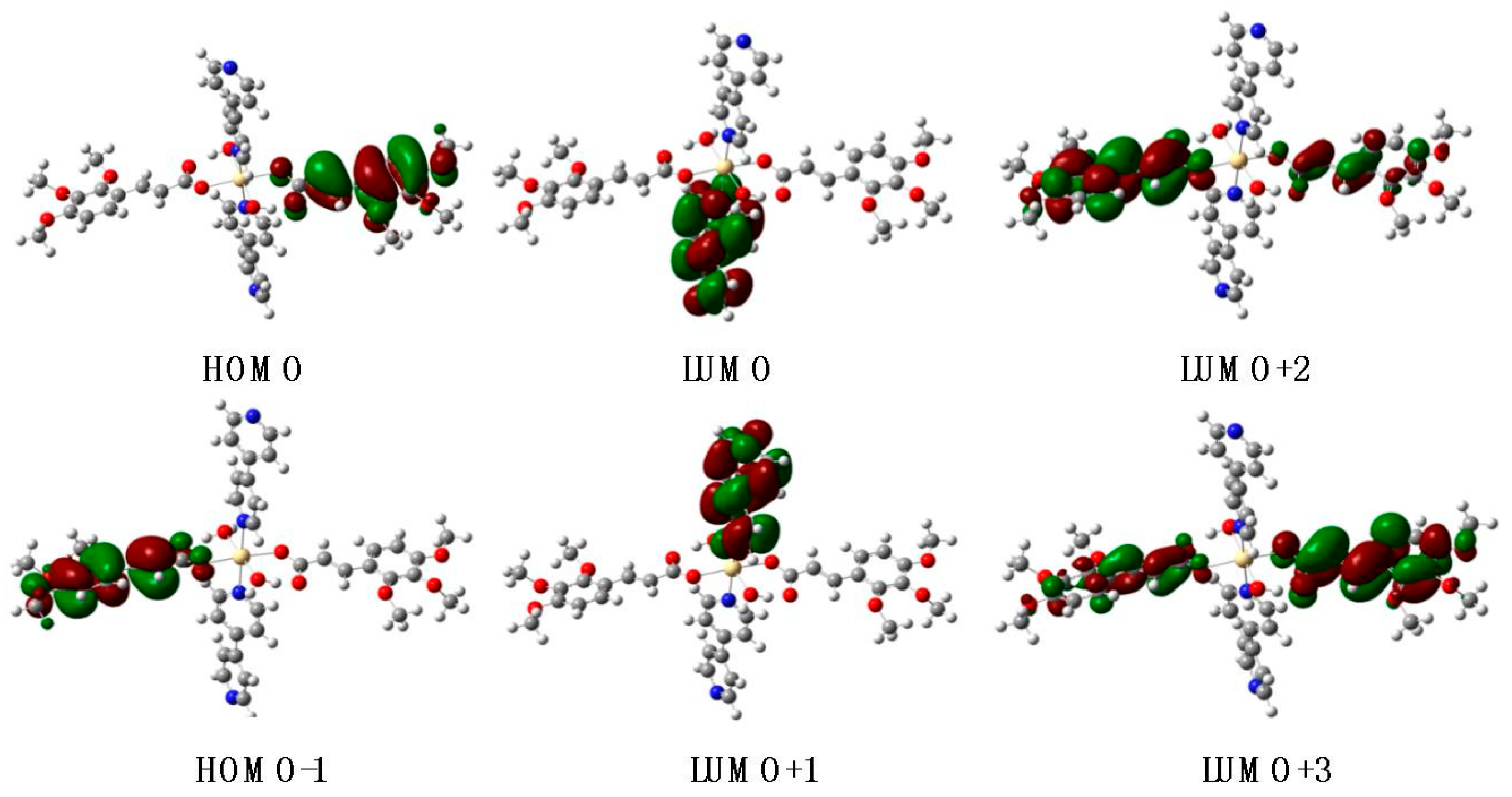
3.7. UV–Visible Absorption Spectra and TD-DFT Calculations
3.8. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, K.; Jie, G.A.; Liu, J.H.; Fu, Y.H.; Ma, R.; Lu, X.Q.; Zhang, F.M.; Zhu, W.D.; Fan, M.H. Visible-light-driven photocatalytic selective oxidation of amines and sulfides over a vanadium metal-organic framework. Sustain. Energ. Fuels 2022, 6, 5261–5267. [Google Scholar] [CrossRef]
- Ahmed, I.; Mondol, M.H.; Jung, M.-J.; Lee, G.H.; Jhung, S.H. MOFs with bridging or terminal hydroxo ligands: Applications in adsorption, catalysis, and functionalization. Coordin. Chem. Rev. 2023, 475, 214912. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Liu, M.; Lang, F.F.; Pang, J.D.; Bu, X.H. Recent progress in metal-organic frameworks (MOFs) for electrocatalysis. Ind. Chem. Mater. 2023, 1, 9–38. [Google Scholar] [CrossRef]
- Li, H.; Li, L.B.; Lin, R.B.; Zhou, W.; Zhang, Z.J.; Xiang, S.C.; Chen, B.L. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Paz, F.A.A. Metal-organic frameworks: A future toolbox for biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Shanmuga, M.; Agamendran, N.; Sekar, K.; Natarajan, T.S. Metal-organic frameworks (MOFs) for energy production and gaseous fuel and electrochemical energy storage applications. Phys. Chem. Chem. Phys. 2023, 25, 30116–30144. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mandal, M.; Maiti, D.K. Smart Metal-Organic Frameworks for Biotechnological Applications: A Mini-Review. ACS Appl. Bio Mater. 2021, 4, 8159–8171. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-free antibacterial strategies enabled by nanomaterials: Progress and perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef]
- Willyard, C. Drug-resistant bacteria ranked. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M. Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert Rev. Anti-Infect. Ther. 2019, 17, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Karimi Alavijeh, R.; Beheshti, S.; Akhbari, K.; Morsali, A. Investigation of reasons for metal-organic framework’s antibacterial activities. Polyhedron 2018, 156, 257–278. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal-organic framework-based stimuli-responsive systems for drug delivery. Adv. Sci. 2019, 6, 1801526. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bhattacharya, A.; Dutta, T.; Nag, S.; Wulferding, D.; Lemmens, P.; Pal, S.K. Nano MOF entrapping hydrophobic photosensitizer for dual-stimuli-responsive unprecedented therapeutic action against drug-resistant bacteria. ACS Appl. Bio Mater. 2019, 2, 1772–1780. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Bagchi, D.; Katouah, H.A.; Hasan, M.N.; Altass, H.M.; Pal, S.K. Enhanced water stability and photoresponsivity in metal-organic framework (MOF): A potential tool to combat drug-resistant bacteria. Sci. Rep. 2019, 9, 19372. [Google Scholar] [CrossRef]
- Kaur, N.; Tiwari, P.; Kapoor, K.S.; Saini, A.K.; Sharma, V.; Mobin, S.M. Metal−organic framework based antibiotic release and antimicrobial response: An overview. CrystEngComm 2020, 22, 7513–7527. [Google Scholar] [CrossRef]
- Sánchez-Férez, F.; Rius-Bartra, J.M.; Ayllón, J.; Calvet, T.; Font-Bardia, M.; Pons, J. Tuning Photophysical Properties by p-Functional Groups in Zn(II) and Cd(II) Complexes with Piperonylic Acid. Molecules 2022, 27, 1365. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Tupaeva, I.O.; Demidov, O.P.; Sayapin, Y.A.; Gusakov, E.A.; Minkin, V.I.; Metelitsa, A.V. Dual-state emission properties of 2-(2-carboalkoxy-3,4-dichloro-6-hydroxyphenyl)benzoxazoles and its Zn(II) and Cd(II) complexes. J. Lumin. 2023, 263, 120111. [Google Scholar] [CrossRef]
- Vlasenko, V.G.; Burlov, A.S.; Milutka, M.S.; Koshchienko, Y.V.; Uraev, A.I.; Lazarenko, V.A.; Makarova, N.I.; Metelitsa, A.V.; Zubenko, A.A.; Garnovskii, D.A. Cu(II), Ni(II), Co(II), Zn(II), and Pd(II) Complexes with (4Z)-4-[(2-Furylmethylamino)methylene]-5-methyl-2-phenylpyrazol-3-one: Synthesis, Structures, and Properties. Russ. J. Coord. Chem+ 2023, 49, 148–157. [Google Scholar]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Chen, Y.T.; Li, Z.X.; Pan, P.; Lao, Z.Z.; Xu, J.T.; Li, Z.H.; Zhan, S.F.; Liu, X.H.; Wu, Y.N.; Wang, W.B.; et al. Cinnamic acid inhibits Zika virus by inhibiting RdRp activity. Antivir. Res. 2021, 192, 105117. [Google Scholar] [CrossRef] [PubMed]
- Annuur, R.M.; Triana, D.; Ernawati, T.; Murai, Y.; Aswad, M.; Hashimoto, M.; Tachrim, Z.P. A Review of Cinnamic Acid’s Skeleton Modification: Features for Antibacterial-Agent-Guided Derivatives. Molecules 2024, 29, 3929. [Google Scholar] [CrossRef] [PubMed]
- Safinejad, M.; Rigi, A.; Zeraati, M.; Heidary, Z.; Jahani, S.; Chauhan, N.P.S.; Sargazi, G. Lanthanum-based metal organic framework (La-MOF) use of 3,4-dihydroxycinnamic acid as drug delivery system linkers in human breast cancer therapy. BMC Chem. 2022, 16, 93. [Google Scholar] [CrossRef]
- Graminha, A.E.; Honorato, J.; Dulcey, L.L.; Godoy, L.R.; Barbosa, M.F.; Cominetti, M.R.; Menezes, A.C.; Batista, A.A. Evaluation of the biological potential of ruthenium(II) complexes with cinnamic acid. J. Inorg. Biochem. 2020, 206, 111021. [Google Scholar] [CrossRef]
- Batool, S.S.; Gilani, S.R.; Zainab, S.S.; Tahir, M.N.; Harrison, W.T.; Haider, M.S.; Syed, Q.; Mazhar, S.; Shoaib, M. Synthesis, crystal structure, thermal studies and antimicrobial activity of a mononuclear Cu(II)-cinnamate complex with N,N,N′,N′-tetramethylethylenediamine as co-ligand. Polyhedron 2020, 178, 114346. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian09, Revision A.02; Gaussian, Inc.: Wallingford, UK, 2009.
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. A half-half theory of density functionals. J. Chem. Phys. 1993, 98, 1372. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Frisch, J.; Glowatzki, H.; Janietz, S.; Koch, N. Solution-based metal electrode modification for improved charge injection in polymer field-effect transistors. Org. Electron. 2009, 10, 1459–1465. [Google Scholar] [CrossRef]
- Jacquemin, D.; Perpete, E.A.; Scuseria, G.E.; Ciofini, I.; Adamo, C. TD-DFT Performance for the Visible Absorption Spectra of Organic Dyes: Conventional versus Long-Range Hybrids. J. Chem. Theory Comput. 2007, 4, 123–135. [Google Scholar] [CrossRef]
- Liu, Q. Theoretical research on the dye molecules with different π-bridge structures. J. Mol. Model. 2023, 29, 248. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Phys. Chem. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. 1993, 5, 799–805. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, S.Y.; Lin, X.L.; Zhou, J.X.; Gao, X.J.; Zhen, Y.Q.; Ma, X.D.; Jin, S.W.; Shi, L.F.; Liu, H.; et al. Nine Cd2+ complexes containing 3,5-dimethylpyrazole and carboxylic acids: Synthesis, spectroscopic and crystallographic characterization. J. Mol. Struc. 2024, 1297, 136930. [Google Scholar] [CrossRef]
- Yang, X.M.; Wang, A.R.; Li, J.; Huang, P.L.; Lu, Z.F.; Li, S.Y.; Li, J.M. Synthesis, crystal structures and fluorescence properties of two 1D Zn(II) homologous coordination polymers. Z. Naturforschung B 2023, 78, 43–50. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.J.; Fan, G.; Tang, L.; Yue, E.L.; Bai, C.; Wang, X.; Zhang, Y.Q. A highly stable cadmium(II) metal-organic framework for detecting tetracycline and p-nitrophenol. Chin. J. Inorg. Chem. 2024, 40, 646–654. [Google Scholar]
- Braiman, M.S.; Briercheck, D.M.; Kriger, K.M. Modeling Vibrational Spectra of Amino Acid Side Chains in Proteins: Effects of Protonation State, Counterion, and Solvent on Arginine CN Stretch Frequencies. J. Phys. Chem. B 1999, 103, 4744–4750. [Google Scholar] [CrossRef]
- McNair, R.; Cseri, L.; Szekely, G.; Dryfe, R. Asymmetric Membrane Capacitive Deionization Using Anion-Exchange Membranes Based on Quaternized Polymer Blends. ACS Appl. Polym. Mater. 2020, 2, 2946–2956. [Google Scholar] [CrossRef]
- Najafi, E.; Janghouri, M.; Hashemzadeh, A.; Ng, S.W. Mixed ligand Cd(II) coordination architectures based on bulky anthracene-9-carboxylate ligand: Crystal structures and optical properties. Inorg. Chim. Acta. 2023, 552, 121482. [Google Scholar] [CrossRef]
- Mandal, S.; Saha, R.; Saha, M.; Pradhan, R.; Butcher, R.J.; Saha, N.C. Synthesis, crystal structure, spectral characterization and photoluminescence property of three Cd(II) complexes with a pyrazole based Schiff-base ligand. J. Mol. Struct. 2016, 1110, 11–18. [Google Scholar] [CrossRef]
- Hu, T.; Liu, L.; Lv, X.; Chen, X.; He, H.; Dai, F.; Zhang, G.; Sun, D. Luminescent zinc and cadmium metal-organic frameworksbased on tetrazole ligands. Polyhedron 2010, 29, 296–302. [Google Scholar] [CrossRef]
- Li, K.H.; Xu, K.; Mei, S.L.; Rao, D.L.; Mei, C.Z. Synthesis, Structure and Luminescence Property of Cd(II) Coordination Polymer Based on 4-Methylphthalic Acid and 4,4′-Bipyridine Ligand. Chin. J. Inorg. Chem. 2013, 29, 1312–1318. [Google Scholar]
- Sun, S.W.; Wang, G.F. Two cadmium coordination polymers constructed by varying V-shaped co-ligands: Syntheses, structures, and fluorescence properties. Chin. J. Inorg. Chem. 2024, 40, 613–620. [Google Scholar]
- Gao, L.J.; Cao, P.X.; Wang, J.J.; Wu, Y.P.; Fu, F.; Zhang, M.L.; Ren, Y.X.; Hou, X.Y. Syntheses, crystal structures, and luminescence of two new coordination polymers based on biphenyl-2,2′,4,4′-tetracarboxylate. J. Coord. Chem. 2011, 64, 1299–1308. [Google Scholar] [CrossRef]
- Konar, S.; Datta, S.K.; Dolai, M.; Das, A.; Pathak, S.; Chatterjee, S.; Das, K. Synthetic and structural investigations of Cd(II) complexes of tetradentate pyrimidine based Schiff base ligand: Insight through non covalent interactions, TDDFT calculation and Hirshfeld surface analysis. J. Mol. Struct. 2019, 1178, 682–691. [Google Scholar] [CrossRef]
- Yadav, A.; Dindorkar, S.S.; Patel, V. Untangling the effect of π-conjugation length on the opto-electronic properties of reactive dye-based sensitizer. J. Comput. Electron. 2023, 22, 1706–1714. [Google Scholar] [CrossRef]
- Mansour, M.; Issa, T.B.; Issaoui, N.; Harchani, A.; Puebla, E.G.; Ayed, B. Synthesis, crystal structure, vibrational spectroscopy, optical investigation and DFT study of a novel hybrid material: 4,4′-diammoniumdiphenylsulfone iodobusmuthate. J. Mol. Struct. 2019, 1186, 31–38. [Google Scholar] [CrossRef]
- Li, J.; Clark, B.R. Synthesis of Natural and Unnatural Quinolones Inhibiting the Growth and Motility of Bacteria. J. Nat. Prod. 2020, 83, 3181–3190. [Google Scholar] [CrossRef] [PubMed]




| Cd-Tmca-Bpy | |
|---|---|
| Empirical formula | C17H19Cd0.5NO6 |
| Formula weight | 389.53 |
| Temperature/K | 293(2) |
| Crystal system | monoclinic |
| Space group | C2/c |
| a/Å | 28.753(12) |
| b/Å | 11.848(5) |
| c/Å | 10.632(5) |
| α/° | 90 |
| β/° | 105.513(13) |
| γ/° | 90 |
| Volume/Å3 | 3490(3) |
| Z | 8 |
| ρcalcg/cm3 | 1.483 |
| μ/mm−1 | 5.623 |
| F(000) | 1600.0 |
| Crystal size/mm3 | 0.18 × 0.18 × 0.17 |
| Radiation | CuKα(λ = 1.5478) |
| 2Θ range for data collection/° | 12.516 to 177.626 |
| Index ranges | −37 ≤ h ≤ 37, −15 ≤ k ≤ 15, −13 ≤ l ≤ 13 |
| Reflections collected | 35,204 |
| Independent reflections | 3936 [Rint = 0.0243, Rsigma = 0.0130] |
| Data/restraints/parameters | 3936/0/228 |
| Goodness-of-fit on F2 | 1.146 |
| Final R indexes [I >= 2σ(I)] | R1 = 0.0257, wR2 = 0.0674 |
| Final R indexes [all data] | R1 = 0.0280, wR2 = 0.0693 |
| Largest diff. peak/hole/e Å−3 | 0.76/−0.44 |
| Bond | Length/Å | Bond | Length/Å |
|---|---|---|---|
| Cd1-O1 1 | 2.2967(16) | Cd1-N1 | 2.375(3) |
| Cd1-O1 | 2.2968(16) | Cd1-N2 2 | 2.400(3) |
| Cd1-O6 | 2.2958(17) | Cd1-O6 1 | 2.2957(17) |
| Atoms | Angle/° | Atoms | Angle/° |
|---|---|---|---|
| O11-Cd1-O1 | 179.61(8) | O61-Cd1-O6 | 172.68(8) |
| O11-Cd1-N1 | 90.19(4) | O1-Cd1-N1 | 90.20(4) |
| O1-Cd1-N2 2 | 89.80(4) | O11-Cd1-N22 | 89.81(4) |
| O61-Cd1-O1 1 | 89.90(6) | O6-Cd1-O11 | 90.07(6) |
| O6-Cd1-O1 | 89.90(6) | O61-Cd1-O1 | 90.07(6) |
| O6-Cd1-N1 | 93.66(4) | O61-Cd1-N1 | 93.66(4) |
| O6-Cd1-N22 | 86.34(4) | O61-Cd1-N22 | 86.34(4) |
| N1-Cd1-N22 | 180.0 |
| Hydrogen Bonds | Length/Å | Angle/° |
|---|---|---|
| O6-H6A-O1 4 | 1.946 | 148.54 |
| O6-H6B-O2 | 1.844 | 147.88 |
| C10-H10A-O2 5 | 2.538 | 152.16 |
| C11-H11B-O5 | 2.491 | 114.68 |
| C13-H13-O1 6 | 2.621 | 170.82 |
| Complex | Cd-Tmca-bpy |
|---|---|
| Staphylococcus epidermidis | 15 |
| Bacillus subtilis subsp. Spizizenii | 13 |
| Escherichia coli | 26 |
| Staphylococcus aureus | 22 * |
| Pseudomonas aeruginosa | 18 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Han, X.; Liu, Q.; Li, J.; He, Z. Crystal Structure, Photophysical Properties and Antibacterial Activity of a Cd(II) Complex with Trans-2,3,4-Trimethoxycinnamic Acid and 4,4′-Bipyridine Ligands. Polymers 2024, 16, 2643. https://doi.org/10.3390/polym16182643
Wang L, Han X, Liu Q, Li J, He Z. Crystal Structure, Photophysical Properties and Antibacterial Activity of a Cd(II) Complex with Trans-2,3,4-Trimethoxycinnamic Acid and 4,4′-Bipyridine Ligands. Polymers. 2024; 16(18):2643. https://doi.org/10.3390/polym16182643
Chicago/Turabian StyleWang, Linyu, Xiao Han, Qun Liu, Jianye Li, and Zhifang He. 2024. "Crystal Structure, Photophysical Properties and Antibacterial Activity of a Cd(II) Complex with Trans-2,3,4-Trimethoxycinnamic Acid and 4,4′-Bipyridine Ligands" Polymers 16, no. 18: 2643. https://doi.org/10.3390/polym16182643
APA StyleWang, L., Han, X., Liu, Q., Li, J., & He, Z. (2024). Crystal Structure, Photophysical Properties and Antibacterial Activity of a Cd(II) Complex with Trans-2,3,4-Trimethoxycinnamic Acid and 4,4′-Bipyridine Ligands. Polymers, 16(18), 2643. https://doi.org/10.3390/polym16182643





