Synthesis, Morphology, and Biomedical Applications of Plasma-Based Polymers: Recent Trends and Advances
Abstract
:1. Introduction
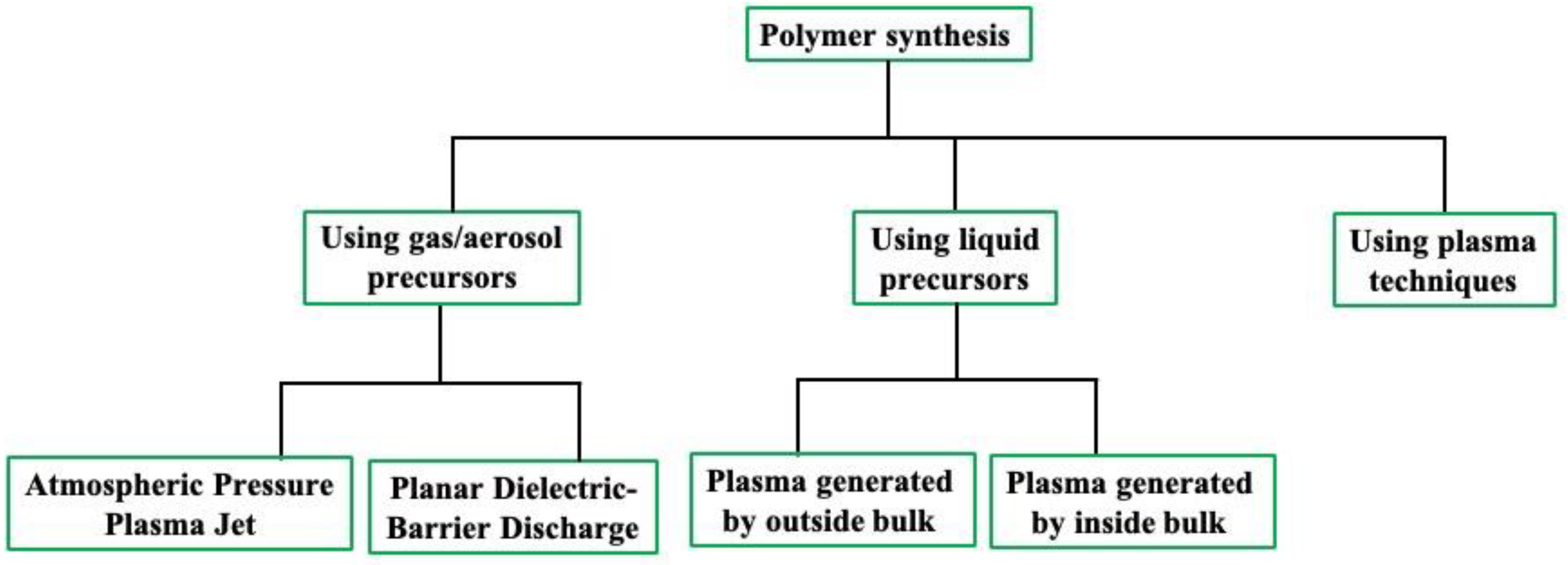
2. Synthesis Approaches Used for Polymer Synthesis Using Plasma
2.1. Polymer Synthesis Using Gas/Aerosol-Type Precursors
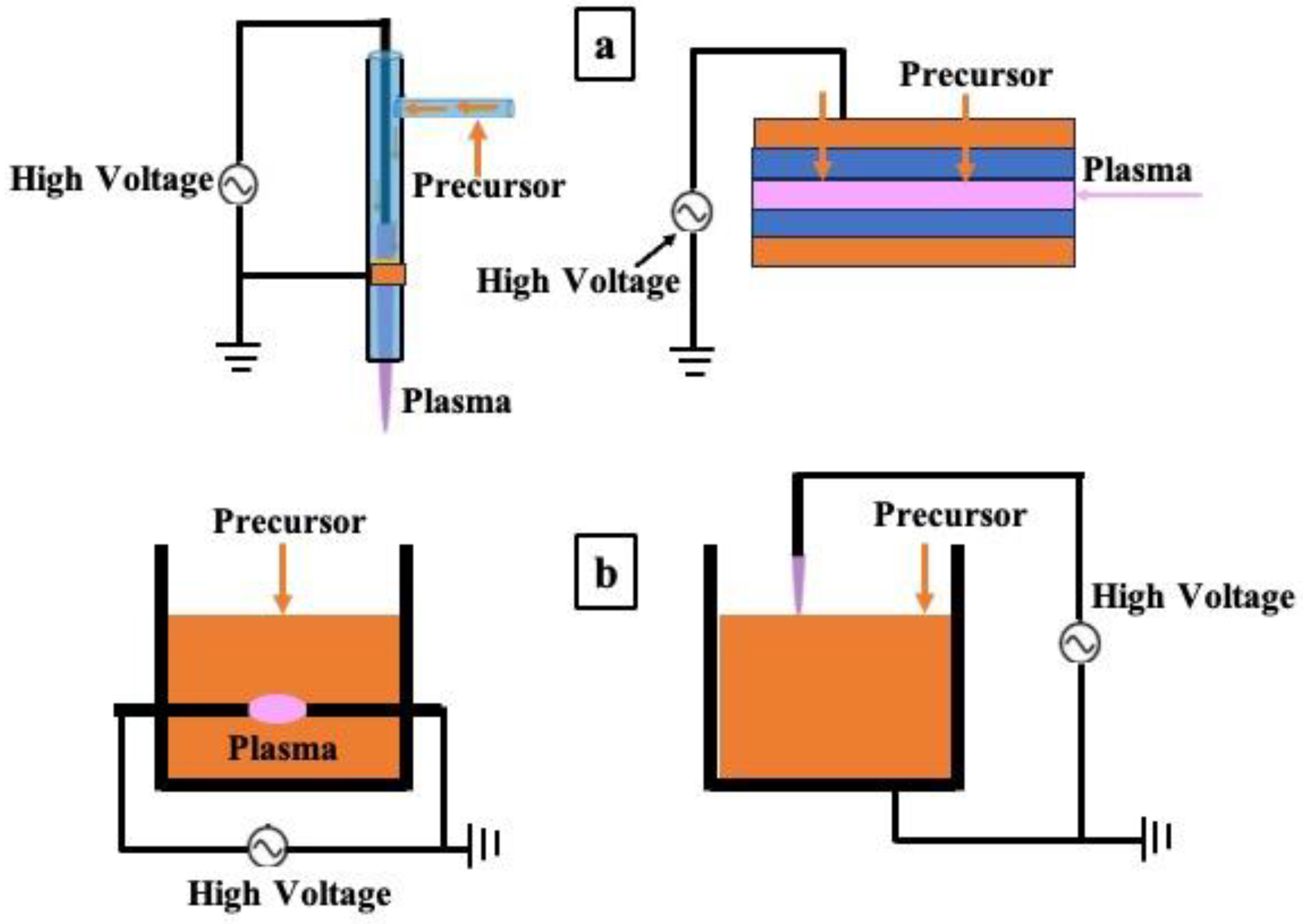
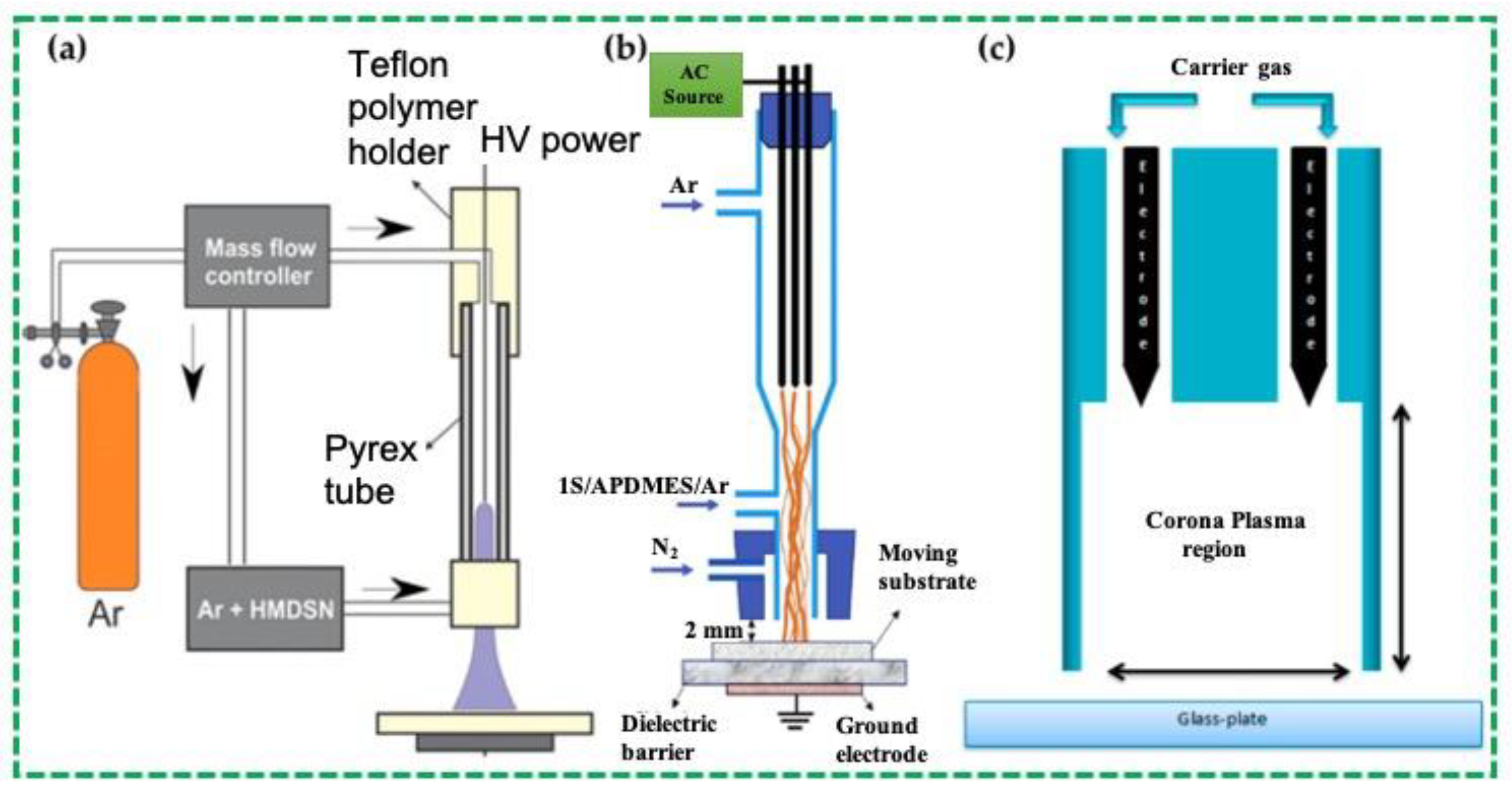
2.1.1. Atmospheric Pressure Plasma Jet Method
2.1.2. Dielectric-Barrier Discharge Method
2.2. Polymer Synthesis Using Liquid-Type Precursors
2.2.1. Plasma Generated by the Outside Bulk Liquid Precursor
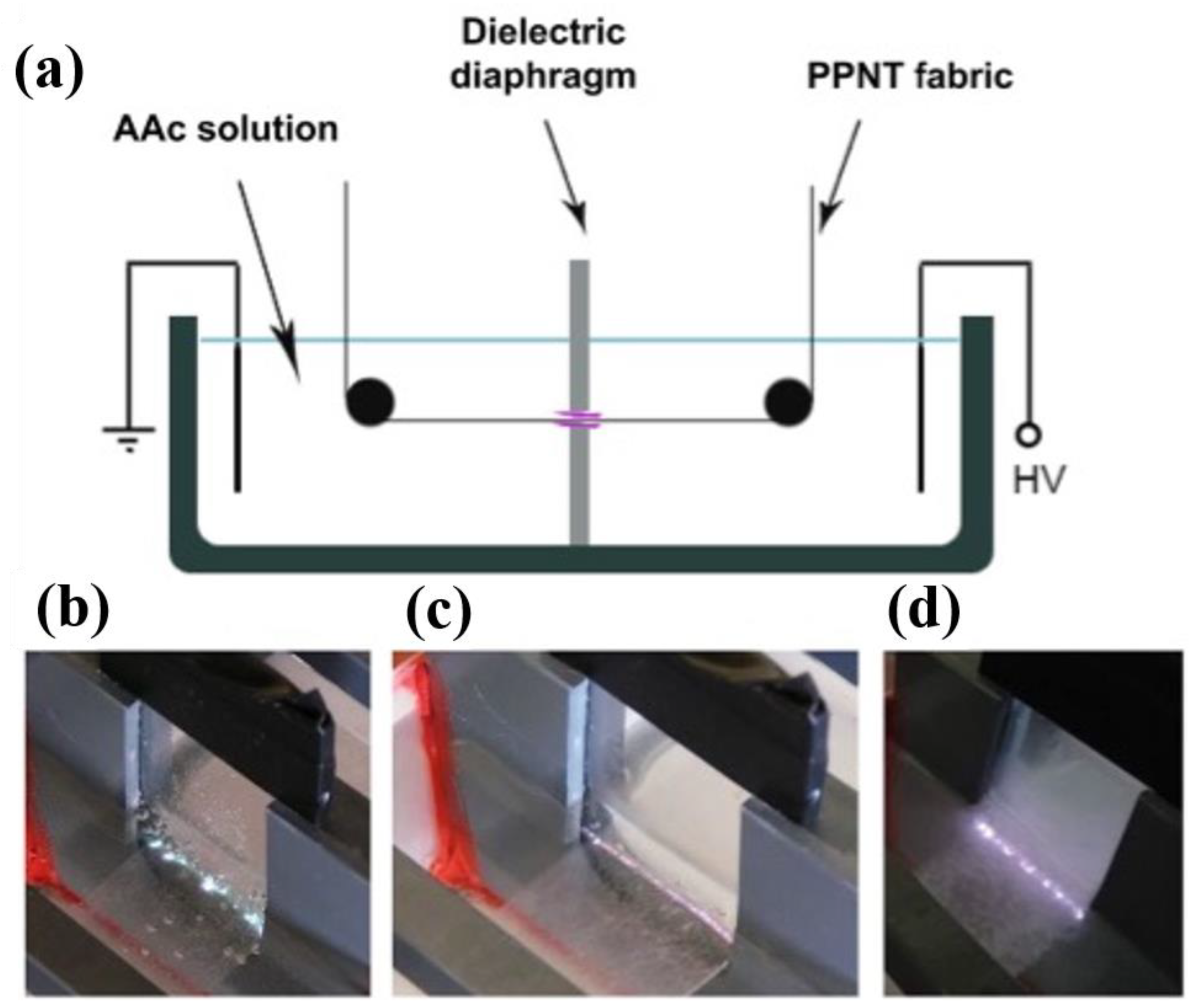
2.2.2. Plasma Generated by the Inside Bulk Liquid Precursor
2.3. Polymer Synthesis Using Plasma Techniques
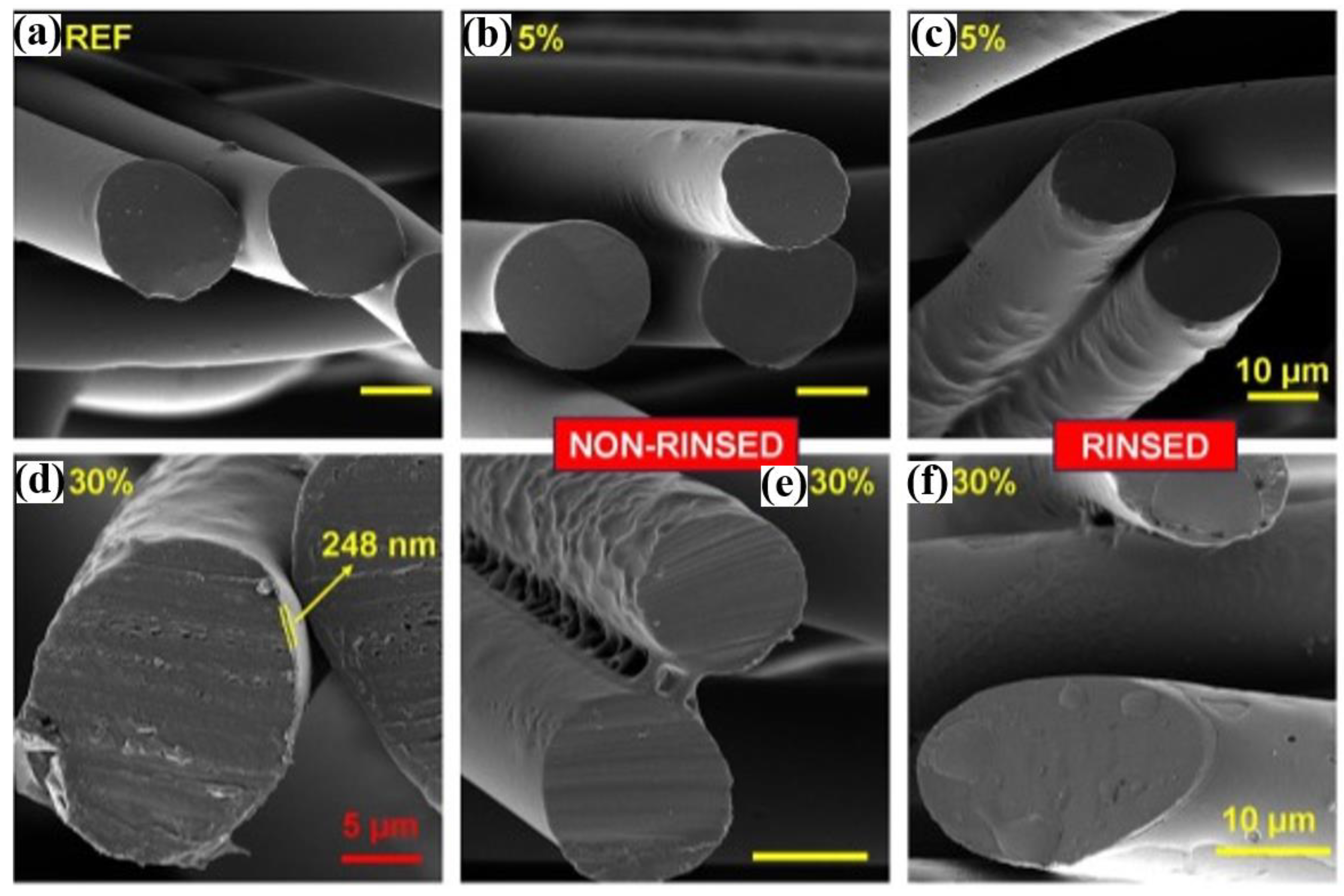
3. Morphological Features
4. Biomedical Applications
4.1. Biocompatibility Enhancement
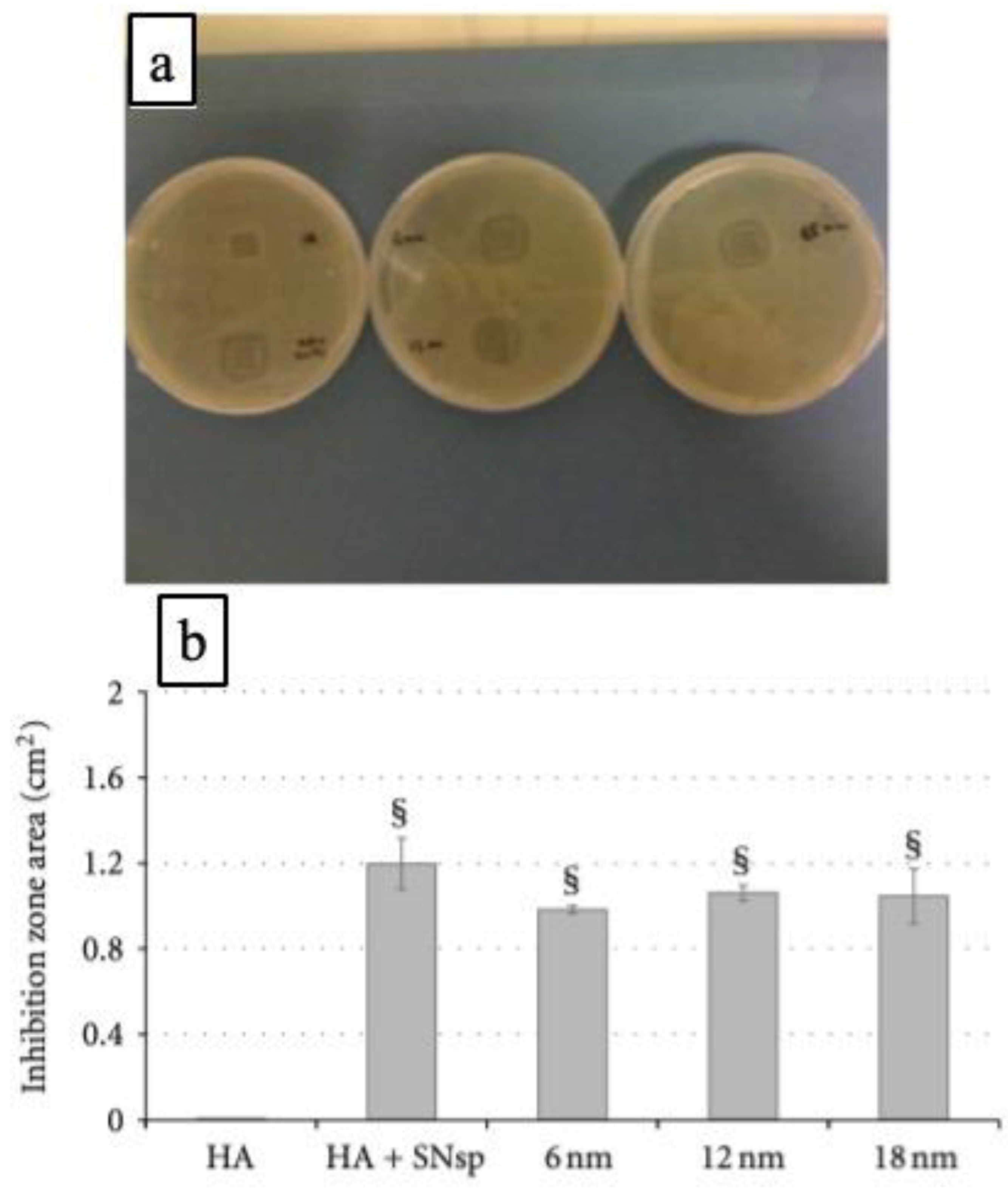
4.2. Uses as Antimicrobial Coatings
4.3. Uses in Attachment of Mammalian Cells
4.4. Silver Coating Using Plasma-Based Polymer
5. Summary and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langmuir, I. Oscillations in ionized gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Khelifa, F.; Ershov, S.; Habibi, Y.; Snyders, R.; Dubois, P. Free-radical-induced grafting from plasma polymer surfaces. Chem. Rev. 2016, 116, 3975–4005. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Jung, E.Y.; Parsons, T.; Tae, H.-S.; Park, C.-S. A review of plasma synthesis methods for polymer films and nanoparticles under atmospheric pressure conditions. Polymers 2021, 13, 2267. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.D.; Kolb, J.F.; Holub, M.; Uhrlandt, D.; Simek, M.; Ostrikov, K.; Hamaguchi, S.; Cvelbar, U.; Cernak, M.; Locke, B.; et al. The future for plasma science and technology. Plasma. Process. Polym. 2019, 16, 1800118. [Google Scholar] [CrossRef]
- Zhou, H.P.; Ye, X.; Huang, W.; Wu, M.Q.; Mao, L.N.; Yu, B.; Xu, S.; Levchenko, I.; Bazaka, K. Wearable, Flexible, Disposable Plasma-Reduced graphene oxide stress sensors for monitoring activities in austere environments. ACS Appl. Mater. Interfaces 2019, 11, 15122–15132. [Google Scholar] [CrossRef]
- Levchenko, I.; Bazaka, K.; Belmonte, T.; Keidar, M.; Xu, S. Advanced materials for next-generation spacecraft. Adv. Mater. 2018, 30, e1802201. [Google Scholar] [CrossRef]
- Levchenko, I.; Xu, S.; Wu, Y.-L.; Bazaka, K. Hopes and concerns for astronomy of satellite constellations. Nat. Astron. 2020, 4, 1012–1014. [Google Scholar] [CrossRef]
- Levchenko, I.; Baranov, O.; Fang, J.; Cherkun, O.; Xu, S.; Bazaka, K. Focusing plasma jets to achieve high current density: Feasibility and opportunities for applications in debris removal and space exploration. Aerosp. Sci. Technol. 2021, 108, 106343. [Google Scholar] [CrossRef]
- Coad, B.R.; Favia, P.; Vasilev, K.; Griesser, H.J. Plasma polymerization for biomedical applications: A review. Plasma Process Polym. 2022, 19, e2200121. [Google Scholar] [CrossRef]
- Jorg, F.F. Plasma Polymerization. In The Plasma Chemistry of Polymer Surfaces: Advanced Techniques for Surface Design, 1st ed.; Wiley-VCH: Weinheim, Germany, 2021; pp. 337–375. [Google Scholar]
- Goodman, J. The formation of thin polymer films in the gas discharge. J. Polym. Sci. 1960, 44, 551–552. [Google Scholar] [CrossRef]
- Lawton, E.L. Adhesion improvement of tire cord induced by gas plasma. J. Appl. Polym. Sci. 1974, 18, 1557–1574. [Google Scholar]
- Stille, J.K.; Rix, C.E. The reaction of halobenzenes in a radiofrequency glow discharge. J. Org. Chem. 1966, 31, 1591–1594. [Google Scholar] [CrossRef]
- Denaro, A.R.; Owens, P.A.; Crawshaw, A. Glow discharge polymerization-II α-methylstyrene, ω-methylstyrene and allylbenzene. Eur. Polym. J. 1969, 5, 471–482. [Google Scholar] [CrossRef]
- Kobayashi, H.; Bell, A.T.; Shen, M. Formation of an amorphous powder during the polymerization of ethylene in a radio-frequency discharge. J. Appl. Polym. Sci. 1973, 17, 885–892. [Google Scholar] [CrossRef]
- Coad, B.R.; Scholz, T.; Vasilev, K.; Hayball, J.D.; Short, R.D.; Griesser, H.J. Functionality of proteins bound to plasma polymer surfaces. ACS Appl. Mater. Interfaces. 2012, 4, 2455–2463. [Google Scholar]
- Truica-Marasescu, F.; Wertheimer, M.R. Nitrogen-rich plasma-polymer films for biomedical applications. Plasma Process. Polym. 2008, 5, 44–57. [Google Scholar] [CrossRef]
- Vasani, R.B.; Szili, E.J.; Rajeev, G.; Voelcker, N.H. On-demand antimicrobial treatment with antibiotic-loaded porous silicon capped with a pH-responsive dual plasma polymer barrier. Chem. Asian J. 2017, 12, 1605–1614. [Google Scholar] [CrossRef]
- Seo, H.J.; Gil, Y.E.; Hwang, K.-H.; Ananth, A.; Boo, J.-H. Synthesis and characterization of plasma-polymer gate dielectric films for graphene field effect transistor devices. Electron. Mater. Lett. 2019, 15, 396–401. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Ikeda, Y. Plasma polymerization of copper phthalocyanines and application of the plasma polymer films to NO2 gas sensor device. J. Appl. Polym. Sci. 1995, 55, 1451–1464. [Google Scholar] [CrossRef]
- He, J.-H.; Singamaneni, S.; Ho, C.H.; Lin, Y.-H.; McConney, M.E.; Tsukruk, V.V. A thermal sensor and switch based on a plasma polymer/ZnO suspended nanobelt bimorph structure. Nanotechnology 2009, 20, 065502. [Google Scholar] [CrossRef]
- Bhatt, S.; Pulpytel, J.; Aref-Khonsari, F. Low and atmospheric plasma polymerisation of nanocoatings for bio-applications. Surf. Innov. 2015, 3, 63–83. [Google Scholar] [CrossRef]
- Hegemann, D.; Lorusso, E.; Butron-Garcia, M.-I.; Blanchard, N.E.; Rupper, P.; Favia, P.; Heuberger, M.; Vandenbossche, M. Suppression of hydrophobic recovery by plasma polymer films with vertical chemical gradients. Langmuir 2016, 32, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Bao, L.; Wang, B.; Fan, M.; Feo, L. Plasma surface modification and bonding enhancement for bamboo composites. Compos. Part B Eng. 2018, 138, 157–167. [Google Scholar] [CrossRef]
- Ishijima, T.; Nosaka, K.; Tanaka, Y.; Uesugi, Y.; Goto, Y.; Horibe, H. A high-speed photoresist removal process using multibubble microwave plasma under a mixture of multiphase plasma environment. Appl. Phys. Lett. 2013, 103, 142101. [Google Scholar] [CrossRef]
- Bitar, R.; Cools, P.; Geyter, N.D.; Morent, R. Acrylic acid plasma polymerization for biomedical use. Appl. Surf. Sci. 2018, 448, 168–185. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef]
- Mun, M.K.; Lee, W.O.; Park, J.W.; Kim, D.S.; Yeom, G.Y.; Kim, D.W. Nanoparticles synthesis and modification using solution plasma process. Appl. Sci. Converg. Technol. 2017, 26, 164–173. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, L.; Chen, J.; Gal, J.-Y.; Wu, H. Synthesis and characterization of aniline and aniline-o-sulfonic acid copolymers. Eur. Polym. J. 2007, 43, 2072–2079. [Google Scholar] [CrossRef]
- Mariotti, D.; Sankaran, R.M. Microplasmas for nanomaterials synthesis. J. Phys. D Appl. Phys. 2010, 43, 323001. [Google Scholar] [CrossRef]
- Özçiçek, N.P.; Pekmez, K.; Holze, R.; Yildiz, A. Spectroelectrochemical investigations of aniline-thiophene copolymers in acetonitrile. J. Appl. Polym. Sci. 2003, 90, 3417–3423. [Google Scholar] [CrossRef]
- Muzammil, I.; Li, Y.; Lei, M. Tunable wettability and pH-responsiveness of plasma copolymers of acrylic acid and octafluorocy-clobutane. Plasma Process. Polym. 2017, 14, 1700053. [Google Scholar] [CrossRef]
- Liu, T.; Yang, F.; Li, Y.; Ren, L.; Zhang, L.; Xu, K.; Wang, X.; Xu, C.; Gao, J. Plasma synthesis of carbon nanotube-gold nanohybrids: Efficient catalysts for green oxidation of silanes in water. J. Mater. Chem. A. 2014, 2, 245–250. [Google Scholar] [CrossRef]
- Fauchais, P.; Etchart-Salas, R.; Rat, V.; Coudert, J.F.; Caron, N.; Wittmann-Ténèze, K. Parameters controlling liquid plasma spraying: Solutions, sols, or suspensions. J. Therm. Spray Technol. 2008, 17, 31–59. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Guo, Y. Synthesis of intrinsic fluorescent polypyrrole nanoparticles by atmospheric pressure plasmapolymerization. Appl. Surf. Sci. 2009, 255, 6924–6929. [Google Scholar] [CrossRef]
- Teslaru, T.; Topala, I.; Dobromir, M.; Pohoata, V.; Curecheriu, L.; Dumitrascu, N. Polythiophene films obtained by polymerization under atmospheric pressure plasma conditions. Mater. Chem. Phys. 2016, 169, 120–127. [Google Scholar] [CrossRef]
- Saito, G.; Akiyama, T. Nanomaterial synthesis using plasma generation in liquid. J. Nanomater. 2015, 2015, 123696. [Google Scholar] [CrossRef]
- Rezaei, F.; Vanraes, P.; Nikiforov, A.; Morent, R.; Geyter, N.D. Applications of plasma-liquid systems: A review. Materials 2019, 12, 2751. [Google Scholar] [CrossRef]
- Seebock, R.; Esrom, H.; Charbonnier, M.; Romand, M.; Kogelschatz, U. Surface modification of polyimide using dielectric barrier discharge treatment. Surf. Coat. Technol. 2001, 142144, 455459. [Google Scholar] [CrossRef]
- Mittal, K.L. Adhesion aspects of metallization of organic polymer surfaces. J. Vac. Sci. Technol. 1976, 13, 19–25. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, S.; Kong, F.; Zhang, C.; Dong, P.; Yan, P.; Cheng, X.; Ostrikov, K.K.; Shao, T. Atmospheric-pressure plasma jet deposition of bumpy coating improves polypropylene surface flashover performance in vacuum. Surf. Coat. Technol. 2020, 387, 125511. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Ramkumar, M.C.; Kumar, A.A.; Vasu, D.; Padmanabhan, P.V.A.; Tabaei, P.S.E.; Cools, P.; Geyter, N.D.; Morent, R.; Jaganathan, S.K. Development of phosphor containing functional coatings via cold atmospheric pressure plasma jet—Study of various operating parameters. Appl. Surf. Sci. 2019, 488, 343–350. [Google Scholar] [CrossRef]
- Hossain, M.M.; Trinh, Q.H.; Nguyen, D.B.; Sudhakaran, M.S.P.; Mok, Y.S. Formation of plasma-polymerized superhydrophobic coating using an atmospheric-pressure plasma jet. Thin Solid Film. 2019, 675, 34–42. [Google Scholar] [CrossRef]
- Jang, H.J.; Park, C.-S.; Jung, E.Y.; Bae, G.T.; Shin, B.J.; Tae, H.-S. Synthesis and properties of thiophene and aniline copolymer using atmospheric pressure plasma jets copolymerization technique. Polymers 2020, 12, 2225. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-S.; Jung, E.Y.; Jang, H.J.; Bae, G.T.; Shin, B.J.; Tae, H.-S. Synthesis and properties of plasma-polymerized methyl methacrylate via the atmospheric pressure plasma polymerization technique. Polymers 2019, 11, 396. [Google Scholar] [CrossRef]
- Doherty, K.G.; Oh, J.S.; Unsworth, P.; Sheridan, C.M.; Weightman, P.; Bradley, J.W.; Williams, R.L. Plasma polymerization using helium atmospheric-pressure plasma jet with heptylamine monomer. Plasma Process. Polym. 2019, 16, e1800185. [Google Scholar] [CrossRef]
- Kim, J.Y.; Iqbal, S.; Jang, H.J.; Jung, E.Y.; Bae, G.T.; Park, C.-S.; Tae, H.-S. In-situ iodine doping characteristics of conductive polyaniline film polymerized by low-voltage-driven atmospheric pressure plasma. Polymers 2021, 13, 418. [Google Scholar] [CrossRef]
- Yan, X.; Liu, G.-S.; Yang, J.; Pu, Y.; Chen, S.; He, H.-W.; Wang, C.; Long, Y.-Z.; Jiang, S. In situ surface modification of paper-based relics with atmospheric pressure plasma treatment for preservation purposes. Polymers 2019, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Karl, C.W.; Rahimi, W.; Kubowicz, S.; Lang, A.; Geisler, H.; Giese, U. Surface modification of ethylene propylene diene terpolymer rubber by plasma polymerization using organosilicon precursors. ACS Appl. Polym. Mater. 2020, 2, 3789–3796. [Google Scholar] [CrossRef]
- Yang, J.; Pu, Y.; Miao, D.; Ning, X. Fabrication of durably superhydrophobic cotton fabrics by atmospheric pressure plasma treatment with a siloxane precursor. Polymers 2018, 10, 460. [Google Scholar] [CrossRef]
- Mertens, J.; Nisol, B.; Hubert, J.; Reniers, F. Use of remote atmospheric mass spectrometry in atmospheric plasma polymerization of hydrophilic and hydrophobic coatings. Plasma Process. Polym. 2020, 17, 1900250. [Google Scholar] [CrossRef]
- Getnet, T.G.; da Silva, G.F.; Duarte, I.S.; Kayama, M.E.; Rangel, E.C.; Cruz, N.C. Atmospheric pressure plasma chemical vapor deposition of carvacrol thin films on stainless steel to reduce the formation of E. coli and S. aureus biofilms. Materials 2020, 13, 3166. [Google Scholar] [CrossRef] [PubMed]
- Sťahel, P.; Mazánková, V.; Tomečková, K.; Matoušková, P.; Brablec, A.; Prokeš, L.; Jurmanová, J.; Buršíková, V.; Přibyl, R.; Lehocký, M.; et al. Atmospheric pressure plasma polymerized oxazoline-based thin films-antibacterial properties and cytocompatibility performance. Polymers 2019, 11, 2069. [Google Scholar] [CrossRef]
- Bardon, J.; Martin, A.; Fioux, P.; Amari, T.; Mertz, G.; Delmée, M.; Ruch, D.; Roucoules, V. Reinforcement of a dodecylacrylate plasma polymer by admixture of a diacrylate or a dimethacrylate cross-linker. Plasma Process. Polym. 2018, 15, 1800031. [Google Scholar] [CrossRef]
- Demaude, A.; Poleunis, C.; Goormaghtigh, E.; Viville, P.; Lazzaroni, R.; Delcorte, A.; Gordon, M.; Reniers, F. Atmospheric pressure plasma deposition of hydrophilic/phobic patterns and thin film laminates on any surface. Langmuir 2019, 35, 9677–9683. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, L.; Nikiforov, A.; Onyshchenko, Y.; Cools, P.; Ostrikov, K.; Geyter, N.D.; Morent, R. Atmospheric-pressure plasma assisted engineering of polymer surfaces: From high hydrophobicity to superhydrophilicity. Appl. Surf. Sci. 2021, 535, 147032. [Google Scholar] [CrossRef]
- Loyer, F.; Bengasi, G.; Frache, G.; Choquet, P.; Boscher, N.D. Insights in the initiation and termination of poly (alkyl acrylates) synthesized by atmospheric pressure plasma-initiated chemical vapor deposition (AP-PiCVD). Plasma Process. Polym. 2018, 15, 1800027. [Google Scholar] [CrossRef]
- Loyer, F.; Combrisson, A.; Omer, K.; Moreno-Couranjou, M.; Choquet, P.; Boscher, N.D. Thermoresponsive water-solublepolymer layers and water-stable copolymer layers synthesized by atmospheric plasma initiated chemical vapor deposition. ACS Appl. Mater. Interfaces 2019, 11, 1335–1343. [Google Scholar] [CrossRef]
- Abessolo Ondo, D.; Loyer, F.; Werner, F.; Leturcq, R.; Dale, P.J.; Boscher, N.D. Atmospheric-pressure synthesis of atomically smooth, conformal, and ultrathin low-k polymer insulating layers by plasma-initiated chemical vapor deposition. ACS Appl. Polym. Mater. 2019, 1, 3304–3312. [Google Scholar] [CrossRef]
- Khoo, Y.S.; Lau, W.J.; Liang, Y.Y.; Karaman, M.; Gürsoy, M.; Lai, G.S.; Ismail, A.F. Rapid and eco-friendly technique for surface modification of TFC RO membrane for improved filtration performance. J. Environ. Chem. Eng. 2021, 9, 105227. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.J.; Park, C.-S.; Shin, B.J.; Seo, J.H.; Tae, H.-S. Atmospheric pressure plasma polymerization using double grounded electrodes with He/Ar mixture. AIP Adv. 2015, 5, 097137. [Google Scholar] [CrossRef]
- Kodaira, F.V.P.; Ricci Castro, A.H.; Prysiazhnyi, V.; Mota, R.P.; Quade, A.; Kostov, K.G. Characterization of plasma polymerized HMDSN films deposited by atmospheric plasma jet. Surf. Coat. Technol. 2017, 312, 117–122. [Google Scholar] [CrossRef]
- Malinowski, S.; Herbert, P.A.F.; Rogalski, J.; Jaroszyńska-Wolińska, J. Laccase enzyme polymerization by soft plasma jet for durable bioactive coatings. Polymers 2018, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-S.; Kim, D.Y.; Kim, D.H.; Lee, H.-K.; Shin, B.J.; Tae, H.-S. Humidity-independent conducting polyaniline films synthe sized using advanced atmospheric pressure plasma polymerization with in-situ iodine doping. Appl. Phys. Lett. 2017, 110, 033502. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.-S.; Kim, W.H.; Shin, B.J.; Hong, J.G.; Park, T.S.; Seo, J.H.; Tae, H.-S. Influences of guide-tube and bluff-body on advanced atmospheric pressure plasma source for single-crystalline polymer nanoparticle synthesis at low temperature. Phys. Plasmas 2017, 24, 023506. [Google Scholar] [CrossRef]
- Moosburger-Will, J.; Bauer, M.; Schubert, F.; Kunzmann, C.; Lachner, E.; Zeininger, H.; Maleika, M.; Hönisch, B.; Küpfer, J.; Zschoerper, N.; et al. Methyltrimethoxysilane plasma polymerization coating of carbon fiber surfaces. Surf. Coat. Technol. 2017, 311, 223–230. [Google Scholar] [CrossRef]
- Ibrahim, J.; Al-Babtaineh, S.A.; Cousens, S.; Michelmore, A.; Corr, C.; Whittle, J. A surface dielectric barrier discharge for deposition of allylamine polymer costings. Appl. Surf. Sci. 2021, 544, 148826. [Google Scholar] [CrossRef]
- Ramkumar, M.C.; Navaneetha Pandiyaraj, K.; Arun Kumar, A.; Padmanabhan, P.V.A.; Cools, P.; Geyter, N.D.; Morent, R.; Uday Kumar, S.; Kumar, V.; Gopinath, P.; et al. Atmospheric pressure non-thermal plasma assisted polymerization of poly (ethylene glycol) methylether methacrylate (PEGMA) on low density polyethylene (LDPE) films for enhancement of biocompatibility. Surf. Coat. Technol. 2017, 329, 55–67. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Ramkumar, M.C.; Arun Kumar, A.; Padmanabhan, P.V.A.; Pichumani, M.; Bendavid, A.; Cools, P.; Geyter, N.D.; Morent, R.; Kumar, V.; et al. Evaluation of surface properties of low-density polyethylene (LDPE) films tailored by atmospheric pressure non-thermal plasma (APNTP) assisted co-polymerization and immobilization of chitosan for improvement of antifouling properties. Mater. Sci. Eng. C 2019, 94, 150–160. [Google Scholar] [CrossRef]
- Dvořáková, H.; Čech, J.; Stupavská, M.; Prokeš, L.; Jurmanová, J.; Buršíková, V.; Ráheľ, J.; Sťahel, P. Fast surface hydrophilization via atmospheric pressure plasma polymerization for biological and technical applications. Polymers 2019, 11, 1613. [Google Scholar] [CrossRef]
- Manakhov, A.; Michlícek, M.; Necas, D.; Polcák, J.; Makhneva, E.; Eliáš, M.; Zajíčková, L. Carboxyl-rich coatings deposited by atmospheric plasma co-polymerization of maleic anhydride and acetylene. Surf. Coat. Technol. 2016, 295, 37–45. [Google Scholar]
- Jalaber, V.; Del Frari, D.; De Winter, J.; Mehennaoui, K.; Planchon, S.; Choquet, P.; Detrembleur, C.; Moreno-Couranjou, M. Atmospheric aerosol assisted pulsed plasma polymerization: An environmentally friendly technique for tunable catechol-bearing thin films. Front. Chem. 2019, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.; Fricke, K.; Mika, F.; Pokorná, Z.; Zajíčková, L.; Foest, R. Liquid assisted plasma enhanced chemical vapour deposition with a non-thermal plasma jet at atmospheric pressure. Thin Solid Film. 2017, 630, 71–78. [Google Scholar] [CrossRef]
- Tan, P.E.C.; Mahinay, C.L.S.; Culaba, I.B.; Streeter, O.K.M.; Hilario, M.R.A. Plasma polymerization of styrene using an argon-fed atmospheric pressure plasma jet. J. Vac. Sci. Technol. B 2018, 36, 04I102. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Sun, D.; Zhang, R.; Lin, W.-F.; Macias-Montero, M.; Patel, J.; Askari, S.; McDonald, C.; Mariotti, D.; Maguire, P. Gold nanoparticle-polymer nanocomposites synthesized by room temperature atmospheric pressure plasma and their potential for fuel cell electrocatalytic application. Sci. Rep. 2017, 7, 46682. [Google Scholar] [CrossRef]
- Gamaleev, V.; Kajikawa, K.; Takeda, K.; Hiramatsu, M. Investigation of nanographene produced by in-liquid plasma for development of highly durable polymer electrolyte fuel cells. J. Carbon Res. 2018, 4, 65. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef]
- Omurzak, E.; Shimokawa, W.; Taniguchi, K.; Chen, L.; Okamoto, M.; Iwasaki, H.; Yamasaki, M.; Kawamura, Y.; Sulaimankulova, S.; Mashimo, T. Synthesis of wurtzite-type ZnMgS by the pulsed plasma in liquid. Jpn. J. Appl. Phys. 2011, 50, 01AB09. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. In-liquid plasma: A novel tool in the fabrication of nanomaterials and in the treatment of wastewaters. RSC Adv. 2017, 7, 47196. [Google Scholar] [CrossRef]
- Hyun, K.Y.; Ueno, T.; Li, O.L.; Saito, N. Synthesis of heteroatom-carbon nanosheets by solution plasma processing using N-methyl-2-pyrrolidone as precursor. RSC Adv. 2016, 6, 6990–6996. [Google Scholar] [CrossRef]
- Lee, S.; Heo, Y.K.; Bratescu, M.A.; Ueno, T.; Saito, N. Solution plasma synthesis of a boron-carbon-nitrogen catalyst with a controllable bond structure. Phys. Chem. Chem. Phys. 2017, 19, 15264–15272. [Google Scholar] [CrossRef]
- Tipplook, M.; Pornaroontham, P.; Watthanaphanit, A.; Saito, N. Liquid-phase plasma-assisted in situ synthesis of amino-rich nanocarbon for transition metal ion adsorption. ACS Appl. Nano Mater. 2020, 3, 218–228. [Google Scholar] [CrossRef]
- Shin, J.-G.; Park, C.-S.; Jung, E.Y.; Shin, B.J.; Tae, H.S. Synthesis of a polyaniline nanoparticle using a solution plasma process with an Ar gas bubble channel. Polymers 2019, 11, 105. [Google Scholar] [CrossRef]
- Shin, J.-G.; Shin, B.J.; Jung, E.Y.; Park, C.-S.; Kim, J.Y.; Tae, H.-S. Effects of a dielectric barrier discharge (DBD) on characteristics of polyaniline nanoparticles synthesized by a solution plasma process with an Ar gas bubble channel. Polymers 2020, 12, 1939. [Google Scholar] [CrossRef]
- Kováčik, D.; Šrámková, P.; Multáňová, P.; Stupavská, M.; Siadati, S.; Ďurina, P.; Zahoranov, A. Plasma-induced polymerization and grafting of acrylic acid on the polypropylene nonwoven fabric using pulsed underwater diaphragm electrical discharge. Plasma Chem. Plasma Process. 2024, 44, 983–1001. [Google Scholar] [CrossRef]
- Asandulesa, M.; Topala, I.; Pohoata, V.; Legrand, Y.M.; Dobromir, M.; Totolin, M.; Dumitrascu, N. Chemically polymerization mechanism of aromatic compounds under atmospheric pressure plasma conditions. Plasma Chem. Plasma Process. 2013, 10, 469–480. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Hegemann, D. Recent approaches to reduce aging phenomena in oxygen-and nitrogen-containing plasma polymer films: An overview. Curr. Opin. Solid State Mater. Sci. 2018, 22, 26–38. [Google Scholar] [CrossRef]
- Hegemann, D.; Hossain, M.M.; Korner, E.; Balazs, D.J. Macroscopic description of plasma polymerization. Plasma Process. Polym. 2007, 4, 229–238. [Google Scholar] [CrossRef]
- Ligot, S.; Bousser, E.; Cossement, D.; Klemberg Sapieha, J.; Viville, P.; Dubois, P.; Snyders, R. Correlation between mechanical properties and cross-linking degree of ethyl lactate plasma polymer films. Plasma Process. Polym. 2015, 12, 508–518. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, N.; Kim, U.Y.; Hong, S.I.; Sasabe, H. Plasma polymerization of hexamethyldisilazane. Polym. J. 1990, 22, 242–249. [Google Scholar] [CrossRef]
- Cruz, G.J.; Morales, J.; Castillo-Ortega, M.M.; Olayo, R. Synthesis of polyaniline films by plasma polymerization. Synth. Met. 1997, 88, 213–218. [Google Scholar] [CrossRef]
- Kim, J.Y.; Iqbal, S.; Jang, H.J.; Jung, E.Y.; Bae, G.T.; Park, C.S.; Shin, B.J.; Tae, H.S. Transparent polyaniline thin film synthesized using a low-voltage-driven atmospheric pressure plasma reactor. Materials 2021, 14, 1278. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Kumar, V.; Subramaniyan, V.; Nagarajan, K.; Sekar, M.; Chinni, S.V.; Ramachawolran, G. Plasma modification techniques for natural polymer-based drug delivery systems. Pharmaceutics 2023, 15, 2066. [Google Scholar] [CrossRef] [PubMed]
- Baki, A.; Rahman, C.V.; White, L.J.; Scurr, D.J.; Qutachi, O.; Shakesheff, K.M. Surface modification of PDLLGA microspheres with gelatine methacrylate: Evaluation of adsorption, entrapment, and oxygen plasma treatment approaches. Acta Biomater. 2017, 53, 450–459. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 9780471724254. [Google Scholar]
- Farr, N.; Thanarak, J.; Schäfer, J.; Quade, A.; Claeyssens, F.; Green, N.; Rodenburg, C. Understanding surface modifications induced via argon plasma treatment through secondary electron hyperspectral imaging. Adv. Sci. 2021, 8, 2003762. [Google Scholar] [CrossRef]
- Ghobeira, R.; Philips, C.; Declercq, H.; Cools, P.; De Geyter, N.; Cornelissen, R.; Morent, R. Effects of different sterilization methods on the physico-chemical and bioresponsive properties of plasma-treated polycaprolactone Films. Biomed. Mater. 2017, 12, 015017. [Google Scholar] [CrossRef]
- Bullard, K.K.; Srinivasarao, M.; Gutekunst, W.R. Modification of cellulose nanocrystal surface chemistry with diverse nucleo philes for materials integration. J. Mater. Chem. A 2020, 8, 18024–18031. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Nagashima, S.; Hasebe, T.; Tsuya, D.; Horikoshi, T.; Ochiai, M.; Tanigawa, S.; Koide, Y.; Hotta, A.; Suzuki, T. Controlled formation of wrinkled diamond-like carbon (DLC) film on grooved poly(dimethylsiloxane) substrate. Diam. Relat. Mater. 2012, 22, 48–51. [Google Scholar] [CrossRef]
- Tan, G.; Chen, R.; Ning, C.; Zhang, L.; Ruan, X.; Liao, J. effects of argon plasma treatment on surface characteristic of photopolymerization PEGDA–HEMA hydrogels. J. Appl. Polym. Sci. 2012, 124, 459–465. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Han, S.; Kim, K.-J. Improvement of hydrophobic properties of polymer surfaces by plasma source ion implantation. Surf. Coat. Technol. 2006, 200, 4763–4769. [Google Scholar] [CrossRef]
- Michael, P.; Lam, Y.T.; Filipe, E.C.; Tan, R.P.; Chan, A.H.P.; Lee, B.S.L.; Feng, N.; Hung, J.; Cox, T.R.; Santos, M.; et al. Plasma polymerized nanoparticles effectively deliver dual SiRNA and drug therapy in vivo. Sci. Rep. 2020, 10, 12836. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Griesser, S.S.; Griesser, H.J. Antibacterial surfaces and coatings produced by plasma techniques. Plasma. Process. Polym. 2011, 8, 1010. [Google Scholar] [CrossRef]
- Nikiforov, A.; Deng, X.L.; Xiong, Q.; Cvelbar, U.; De Geyter, N.; Morent, R.; Leys, C. Non-thermal plasma technology for the development of antimicrobial surfaces: A review. J. Phys. D Appl. Phys. 2016, 49, 204002. [Google Scholar] [CrossRef]
- Sardella, E.; Palumbo, F.; Camporeale, G.; Favia, P. Nonequilibrium plasma processing for the preparation of antibacterial surfaces. Materials 2016, 9, 515. [Google Scholar] [CrossRef]
- Michl, T.D.; Coad, B.R.; Husler, A.; Valentin, J.D.P.; Vasilev, K.; Griesser, H.J. Effects of precursor and deposition conditions on prevention of bacterial biofilm growth on chlorinated plasma polymers. Plasma Process. Polym. 2016, 13, 654. [Google Scholar] [CrossRef]
- Michl, T.D.; Coad, B.R.; Doran, M.; Osiecki, M.; Kafshgari, M.H.; Voelcker, N.H.; Husler, A.; Vasilev, K.; Griesser, H.J. Nitric oxide releasing plasma polymer coating with bacteriostatic properties and no cytotoxic side effects. Chem. Commun. 2015, 51, 7058. [Google Scholar] [CrossRef]
- Pegalajar-Jurado, A.; Easton, C.D.; Styan, K.E.; McArthur, S.L. Antibacterial activity studies of plasma polymerised cineole films. J. Mater. Chem. B 2014, 2, 4993. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Bazaka, K.; Jacob, M.V. Review on the antimicrobial properties of carbon nanostructures. Nanomaterials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Kumar, A.; Al-Jumaili, A.; Prasad, K.; Bazaka, K.; Mulvey, P.; Warner, J.; Jacob, M.V. Pulse plasma deposition of Terpinen-4-ol: An insight into polymerization mechanism and enhanced antibacterial response of developed thin films. Plasma Chem. Plasma Process. 2020, 40, 339. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.N.; Cavallaro, A.A.; Vasilev, K. Properties and reactivity of polyoxazoline plasma polymer films. J. Mater. Chem. B 2015, 3, 6327. [Google Scholar] [CrossRef] [PubMed]
- Ramiasa, M.; Cavallaro, A.; Mierczynska, A.; Christo, S.; Gleadle, J.; Hayball, J.; Vasilev, K. Plasma polymerised polyoxazoline thin films for biomedical applications. Chem. Commun. 2015, 51, 4279. [Google Scholar] [CrossRef]
- Cavallaro, A.A.; Macgreggor-Ramiasa, M.N.; Vasilev, K.A. Plasma Polymerised Oxazoline Coatings and Uses Thereof. WO2017035566A1, 9 March 2017. [Google Scholar]
- Cavallaro, A.A.; Macgregor-Ramiasa, M.N.; Vasilev, K. Antibiofouling properties of plasma-deposited oxazoline-based thin films. ACS Appl. Mater. Interfaces 2016, 8, 6354–6362. [Google Scholar] [CrossRef]
- Chan, Y.W.; Siow, K.S.; Ng, P.Y.; Gires, U.; Yeop Majlis, B. Plasma polymerized carvone as an antibacterial and biocompatible coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 861. [Google Scholar] [CrossRef]
- Kaleli-Can, G.; OOzguzar, H.F.; Kahriman, S.; Turkal, M.; Gocmen, J.S.; Yurtcu, E.; Mutlu, M. Improvement in antimicrobial properties of titanium by diethyl phosphite plasma-based surface modification. Mater. Today Commun. 2020, 25, 101565. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Hoffman, A.S. Gas-discharge techniques for biomaterial modification. Crit. Rev. Biocompat. 1987, 4, 1–42. [Google Scholar]
- Barry, J.J.A.; Howard, D.; Shakesheff, K.M.; Howdle, S.M.; Alexander, M.R. Using a core-sheath distribution of surfacechemistry through 3d tissue engineering scaffolds to control cell ingress. Adv. Mater. 2006, 18, 1406–1410. [Google Scholar] [CrossRef]
- Intranuovo, F.; Gristina, R.; Brun, F.; Mohammadi, S.; Ceccone, G.; Sardella, E.; Rossi, F.; Tromba, G.; Favia, P. Plasma modification of pcl porous scaffolds fabricated by solvent casting/particulate-leaching for tissue engineering. Plasma. Process. Polym. 2014, 11, 184. [Google Scholar] [CrossRef]
- Sardella, E.; Salama, R.A.; Waly, G.H.; Habib, A.N.; Favia, P.; Gristina, R. Improving internal cell colonization of porous scaffolds with chemical gradients produced by plasma assisted approaches. ACS Appl. Mater. Interfaces 2017, 9, 4966. [Google Scholar] [CrossRef]
- Barry, J.J.; Silva, M.M.; Shakesheff, K.M.; Howdle, S.M.; Alexander, M.R. Using plasma deposits to promote cell population of the porous interior of three-dimensional Poly (D, L-Lactic Acid) tissue-engineering scaffolds. Adv. Funct. Mater. 2005, 15, 1134–1140. [Google Scholar] [CrossRef]
- Intranuovo, F.; Sardella, E.; Gristina, R.; Nardulli, M.; White, L.; Howard, D.; Shakesheff, K.M.; Alexander, M.R.; Favia, P. E-CVD processes improve cell affinity of polymer scaffolds for tissue engineering. Surf. Coat. Technol. 2011, 205, S548. [Google Scholar] [CrossRef]
- Sardella, E.; Fisher, E.R.; Shearer, J.C.; Garzia Trulli, M.; Gristina, R.; Favia, P. N2/H2O plasma assisted functionalization of poly(ε-caprolactone) porous scaffolds: Acidic/basic character versus cell behavior. Plasma. Process. Polym. 2015, 12, 786. [Google Scholar] [CrossRef]
- Armenise, V.; Gristina, R.; Favia, P.; Cosmai, S.; Fracassi, F.; Sardella, E. Plasma-assisted deposition of magnesium-containing coatings on porous scaffolds for bone tissue engineering. Coatings 2020, 10, 356. [Google Scholar] [CrossRef]
- Ploux, L.; Mateescu, M.; Anselme, K.; Vasilev, K. Antibacterial properties of silver-loaded plasma polymer coatings. J. Nanomater. 2012, 2012, 674145. [Google Scholar] [CrossRef]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Haidari, H.; Garg, S.; Vasilev, K.; Kopecki, Z.; Cowin, A.J. Silver-based wound dressings: Current issues and future developments for treating bacterial infections. Wound Pract. Res. 2020, 28, 173–180. [Google Scholar] [CrossRef]
- Vasilev, K. Nanoengineered antibacterial coatings and aaterials: A perspective. Coatings 2019, 9, 654. [Google Scholar] [CrossRef]




| Polymer | Plasma Treatment | Significance and Particularity | Ref. |
|---|---|---|---|
| Starch in film form | N2 plasma | Enhanced cell adhesion | [98] |
| Cellulose in nanocrystal form | O2 plasma | Enhanced cell viability | [99] |
| Polycarbonate (coated with diamond-like carbon film) | Radiofrequency (RF) plasma | Inhibition of protein adsorption, less platelet adhesion | [100] |
| Poly(dimethylsiloxane) (coated with diamond-like carbon film) | Ar plasma | Cellular responses in mammalian cells. | [101] |
| Pectin hydrogels | Ar plasma | Enhanced cell adhesion | [102] |
| Low-density polyethylene | Plasma source ion implantation | Enhanced surface hydrophobic features | [103] |
| Polymerized nanoparticles | Reactive gas emissions plasma | Considerably lessened tumor growth in mice | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman Khan, M.M.; Asrafali, S.P.; Periyasamy, T. Synthesis, Morphology, and Biomedical Applications of Plasma-Based Polymers: Recent Trends and Advances. Polymers 2024, 16, 2701. https://doi.org/10.3390/polym16192701
Rahman Khan MM, Asrafali SP, Periyasamy T. Synthesis, Morphology, and Biomedical Applications of Plasma-Based Polymers: Recent Trends and Advances. Polymers. 2024; 16(19):2701. https://doi.org/10.3390/polym16192701
Chicago/Turabian StyleRahman Khan, Mohammad Mizanur, Shakila Parveen Asrafali, and Thirukumaran Periyasamy. 2024. "Synthesis, Morphology, and Biomedical Applications of Plasma-Based Polymers: Recent Trends and Advances" Polymers 16, no. 19: 2701. https://doi.org/10.3390/polym16192701
APA StyleRahman Khan, M. M., Asrafali, S. P., & Periyasamy, T. (2024). Synthesis, Morphology, and Biomedical Applications of Plasma-Based Polymers: Recent Trends and Advances. Polymers, 16(19), 2701. https://doi.org/10.3390/polym16192701







