Assessment of Clayey Freshwater Sediments as Suitable Precursors for Alkaline Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Samples Preparation
2.3. Employed Experimental Setup
3. Results and Discussion
3.1. Materials Characterization
3.2. AAMs Characterization
4. Conclusions
- The concentration of the most hazardous elements identified in the studied samples of FWS were very similar compared to alternative cementitious materials such as coal fly ash.
- Since the FWS contains a substantial portion of clay minerals such as kaolinite and illite, the elevated temperature treatment may result in a significant improvement in the material reactivity to meet the requirements for use as an alternative binder system.
- The PSD results present an average range of particle sizes between 27.198 µm and 50.78 µm. This enhanced workability is counterbalanced by a decrease in strength, attributed to the material’s lower reactivity.
- FWS-based AAMs demonstrated sufficient mechanical strength, and a compressive strength of 14.59 MPa to 37.09 MPa was obtained across the samples. This strength enhancement can be attributed to the effective dissolution of aluminosilicate during the alkali activation reaction.
- The development in mechanical performance is related to the content of the amorphous phase and the Si/Al ratio. Specifically, the alkali activation of FWS through the use of potassium water glass and sodium hydroxide provides better performance due to a better-balanced Si/Al ratio and the availability of Na+ and K+ ions.
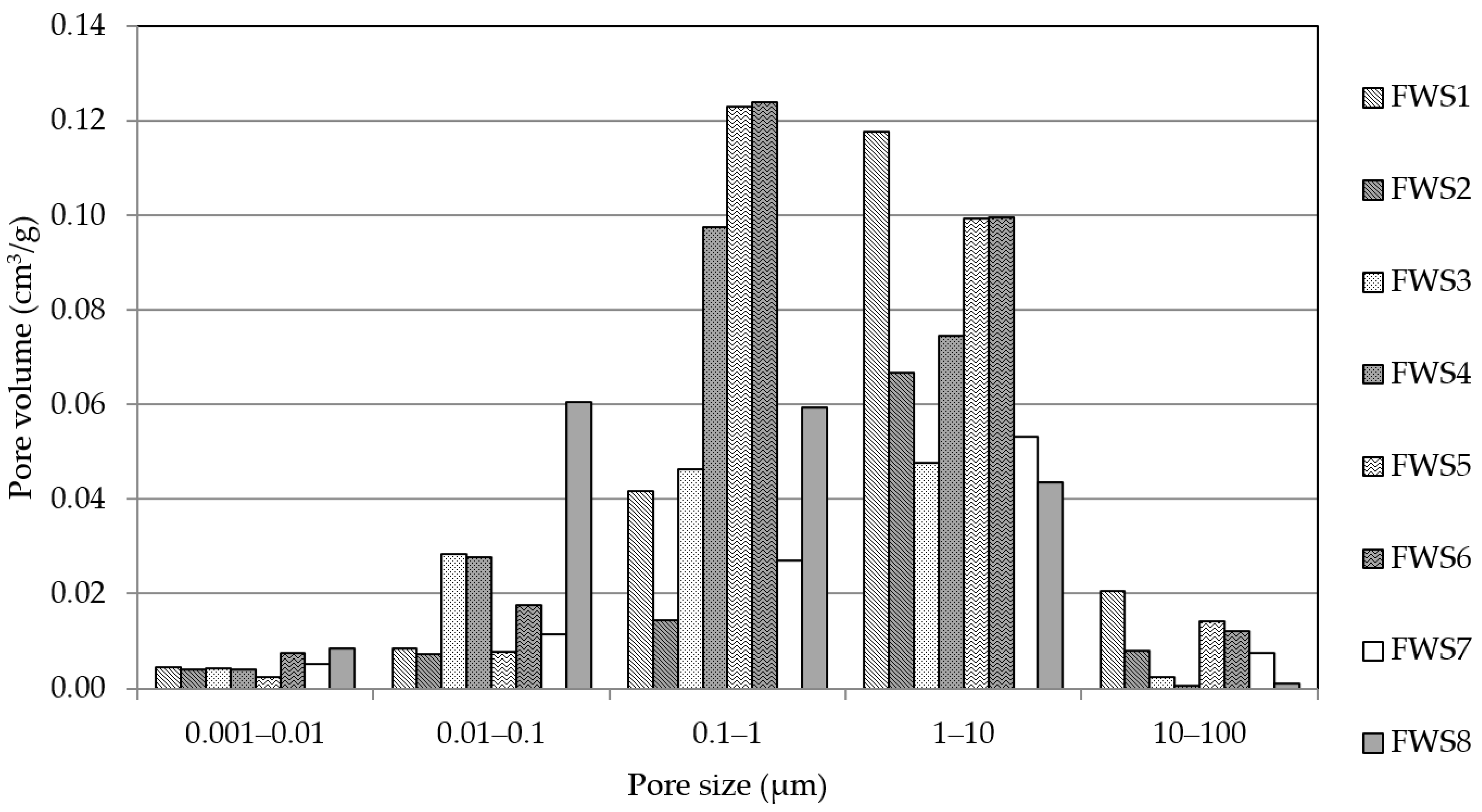
- The SEM analysis and MIP results point to the formation of more dense structures for sediments with fine particles, as well as the formation of more rugged surfaces with distinct cracks. More amorphous sediments with lower Si/Al ratios tend to a higher share of pores in the range of 0.01–0.1 µm.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Amran, M.; Makul, N.; Fediuk, R.; Lee, Y.H.; Vatin, N.I.; Lee, Y.Y.; Mohammed, K. Global carbon recoverability experiences from the cement industry. Case Stud. Constr. Mater. 2022, 17, e01439. [Google Scholar] [CrossRef]
- Sandanayake, M.; Law, D.; Sargent, P. A new framework for assessing the environmental impacts of circular economy friendly soil waste-based geopolymer cements. Build. Environ. 2022, 210, 108702. [Google Scholar] [CrossRef]
- Lanjewar, B.A.; Chippagiri, R.; Dakwale, V.A.; Ralegaonkar, R.V. Application of Alkali-Activated Sustainable Materials: A Step towards Net Zero Binder. Energies 2023, 16, 969. [Google Scholar] [CrossRef]
- de Brito, J.; Kurda, R. The past and future of sustainable concrete: A critical review and new strategies on cement-based materials. J. Clean. Prod. 2021, 281, 72. [Google Scholar] [CrossRef]
- Gottinger, A.; Ladu, L.; Quitzow, R. Studying the Transition towards a Circular Bioeconomy-A Systematic Literature Review on Transition Studies and Existing Barriers. Sustainability 2020, 12, 8990. [Google Scholar] [CrossRef]
- Chougan, M.; Ghaffer, S.H.; Sikora, P.; Mijowska, E.; Kukulka, W.; Stephan, D. Boosting Portland cement-free composite performance via alkali-activation and reinforcement with pre-treated functionalised wheat straw. Ind. Crops Prod. 2022, 178, 114648. [Google Scholar] [CrossRef]
- Fort, J.; Vejmelkova, E.; Keppert, M.; Rovnanikova, P.; Bezdicka, P.; Cerny, R. Alkaline activation of low-reactivity ceramics: Peculiarities induced by the precursors’ dual character. Cem. Concr. Compos. 2020, 105, 103440. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 27. [Google Scholar] [CrossRef]
- Yamchelou, M.T.; Law, D.W.; Patnaikuni, I.; Li, J. Alkali activation of mechanically activated low-grade clay. J. Sustain. Cem.-Based Mater. 2021, 10, 272–288. [Google Scholar] [CrossRef]
- Ilcan, H.; Sahin, O.; Kul, A.; Yildirim, G.; Sahmaran, M. Rheological properties and compressive strength of construction and demolition waste-based geopolymer mortars for 3D-Printing. Constr. Build. Mater. 2022, 328, 127114. [Google Scholar] [CrossRef]
- Ojha, A.; Aggarwal, P. Fly Ash Based Geopolymer Concrete: A Comprehensive Review. Silicon 2022, 14, 2453–2472. [Google Scholar] [CrossRef]
- Segura, I.P.; Ranjbar, N.; Damo, A.J.; Jensen, L.S.; Canut, M.; Jensen, P.A. A review: Alkali-activated cement and concrete production technologies available in the industry. Heliyon 2023, 9, e15718. [Google Scholar] [CrossRef] [PubMed]
- Keppert, M.; Vejmelkova, E.; Bezdicka, P.; Dolezelova, M.; Cachova, M.; Scheinherrova, L.; Pokorny, J.; Vysvaril, M.; Rovnanikova, P.; Cerny, R. Red-clay ceramic powders as geopolymer precursors: Consideration of amorphous portion and CaO content. Appl. Clay Sci. 2018, 161, 82–89. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 14. [Google Scholar] [CrossRef] [PubMed]
- Konig, K.; Traven, K.; Pavlin, M.; Ducman, V. Evaluation of locally available amorphous waste materials as a source for alternative alkali activators. Ceram. Int. 2021, 47, 10. [Google Scholar] [CrossRef]
- Athira, V.S.; Charitha, V.; Athira, G.; Bahurudeen, A. Agro-waste ash based alkali-activated binder: Cleaner production of zero cement concrete for construction. J. Clean. Prod. 2021, 286, 18. [Google Scholar] [CrossRef]
- Farooq, F.; Xin, J.; Javed, M.F.; Akbar, A.; Shah, M.I.; Aslam, F.; Alyousef, R. Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- Cong, P.L.; Cheng, Y.Q. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng.-Engl. Ed. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean. Prod. 2020, 263, 13. [Google Scholar] [CrossRef]
- Sun, B.B.; Sun, Y.B.; Ye, G.; De Schutter, G. A mix design methodology of slag and fly ash-based alkali-activated paste. Cem. Concr. Compos. 2022, 126, 104368. [Google Scholar] [CrossRef]
- Fort, J.; Mildner, M.; Keppert, M.; Pommer, V.; Cerny, R. Experimental and Environmental Analysis of High-Strength Geopolymer Based on Waste Bricks and Blast Furnace Slag. Polymers 2023, 15, 3092. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinia, A.; Arulrajah, A.; D’Amico, A.; Horpibulsuk, S. Alkali activation of lime kiln dust and fly ash blends for the stabilisation of demolition wastes. Road Mater. Pavement Des. 2020, 21, 1514–1528. [Google Scholar] [CrossRef]
- Shi, C.J.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Kaze, C.R.; Djobo, J.N.Y.; Nana, A.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Leonelli, C.; Rahier, H. Effect of silicate modulus on the setting, mechanical strength and microstructure of iron-rich aluminosilicate (laterite) based-geopolymer cured at room temperature. Ceram. Int. 2018, 44, 21442–21450. [Google Scholar] [CrossRef]
- Sumesh, M.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H.; Singh, R.; Nayaka, R.R.; Srinivas, K. Chemo-physico-mechanical characteristics of high-strength alkali-activated mortar containing non-traditional supplementary cementitious materials. J. Build. Eng. 2021, 44, 103368. [Google Scholar] [CrossRef]
- He, J.; Kawasaki, S.; Achal, V. The Utilization of Agricultural Waste as Agro-Cement in Concrete: A Review. Sustainability 2020, 12, 6971. [Google Scholar] [CrossRef]
- Komnitsas, K. Co-valorization of marine sediments and construction & demolition wastes through alkali activation. J. Environ. Chem. Eng. 2016, 4, 4661–4669. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, O.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 28. [Google Scholar] [CrossRef]
- Mostefa, F.; Bouhamou, N.E.; Aggoune, S.; Mesbah, H. Elaboration of geopolymer cement based on dredged sediment. J. Mater. Eng. Struct. 2019, 6, 39–51. [Google Scholar]
- Li, X.L.; Lv, X.R.; Zhou, X.T.; Meng, W.N.; Bao, Y. Upcycling of waste concrete in eco-friendly strain-hardening cementitious composites: Mixture design, structural performance, and life-cycle assessment. J. Clean. Prod. 2022, 330, 129911. [Google Scholar] [CrossRef]
- Krasa, J.; Dostal, T.; Van Rompaey, A.; Vaska, J.; Vrana, K. Reservoirs’ siltation measurments and sediment transport assessment in the Czech Republic, the Vrchlice catchment study. Catena 2005, 64, 348–362. [Google Scholar] [CrossRef]
- Zibret, L.; Wisniewski, W.; Horvat, B.; Bozic, M.; Gregorc, B.; Ducman, V. Clay rich river sediments calcined into precursors for alkali activated materials. Appl. Clay Sci. 2023, 234, 106848. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, M.Y.; Li, X.C.; Wang, J.X.; Wang, Z.M.; Li, L.; Lyu, X.J. Alkali-hydrothermal activation of mine tailings to prepare one-part geopolymer: Activation mechanism, workability, strength, and hydration reaction. Ceram. Int. 2022, 48, 30407–30417. [Google Scholar] [CrossRef]
- Lafhaj, Z.; Samara, M.; Agostini, F.; Boucard, L.; Skoczylas, F.; Depelsenaire, G. Polluted river sediments from the North region of France: Treatment with Novosol (R) process and valorization in clay bricks. Constr. Build. Mater. 2008, 22, 755–762. [Google Scholar] [CrossRef]
- Gharzouni, A.; Ouamara, L.; Sobrados, I.; Rossignol, S. Alkali-activated materials from different aluminosilicate sources: Effect of aluminum and calcium availability. J. Non-Cryst. Solids 2018, 484, 14–25. [Google Scholar] [CrossRef]
- Snellings, R.; Cizer, O.; Horckmans, L.; Durdzinski, P.T.; Dierckx, P.; Nielsen, P.; Van Balen, K.; Vandewalle, L. Properties and pozzolanic reactivity of flash calcined dredging sediments. Appl. Clay Sci. 2016, 129, 35–39. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Molino, A.; Roviello, G.; Colangelo, F.; Molino, B.; Cioffi, R. Synergistic recycling of calcined clayey sediments and water potabilization sludge as geopolymer precursors: Upscaling from binders to precast paving cement-free bricks. Constr. Build. Mater. 2017, 133, 14–26. [Google Scholar] [CrossRef]
- Pachideh, G.; Gholhaki, M.; Ketabdari, H. Effect of pozzolanic wastes on mechanical properties, durability and microstructure of the cementitious mortars. J. Build. Eng. 2020, 29, 101178. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andres, J.M.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicova, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef]
- Shen, J.L.; Li, Y.; Lin, H.; Lv, J.F.; Feng, S.; Ci, J.C. Early properties and chemical structure analysis of alkali-activated brick geopolymer with varied alkali dosage. J. Build. Eng. 2022, 60, 105186. [Google Scholar] [CrossRef]
- Eleutério, R.V.; Simao, L.; Lemes, P.; Hotza, D. Evaluation of As-Received Green Liquor Dregs and Biomass Ash Residues from a Pulp and Paper Industry as Raw Materials for Geopolymers. Minerals 2023, 13, 1158. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Manzi, S.; Lancellotti, I.; Kamseu, E.; Barbieri, L.; Leonelli, C. Mix-design and characterization of alkali activated materials based on metakaolin and ladle slag. Appl. Clay Sci. 2013, 73, 78–85. [Google Scholar] [CrossRef]
- Pavlin, M.; Horvat, B.; Frankovi, A.; Ducman, V. Mechanical, microstructural and mineralogical evaluation of alkali-activated waste glass and stone wool. Ceram. Int. 2021, 47, 15102–15113. [Google Scholar] [CrossRef]
- Khedmati, M.; Alanazi, H.; Kim, Y.R.; Nsengiyumva, G.; Moussavi, S. Effects of Na2O/SiO2 molar ratio on properties of aggregate-paste interphase in fly ash-based geopolymer mixtures through multiscale measurements. Constr. Build. Mater. 2018, 191, 564–574. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoo, S.W.; Jung, S.H.; Lee, K.M.; Kwon, S.J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Lahoti, M.; Narang, P.; Tan, K.H.; Yang, E.H. Mix design factors and strength prediction of metakaolin-based geopolymer. Ceram. Int. 2017, 43, 11433–11441. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, X.M.; Zhang, W.; Li, Z.P.; Zhang, Y.L.; Li, Y.; Ren, Y.Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yehualaw, M.D.; Vo, D.H.; Huyn, T.P. Development of high-strength alkali-activated pastes containing high volumes of waste brick and ceramic powders. Constr. Build. Mater. 2019, 218, 519–529. [Google Scholar] [CrossRef]
- Vinai, R.; Soutsos, M. Production of sodium silicate powder from waste glass cullet for alkali activation of alternative binders. Cem. Concr. Res. 2019, 116, 45–56. [Google Scholar] [CrossRef]
- Ye, T.H.; Xiao, J.Z.; Duan, Z.H.; Li, S.S. Geopolymers made of recycled brick and concrete powder—A critical review. Constr. Build. Mater. 2022, 330, 127232. [Google Scholar] [CrossRef]
- Fletcher, R.A.; MacKenzie, K.J.D.; Nicholson, C.L.; Shimada, S. The composition range of aluminosilicate geopolymers. J. Eur. Ceram. Soc. 2005, 25, 1471–1477. [Google Scholar] [CrossRef]
- Slosarczyk, A.; Fort, J.; Klapiszewska, I.; Thomas, M.; Klapiszewski, L.; Cerny, R. A literature review of the latest trends and perspectives regarding alkali-activated materials in terms of sustainable development. J. Mater. Res. Technol.-JMRT 2023, 25, 5394–5425. [Google Scholar] [CrossRef]
- Fort, J.; Vejmelkova, E.; Konakova, D.; Alblova, N.; Cachova, M.; Keppert, M.; Rovnanikova, P.; Cerny, R. Application of waste brick powder in alkali activated aluminosilicates: Functional and environmental aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
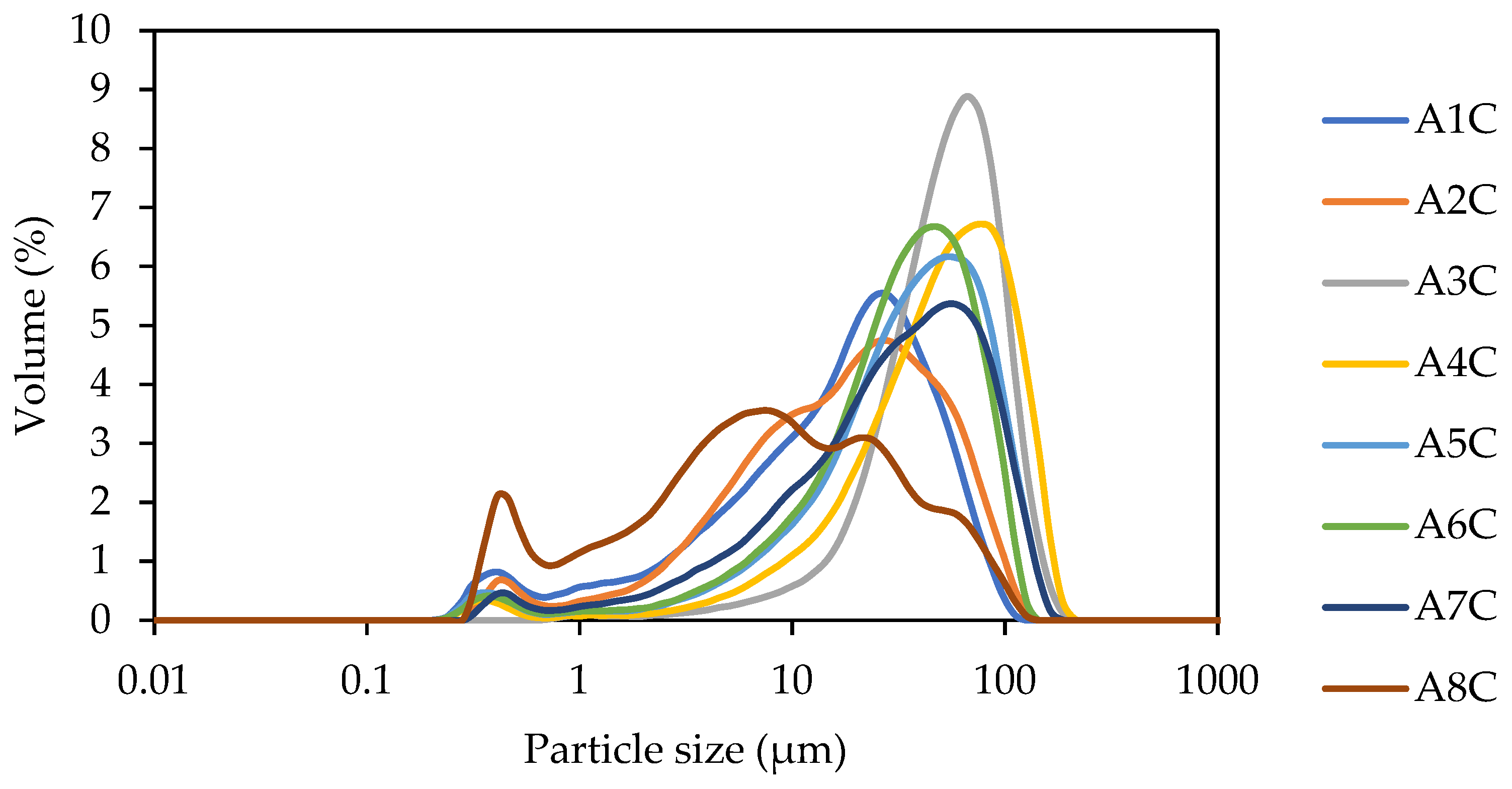
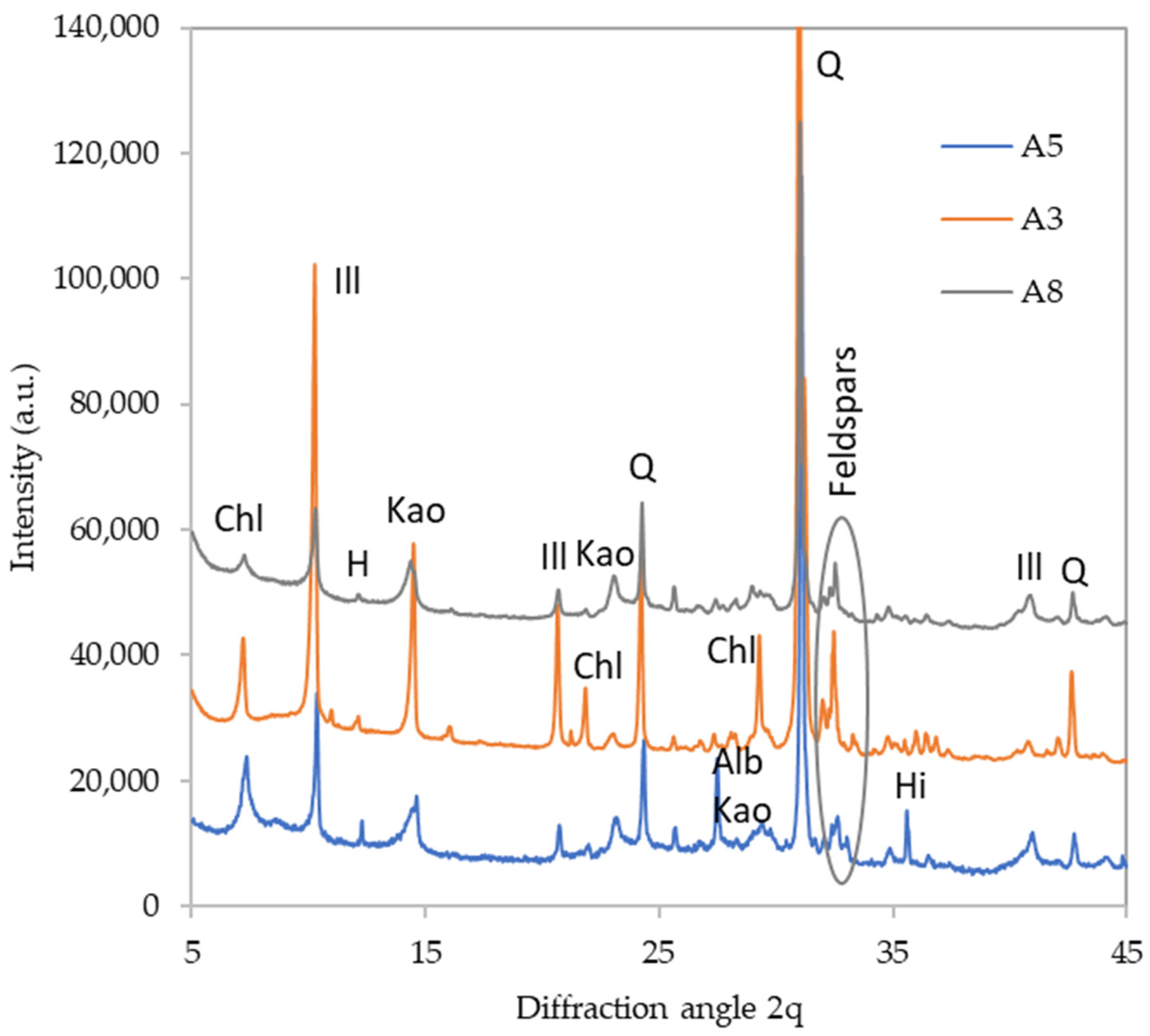

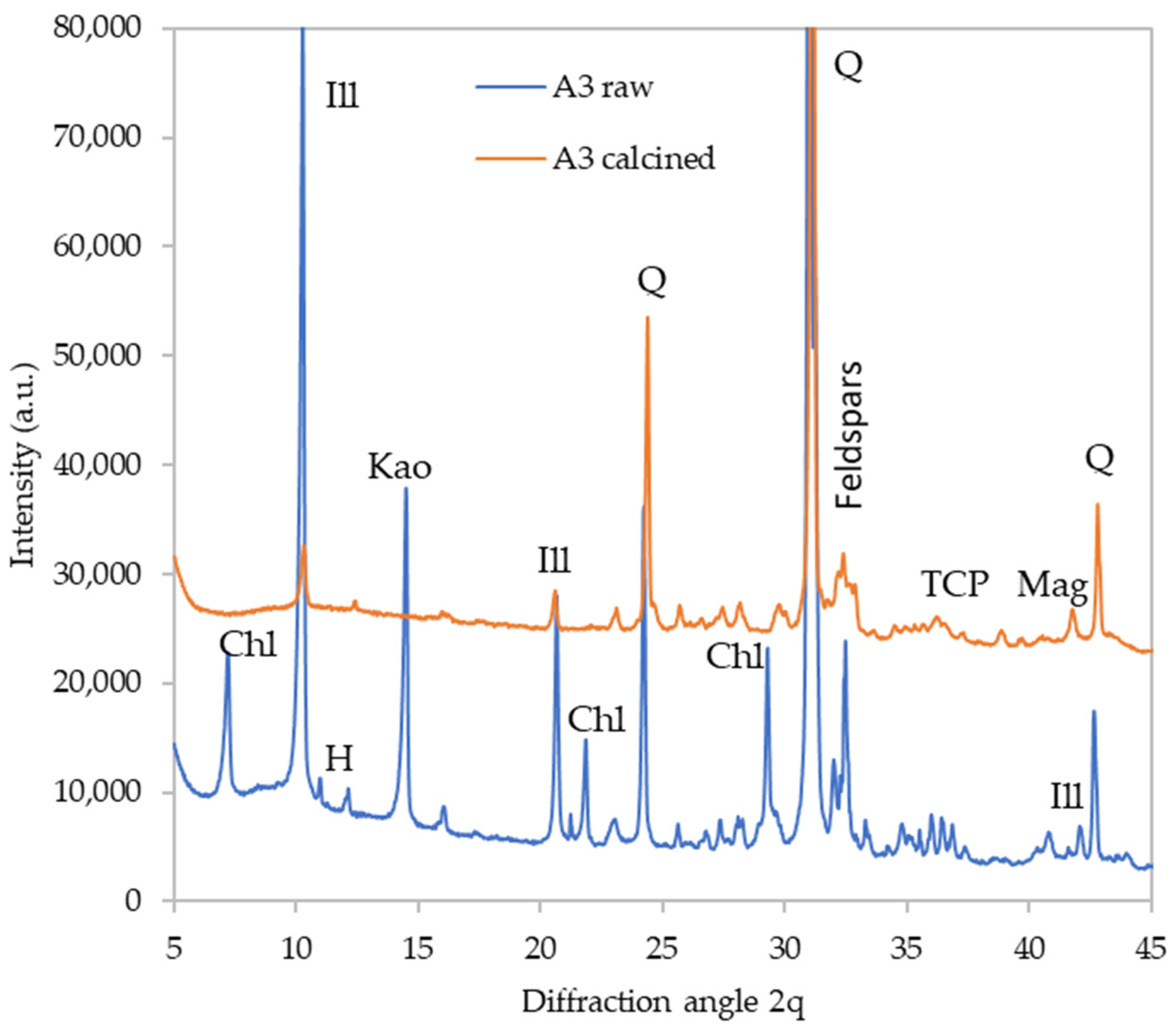

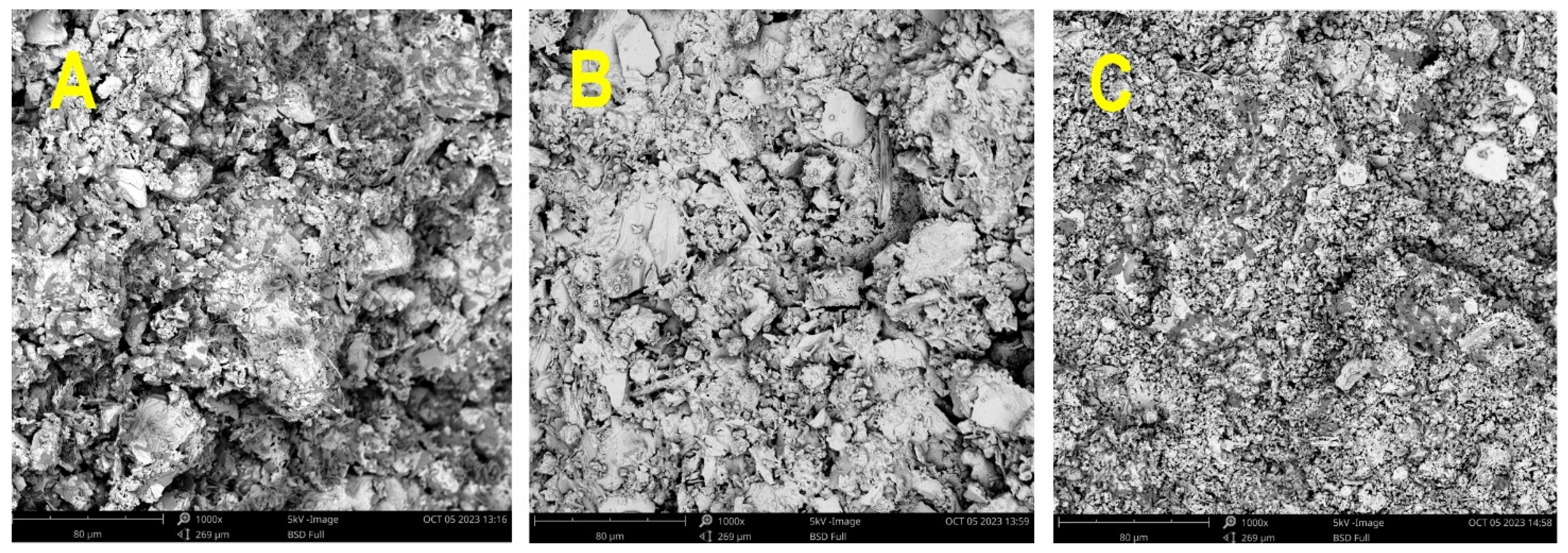
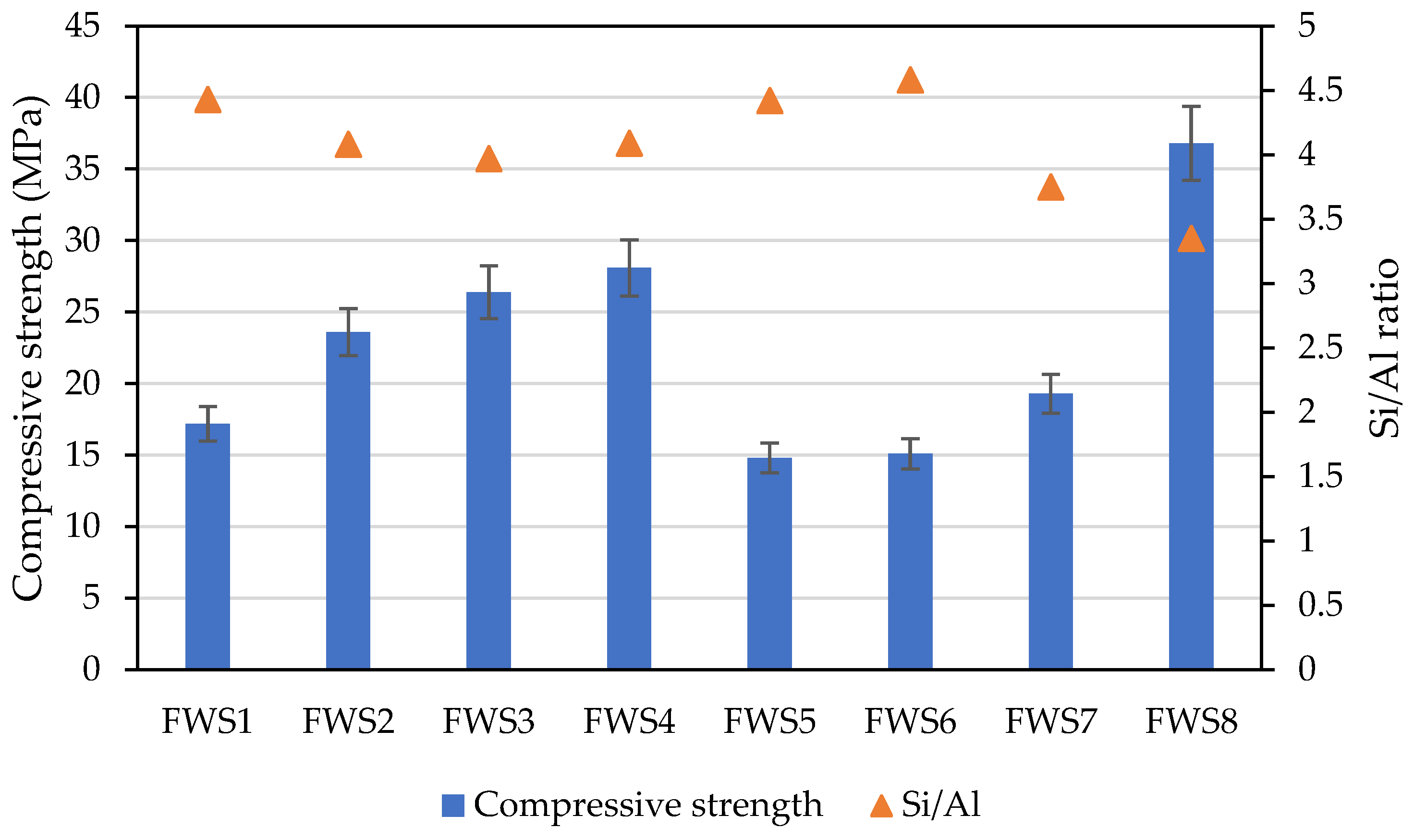
| Sample | FWS (g) | Potassium Silicate (g) | NaOH (g) | Water (g) | Si/Al Ratio |
|---|---|---|---|---|---|
| FWS1 | 900 | 280 | 20 | 30 | 4.43 |
| FWS2 | 900 | 280 | 20 | 35 | 4.08 |
| FWS3 | 900 | 280 | 20 | 25 | 3.97 |
| FWS4 | 900 | 280 | 20 | 30 | 4.09 |
| FWS5 | 900 | 280 | 20 | 45 | 4.42 |
| FWS6 | 900 | 280 | 20 | 40 | 4.58 |
| FWS7 | 900 | 280 | 20 | 20 | 3.75 |
| FWS8 | 900 | 280 | 20 | 55 | 3.35 |
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | ||
|---|---|---|---|---|---|---|---|---|---|
| Clay minerals | Kaolinite | ++ | ++ | + | ++ | ++ | ++ | + | +++ |
| Chlorite | ++ | ++ | + | + | 0 | + | ++ | 0 | |
| Illite | ++ | +++ | ++ | ++ | ++ | ++ | +++ | ++ | |
| Quartz | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| Feldspars | Albite | + | 0 | 0 | + | ++ | + | 0 | + |
| Plagioclase | 0 | + | + | + | 0 | 0 | + | + | |
| Microcline | + | + | 0 | ++ | + | 0 | 0 | 0 | |
| Anorthite | + | 0 | 0 | + | 0 | 0 | 0 | 0 | |
| Hornblende | + | + | + | 0 | + | + | 0 | + | |
| Hillebrandite | + | 0 | 0 | + | 0 | 0 | + | 0 | |
| Major Oxides (% by Mass) | A1C | A2C | A3C | A4C | A5C | A6C | A7C | A8C |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 64.53 | 66.95 | 64.02 | 63.99 | 63.14 | 58.59 | 57.97 | 60.65 |
| Al2O3 | 20.11 | 18.64 | 19.96 | 20.17 | 20.55 | 28.57 | 23.18 | 22.21 |
| Fe2O3 | 4.62 | 3.84 | 5.04 | 5.04 | 5.4 | 6.31 | 8.84 | 7.45 |
| CaO | 1.6 | 1.7 | 1.71 | 1.59 | 1.66 | 0.37 | 1.69 | 1.28 |
| MgO | 2.25 | 1.85 | 2.24 | 2.24 | 2.31 | 0.75 | 1.98 | 1.89 |
| Na2O | 1.16 | 1.48 | 1.29 | 1.4 | 1.25 | 0.22 | 0.68 | 1.07 |
| K2O | 4.03 | 3.81 | 3.54 | 3.55 | 3.63 | 3.37 | 3.07 | 2.97 |
| TiO2 | 0.8 | 0.8 | 0.9 | 0.91 | 0.93 | 1.04 | 1.1 | 1.11 |
| SO3 | 0.26 | 0.31 | 0.27 | 0.35 | 0.25 | 0.11 | 0.39 | 0.39 |
| P2O5 | 0.33 | 0.36 | 0.68 | 0.43 | 0.54 | 0.27 | 0.65 | 0.57 |
| Cl | 0.01 | 0.01 | 0 | 0 | 0.01 | 0 | 0.01 | 0.01 |
| Sum | 99.7 | 99.75 | 99.65 | 99.67 | 99.67 | 99.6 | 99.56 | 99.6 |
| Heavy Metals (mg/g) | A1C | A2C | A3C | A4C | A5C | A6C | A7C | A8C |
|---|---|---|---|---|---|---|---|---|
| V | 0.26 | 0.25 | 0.29 | 0.27 | 0.28 | 0.21 | 0.41 | 0.36 |
| Cr | 0.21 | 0.16 | 0.22 | 0.21 | 0.22 | 0.23 | 0.28 | 0.26 |
| Mn | 1.42 | 1.34 | 1.66 | 1.81 | 1.41 | 1.08 | 2.95 | 2.51 |
| Co | 0.13 | 0 | 0.13 | 0.14 | 0.12 | 0 | 0.16 | 0.15 |
| Ni | 0 | 0.07 | 0.06 | 0.12 | 0.14 | 0 | 0.18 | 0.13 |
| Cu | 0.13 | 0.12 | 0.18 | 0.18 | 0.22 | 0 | 0.2 | 0.16 |
| Zn | 0.76 | 0.51 | 0.9 | 0.75 | 1 | 0.22 | 1.29 | 1.24 |
| Ga | 0.08 | 0 | 0 | 0 | 0.06 | 0 | 0.07 | 0 |
| Rb | 0.39 | 0.27 | 0.25 | 0.28 | 0.29 | 0.51 | 0.27 | 0.25 |
| Sr | 0.33 | 0.36 | 0.38 | 0.4 | 0.4 | 0.52 | 0.27 | 0.3 |
| Zr | 1.02 | 1.16 | 1.33 | 1.14 | 1.12 | 2.31 | 0.67 | 0.99 |
| Nb | 0 | 0 | 0.11 | 0 | 0.09 | 0 | 0 | 0.09 |
| Ba | 1.37 | 1.45 | 1.38 | 1.53 | 1.72 | 3.17 | 1.96 | 1.77 |
| Pb | 0.19 | 0.17 | 0.23 | 0.22 | 0.2 | 0.17 | 0.22 | 0.2 |
| Material | Amorphous | Quartz | Plagioclase | Anorthoclase | Microcline | Spinel Phase | Maghemite | Ca3(PO4)2 | Mica |
|---|---|---|---|---|---|---|---|---|---|
| A1C | 8 | 54 | 20 | 3 | 7 | 5 | 0 | 0 | 5 |
| A2C | 12 | 49 | 10 | 5 | 8 | 6 | 0 | 5 | 4 |
| A3C | 16 | 34 | 11 | 4 | 13 | 7 | 1 | 6 | 10 |
| A4C | 18 | 43 | 8 | 9 | 9 | 2 | 0 | 5 | 9 |
| A5C | 6 | 49 | 12 | 11 | 8 | 3 | 1 | 3 | 6 |
| A6C | 7 | 53 | 9 | 9 | 7 | 4 | 2 | 4 | 6 |
| A7C | 10 | 39 | 5 | 18 | 11 | 4 | 1 | 4 | 8 |
| A8C | 38 | 28 | 7 | 3 | 11 | 7 | 1 | 3 | 2 |
| Sample No. | Bulk Density (kg/m3) | Matrix Density (kg/m3) | Total Open Porosity (%) |
|---|---|---|---|
| FWS1 | 1.83 | 2.48 | 29% |
| FWS2 | 1.86 | 2.47 | 24% |
| FWS3 | 1.64 | 2.49 | 34% |
| FWS4 | 1.88 | 2.49 | 35% |
| FWS5 | 1.89 | 2.49 | 24% |
| FWS6 | 1.72 | 2.56 | 33% |
| FWS7 | 1.83 | 2.53 | 30% |
| FWS8 | 1.86 | 2.52 | 26% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fořt, J.; Afolayan, A.; Mildner, M.; Hotěk, P.; Keppert, M.; Černý, R. Assessment of Clayey Freshwater Sediments as Suitable Precursors for Alkaline Activation. Polymers 2024, 16, 175. https://doi.org/10.3390/polym16020175
Fořt J, Afolayan A, Mildner M, Hotěk P, Keppert M, Černý R. Assessment of Clayey Freshwater Sediments as Suitable Precursors for Alkaline Activation. Polymers. 2024; 16(2):175. https://doi.org/10.3390/polym16020175
Chicago/Turabian StyleFořt, Jan, Ayodele Afolayan, Martin Mildner, Petr Hotěk, Martin Keppert, and Robert Černý. 2024. "Assessment of Clayey Freshwater Sediments as Suitable Precursors for Alkaline Activation" Polymers 16, no. 2: 175. https://doi.org/10.3390/polym16020175
APA StyleFořt, J., Afolayan, A., Mildner, M., Hotěk, P., Keppert, M., & Černý, R. (2024). Assessment of Clayey Freshwater Sediments as Suitable Precursors for Alkaline Activation. Polymers, 16(2), 175. https://doi.org/10.3390/polym16020175









