Syndiotactic Polyolefins by Hydrogenation of Highly Stereoregular 1,2 Polydienes: Synthesis and Structural Characterization
Abstract
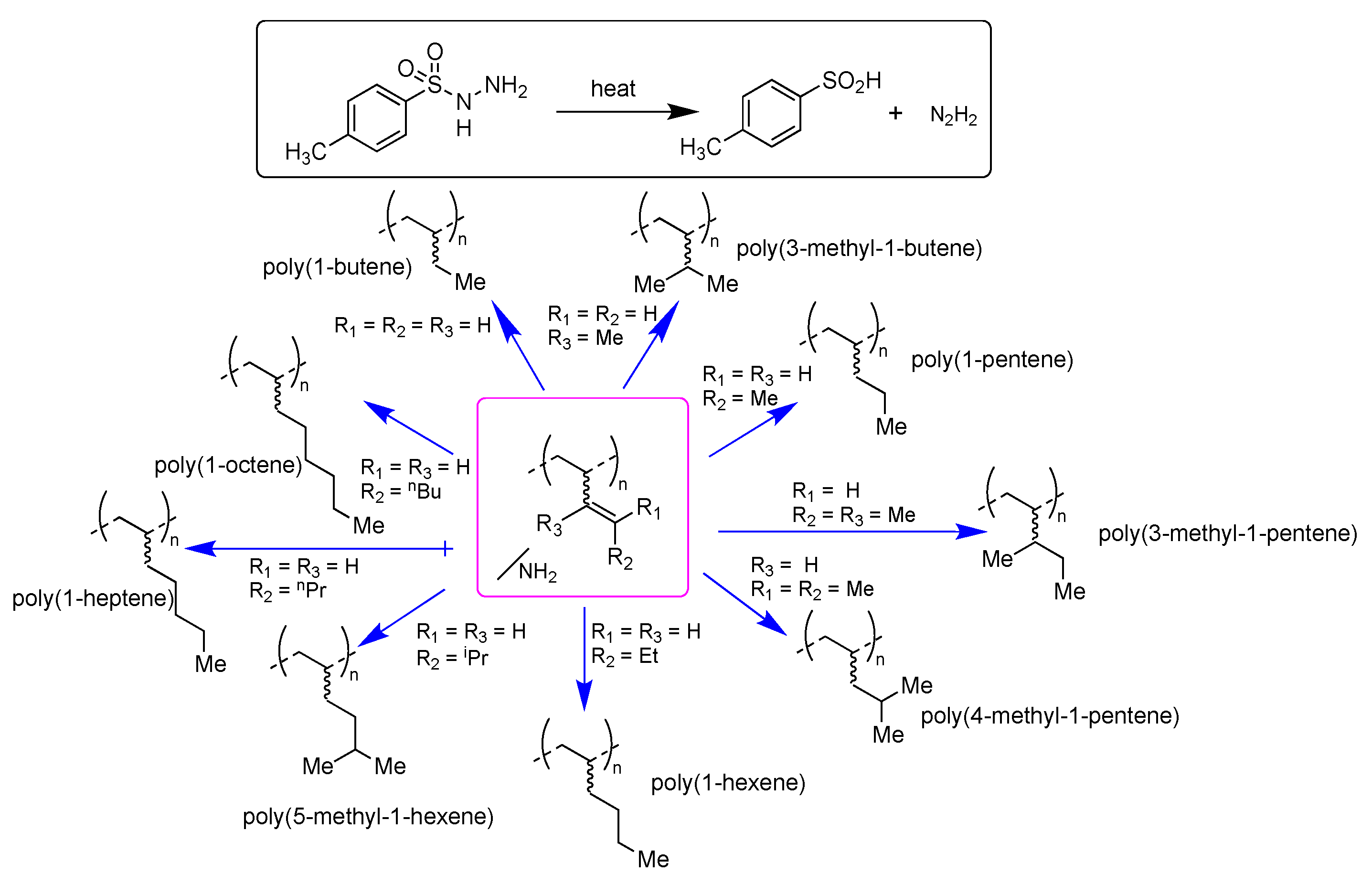
1. Introduction
2. Materials and Methods
2.1. General Procedures and Materials
2.2. Hydrogenation Procedure
2.3. Polymer Characterization
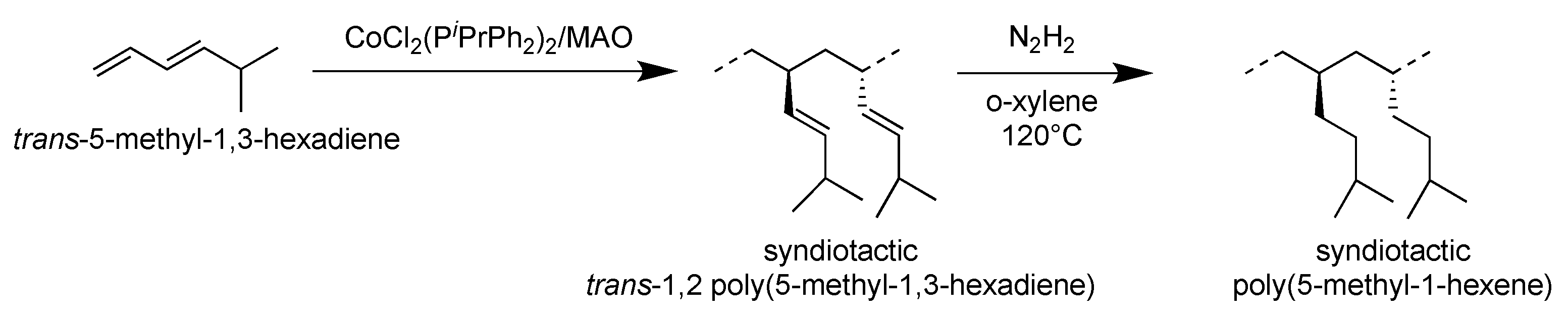
3. Results and Discussion
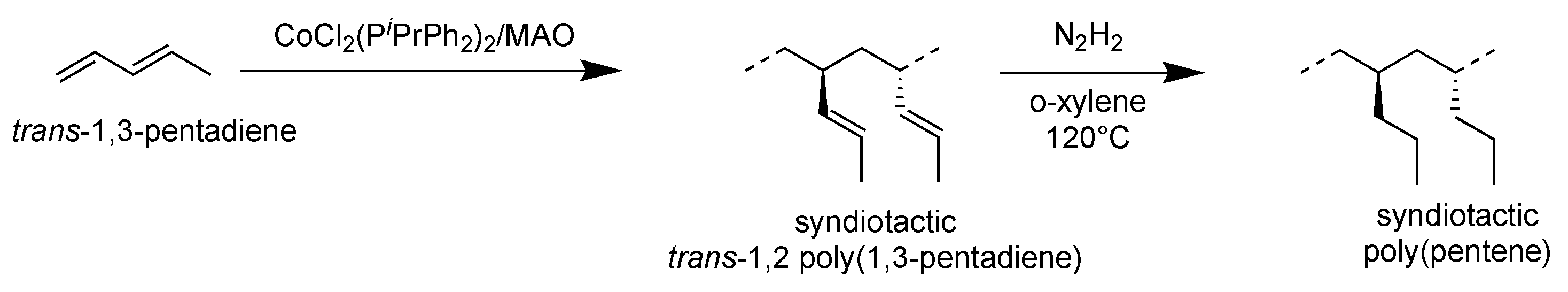
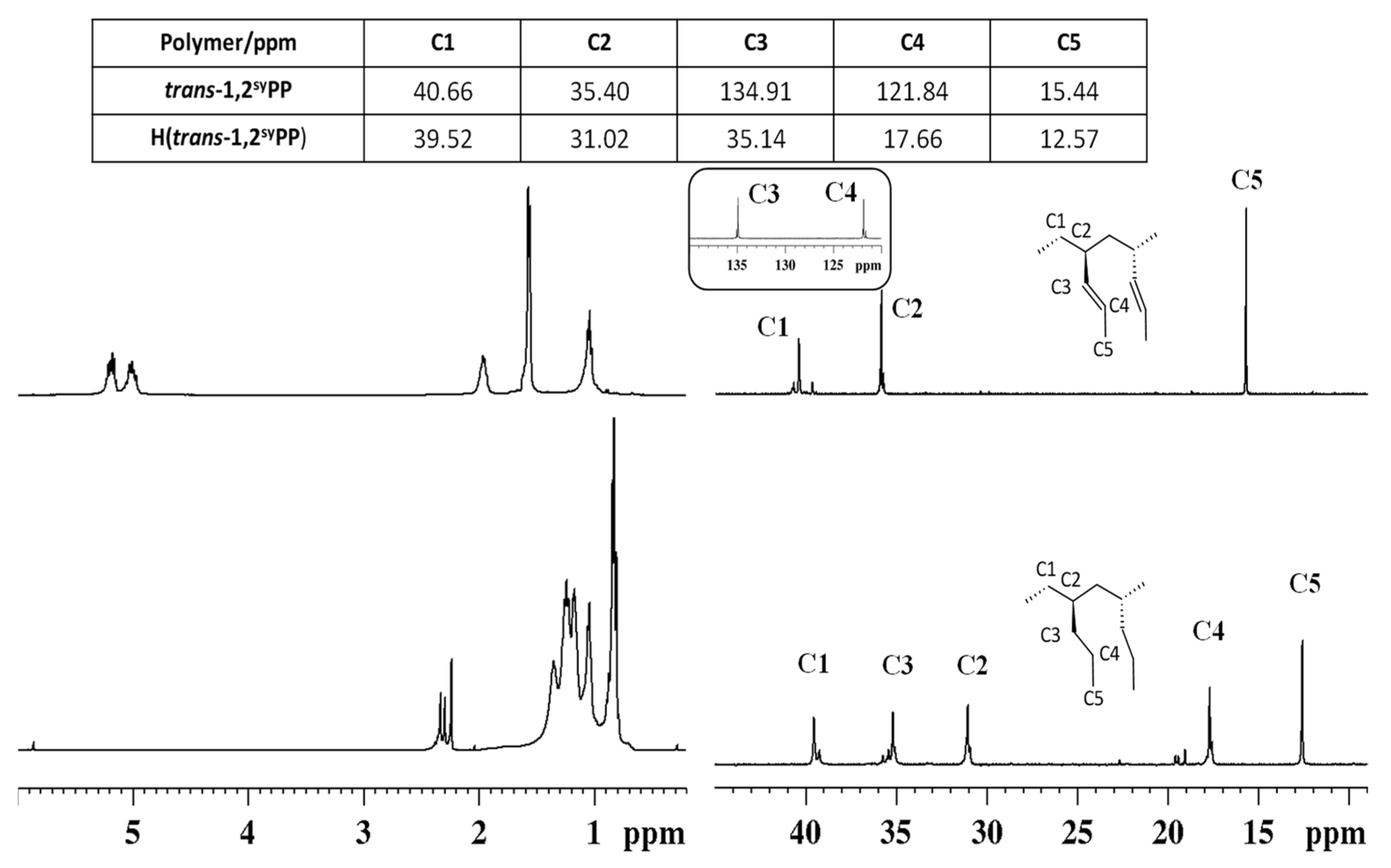
3.1. Syndiotactic Poly(1-pentene)_H(1,2syPP)
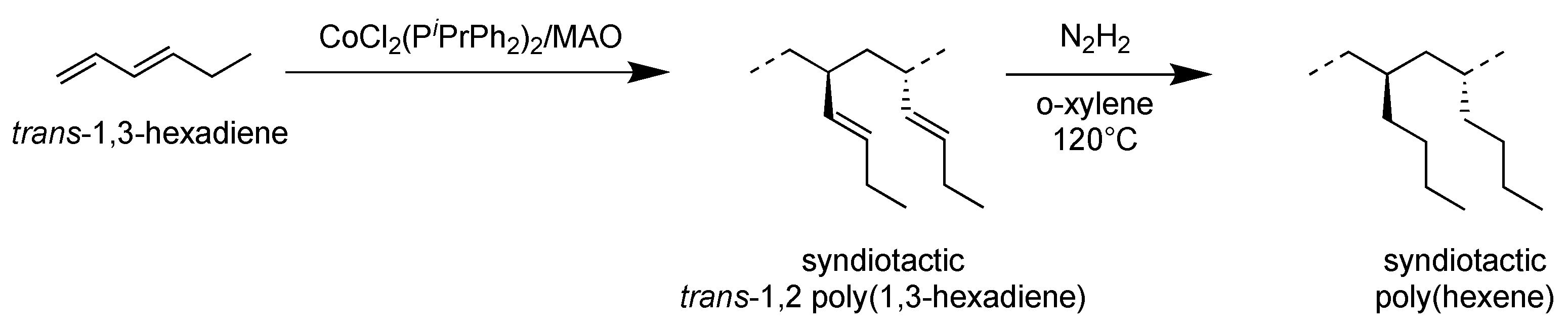
3.2. Syndiotactic Poly(1-hexene)_H(1,2syPHX)
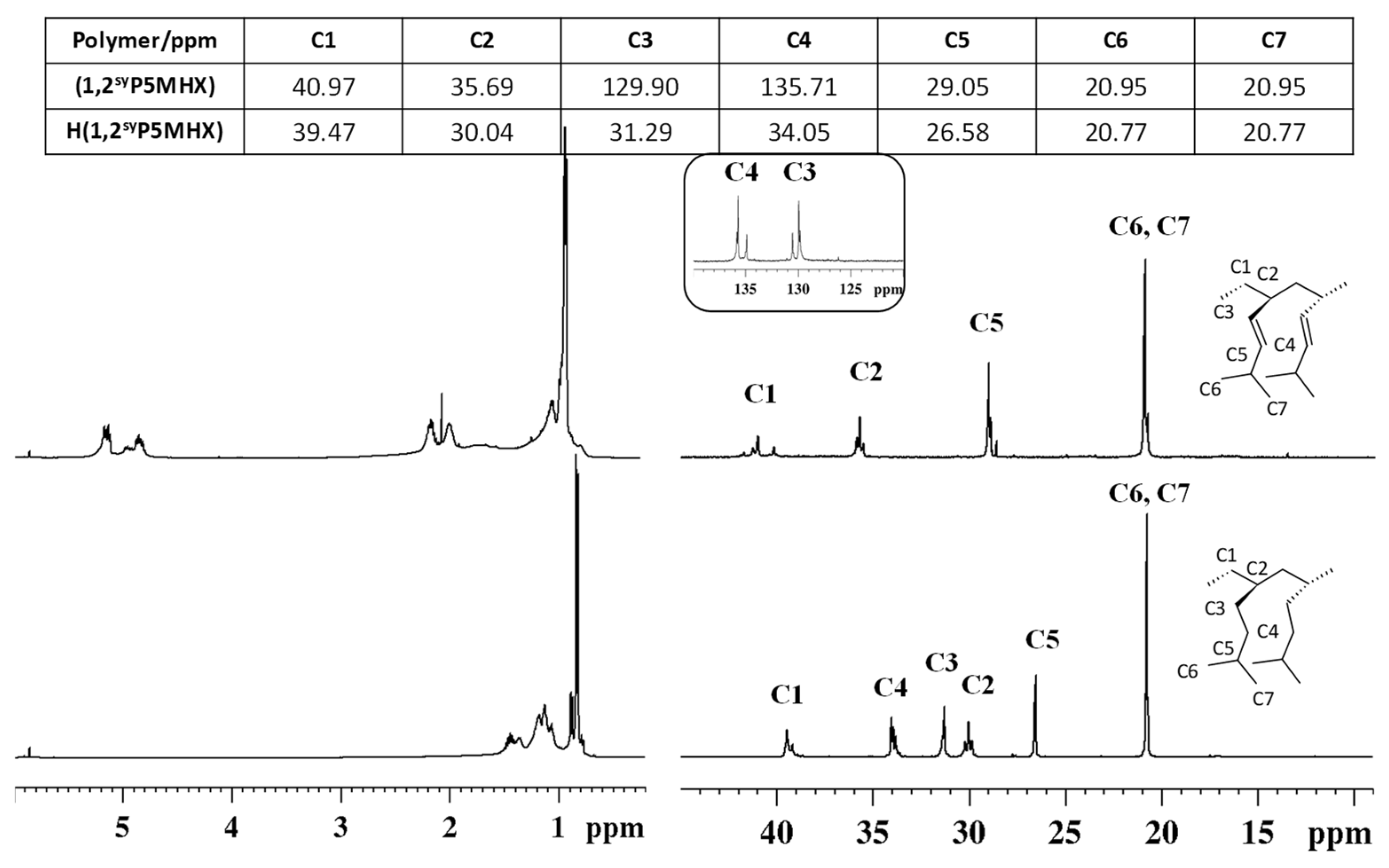
3.3. Syndiotactic Poly(5-methyl-1-hexene) [H(1,2syP5MHX)]
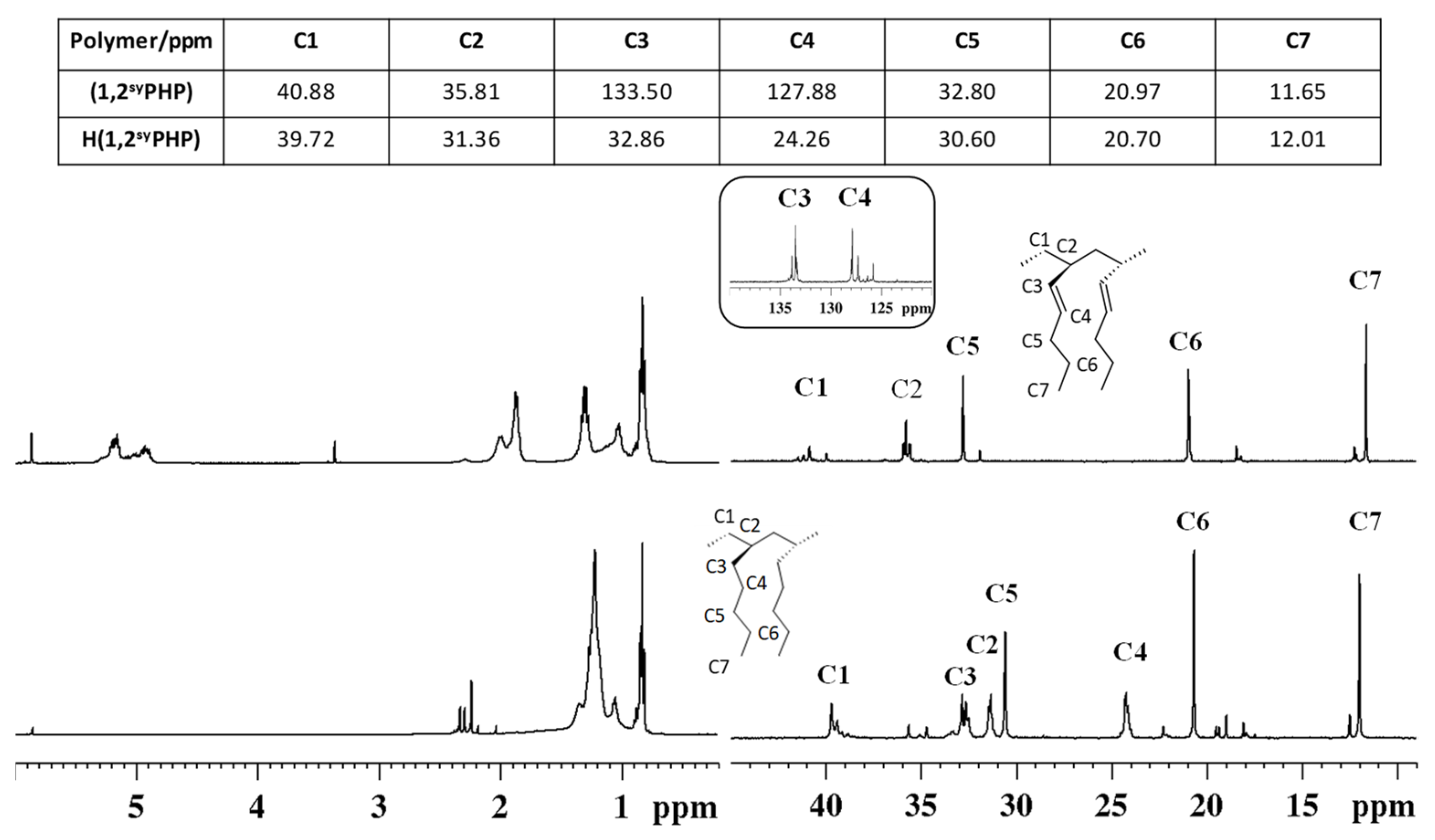
3.4. Syndiotactic Poly(1-heptene)_H(1,2syPHP)
3.5. Syndiotactic Poly(1-octene)_[H(1,2syPO)]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porri, L.; Giarrusso, A. Conjugated Diene Polymerization. In Comprehensive Polymer Science; Eastmond, G., Edwith, A., Russo, S., Sigwalt, P., Eds.; Pergamon Press Ltd: Oxford, UK, 1989; Volume 4, Part II, pp. 53–108. [Google Scholar]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well Defined Transition Metal Complexes with Phosphorus and Nitrogen Ligands for 1,3-Dienes Polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes polymerization: Where we are and what lies ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Porri, L.; Giarrusso, A.; Ricci, G. Recent views on the mechanism of diolefin polymerization with transition metal initiator systems. Prog. Polym. Sci. 1991, 16, 405–441. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Porri, L. Polymerization of (Z)-1,3-pentadiene with CpTiCl3/MAO. Effect of temperature on polymer structure and mechanistic implications. Macromolecules 1994, 27, 868–869. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Giarrusso, A.; Porri, L. Polymerization of 1,3-dienes with the soluble catalyst system methylaluminoxanes-[CpTiCl3]. Influence of monomer structure on polymerization stereospecificity. J. Organomet. Chem. 1993, 451, 67–72. [Google Scholar] [CrossRef]
- Ricci, G.; Battistella, M.; Porri, L. Chemoselectivity and stereospecificity of Cr(II) catalysts for 1,3-diene polymerization. Macromolecules 2001, 34, 5766–5769. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Porri, L. Polymerization of butadiene to 1,2-syndiotactic polymer with (η3-C8H13)(η4-C4H6)Co. Some observations on the factors that determine the stereospecificity. Polymer Commun. 1988, 29, 305–307. [Google Scholar]
- Ricci, G.; Morganti, D.; Sommazzi, A.; Santi, R.; Masi, F. Polymerization of 1,3-dienes with iron complexes based catalysts. Influence of the ligand on catalyst activity and stereospecificity. J. Mol. Cat. A Chem. 2003, 204/205, 287–293. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Zanchin, G.; Palucci, B.; Boccia, A.C.; Sommazzi, A.; Masi, F.; Zacchini, S.; Guelfi, M.; Pampaloni, G. Highly Stereoregular 1,3-Butadiene and Isoprene Polymers through Monoalkyl−N−Aryl Substituted Iminopyridine Iron Complex-Based Catalysts: Synthesis and Characterization. Macromolecules 2021, 54, 9947–9959. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Motta, T.; Zannoni, G.; Canetti, M.; Bertini, F. Synthesis and X-Ray structure of CoCl2(PiPrPh2)2. A new highly active and stereospecific catalyst for 1,2 polymerization of conjugated dienes when used associated with MAO. Macromolecules 2005, 38, 1064–1070. [Google Scholar] [CrossRef]
- Boccia, A.C.; Leone, G.; Boglia, A.; Ricci, G. Novel Stereoregular cis-1,4 and trans-1,2 Poly(diene)s: Synthesis, Characterization, and Mechanistic Considerations. Polymer 2013, 54, 3492–3503. [Google Scholar] [CrossRef]
- Ricci, G.; Boglia, A.; Motta, T.; Bertini, F.; Boccia, A.C.; Zetta, L.; Alberti, E.; Famulari, A.; Arosio, P.; Meille, S.V. Synthesis and Structural Characterization of Syndiotactic trans-1,2 and cis-1,2 Polyhexadienes. J. Polym. Sci Part A Polym. Chem. 2007, 45, 5339–5353. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Bertini, F.; Boccia, A.C.; Zetta, L. Synthesis and Characterization of Isotactic 1,2-Poly(E-3-methyl-1,3-pentadiene). Some Remarks about the Influence of Monomer Structure on Polymerization Stereoselectivity. Macromolecules 2009, 42, 3048–3056. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L. Diethylbis(2,2-bipyridine)iron/MAO. A Very Active and Stereospecific Catalyst for 1,3-Diene Polymerization. Macromol. Rapid Commun. 2002, 23, 922–927. [Google Scholar] [CrossRef]
- Pirozzi, B.; Napolitano, R.; Petraccone, V.; Esposito, S. Determination of the Crystal Structure of Syndiotactic 3,4-Poly(2-methyl-1,3-butadiene) by Molecular Mechanics and X-Ray Diffraction. Macromol. Chem. Phys. 2004, 205, 1343–1350. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Zanchin, G.; Masi, F.; Guelfi, M.; Pampaloni, G. Dichloro(2,2′-bipyridine)copper/MAO: An Active and Stereospecific Catalyst for 1,3-Diene Polymerization. Molecules 2023, 28, 374. [Google Scholar] [CrossRef]
- Ricci, G.; Bertini, F.; Boccia, A.C.; Zetta, L.; Alberti, E.; Pirozzi, B.; Giarrusso, A.; Porri, L. Synthesis and Characterization of Syndiotactic 1,2 Poly(3-Methyl-1,3-Pentadiene). Macromolecules 2007, 40, 7238–7243. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Santillo, C.; Di Girolamo, R.; Leone, G.; Ricci, G. Chirality, entropy and crystallization in polymers: Isotactic poly(3-methyl-1-pentene) as an example of influence of chirality and entropy on the ctystal structure. CrystEngComm 2015, 17, 6006–6013. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Santillo, C.; Di Girolamo, R.; Leone, G.; Boccia, A.C.; Ricci, G. Crystal structure of isotactic poly((R,S)-3-methyl-1-pentene). Macromolecules 2015, 48, 5251–5266. [Google Scholar] [CrossRef]
- De Rosa, C.; Malafronte, A.; Scoti, M.; Auriemma, F.; Pierro, I.; Leone, G.; Ricci, G. Synthesis and Structure of Syndiotactic Poly(3-Methyl- 1-Butene): A Case of 3/1 Helical Conformation for Syndiotactic Polymers. Macromolecules 2018, 51, 8574–8584. [Google Scholar] [CrossRef]
- Samran, J.; Phinyocheep, P.; Daniel, P.; Kittipoom, S. Hydrogenation of unsaturated rubbers using diimide as a reducing agent. J. App. Polym. Sci. 2005, 95, 16–27. [Google Scholar] [CrossRef]
- Mango, L.A.; Lenz, R.W. Hydrogenation of unsaturated polymers with diimide. Makromol. Chem. 1973, 163, 13. [Google Scholar] [CrossRef]
- Harwood, H.J.; Russel, D.B.; Verthe, J.J.A.; Zymonas, J. Diimide as a reagent for the hydrogenation of unsaturated polymers. Makromol. Chem. 1973, 163, 1–12. [Google Scholar] [CrossRef]
- Santin, C.K.; Jacobi, M.M.; Schuster, R.H. Influence of 1,2 units content on the hydrogenation of polydienes by TSH. J. Appl. Polym. Sci. 2011, 119, 1195–1203. [Google Scholar] [CrossRef]
- Singha, N.K.; Bhattacharjee, S.; Sivaram, S. Hydrogenation of Diene Elastomers, Their Properties and Applications: A Critical Review. Rubber Chem. Technol. 1997, 70, 309–367. [Google Scholar] [CrossRef]
- Sozzani, P.; Simonutti, R.; Galimberti, M. MAS NMR Characterization of Syndiotactic Polypropylene: Crystal Structure and Amorphous Phase Conformation. Macromolecules 1993, 26, 5782–5789. [Google Scholar] [CrossRef]
- Asakura, T.; Demura, M.; Nishiyama, Y. Carbon-13 NMR Spectral Assignment of Five Polyolefins Determined from the Chemical Shift Calculation and the Polymerization Mechanism. Macromolecules 1991, 24, 2334–2340. [Google Scholar] [CrossRef]
- Saito, J.; Suzuki, Y.; Makio, H.; Tanaka, H.; Onda, M.; Fujita, T. Polymerization of Higher R-Olefins with a Bis(Phenoxyimine)Ti Complex/iBu3Al/Ph3CB(C6F5)4: Formation of Stereo- and Regioirregular High Molecular Weight Polymers with High Efficiency. Macromolecules 2006, 39, 4023–4031. [Google Scholar] [CrossRef]
- Janas, Z.; Godbole, D.; Nerkowski, T.; Szezegot, K. Zirconium and hafnium complexes of the thio(bisphenolato) ligand: Synthesis, structural characterization and testing as 1-hexene polymerization catalyst. Dalton Trans. 2009, 8846–8863. [Google Scholar] [CrossRef]
- Lukesova, L.; Ward, B.D.; Bellemin-Laponnaz, S.; Wadepohl, H.; Gade, L.H. High tacticity control in organolanthanide polymerization catalysis: Formation of isotactic poly(α-alkenes) with a chiral C3-symmetric thulium complex. Dalton Trans. 2007, 920–922. [Google Scholar] [CrossRef]
- Delfini, M.; Di Cocco, M.E.; Paci, M.; Aglietto, M.; Carlini, C.; Crisci, L.; Ruggeri, G. 13C n.m.r. study of poly[(S)-5-metrhyl-1-heptene] and poly(1-heptene) prepared by Ziegler-Natta catalyst. Polymer 1985, 26, 1459–1462. [Google Scholar] [CrossRef]




| Polymer | Monomer | Time (h) | Yield (%) | 1,2 a (%) | [rrrr] b (%) | m.p. c (°C) | Mw d (kg/mol) | Mw/Mn d |
|---|---|---|---|---|---|---|---|---|
| 1,2syPP | EP | 168 | 89 | 99 | 60 | 168 | 167 | 2.5 |
| 1,2syPHX | HX | 48 | 49 | 99 | 58 | 109 | 74 | 1.6 |
| 1,2syP5MHX | 5MHX | 236 | 74 | 99 | 50 | - | 69 | 4.3 |
| 1,2syPHP | HP | 336 | 73 | 90 | 43 | - | 29 | 2.7 |
| 1,2syPO | O | 144 | 76 | 92 | 44 | - | 30 | 2.3 |
| Polymer | [rrrr] a | Mw b | Mw/Mn b | Tg c |
|---|---|---|---|---|
| (%) | (kg/mol) | (°C) | ||
| H(1,2syPP)_syndiotactic poly(1-pentene) | 62 | 175 | 2.6 | −46 |
| H(1,2syPHX)_syndiotactic poly(1-hexene) | 59 | 85 | 1.4 | −50 |
| H(1,2syP5MH)_syndiotactic poly(5-methyl-1-hexene) | 50 | 77 | 2.9 | −55 |
| H(1,2syPHP)_syndiotactic poly(1-heptene) | 42 | 38 | 2.0 | −56 |
| H(1,2syPO)_syndiotactic poly(1-octene) | 45 | 35 | 2.1 | −60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, G.; Pierro, I.; Boccia, A.C. Syndiotactic Polyolefins by Hydrogenation of Highly Stereoregular 1,2 Polydienes: Synthesis and Structural Characterization. Polymers 2024, 16, 2711. https://doi.org/10.3390/polym16192711
Ricci G, Pierro I, Boccia AC. Syndiotactic Polyolefins by Hydrogenation of Highly Stereoregular 1,2 Polydienes: Synthesis and Structural Characterization. Polymers. 2024; 16(19):2711. https://doi.org/10.3390/polym16192711
Chicago/Turabian StyleRicci, Giovanni, Ivana Pierro, and Antonella Caterina Boccia. 2024. "Syndiotactic Polyolefins by Hydrogenation of Highly Stereoregular 1,2 Polydienes: Synthesis and Structural Characterization" Polymers 16, no. 19: 2711. https://doi.org/10.3390/polym16192711
APA StyleRicci, G., Pierro, I., & Boccia, A. C. (2024). Syndiotactic Polyolefins by Hydrogenation of Highly Stereoregular 1,2 Polydienes: Synthesis and Structural Characterization. Polymers, 16(19), 2711. https://doi.org/10.3390/polym16192711










