Synthesis and Redox Activity of Polyenaminones for Sustainable Energy Storage Applications
Abstract
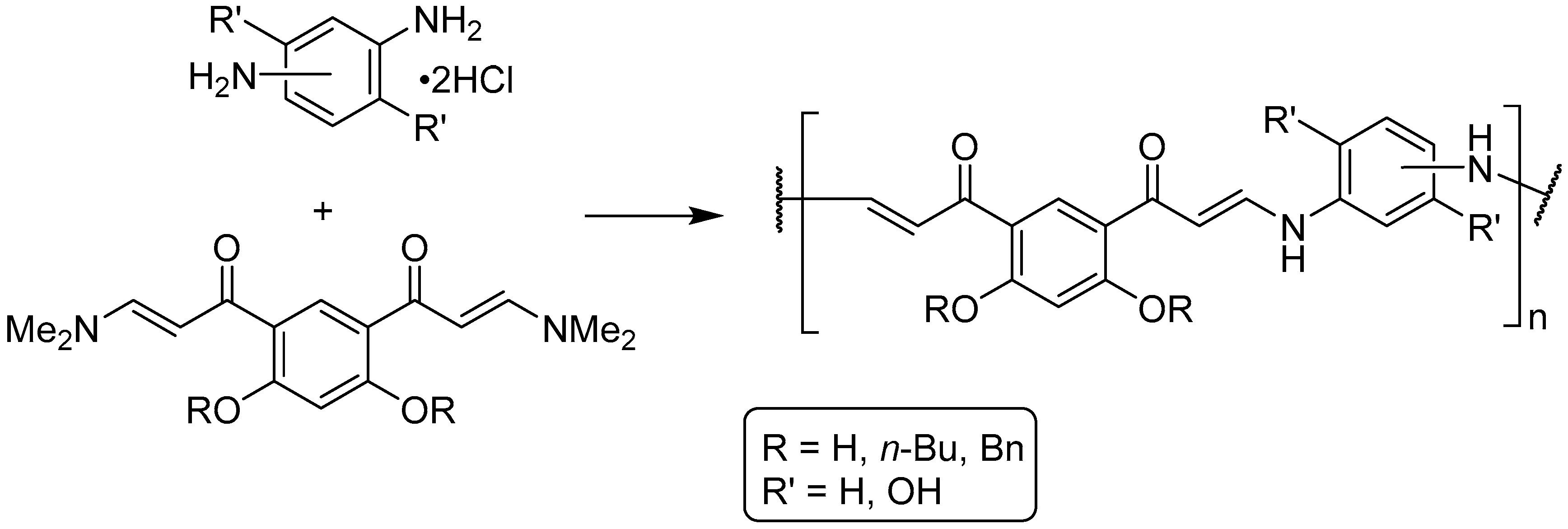
:1. Introduction
2. Materials and Characterizations
2.1. Materials
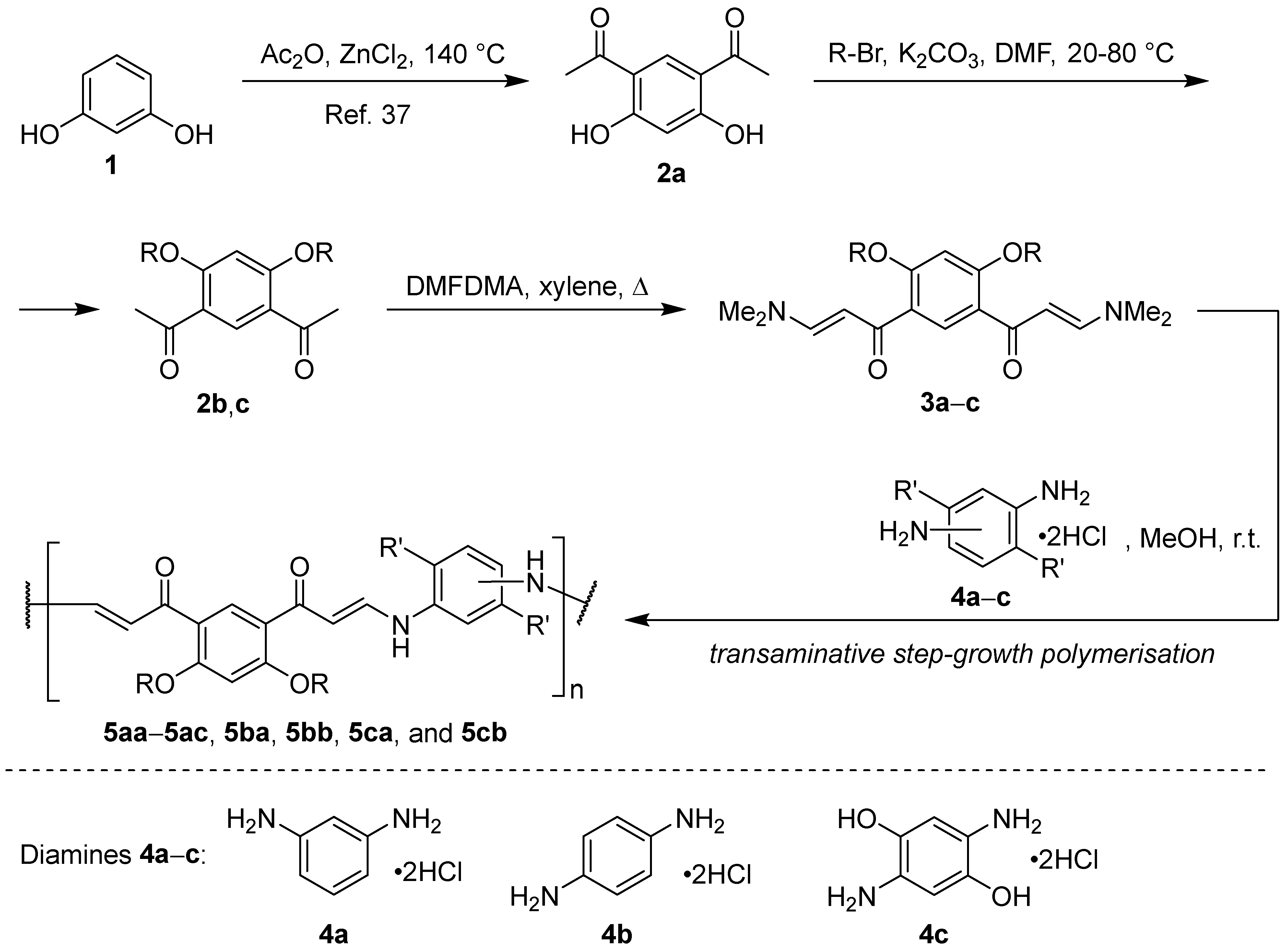
2.2. Synthesis of 4,6-Diacetylresorcinol (2a) [37,38]
2.3. General Procedure for the Synthesis of Dialkylated 4,6-Diacetylresorcinols 2b and 2c [39]
2.3.1. 1,1′-(4,6-Dibutoxy-1,3-phenylene)bis(ethan-1-one) (2b)
2.3.2. 1,1′-(4,6-Dibenzyloxy-1,3-phenylene)bis(ethan-1-one) (2c) [39]
2.4. General Procedure for the Synthesis of Bis-Enaminones 3a–c
2.4.1. (2E,2′E)-1,1′-(4,6-dihydroxy-1,3-phenylene)bis [3-(dimethylamino)prop-2-en-1-one] (3a) [40]
2.4.2. (2E,2′E)-1,1′-(4,6-dibutoxy-1,3-phenylene)bis [3-(dimethylamino)prop-2-en-1-one] (3b)
2.4.3. (2E,2′E)-1,1′-(4,6-dibenzyloxy-1,3-phenylene)bis [3-(dimethylamino)prop-2-en-1-one] (3c)
2.5. Synthesis of Compounds 5aa–5ac, 5ba, 5bb, 5ca, and 5cb
2.5.1. Poly{(EZ)-3-{[3-(λ2-azaneyl)phenyl]amino}-1-[2,4-dihydroxy-5-(3λ3-prop-2-enoyl)phenyl]prop-2-en-1-one} (5aa)
2.5.2. Poly{(EZ)-3-{[4-(λ2-azaneyl)phenyl]amino}-1-[2,4-dihydroxy-5-(3λ3-prop-2-enoyl)phenyl]prop-2-en-1-one} (5ab)
2.5.3. Poly{(EZ)-3-{[4-(λ2-azaneyl)-2,5-dihydroxyphenyl]amino}-1-[2,4-dihydroxy-5-(3λ3-prop-2-enoyl)phenyl]prop-2-en-1-one} (5ac)
2.5.4. Poly{(EZ)-3-{[3-(λ2-azaneyl)phenyl]amino}-1-[2,4-bis(butoxy)-5-(3λ3-prop-2-enoyl)phenyl]prop-2-en-1-one} (5ba)
2.5.5. Poly{(EZ)-3-{[4-(λ2-azaneyl)phenyl]amino}-1-[2,4-bis(butoxy)-5-(3λ3-prop-2-enoyl)phenyl]prop-2-en-1-one} (5bb)
2.5.6. Poly{(EZ)-3-{[3-(λ2-azaneyl)phenyl]amino}-1-[2,4-bis(benzyloxy)-5-(3λ3-prop-2-enoyl)phenyl]prop-2-en-1-one} (5ca)
2.5.7. Poly{(EZ)-3-{[4-(λ2-azaneyl)phenyl]amino}-1-[2,4-bis(benzyloxy)-5-(3λ3-prop-2-enoyl)phenyl]prop-2-en-1-one} (5cb)
3. Results
3.1. Synthesis
3.2. Characterization
3.2.1. Characterization by NMR
3.2.2. Characterization by FTIR
3.2.3. Characterization by SEM
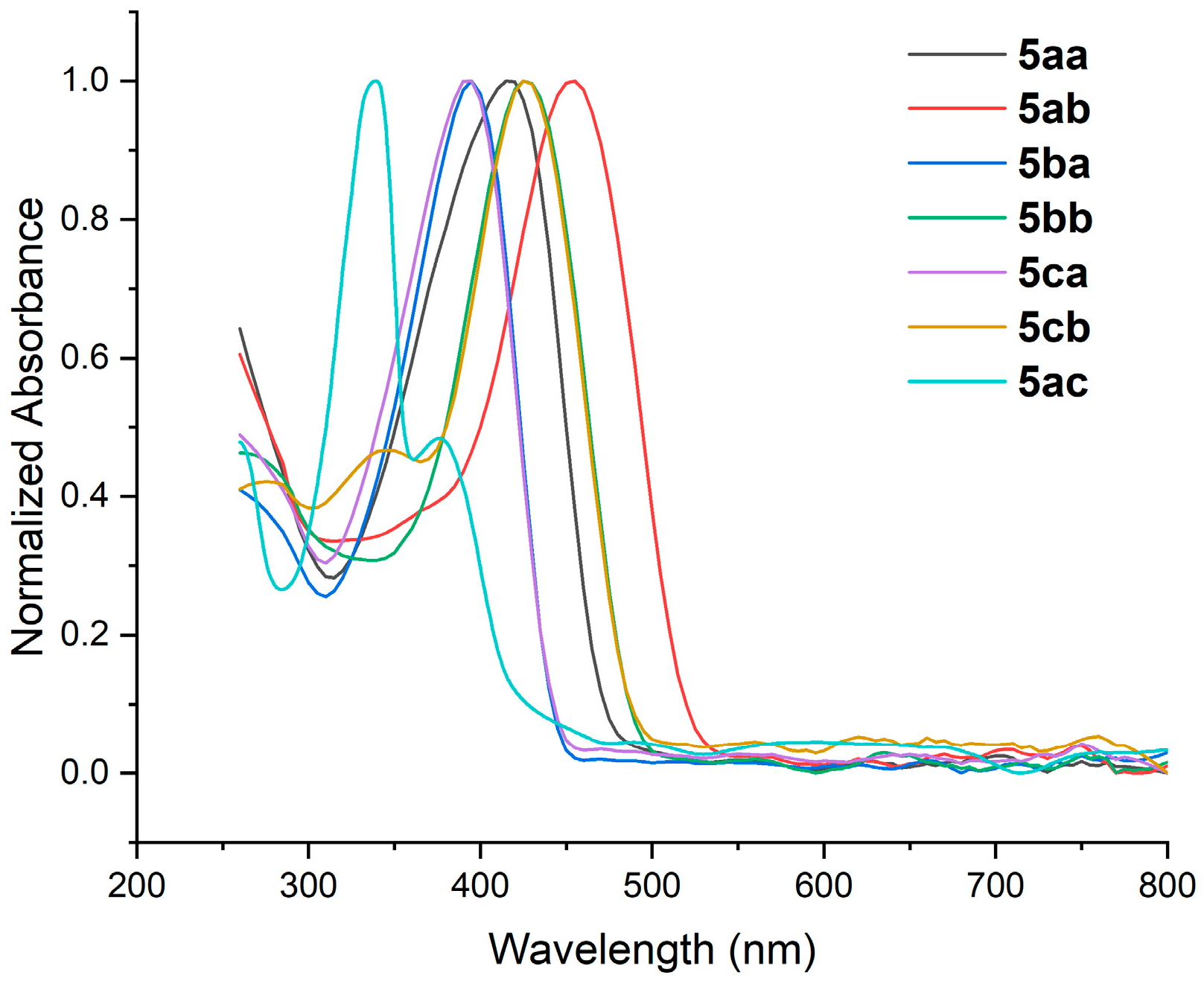
3.2.4. Characterization by UV-Vis Spectroscopy
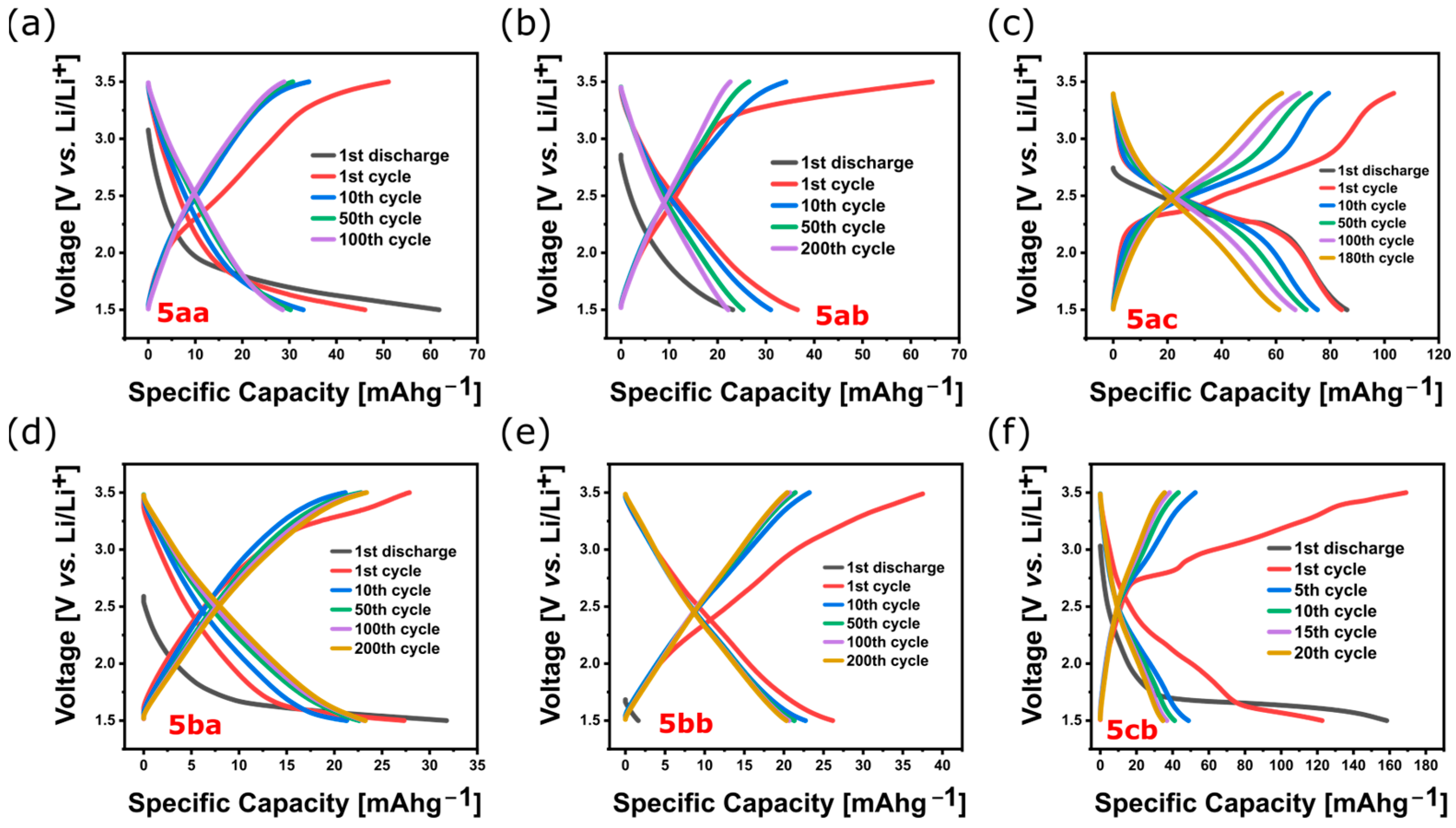
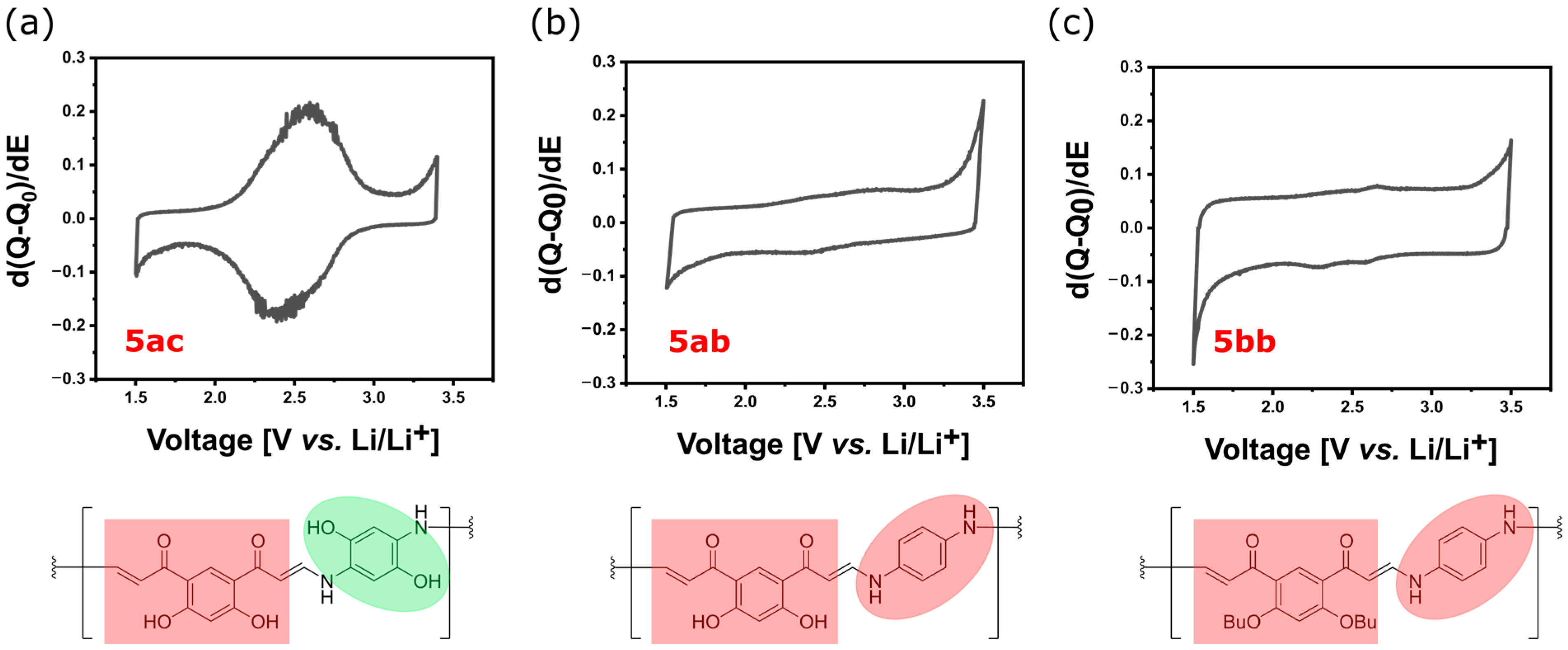
3.2.5. Determination of Redox Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koltzenburg, S.; Maskos, M.; Nuyken, O. Polymer Chemistry; Springer: Berlin, Germany, 2017; pp. 1–584. [Google Scholar] [CrossRef]
- Carraher, C.E., Jr. Polymer Chemistry, 10th ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–794. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Snyder, R.L.; Fortman, D.J.; De Hoe, D.X.; Hillmyer, M.A.; Dichtel, W.R. Reprocessable acid-degradable polycarbonate vitrimers. Macromolecules 2018, 51, 389–397. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Tapper, R.J.; Longana, M.L.; Norton, A.; Potter, K.D.; Hamerton, I. An Evaluation of Life Cycle Assessment and its Application to the Closed-Loop Recycling of Carbon Fibre Reinforced Polymers. Compos. Part B 2020, 184, 107665. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Z.; Liu, X.; Mei, P.; Yang, Y. Redox-active polymers as organic electrode materials for sustainable supercapacitors. Renew. Sustain. Energy Rev. 2024, 147, 111247. [Google Scholar] [CrossRef]
- Tour, J.M.; Kittrell, C.; Colvin, V.L. Green carbon as a bridge to renewable energy. Nat. Mater. 2010, 9, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Bitenc, J.; Pirnat, K.; Lužanin, O.; Dominko, R. Organic Cathodes, a Path toward Future Sustainable Batteries: Mirage or Realistic Future? Chem. Mater. 2024, 36, 1025–1040. [Google Scholar] [CrossRef]
- Jabeen, N.; Hussain, A.; Ullah, Z.; Haq, T.U.; Elsharkawy, E.M.; El-Bahy, Z.M.; Hessein, M.M. Design and performance evaluation of hierarchical ZnCo2S4@PANI core-shell nanorod arrays for high-efficiency supercapacitor applications. Mater. Chem. Phys. 2024, 320, 129462. [Google Scholar] [CrossRef]
- Hussain, A.; Jabeen, N.; Tabassum, A.; Ali, J. 3D-Printed Conducting Polymers for Solid Oxide Fuel Cells. In 3D-Printed Conducting Polymers: Fundamentals, Advances, Challenges, 1st ed.; Gupta, R.K., Ed.; CRC Press: Boca Raton, FL, USA, 2024; pp. 179–195. [Google Scholar] [CrossRef]
- Guin, P.S.; Das, S.; Mandal, P.C. Electrochemical Reduction of Quinones in Different Media: A Review. Int. J. Electrochem. 2011, 2011, 816202. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Li, J.; Li, B. Organic quinones towards advanced electrochemical energy storage: Recent advances and challenges. J. Mater. Chem. A 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Piro, B.; Reisberg, S.; Anquetin, G.; Duc, H.-T.; Pham, M.-C. Quinone-Based Polymers for Label-Free and Reagentless Electrochemical Immunosensors: Application to Proteins, Antibodies and Pesticides Detection. Biosensors 2013, 3, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Wang, X.; Zhang, J.; Yang, Y. One-pot solvothermal incorporation of graphene into chain-engineered polyquinones for metal-free supercapacitors. Chem. Commun. 2020, 56, 11191–11194. [Google Scholar] [CrossRef] [PubMed]
- Šenica, L.; Grošelj, U.; Kasunič, M.; Kočar, D.; Stanovnik, B.; Svete, J. Synthesis of Enaminone-Based Vinylogous Peptides. Eur. J. Org. Chem. 2014, 2014, 3067–3071. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, B.; Liu, Y.; Wan, J.-P. Recent Advances in Reactions Using Enaminone in Water or Aqueous Medium. Adv. Synth. Catal. 2022, 364, 1508–1521. [Google Scholar] [CrossRef]
- Huang, J.; Yu, F. Recent Advances in Organic Synthesis Based on N,N-Dimethyl Enaminones. Synthesis 2021, 53, 587–610. [Google Scholar] [CrossRef]
- Gaber, H.M.; Bagley, M.C.; Muhammad, Z.A.; Gomha, S.M. Recent developments in chemical reactivity of N,N-dimethylenamino ketones as synthons for various heterocycles. RSC Adv. 2017, 7, 14562–14610. [Google Scholar] [CrossRef]
- Stanovnik, B.; Svete, J. Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones. Chem. Rev. 2004, 104, 2433. [Google Scholar] [CrossRef]
- Wang, L.-J.; Dong, P.-J.; Zhang, G.; Zhang, F.-M. Review and Perspectives of β-Keto-enamine-Based Covalent Organic Framework for Photocatalytic Hydrogen Evolution. Energy Fuels 2023, 37, 6323–6347. [Google Scholar] [CrossRef]
- Edelman, P.G.; Mathisen, R.J.; Huang, S.J. Poly(amide-enamines). In Advances in Polymer Synthesis; Culbertson, B.M., McGrath, J.E., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 275–290. [Google Scholar] [CrossRef]
- Kimura, S. Preparation of Polyquinolones. Makromol. Chem. 1968, 117, 203–209. [Google Scholar] [CrossRef]
- Higashi, F.; Tai, A.; Adachi, K. The Reaction Between Diethyl Succinylsuccinate (1,4-Diethoxycarbonyl-2,5-dihydroxy-l,4-cyclohexadiene) and Amines and Its Application to Polymer Synthesis. J. Polym. Sci. Part A-1 1970, 8, 2563–2577. [Google Scholar] [CrossRef]
- Moore, J.A.; Kochanowski, J.E. Poly(amine esters) Derived from Diethyl 1,4-Cyclohexanedione-2,5-dicarboxylate. Macromolecules 1975, 8, 121–127. [Google Scholar] [CrossRef]
- Ueda, M.; Kino, K.; Hirono, T.; Imai, Y. Synthesis of Polyenamines by Vinylogous Nucleophilic Substitution Polymerisation of 2,2′-Disubstituted Bis(4-ethoxymethylene-5-Oxazolone) with Diamines. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 931–938. [Google Scholar] [CrossRef]
- Ueda, M.; Otaira, K.; Imai, Y. Synthesis of Polyenamines by Vinylogous Nucleophilic Substitution Polymerisation of 1,6-Diethoxy-1,5-hexadiene-3,4-dione with Diamines. J. Polym. Sci. Polym. Chem. Ed. 1978, 16, 2809–2815. [Google Scholar] [CrossRef]
- Ueda, M.; Funayama, M.; Imai, Y. Synthesis of Polyenamines with Pendant Hydroxyl Groups by Ring-Opening Polyaddition of 5,5′-Oxalylbis(3,4-dihydro-2H-pyran) with Diamines. Polym. J. 1979, 11, 491–495. [Google Scholar] [CrossRef]
- Christensen, P.R.; Schuermann, A.M.; Loeffler, K.E.; Helms, B.A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem. 2019, 11, 442–448. [Google Scholar] [CrossRef]
- Deng, H.; Hu, R.; Leung, A.C.S.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. Construction of regio- and stereoregular poly(enaminone)s by multicomponent tandem polymerisations of diynes, diaroyl chloride and primary amines. Polym. Chem. 2015, 6, 4436–4446. [Google Scholar] [CrossRef]
- Deng, H.; He, Z.; Lam, J.W.Y.; Tang, B.Z. Regio- and stereoselective construction of stimuli-responsive macromolecules by a sequential coupling-hydroamination polymerisation route. Polym. Chem. 2015, 6, 8297–8305. [Google Scholar] [CrossRef]
- He, B.; Zhen, S.; Wu, Y.; Hu, R.; Zhao, Z.; Qin, A.; Tang, B.Z. Cu(I)-Catalyzed amino-yne click polymerisation. Polym. Chem. 2016, 7, 7375–7382. [Google Scholar] [CrossRef]
- Imai, Y.; Sakai, N.; Sasaki, J.; Ueda, M. Synthesis of Polyenamines by Vinylogous Nucleophilic Substitution Polymerisation of 1,1′-(3,4-Dioxo-1,5-hexadienylene)di-2-pyrrolidone with Diamines. Makromol. Chem. 1979, 180, 1797–1800. [Google Scholar] [CrossRef]
- Tomažin, U.; Alič, B.; Kristl, A.; Ručigaj, A.; Grošelj, U.; Požgan, F.; Krajnc, M.; Štefane, B.; Šebenik, U.; Svete, J. Synthesis of polyenaminones by acid-catalysed amino–enaminone ‘click’ polymerization. Eur. Polym. J. 2018, 108, 603–616. [Google Scholar] [CrossRef]
- Zupanc, A.; Kotnik, T.; Štanfel, U.; Brodnik Žugelj, H.; Kristl, A.; Ručigaj, A.; Matoh, L.; Pahovnik, D.; Grošelj, U.; Opatz, T.; et al. Chemical recycling of polyenaminones by transamination reaction via amino–enaminone polymerisation/depolymerization. Eur. Polym. J. 2019, 121, 109282. [Google Scholar] [CrossRef]
- Štanfel, U.; Kotnik, T.; Ričko, S.; Grošelj, U.; Štefane, B.; Pirnat, K.; Žagar, E.; Genorio, B.; Svete, J. Synthesis of Optically and Redox Active Polyenaminones from Diamines and α,α′-Bis[(dimethylamino)methylidene]cyclohexanediones. Polymers 2022, 14, 4120. [Google Scholar] [CrossRef]
- Mujahid, M.; Yogeeswari, P.; Sriram, D.; Basavanag, U.M.V.; Díaz-Cervantes, E.; Córdoba-Bahena, L.; Robles, J.; Gonnade, R.G.; Karthikeyan, M.; Vyas, R.; et al. Spirochromone-chalcone conjugates as antitubercular agents: Synthesis, bio evaluation and molecular modeling studies. RSC Adv. 2015, 5, 106448–106460. [Google Scholar] [CrossRef]
- Baker, W. 21. 4: 6- and 2: 4-Diacetylresorcinol. J. Chem. Soc. 1934, 1934, 71–73. [Google Scholar] [CrossRef]
- Shishmakova, T.G.; Bardamova, M.I.; Kotlyarevskii, I.L. Di- and tetraacetylenic amines of resorcinol series. Russ. Chem. Bull. 1972, 21, 1969–1972. [Google Scholar] [CrossRef]
- Assiri, M.A.; Ali, T.E.; Ibrahim, M.A.; El-Amin, E.M.; Yahia, I.S. 4,6-Diacetylresorcinol in Heterocyclic Synthesis Part II: Synthesis of Some Novel 4,6-Bis(azolyl/azinyl/azepinyl)resorcinols. Heterocycles 2019, 98, 114–125. [Google Scholar] [CrossRef]
- Bensalah; Gadri; Cañizares, P.; Sáez, C.; Lobato, J.; Rodrigo, M.A. Electrochemical Oxidation of Hydroquinone, Resorcinol, and Catechol on Boron-Doped Diamond Anodes. Environ. Sci. Technol. 2005, 39, 7234–7239. [Google Scholar] [CrossRef]
- Pirnat, K.; Bitenc, J.; Vizintin, A.; Krajnc, A.; Tcherychova, E. Indirect Synthesis Route toward Cross-Coupled Polymers for High Voltage Organic Positive Electrodes. Chem. Mater. 2018, 30, 5726–5732. [Google Scholar] [CrossRef]
- Liang, K.; Li, X.; Wei, D.; Jin, C.; Liu, C.; Xia, C. Deprotection of benzyl-derived groups via photochemically mesolytic cleavage of C–N and C–O bonds. Chem 2023, 9, 511–522. [Google Scholar] [CrossRef]
- Auger, C.; Gohy, J.-F.; Poizot, P.; Vlad, A. A H-bond stabilized quinone electrode material for Li–organic batteries: The strength of weak bonds. Chem. Sci. 2019, 10, 418–426. [Google Scholar] [CrossRef]
- Bitenc, J.; Lindahl, N.; Vizintin, A.; Abdelhamid, M.E.; Dominko, R.; Johansson, P. Concept and electrochemical mechanism of an Al metal anode–organic cathode battery. Energy Storage Mater. 2020, 24, 379–383. [Google Scholar] [CrossRef]
- Narayan, R.; Blagojević, A.; Mali, G.; Vélez Santa, J.F.; Bitenc, J.; Randon-Vitanova, A.; Dominko, R. Nanostructured Poly(hydroquinonyl-benzoquinonyl sulfide)/Multiwalled Carbon Nanotube Composite Cathodes: Improved Synthesis and Performance for Rechargeable Li and Mg Organic Batteries. Chem. Mater. 2022, 34, 6378–6388. [Google Scholar] [CrossRef]


| Entry | Transformation | R | R′ | Yield (%) |
|---|---|---|---|---|
| 1 | 1 → 2a | - | 88 | |
| 2 | 2a → 2b | n-Bu | 72 | |
| 3 | 2a → 2c | CH2Ph | 81 | |
| 4 | 2a → 3a | H | 82 | |
| 5 | 2b → 3b | n-Bu | 62 | |
| 6 | 2c → 3c | CH2Ph | 84 | |
| 7 | 3a + 4a → 5aa | H | H | 95 |
| 8 | 3a + 4b → 5ab | H | H | 100 |
| 9 | 3a + 4c → 5ac | H | OH | 100 |
| 10 | 3b + 4a → 5ba | n-Bu | H | 88 |
| 11 | 3b + 4b → 5bb | n-Bu | H | 92 |
| 12 | 3c + 4a → 5ca | CH2Ph | H | 84 |
| 13 | 3c + 4b → 5cb | CH2Ph | H | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotnik, T.; Menart, S.; Adam, Ž.; Bitenc, J.; Ciber, L.; Grošelj, U.; Petek, N.; Štefane, B.; Svete, J.; Genorio, B. Synthesis and Redox Activity of Polyenaminones for Sustainable Energy Storage Applications. Polymers 2024, 16, 2700. https://doi.org/10.3390/polym16192700
Kotnik T, Menart S, Adam Ž, Bitenc J, Ciber L, Grošelj U, Petek N, Štefane B, Svete J, Genorio B. Synthesis and Redox Activity of Polyenaminones for Sustainable Energy Storage Applications. Polymers. 2024; 16(19):2700. https://doi.org/10.3390/polym16192700
Chicago/Turabian StyleKotnik, Tomaž, Svit Menart, Žan Adam, Jan Bitenc, Luka Ciber, Uroš Grošelj, Nejc Petek, Bogdan Štefane, Jurij Svete, and Boštjan Genorio. 2024. "Synthesis and Redox Activity of Polyenaminones for Sustainable Energy Storage Applications" Polymers 16, no. 19: 2700. https://doi.org/10.3390/polym16192700
APA StyleKotnik, T., Menart, S., Adam, Ž., Bitenc, J., Ciber, L., Grošelj, U., Petek, N., Štefane, B., Svete, J., & Genorio, B. (2024). Synthesis and Redox Activity of Polyenaminones for Sustainable Energy Storage Applications. Polymers, 16(19), 2700. https://doi.org/10.3390/polym16192700









