Abstract
Degradable polymers (both biomacromolecules and several synthetic polymers) for biomedical applications have been promising very much in the recent past due to their low cost, biocompatibility, flexibility, and minimal side effects. Here, we present an overview with updated information on natural and synthetic degradable polymers where a brief account on different polysaccharides, proteins, and synthetic polymers viz. polyesters/polyamino acids/polyanhydrides/polyphosphazenes/polyurethanes relevant to biomedical applications has been provided. The various approaches for the transformation of these polymers by physical/chemical means viz. cross-linking, as polyblends, nanocomposites/hybrid composites, interpenetrating complexes, interpolymer/polyion complexes, functionalization, polymer conjugates, and block and graft copolymers, are described. The degradation mechanism, drug loading profiles, and toxicological aspects of polymeric nanoparticles formed are also defined. Biomedical applications of these degradable polymer-based biomaterials in and as wound dressing/healing, biosensors, drug delivery systems, tissue engineering, and regenerative medicine, etc., are highlighted. In addition, the use of such nano systems to solve current drug delivery problems is briefly reviewed.
1. Introduction
A biomaterial is a natural or synthetic substance engineered to interact with biological systems for direct medical treatment. To qualify a substance as a biomaterial, it needs to be biocompatible and should be able to meet its desired function. Also, the physical, chemical, and biological properties of a substance to be used as biomaterial for tissue engineering and drug delivery devices affect the host response and thus need fine-tuning. Polymers and their composites provide an assembly of biomaterials with different physical, chemical, and mechanical properties. The degradable polymers undergo disintegration into low molecular weight products which are reabsorbed or excreted by themselves [1,2,3,4,5,6].
In the case of biodegradable polymers, the changes in the material properties take place by degradation over time in the presence of living organisms. Such changes may cause long-term host responses which are remarkably different from the initial response. Synthetic polymers are often non-degradables that create problems in waste disposal. However, to circumvent these issues, intense efforts have been made to produce degradable polymers. These polymers undergo thermal/photo/oxidative/hydrolytic/enzymatic biodegradation at random places along the macromolecular chain or through unzipping harmless products [7,8,9,10,11].
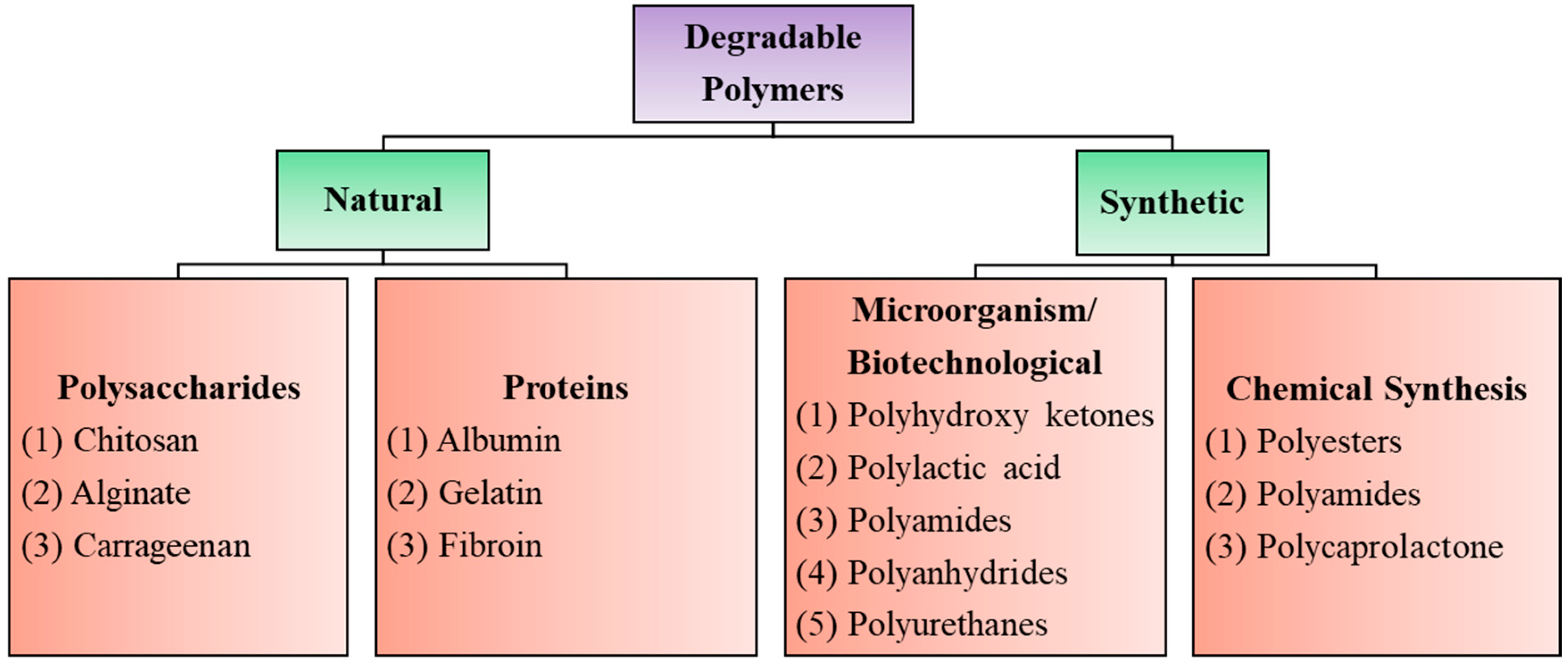
Biodegradable polymers are those that occur in nature such as polysaccharides, proteins, nucleic acids (in plants/animals), those synthesized by bacteria such as polyhydroxyalkanoates, or some semisynthetic materials derived from biopolymers or purely synthetic ones such as polylactic acid, polycaprolactones, etc., (Figure 1). Synthetic degradable polymers often belong to the polyester family, such as polyglycolides and polylactides. However, such polymers are poorly biocompatible, releasing acidic degradation products. Their poor processability and loss of mechanical properties during the initial stages of degradation also limit their usefulness as biomaterials [12,13,14].
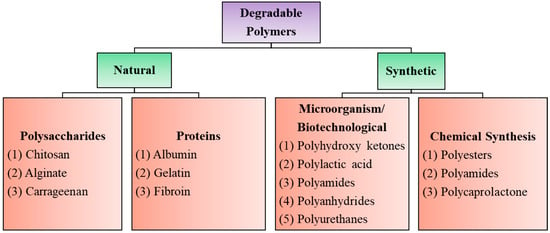
Figure 1.
Degradable polymers of biomedical interest with a few well-known examples.
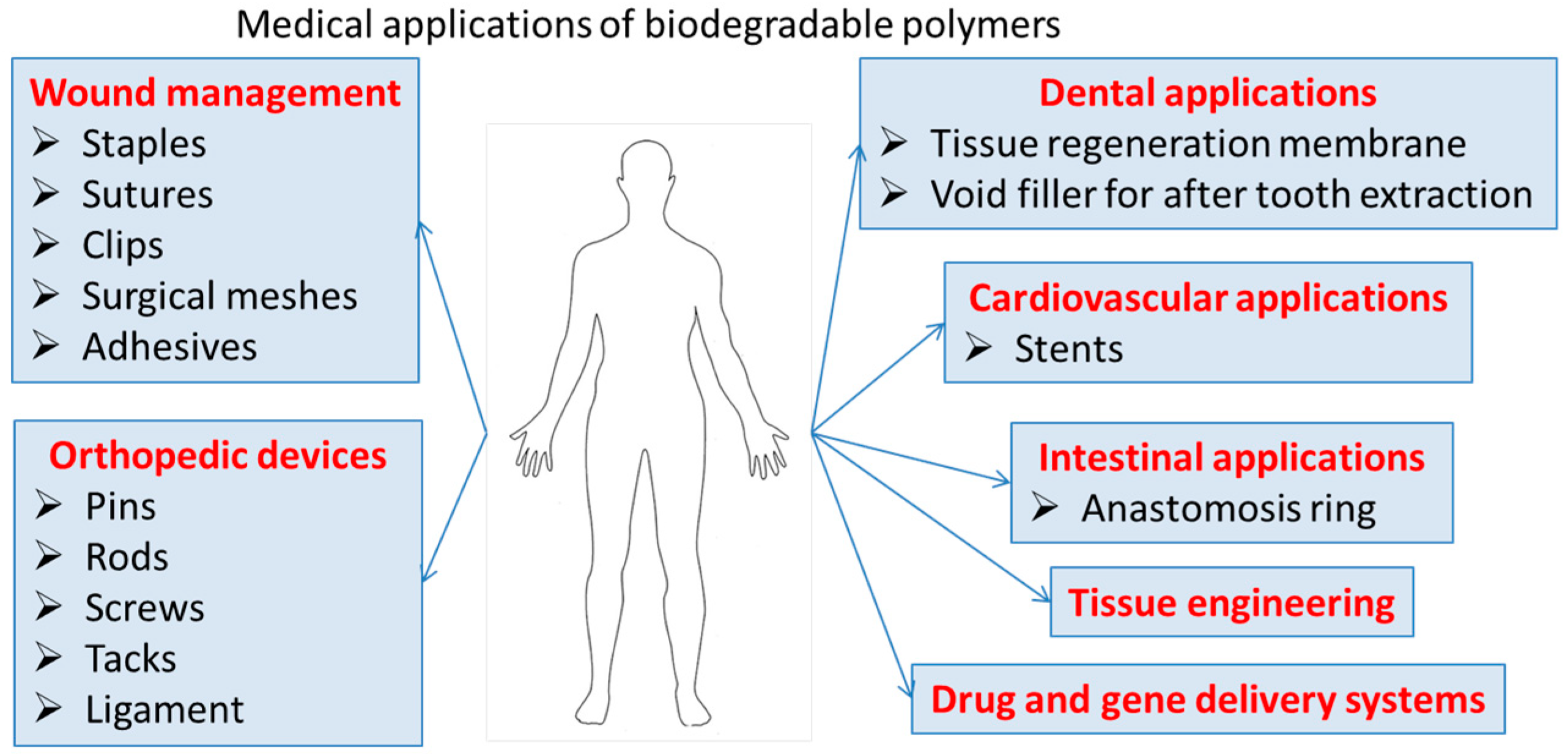
The biocompatibility, low toxicity, and chemical functionalities of these polymers can lead to nanomaterials for a variety of biomedical applications, that ensure clearance from the body and eliminate the need for retrieval or explant. Degradable polymers contain polymer chains that are hydrolytically or enzymatically cleaved, resulting in soluble degradation by-products. These biodegradable polymeric materials with enhanced properties such as bioavailability, stability, and controlled release of bioactive compounds are highly useful in tissue engineering/cartilage scaffolds, creation of skin, blood vessels, temporary prosthetic implants, etc., as illustrated in Figure 2.
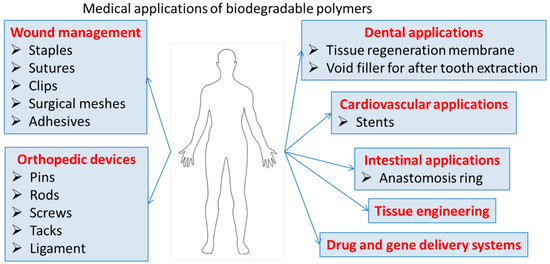
Figure 2.
Medical application of biodegradable polymers.
The clinical applications of these types of materials were reviewed by Stoppel et al. [15]. The chemical, physical, and mechanical properties of the biodegradable polymers might be suitable for their study in different types of medical applications. However, there are not enough data concerning the in vivo tests, especially for the degradation rate, in order to increase their translation to serious clinical assays. In fact, a systematic research strategy related to the modulation of their biodegradability in the presence of highly reactive oxygen species can be useful to widen the application fields of these materials.
Furthermore, the physical, chemical, and biological properties of degradable polymers can be improved and finely tuned through different strategies such as blending with other substances to form polymer alloys or nanocomposites, physical/chemical cross-linking to produce hydrogels, forming interpenetrating networks (IPNs), using their functionalities, and making useful block and graft copolymers as well as self-assembled nanoaggregates. It is therefore not surprising that concrete efforts have been made in recent times to generate such useful macromolecular biomaterials. The thermal and mechanical properties, degradation, and surface characteristics can be optimized on their nanoconstructs using chemical modification strategies. Physicochemical cross-linking, polyion complexes, IPNs, blends/nanocomposites with other materials, coating on to some nanoparticles, and layer-by-layer (l-b-l) assembly may lead to multiphase polymer systems with desired properties for their biomedical applications [12,16,17,18,19,20,21].
Drug delivery systems (DDS) require information on high drug uploading, programmed target-specificity, cellular uptake, clearance, toxicity, metabolism, pharmacokinetics, and excretion. An ideal delivery system should increase the efficiency of the drug and facilitate controlled release from a biocompatible nanocarrier to increase patient compliance. An important factor contributing to DDS efficacy is the passive accumulation of these particles at the desired site through the enhanced permeability and retention (EPR) effect. The conventional DDS have immediate release and sometimes are toxic as the frequency of their administration must be high in order to reach the therapeutic level. In order to avoid these drawbacks and to increase the pharmacokinetics, such as blood circulation, biodistribution, and active targeting, second and third generations of DDS were investigated [22]. The surfaces of these particles were modified in order to improve their stealth effect and also to increase their targeting delivery. A stealth effect can be induced by using PEG as a hydrophilic block as this polymer limits the plasma protein adsorption by steric repulsions and rapid clearance by the reticuloendothelial system (RES). This effect avoids the recognition of the particles by the immune system and thus their blood circulation is enhanced, allowing their accumulation in highly vascularized areas. Besides the particle’s surface, the stealth effect can be also influenced by the shape, size, and core composition [23]. However, until now, the number of formulations based on biodegradable polymers translated to clinical assays is quite limited.
Several nanomaterials from organic (polymers, amphiphiles) and inorganic (nanocarbon, silica, and metal/metal oxide nanoparticles) compounds have been examined as carriers for DDS. These include soft core–shell nanoaggregates such as liposomes, polymer micelles, and polymersomes (resulting from self-assembly of amphiphilic block or graft copolymers), nanoparticles, nanocapsules, nanogels, nanofibers, dendrimers, and nanocomposites which have been of immense use as nanocarriers for drug delivery systems. However, the size and polydispersity/shape/surface charge/surface characteristics/ porosity, etc., need to be fine-tuned for its desired application purpose. Several review articles had already been published on the use of different types of materials for the preparation and characterization of DDS [24,25,26,27,28,29,30,31,32,33,34].
Advances in polymerization techniques in the past two decades have led to the synthesis of tailor-made and well-defined polymers/copolymers of predetermined molecular weight and composition. Several biodegradable, stimuli-responsive, amphiphilic, and double hydrophilic copolymers; and polysaccharide-based graft copolymers, polymer bioconjugates coupled with modern characterization techniques, particularly self-assembled nanoaggregates; have been rather promising in recent years to boost drug nanocarrier research. Biocompatible polymers, due to their characteristic features, have been of immense interest in the scientific community for the diagnosis and treatment of several diseases. This article provides an updated comprehensive overview on all aspects along with discussion on the recent literature studies relevant to degradable polymers in this context.
2. Degradable Polymers
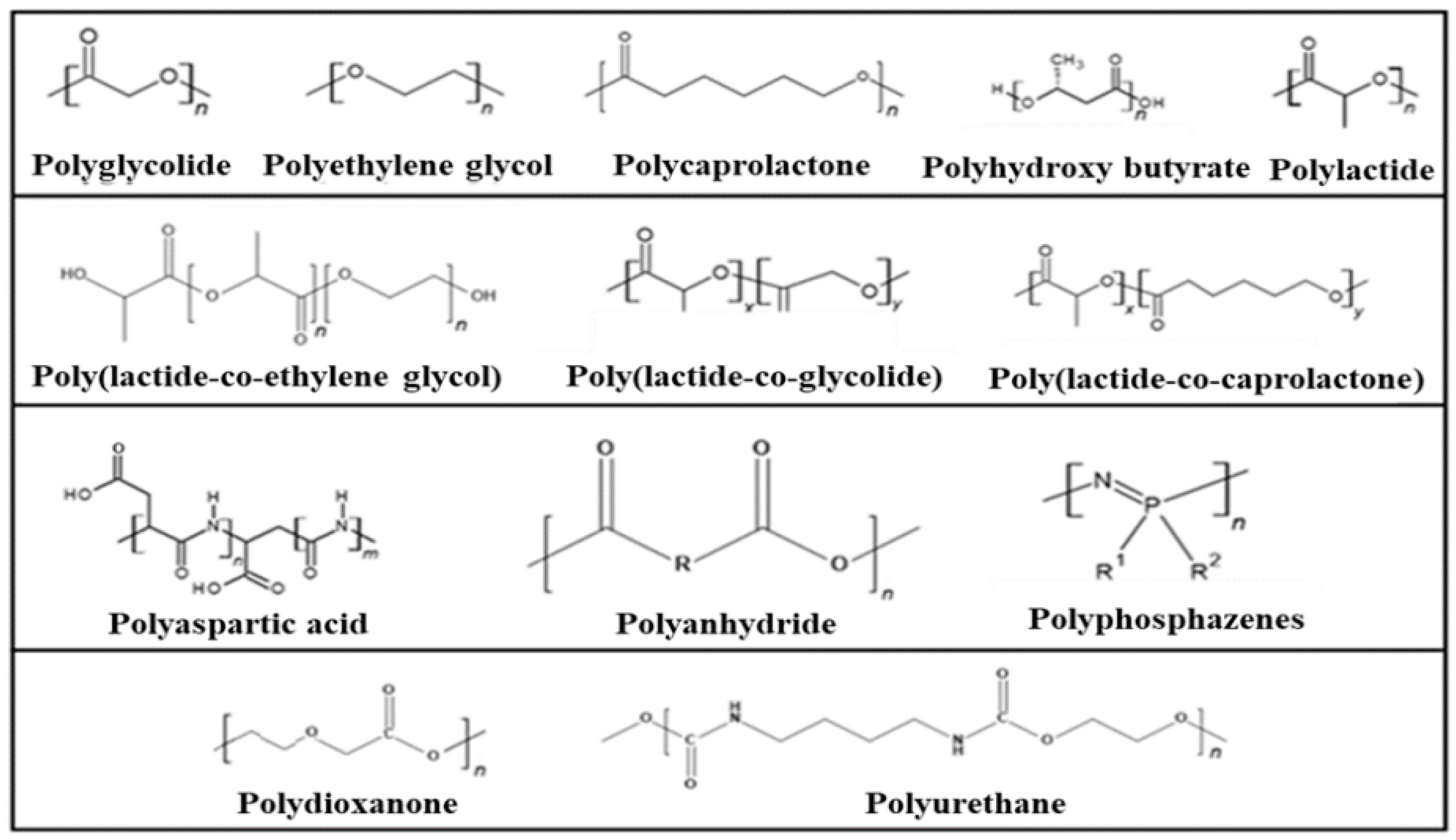
The most important examples of synthetic degradable polymers of biomedical interest are provided in Figure 3.
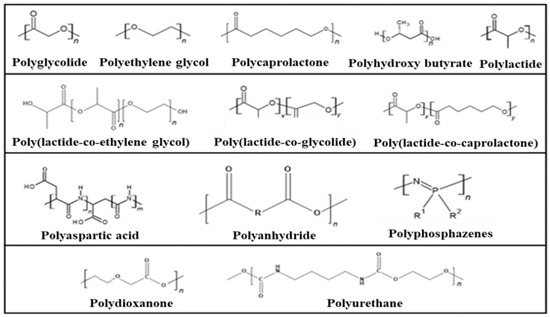
Figure 3.
Synthetic degradable polymers of biomedical interest.
2.1. Bacterial/Synthetic Degradable Polymers
2.1.1. Polyesters
Polyester-based biodegradable polymers have been useful as medical devices such as sutures, plates, stents, screws, bone fixation devices, and tissue repairs, due to their desirable physical and physicochemical properties. A few well-known polyesters are polylactic acid (PLA), poly(lactic-co-glycolic acid) (PLGA), poly(ε-caprolactone) (PCL), poly-3-hydroxybutyrate (or poly-β-hydroxybutyric acid (PHB), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), poly(propylene carbonate) (PPC), poly(butylene succinate) (PBS), and poly(propylene fumarate) (PPF).
Polyhydroxyalkanoates (PHA) polyesters are produced by microorganisms via fermentation. These polymers had been approved by the United States Foods and Drugs Administration (FDA) for clinical applications and used in orthopedics, repair patches, tissue engineering applications, sustained and controlled drug delivery, biomedical devices, and artificial organs [35]. Several bacterial (both gram-positive and gram-negative) species can produce PHA. The degradation of PHA proceeds via an abiotic route through hydrolysis of ester bonds. These novel materials find applications in orthopedics, tissue engineering, drug delivery, biomedical devices, and artificial organs [36,37,38,39,40,41,42,43,44,45,46].
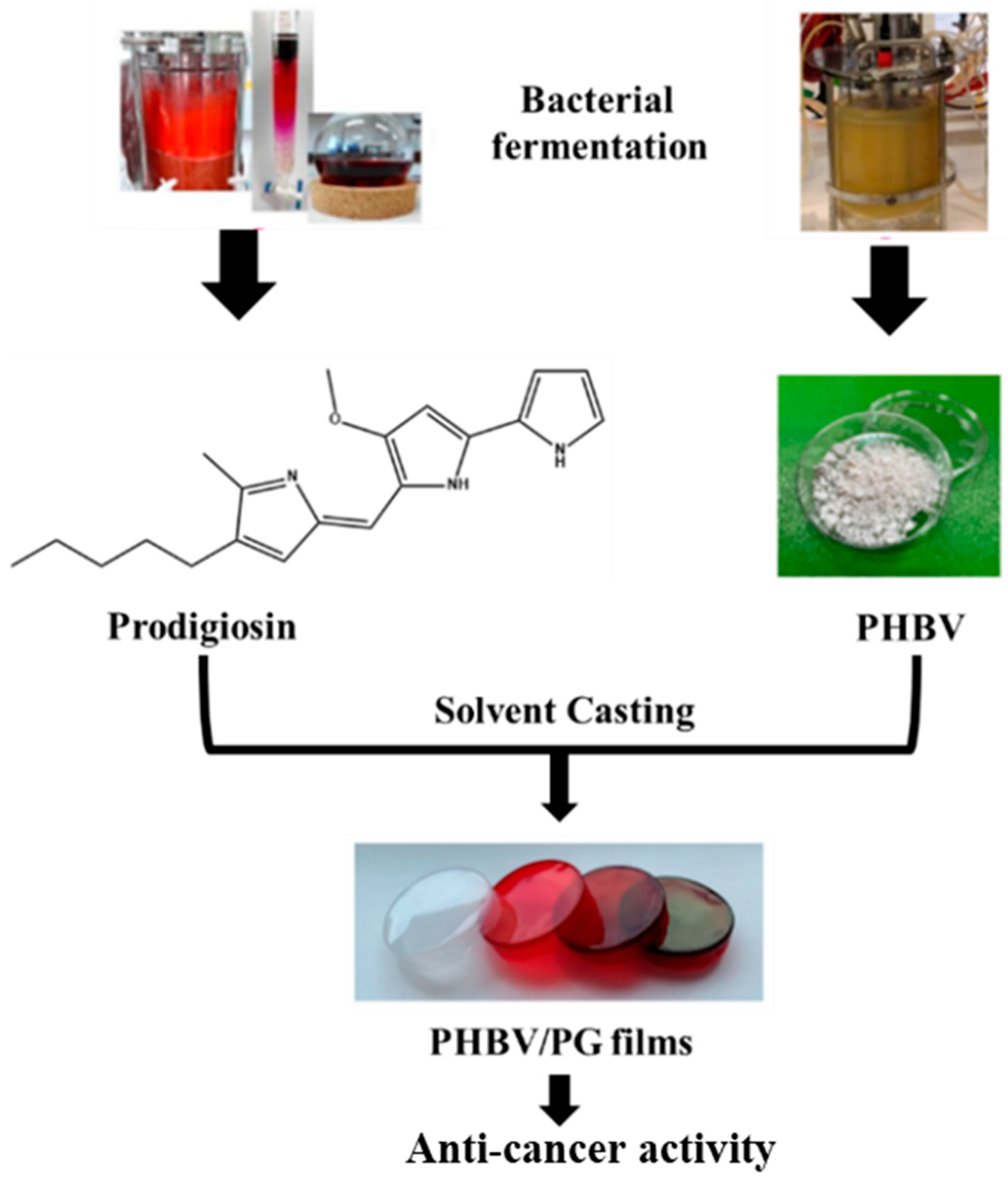
In a very recent study, a bacterial polyester PHBV and bacterial pigment Prodigiosin (PG) were produced using a simple solvent casting method [46], as illustrated in Figure 4.
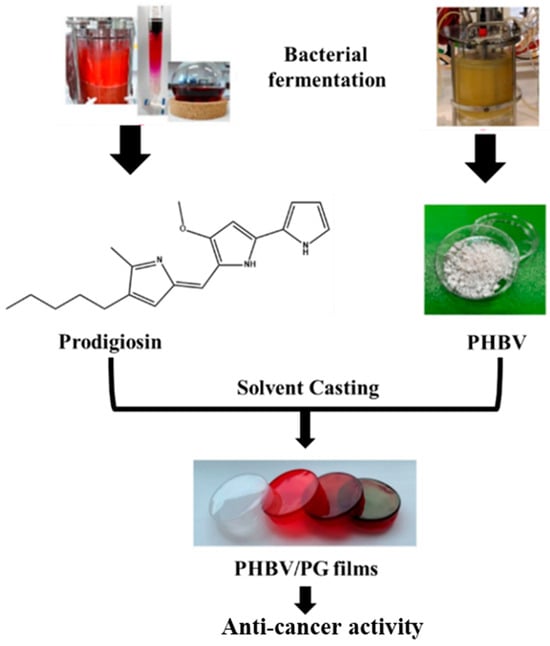
Figure 4.
Study design and potential biomedical application of the PHBV films with incorporated prodigiosin (PG).
Polylactic acid (PLA), polyglycolic acid (PGA), and polylactic acid-co-glycolic acid (PLGA) are FDA-approved polyesters. PLA is a naturally occurring aliphatic polyester obtained from wheat, rice bran, potato starch, corn, and biomass. PLA’s monomer is 2-hydroxypropionic acid, a chiral molecule existing in D- and L- and DL form, and therefore its properties depend on stereoregularity in the polymeric chain. Its biocompatibility, low cost, and biodegradability make it a widely explored biomaterial for various biomedical applications such as therapeutics, surgical implants, prosthetic devices, tissue culture, resorbable surgical sutures, drug delivery systems, etc. [47,48,49,50,51,52,53,54,55,56].
PGA is highly hydrophilic and can be produced by ring-opening polymerization of glycoside (a cyclic diester of GA) or by polycondensation. It is employed as a filler with other biodegradable polymers or in tissue scaffold application [57].
PLGA is a copolymer and is also important biomedically. Copolymerization by changing the monomer ratio of two monomers can result in a product with better mechanical and chemical properties without affecting biodegradability and biocompatibility.
Poly(ε-caprolactone) (PCL), a semi-crystalline degradable polymer, is of interest for long-term implantable/drug delivery systems as it is used in many FDA-approved surgical implants and drug delivery devices for tissue engineering and regenerative medicine applications. For example, the FDA has approved PCL for sutures with a trade name of Maxon™. It can be produced in high yield by ring-opening polymerization of ϵ-caprolactone. Being hydrophobic, PCL has a glass-transition temperature of −60 °C and melting point of 63 °C [58,59,60]. PCL copolymers have been good in accelerating the rate of biosorption. For example, copolymers of ϵ-caprolactone with dl-lactide are more flexible and have a higher degradation rate than PCL. It has been widely used in tissue engineering due to its availability, and the cheap and simple chemical modification approach by which physical/chemical/biological properties can be adjusted. By copolymerization with other esters, the biodegradability rate can also be adjusted [61,62]. Also, its high permeability to various agents has made it an important precursor for the development of drug delivery systems [63,64,65,66,67].
Polydioxanone (PDX) is a poly(ether-ester), has a glass-transition temperature in the range of 10 °C and 0 °C, and about 55% crystallinity. It is a commercially available biodegradable polymer that can be spun into fibers and is thus useful as monofilament suture, medical devices, in tissue engineering, and in drug delivery applications. These electrospun fibers are tough and possess mechanical properties similar to structural components of native vascular extracellular matrix (ECM). PDX sutures are non-antigenic and non-pyrogenic, possessing often greater pliability and strength than polypropylene and other sutures. Moreover, they retain their strength for longer periods than other synthetic absorbable sutures. The presence of an ether oxygen group in the polymer backbone renders PDX-based sutures with good flexibility. They have a unique shape-memory property and so have emerged as important biomaterials [68,69,70,71].
Polyphosphoesters are composed of phosphorus-incorporated monomers and consist of phosphates with two (one in the backbone and one in the side) groups. Polyphosphoesters are biocompatible like natural polymers. The hydrolytic cleavage of the phosphate bonds in the backbone occurs easily. These polymers have high potential for drug and gene delivery as well as scaffolds in bone tissue engineering [72,73,74,75].
Aliphatic Polyester Nanoparticles: The case of polylactic-acid-based nanoparticles.
Nanoparticles (NPs) from degradable polymers are promising as drug delivery carriers for controlled/sustained release due to their improved pharmacokinetics by maintaining the drug concentration at a therapeutic level with minimal side effects. The polyesters used as NPs for drug encapsulation/entrapment are FDA approved. Aliphatic polyesters contain several hydrolytically degradable ester linkages under physiological conditions; non-toxicity to human connective tissue degradation products make these nanoparticles biocompatible. The drug release profile resulting from the degradation of NPs depends on the crystallinity, glass-transition temperature, hydrophobicity, and molecular weight of the polymer and the mechanism can be explained using different models for predicting the kinetics of drug release [76,77,78].
Small sizes of NPs lead to a high surface area to volume ratio and thus enhance the absorption potential and cytotoxicity. However, aliphatic-polyester-based NPs may possibly lead to a reduction in cytotoxicity due to their high biocompatibility and biodegradability. These improve their bioavailability, drug payload and targeted delivery, and stability in the bloodstream.
NPs of aliphatic polyesters are commonly prepared via an emulsion process. Polymeric NPs refer to both nanospheres and nanocapsules. The drug molecules are uniformly distributed in the polymeric matrix of nanospheres, while the drug gets encapsulated inside the core cavity which is surrounded by a polymeric membrane that acts as a thin shell.
The preparation of such nano templates depends upon the hydrophilic/hydrophobic nature of the drug as well as the polymer solubility. Polyesters are hydrophobic and therefore drug solubility is often correlated to the formulation of nanoparticle type. Water-soluble hydrophilic drugs may produce nanocapsules while the hydrophobic ones will form nanospheres. The preparation of NPs is simple. Basically, here both the drug and polyester are mixed to uniformly distribute the drug in the polymeric matrix. The main challenges are the control of particle size and efficient drug loading. The commonly used methods to obtain NPs are briefly described here. In a single-emulsion/solvent-extraction method, usually polyester and the hydrophobic drug are dissolved in a water-immiscible organic solvent and emulsified with water containing surfactant. The organic phase is removed through evaporation at low pressure or vacuum, or by solvent extraction. Nanoparticles are recovered by centrifugation and washed with water or buffer solutions to remove any traces of solvent, stabilizer, and free drug prior to lyophilization. However, for hydrophilic drugs, the method is not suitable. Another approach involves double emulsions which also offer nanoparticles loaded with both hydrophobic and hydrophilic drugs. In this method, an aqueous solution of the hydrophilic drug is added to water immiscible organic phase containing the dissolved polymer and the w/o emulsion formed is subsequently added to a second water phase containing an emulsifier which, on mixing, forms a w/o/w emulsion. A similar solvent removal and purification process is then employed. These methods are quite simple but several process parameters such as phase volumes (oil and water), polymer, drug and stabilizer concentration, type of solvents, and stirring rate need to be carefully controlled. Other methods based on nanoprecipitation, salting out effect, dialysis, spray drying, and supercritical fluids are also employed in the preparation of polyester NPs. Supercritical-fluids-based methods are good due to the use of environmentally friendly solvent and providing NPs with no traces of residual solvents. NPs are stabilized by adsorbed amphiphilic molecules on their surface as the encapsulated drugs need to be protected from degradation. The flocculation of dispersed NPs may produce macroscopic lumps that lead to the early diffusion of drug molecules and degradation. The low molecular weight ionic surfactants can lead to high zeta potential to the dispersed particles and stabilize them through electrical repulsion. Amphiphilic block copolymers (BCPs) anchor to the particle surface and result in steric stabilization. However, care needs to be taken of colloid stability by adsorbed polymers as, if not carefully chosen, the adsorbed polymer may exert the opposite effect, causing flocculation by bridging mechanism or through depletion flocculation. Adsorbed polyelectrolytes (natural or synthetic), as well as block/graft copolymers with a polyelectrolyte block, may render stability to electrosteric stabilization. The stability of dispersed systems has been extensively examined quantitatively after the DLVO theory in the 1940s.
Polylactic-acid-based NPs have been extensively studied in the context of their biomedical application as these NPs have shown a great potential as nano-reservoirs for hydrophobic drugs, proteins, and genes, and releasing the active compound at the targeted site at the desired rate (thanks to their interesting properties). This led to a growing interest also in the nanomedicine field for the synthesis of nanoparticles for drug delivery and imaging purposes [79,80,81,82,83,84].
Block and graft copolymers of polylactic acid with a hydrophilic polymeric moiety behave like amphiphiles and thus self-assemble in an aqueous solution to form polymer micelles or polymersomes. These core–shell aggregates from amphiphilic polymers are excellent for solubilization of hydrophobic drugs [85,86,87]. In particular, PLA copolymers, such as those with polyglycolic acid and polyethylene glycol, have been very interesting as these can improve the characteristic properties of nanoparticles and fine-tune their applications. This co-amphiphilic entity may be water soluble if the lactic acid mole ratio is low. The form nanoaggregates and the hydrophobic drug is solubilized in the polylactic acid core that is stabilized by covalently bonded PEG shell [88,89,90,91,92]. The chemical nature adjustment by the proper ratio of comonomer results in interesting physical/chemical/biological properties in the NPs which would be desirable for their biomedical applications. Several studies have reported the NPs of polylactic acid-polyglycolic acid copolymers using varying ratios of lactic acid and glycolic acid to achieve improved results [93,94,95,96,97,98,99,100,101,102]. PLA graft copolymers also form multiphase polymer systems with an enormous possibility of improving the desirable features of polylactic acid in biomedical applications. The PLA-based graft copolymers are categorized on the basis of different monomers/polymers attached to PLA. That can be prepared using different grafting strategies. These PLA-based graft copolymers with biomacromolecules such as chitosan, cellulose, dextran, starch, etc., as well as synthetic polymers like polyethylene glycol, and vinyl-based polymers have been reported in the literature [103,104,105,106,107,108,109].
Polylactic-acid-based nanocomposites with other organic or inorganic nanomaterials have been important as these help improve the properties of PLA and make PLA-based nanocomposites popular in biomedical fields including bone substitute and repair, tissue engineering, and drug delivery system. Nanocomposites with PLA or PLA copolymers as matrixes significantly help improve PLA’s properties and therefore studies have been made to produce useful nanocomposites with clay minerals, carbon nanotubes, and graphene, etc. [110,111,112,113].
2.1.2. Polyamino Acids
Polymers based on amino acid monomers are derived from the polycondensation of amino acids and have been widely investigated in drug delivery applications due to their low toxicity, extreme biocompatibility, and biodegradability that make them attractive biomaterials, and so are employed in medicinal, biomedical, pharmaceutical, and cosmetic applications [114,115,116,117,118].
In particular, the natural polyamino acids such as poly(β-alanine), poly(lysine), poly(γ-glutamic acid), polyaspartic acid, polyhistidine, and polyarginine; and synthetic polyamino acids such as poly(hydroxyethyl-l-asparagine) and poly(hydroxyethyl-l-glutamine) are explored widely for different applications. These polymers offer multiple pendant functional groups for drug attachment, resulting in polymeric prodrugs. For example, polyglutamic acid-texane has been developed for the first-line treatment of non-small cell lung cancer. Poly(l-glutamic acid) has already entered into Phase III clinical trials [119,120]. Natural amide-based polymers such as gelatin and albumin are widely used in the preparation of biodegradable microcapsules and microspheres.
Synthetic poly(amino acid)s are also investigated in biomedical/drug delivery applications as they are structurally similar with naturally occurring proteins. In general, the application of water-soluble polymer conjugates in drug delivery via carrier-based systems offers several advantages such as improved drug pharmacokinetics, minimal toxicity to vital organs, tissues, or cells, and enhanced drug build-ups at the target site. Moreover, the release of the drug from a carrier at the target tissues can be programmed to achieve a predetermined drug delivery.
Low molecular weight drugs are typically delivered using poly(amino acids), which are cleaved by enzymes into non-toxic substances. Poly(amino acid) polymers degrade inside the body, resulting in the amino acids that are biologically active and are often used as drugs. The following section highlights a few widely used poly(amino acid) polymers in drug delivery.
Poly(aspartic acid) is widely used in biomedical applications, especially gene delivery and drug delivery, due to its low toxicity profile, non-antigenic, excellent biocompatibility, and biodegradability. Poly(aspartic acid)s like other poly(amino acid)s are extensively used in several areas such as water treatment, paper processing, and paint additives. In the field of biomedical sciences, poly(aspartic acid) is employed in the design of dialysis membranes, drug delivery systems, hydrogels, orthopedic implants, and artificial skin [121].
2.1.3. Polyanhydrides
Polyanhydrides can be aliphatic, aromatic, unsaturated, hybrid, or blend depending on the desirable physical properties suiting for specific applications. Polyanhydrides used as biomaterials are surface-eroding polymers that contain two carbonyl groups bound together by an ether bond. Polyanhydrides have been used for the delivery of bioactive molecules to various organs (brain, bones, blood vessels, and eyes) of the human body and are often fabricated as micro/nanoparticles to allow for injectable, oral, or aerosol delivery due to superior biocompatibility and lack of toxicity [122].
These are degradable polymer materials that possess hydrolytically labile chemical bonds in their backbone and can be broken down without secondary influence. They easily undergo hydrolytic degradation to their respective diacids, which are completely eliminated from the body within a short period of time. The degradation of the anhydride bond varies with polymer backbone chemistry which is important for their drug delivery applications. It has been found that these biocompatible polymers degrade into non-mutagenic and non-cytotoxic products [123,124].
Polyanhydride-based NPs can be successfully used as drug carriers. Betulin and its derivatives (e.g., betulin disuccinate) have a broad spectrum of biological relevance, including anti-cancer activity, thus these compounds are promising as new, potentially therapeutic agents. The major problem, which limits their potential pharmaceutical uses, is facing the poor aqueous solubility of lupane triterpenes when trying to formulate pharmaceutical compounds from betulin. However, this problem can be solved by obtaining a polymeric form of betulin and forming it into nanospheres, thus nanoparticles prepared from betulin-based polyanhydrides may have significant applications in drug delivery systems [125].
2.1.4. Polyphosphazenes (PPZ)
Polyphosphazenes are inorganic polymers comprising a backbone of repeating phosphorus and nitrogen atoms with alternating single and double bonds. The two electrons of phosphorus can be used for side-chain conjugation, while two electrons of nitrogen remain a lone pair. By incorporating different side groups, or by using two or more co-substituents in the PPZ backbone, derivatized PPZs offer a wide range of properties. These can be hydrophobic/hydrophilic/amphiphilic, crystalline/amorphous, and bioinert/bioactive. Changes in the organic side groups allow substantial alteration in the physical and degradation properties of these polymers which can be rendered as useful and versatile biomaterials with desired mechanical properties and degradability for tissue engineering applications, such as bone tissue or blood vessels in bioimaging, phototherapy, dentistry (restorative composites and dental liners), and medical devices. PPZs incorporated drugs into their structure by physical encapsulation or chemical bonding, form a prodrug delivery system. The possibility of synthesizing materials with tailored degradation kinetics and their structural flexibility makes PPZs an attractive choice as an efficient carrier in drug delivery. However, these polymers are expensive and are used only for limited purposes [126,127,128,129,130].
Compared to other synthetic polymers, such as polylactic acid (PLA) and poly(lactide-co-glycolide) PLGA, the advantages of PPZ are remarkable, due to the controlled tuning of physicochemical properties via the side-chain conjugation. Furthermore, PPZs are hydrolytic and biodegradable materials yielding non-toxic degradation products. Also, a range of PPZ-based biomaterials has been researched for the development of scaffolding materials for use in bone and skeletal regenerative tissue engineering [131,132,133].
2.1.5. Polyurethanes
Polyurethanes are obtained from the reaction of diisocyanates with compounds containing active hydrogen atoms such as diols or diamines. Polyurethanes have been of great interest due to their versatility, biocompatibility, and tunable mechanical properties and porosities. The major applications include flexible and rigid foams, adhesives, and surface coatings. These have long been used in the biomedical field as a thermoplastic elastomeric material for cardiovascular applications. Catheters, heart valves, prostheses, and vascular grafts have all typically been made of biostable and inert polyurethanes. Being a very stable material, there has also been a rise in research interest in recent years in building resorbable/degradable polyurethanes for tissue engineering and drug delivery systems [134,135,136]. It is possible to create biomedical polyurethanes by giving their backbones hydrolysable segments (e.g., polyether, polyester, and polyamide segments). The use of biocompatible aliphatic or cycloaliphatic diisocyanates (ICs) and diisocyanates generated from amino acids is a second method for producing such polyurethanes. Some substances, such as toluene 2,4-diisocyanate and 4,4′-methylene bis(phenyl isocyanate), are less hazardous than conventional ICs. Such polyurethanes have also been shown to increase cell adhesion and proliferation while having no negative side effects.
Depending on their component parts, biomedical polyurethanes can be biodegradable or non-biodegradable polymers with biocompatible properties that can be adjusted to biological systems, such as those of the blood, organs, and tissues. Also, it is very simple to change the mechanical properties of polyurethanes, i.e., materials for implants with the best yield modulus, strength, and resistance to fatigue, wear, or friction can be accounted where the modulus, mechanical strength, and fatigue resistance are very essential and crucial parameters for polyurethanes to be used in reconstructive surgery of soft tissue and cardiovascular surgery. Due to these advantages, polyurethane-based nanoparticles are widely utilized for controlled delivery of proteins, growth factors, antibiotics, antitumor drugs, and other bioactive substances [137,138,139,140].
2.2. Biopolymers
2.2.1. Polysaccharides
Polysaccharides are natural polymers that have gained enormous interest in recent years due to their abundance and having several functional groups such as hydroxyl, carboxylic, and amino groups. These entities are inert, cheap, non-toxic, and biocompatible with good water stability. These can be easily derivatized, cross-linked, or transformed into multiphase polymer systems such as interpenetrating networks (IPNs), polyblends, graft, and block copolymers [141,142]. Charged polysaccharides form useful polyion complexes. Important polysaccharides of biomedical interest such as cellulose, lignin, starch, agarose, CD, guar-gum, hyaluronic acid, chitosan, alginate, carrageenan, fucoidans, chondroitin sulfate, and pullulan are included for discussion herein.
Cellulose
Cellulose is the most abundantly found polysaccharide in nature, with sequences of β-D-glucopyranose units linked by β-(1,4) glycosidic bonds, and is a very promising advanced polymeric material. It can undergo degradation in the presence of bacteria and fungi present in the air, soil, and water. The biocompatibility, low toxicity, and low cost make cellulose and its derivatives a versatile precursor. However, extensive intermolecular hydrogen bonding transforms cellulose into a highly crystalline polymeric material and thus it is insoluble in almost all solvents, leading to difficult processing. Such dissolution of cellulose is circumvented using ionic liquids and deep eutectic solvents. Cellulose can be etherified or esterified to yield useful environment-friendly materials like methylcellulose, hydroxypropyl cellulose, carboxymethylcellulose, cellulose acetate, cellulose nitrate, etc. Cellulose nanomaterials such as cellulose nanofibrils and nanocrystals as well as bacterial nanocellulose have been of great research interest. However, cellulose has limitations such as a lack of thermoplasticity, poor crease resistance, and a tendency to cause antigenicity [143,144,145,146].
Lignin
Lignin is the other most abundantly found biopolymer that constitutes approximately 15–35% of the lignocellulosic biomass. Its high carbon content, greater thermal stability, enhanced adhesive properties, biodegradability, biocompatibility, non-toxicity, cost effective production, and biological activities make lignin and composite materials important for biomedical applications in tissue engineering scaffolds, wound dressing materials, and drug delivery carriers [147,148,149,150].
Starch
Starch is a plant polysaccharide made up of linear chains of amylose (soluble starch) and branched amylopectin components from a-glucose unit and is chiefly found in cereal grains, legumes, roots, and fruits. Amylose has glucose units connected by α-(1,4) linkage while amylopectin contains glucose units by means of α-(1,4) and α-(1,6) bonds. Starch obtained from different plant sources has different properties which also depend on the degree of maturity of the plant. Chemically modified starch and its physical blends or IPNs with improved properties are important biomaterials in bone and tissue engineering. However, starch is brittle and degrades before its melting temperature. Its poor mechanical properties and difficult processability limit its use as a pure material [151,152,153,154].
Agarose
Agarose is an uncharged polysaccharide, is a main constituent of agar (generally extracted from cell walls of certain marine red algae), dissolves in hot water, and also in polar non-aqueous solvents and ionic liquids. Structurally, it is a linear neutral polysaccharide consisting of alternate (1,3)-β-d-galactopyranose and (1,4)-linked 3,6-anhydro-α-l-galactopyranose units. It dissolves in boiling water and forms gels on cooling to ~<40 °C. Agarose forms chains which create flexible fibers. The chains can curl to helix structures and form very strong gels which display a pronounced hysteresis. Agarose is non-toxic and is useful as a gelling agent as commonly used in nucleic acid electrophoresis, affinity chromatography/ion exchange chromatography, gel plates, or overlays for cells in tissue culture, cell culture media, immunodiffusion techniques, etc. It has been used as a bio-ink due to its excellent biocompatibility, mechanical strength, and thermo-reversible low gelling temperature of 32 °C (this depends on concentration). The gelation results in the formation of an infinite network of 3D agarose fibers. Such hydrogel networks disassemble above 85 °C [155,156,157,158].
Cyclodextrins
Cyclodextrins are oligosaccharides composed of glucose units (α-D-glucopyranose) connected by α-(1,4) bonds enzymatically obtained as α-cyclodextrin (6 glucose units), β-cyclodextrin (7 units), and γ-cyclodextrin (8 units). The unique truncated cone-like structure of cyclodextrins possess an internal non-polar hole with polar hydroxyl groups placed on the surface of its molecule, and thus hydrophobic substances like drugs can get encapsulated in its cavity due to hydrophobic interaction and bind through operative forces like the van der Waals interaction and dipole–dipole interaction, thus forming a host–guest supramolecular complex. The chemistry and applications of cyclodextrins in different fields have been reviewed and these inclusion complexes can be derivatized and suitably chemically modified [159,160].
Guar-gum
Guar-gum is a readily water-soluble, high molecular weight polysaccharide extracted from Cyamopsis tetragonolobus seeds of the leguminous plant. It is made up of a main chain of D-Mann pyranose residues connected by β-(1,4) glycosidic bonds, linked to D-galactopyranose residues by α-(1,6) glycosidic bonds. It is a good emulsifier, thickener, and stabilizer in the food, pharmaceutical, and cosmetic industries. Its solubility in cold water increases depending on the galactose/mannose molar ratio. It is functionalized (esterified/hydroxyalkylated/carboxymethylated) and derivatized using different strategies to decrease its aqueous solubility and improve its mechanical properties for its use in biomedical applications. Guar-gum and its derivatives are designed for oral drug delivery due to enhanced stability over a wide pH range [161,162,163,164,165,166,167].
Hyaluronic acid
Hyaluronan or hyaluronic acid is a linear, non-sulfated glycosaminoglycan composed of disaccharide repeat units of β-1,4-D-glucuronic acid and β-1,3-N-acetyl-D-glucosamine linked by ß-1,4-glycosidic bonds. Hyaluronic acid is the major component of the extracellular matrix of vertebrate soft connective tissues where it is present in high concentrations in synovial fluid, vitreous humor, skin, and umbilical cord. It is commercially extracted from rooster combs or through bacterial fermentation. It is an anionic polysaccharide which can absorb a large amount of water and hence acts as lubricant in native extracellular matrixes controlling the viscoelasticity of connective tissues. Hyaluronic acid can be chemically modified by cross-linking and grafting. Hyaluronic acid is a major ligand for CD44 (frequently over-expressed on the tumor cell surface) and CD168 which are over-expressed in various tumor and inflammation conditions. Hyaluronic acid interacts with cells in many biological processes such as tissue homeostasis, cell proliferation, angiogenesis, tumor invasion, matrix organization, and anti-apoptosis. Hence, it is used in skin rejuvenation, tissue engineering, molecular imaging, and drug delivery applications. Unfortunately, hyaluronic acid is susceptible to rapid degradation. The hydrogels based on hyaluronic acid are generally brittle, or easily dissolve in aqueous solutions. Derivatized hyaluronic acid and IPNs/PICs of hyaluronic acid are useful biomaterials, though high cost and poor mechanical properties are major crunches [168,169,170,171,172,173,174,175,176,177,178,179,180,181].
Chitin and Chitosan
Chitin, a major component of the shells of shrimp and other sea crustaceans, is the second most abundant biomacromolecule used in the food and agriculture, textile, and pharma industries. It is biodegradable, biocompatible, non-toxic, mucoadhesive, and can be easily extracted and chemically modified to yield a wide range of products useful as biomaterials. Chitosan, derived from crabs and cell walls of fungi, is produced commercially by deacetylation of chitin. However, the extent of deacetylation, the content of impurities, and the distribution of the molar mass of chitosan depend on the natural source of the primary material as well as the preparation method. It is a cationic linear copolymer polysaccharide made up of β (1→4) linked 2-amino-2-deoxy-D-glucose (D-glucosamine) and 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) units by glycosidic bonds. The primary amino groups in the polymer backbone of chitosan provide a positive charge on its surface which facilitates the capability of forming inter and intramolecular H-bonding. It also possesses antimicrobial (fungi, bacteria, viruses) activity, making it useful in the biomedical field [182,183]. However, poor solubility at physiological pH is one of its major disadvantages [184,185,186,187,188,189,190,191,192,193,194,195]. Even if the synthesis procedure is not always reproducible, chitosan was tested for short periods on humans and no signs of allergic reactions appeared [196].
Alginate
Alginate is a water-soluble anionic polymer composed of α-L-guluronic acid (G) and β-D-mannuronic acid (M) residues linked by 1,4-glycosidic linkages. It is non-toxic, biocompatible, biodegradable, and can be produced at low cost from marine brown algae. Alginate is easily processed and has many different biomedical applications. Alginate-based three-dimensional scaffolding materials can be employed as hydrogels, microspheres, microcapsules, sponges, and foams for tissue engineering. Alginates, in combination with other polymers as chemically modified, are extensively used in food and biomedical processes. Physical or chemical modification can lead to derivatives having various structures, enhanced properties, and improved applications. For example, the mechanical strength, gelation property, and cell affinity can be finely tuned through combination with other biomaterials, immobilization of specific ligands such as peptide and sugar molecules, and physical or chemical cross-linking. However, susceptibility to hydrolysis in acidic environments, and fabrication due to its low solubility are challenging areas [197,198,199,200,201,202,203]. Different clinical investigations have been reported for alginate-based materials. For example, it was reported that sodium alginate in a dose of 60 mg/day decreased blood pressure after 2 weeks of treatment. Also, alginate-based foodstuffs NTCELL® and DIABECELL® are in an advanced stage of clinical investigation [204].
Carrageenans
Carrageenans constitute a family of linear sulfated polysaccharides that can be extracted from red seaweeds, also called Irish moss (belonging to red algae-Rhodophyta). These large and highly flexible molecules can curl to helical structures and thus form highly viscous solutions or viscoelastic/elastic gels. Carrageenans are composed of alternate units of β-d-galactose and 3,6-anhydro-α-d-galactose, linked by α-(1,3) and β-(1,4) glycosidic unions. Depending on the method and the algae from which the carrageenan is extracted, three main types of carrageenans can be obtained. For example, kappa (κ-1 sulfate group per disaccharide), iota (ι-2 sulfate groups per disaccharide), and lambda (λ-3 sulfate groups per disaccharide), although several other types are also reported. Carrageenans play an important role in plant growth promotion and are elicitors of resistance to pathogens and thus are crop protectants against a broad spectrum of plant pathogens. They also have excellent gelling, thickening, protein binding, and colloid stabilizing properties; and so are widely used in the dairy, food, and cosmetic industries; and also have applications in the pharmaceutical industry, as gel base, emulsion stabilization, etc. However, low gel strength, and anti-coagulant activity are its major drawbacks [205,206,207,208].
Fucoidans
Fucoidans refer to ionic polysaccharides which contain substantial percentages of L-fructose and sulfate ester groups, mainly derived from brown seaweed and some marine invertebrates (such as sea urchins and sea cucumbers). They are non-toxic, biodegradable, biocompatible, and FDA-approved compounds and so are used in many nutraceuticals and functional food products. However, the performance of Fucoidan depends on the species from which it is extracted. It can be transformed into nanoparticles and microparticles, films or hydrogels, and polyion complexes with cationic biopolymers, making it useful in pharmaceutical technology. Fucoidan-based particles serve as a vehicle for several drug and protein molecules. Additionally, fucoidans have been extensively studied due to their several interesting biological activities such as antioxidant nature, anti-inflammatory, anticoagulant, antithrombotic, antifungal, antivirus, antitumor and immunomodulatory against hepatopathy, uropathy and renal path, etc., that enable them as pharmaceutically important [209,210,211,212,213].
Chondroitin sulfate
Chondroitin sulfate is the major constituent of hyaline cartilage, located in cartilage and also at the calcification site of the bone. Structurally, it is a sulphated polysaccharide with repeating disaccharide units of β-1,4-linked-d-glucuronic acid and β-1,3-linked N-acetyl galactosamine and ß-1,4-linked-D-glucuronic acid with certain sulfated position(s). The two major chondroitin sulfates differ from one another in the position of the sulfates at position 4 or 6. The presence of polar carboxyl and hydroxyl functional groups facilitates the polymer to interact with other materials covalently/electrostatically. Its antioxidant, antiatherosclerosis, anticoagulant, antithrombosis, and negative immunogenic properties make chondroitin sulfate a useful biomaterial. It is a widely used material in the treatment of osteoarthritis. Chondroitin sulfate can target the CD44 receptors on cancer cells and be used for cancer therapy [214,215,216,217,218,219].
Pullulan
Pullulan is an α-glucose polymer with α 1→4, and a few α, 1→6 glycosidic bonds and is primarily produced from starch by the fungus Aureobasidium pullulans. It is an excellent material because it is biodegradable, biocompatible, and non-toxic, and it is produced from plant-based starch or sugars regenerated by natural photosynthesis of carbon dioxide and water. Pullulan is a water-soluble, odorless, tasteless, edible polysaccharide, and forms stable, viscous non-hygroscopic solutions. Its adhesiveness, non-immunogenic profile, non-mutagenic and non-carcinogenic qualities, and lower permeability of oxygen make it popularly used in food packaging, pharmaceutical, and cosmetics industries. Pullulan’s solubility and other characteristics can be tailor-made via chemical modification. Various derivatives of Pullulan can be conveniently prepared by cross-linking, esterification, or etherification, blending Pullulan with other polymers, forming IPNs and graft copolymers. This entity comes up with a few limitations; it is highly expensive and brittle with low mechanical strength [220,221,222,223].
Ulvans
Ulvans are complex sulphated polysaccharides obtained from the cell walls of the green algae of the Ulva genus which represent up to 50% of the dry mass. These are mainly composed of sulphated L-rhamnose of D-glucuronic acid and its C5-epimer L-iduronic acid (as found in animal glycosaminoglycans), and of a minor fraction of D-xylose. Ulvans display several physicochemical and biological properties that make these polysaccharides important for food applications [224,225].
Dextran
Dextran, a bacterial homopolysaccharide, is a generic term for glucans made by polymerization of α-d-glucopyranosyl moiety of sucrose catalyzed by the enzyme dextransucrase. It contains glucose units connected by α-(1,6) bonds in the main chain and by α-(1,4), α-(1,3), and α-(1,2) bonds in the branches. The degree of branching, molecular weight, and other properties of dextran changes with the microorganism employed. Dextran solutions have excellent rheological behavior and have larger plasma volume expanding efficacy. Chemical modifications to it involve the introduction of aldehyde, (meth)acrylate, thiol, phenol, and maleimide groups. Being a biocompatible and non-toxic polysaccharide, Dextran is widely used as an antithrombolytic agent, a bio-adhesive, in protein and drug delivery, in tissue-engineered scaffolds, in pharmaceutical, and biomedical applications. Dextran-based injectable hydrogels are also put forth as a site-specific, trackable, chemotherapeutic device. Additionally, Dextrans are known as blood plasma expanders. However, it suffers from disadvantages of high cost and non-availability [226,227,228,229].
Gum arabic
Gum arabic is a complex mixture of glycoproteins and polysaccharides (in particular arabinose and galactose). It is a water-soluble neutral polymer and has been used extensively as stabilizer, thickener, and emulsifier in food, cosmetics, and pharmaceutical industries. It has also been widely exploited as an effective excipient in accomplishing various nanoscale scaffolds for drug delivery and biomedical applications. Gum arabic can be cross-linked to hydrogels, blended with other polymers, used to form drug conjugates, and be attached to nanoparticles that may have biomedical applications [230,231,232,233,234,235,236,237,238].
Pectins
Pectins are polysaccharides present in the cell walls of higher plants. Their structures consist of D-galacturonic acid units connected by α-(1,4) bonds, forming a linear chain framework interrupted by highly branched regions. The composition of pectin differs according to its botanical source. However, a poor water-vapor barrier and low mechanical properties are its few limitations [239].
Gellan
Gellan is an extracellular anionic, linear heteropolysaccharide produced by Pseudomonas elodea. It contains two glucoses, one glucuronic acid, and one rhamnose molecules. A commercially formed deacetylated Gellan gum is a firm, thick, and brittle gel and is used in the food industry. This biocompatible polymer, being FDA approved, is used in additives in foods, cosmetics, pharmaceuticals, and drug delivery systems [240,241,242], and has been thought to be relatively expensive [243].
2.2.2. Proteins
Albumins
Albumin is a protein present both in animal and plant physiological fluids/tissues and plays an important role including maintenance of osmotic pressure, neutralization of free radicals, binding, and transport of various substances like hormones, drugs, in blood, etc. Albumins act as an interface between cells to various scaffold materials as collagen, thereby mediating the integration of these two components in tissue engineering. Serum albumin is a non-toxic, stable, and biodegradable protein. Its binding with drugs plays an important role in pharmacokinetics and drug distribution/metabolism. As a consequence, albumins have emerged as a potential drug carrier and find numerous applications as theranostic agents, contrast agents, biosensors, and implants against cancer, diabetes, arthritis, and hepatitis C. The structural domains and functional groups in albumin allow the binding and capping of several inorganic nanoparticles that enhances its bioavailability, compatibility, and circulation times, with selective bioaccumulation and reduced toxicity [244,245].
Silk proteins
Insects like silkworms and spiders produce silk, the toughest protein fiber in nature. Those produced by silkworms are primarily fibroin and sericin and have excellent mechanical properties like high tensile strength, flexibility, and resistance to compression, biodegradability, and minimal immunogenicity, the properties which make them biomedically important. Polymerization of enzyme fibrinogen gives fibrin (a protein that is known for its biodegradability and biocompatibility) which is an excellent source for the regeneration of bone. Fibroin is a glycoprotein which is insoluble, non-toxic, less immunogenic, hydrophobic, and histocompatible. It is extracted from silkworm cocoons and is made up of amino acids (alanine, serine, and glycine) resulting in antiparallel beta-sheet formation. It forms hydrogels, membranes (films), gels, nanoparticles, scaffolds, and fibers. The mechanical strength, malleability, and high surface area coupled with its biodegradability and biocompatibility make fibroin a viable alternative for application in the field of drug delivery. Sericin, a hydrophilic protein, dissolves in water and functions as a glue. It exists in a partial unfolding state comprising a beta-sheet of 35% and random coil of 63%. As a nanoparticle, it offers inherent anti-tumor, and antioxidant activity. It is non-toxic to fibroblast cells [246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264].
Gelatin
Gelatin is derived from collagen via controlled alkaline, acid, or enzymatic hydrolysis. It has excellent biodegradability and biocompatibility and because of its availability at low cost, it is a versatile polymer to meet industrial demand. The mechanical properties, swelling behavior, thermal properties, etc., of gelatin depend upon the collagen source, extraction method, and degree of cross-linking. Gelatin has been used in the biomedical field as a matrix for implants, and as stabilizers in vaccines against measles, mumps, and rubella. Due to its water permeability and solubility and multifunctional properties, gelatin finds its use as a drug-delivery vehicle. It produces a thermo-reversible gel which makes it a very good precursor in targeted drug delivery. Gelatin is available as drug carrier systems in the form of microparticles, nanoparticles, fibers, and hydrogels [265,266,267,268,269].
Elastin
Elastin, a structural protein, is the main component of elastic fibers present in the extracellular matrix (ECM). It imparts structural support to numerous organs and tissues. It is responsible for allowing tissues in the body to “snap back” to their original shape after being stretched or contracted. Elastin-like polypeptides are stimulus-responsive biopolymers derived from human elastin. Their low immunogenicity and control over their structure can lead to unique architectures as nanoparticles, nanofibers, and nanocomposites, and they display bioactive agents for their wide applications in tissue engineering and drug delivery [270,271,272,273,274,275,276,277].
Collagen
Collagen is the protein found in the human body that offers physical support to tissues by residing within the intercellular space. Generally, collagen is a rod-shaped polymer approximately 300 nm long with a molecular weight of approximately 300 kDa. Collagen undergoes enzymatic degradation within the body to yield its corresponding amino acids. Collagen plays a crucial role in preserving the biological and structural integrity of the ECM.
Animal-derived and recombinant collagens are valuable biomaterials for their use in cosmetic surgery and tissue engineering. Its composite with hydroxyapatite and tricalcium phosphate has been FDA-approved as a biodegradable synthetic bone graft substitute. Collagen has been used for the delivery of low molecular weight drugs, proteins, genes, and plasmids [278,279,280,281,282,283].
3. Chemical Transformation/Modified Degradable Polymers
3.1. Cross-Linking
Cross-linking resembles network formation through some physical or chemical means and is often desirable to impart desired mechanical properties and viscoelastic or even elastic behavior in polymer solutions. The labile bonds are frequently introduced through physical cross-linking and can be broken under physiological conditions, either enzymatically or chemically. Chemical cross-linking of polysaccharide using covalent cross-linking agents is a highly versatile procedure as it improves mechanical stability and rheological behavior. However, covalent cross-linking agents have been found to affect the integrity of the original polymer and may add toxicity in the final product [284,285,286,287,288,289].
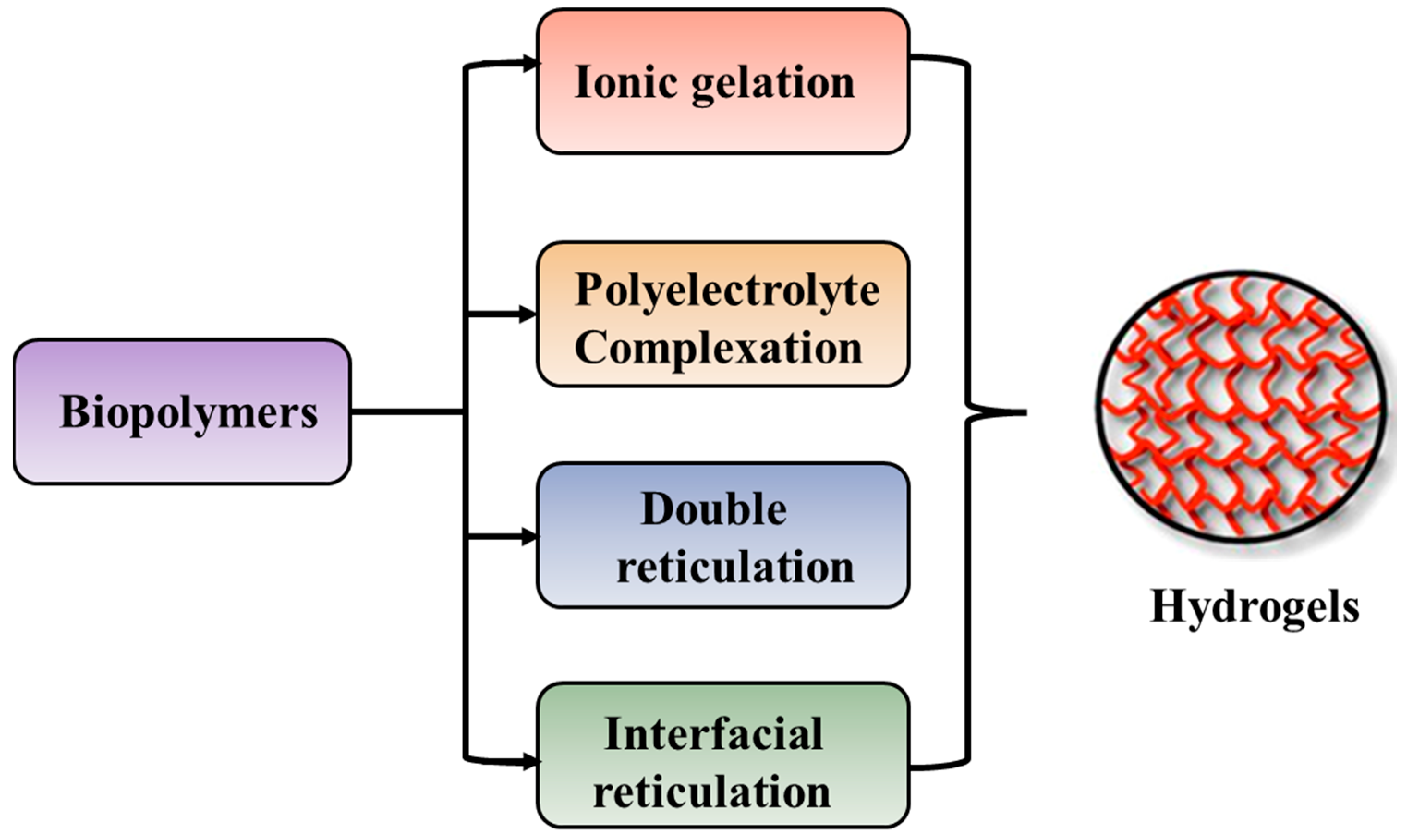
The preparation of hydrogels, by cross-linking, starting from biopolymers such as polysaccharides and proteins, can be achieved by different methods as illustrated in Figure 5.
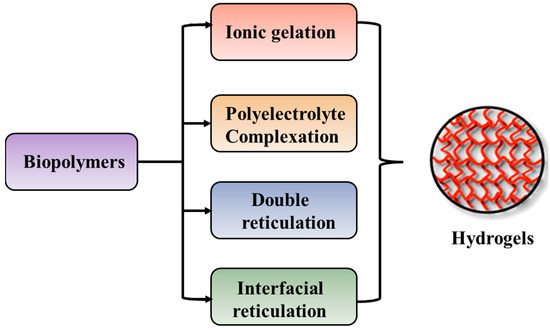
Figure 5.
Preparation techniques specific for preparation of hydrogels starting from biopolymers.
The ionic gelation method is represented by interactions between the positively or negatively charged polymers and cross-linking agents with complementary charges, whereas the polyelectrolyte complexation occurs by electrostatic interaction between oppositely charged polyions, in the absence of cross-linking agents [290,291]. Double reticulation or dual cross-linking is a novel method to apply two cross-linking factors (physical or chemical) to the same material. Generally, an ionic cross-linking is used first followed by a covalent cross-linking [292]. This method decreases the toxicity induced by the covalent cross-linking agents and increases the particle’s stability. The interfacial cross-linking method leads to the preparation of nanocapsules in the absence of any cross-linking agent [293].
Alginate can be easily cross-linked by ionic interactions in the presence of calcium ions at room temperature and physiological pH. Therefore, alginate gels are frequently used as matrixes for the encapsulation and release of bioactive molecules. Cationic polysaccharides, such as chitosan, are cross-linked by glycerol-phosphate disodium salt [294,295,296,297].
3.2. Polyblends and Biocomposites/Nanocomposites
Polyblends are physical mixtures of two or more polymers that are often used to improve performance properties of a material. Polymers can also be mixed with inorganic materials such as metal/metal oxide nanoparticles, silica, clays, etc., and form biocomposite/nanocomposite materials with superior and multifunctional properties. Biopolymers, for example, are beneficial for biomedical applications as they are not very rigid and often have poor mechanical strength as compared to synthetic polymers. In order to overcome these drawbacks, polyblend nanofibers prepared from the mixtures of natural and synthetic polymers represent biomimetic nanostructures that can substitute the native tissue, while providing the topographical and biochemical cues that promote healing. These have desired mechanical, structural, and biological properties suitable for tissue engineering and drug delivery systems.
Polymer-based biocomposites contain two or more different polymers bound together that exhibit superior and unique properties. Currently, a large variety of composites are being developed in order to design novel materials for biomedical and pharmaceutical applications. Nanostructured polymer composites that have superior performance and excellent mechanical properties, along with acceptable biological function, act as important biomaterials. Thus, polyblends and nanocomposites containing polysaccharides as one component, are of immense technological importance [298,299,300,301,302,303,304,305,306,307,308,309,310].
3.3. Interpenetrating Polymer Networks (IPNs)
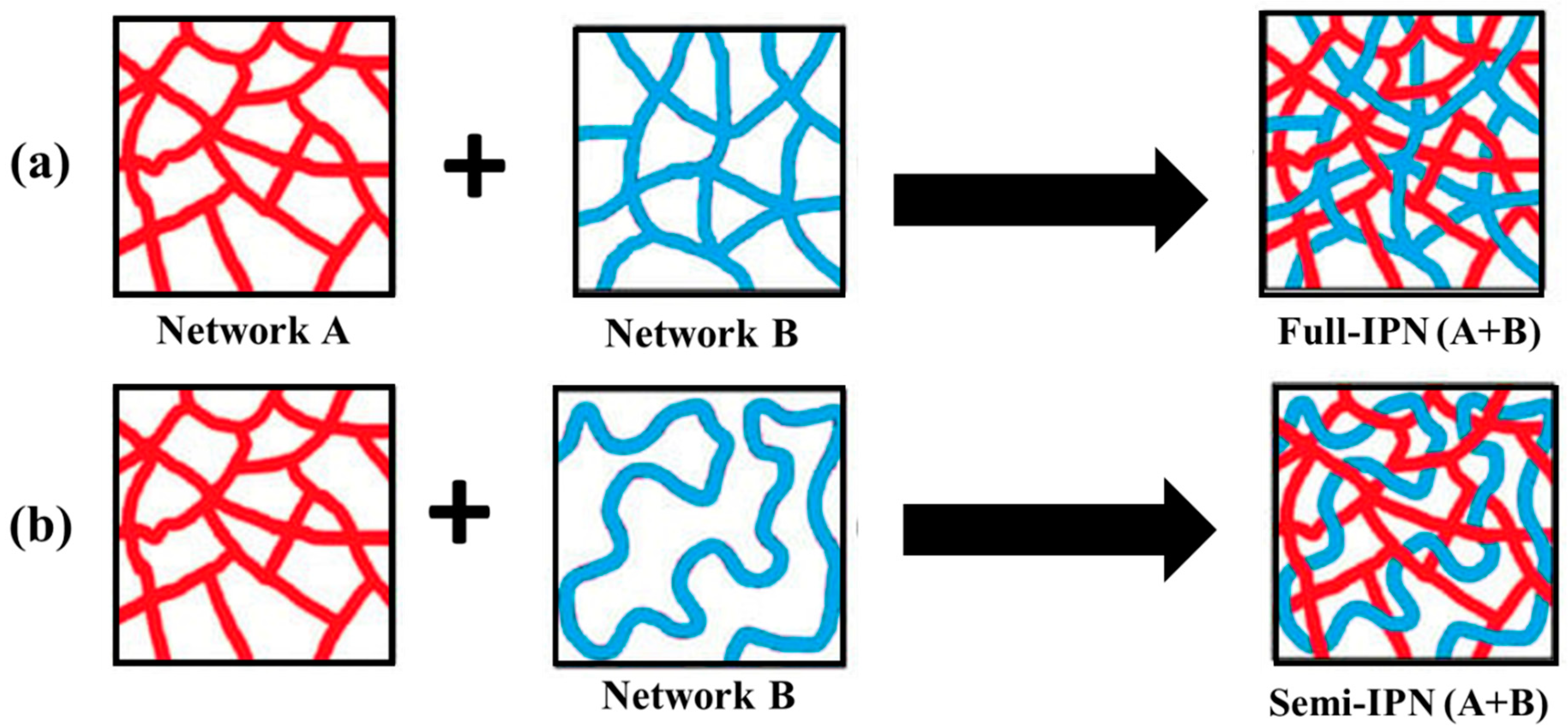
As illustrated in Figure 6, interpenetrating polymer networks (IPNs) are a blend of two or more incompatible polymers in a network with at least one of the systems synthesized in the presence of another. IPNs do form when an aqueous solution of a water-soluble polymer having a monomer is polymerized using an initiator. For example, an aqueous solution of polysaccharide in the presence of a vinyl/acryl monomer can be polymerized in aqueous media forming polymer chains that entangle with the polysaccharide chains [311].
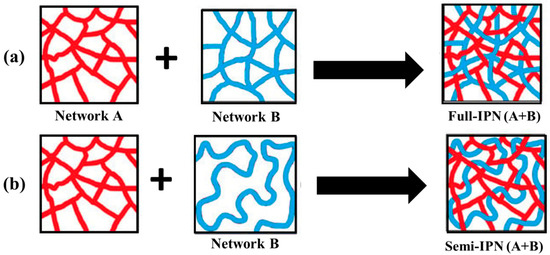
Figure 6.
Schematics of the formation of (a) full interpenetrating polymer network (IPN) and (b) semi-IPN structures [311].
An IPN is a multiphase polymer system that differs from polymer blend where an IPN swells but does not get dissolved in the solvents and, as a consequence, the flow/creep behavior is reduced. These IPNs are different from block and graft copolymers and even interpolymer complexes. In IPNs, only physical forces such as electrostatic, hydrogen bonding, etc., are involved which enable them to be used as a carrier system for drug delivery systems as well as scaffolds for tissue engineering. Also, IPN hydrogels have emerged as innovative biomaterials for drug delivery systems. In this context, polysaccharide-based IPNs are advantageous because of their remarkable properties like excellent swelling capacity, specificity, and mechanical strength. Chitosan and alginates are commonly used polysaccharides to produce IPNs [312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331].
3.4. Functionalization and Polymer Conjugates
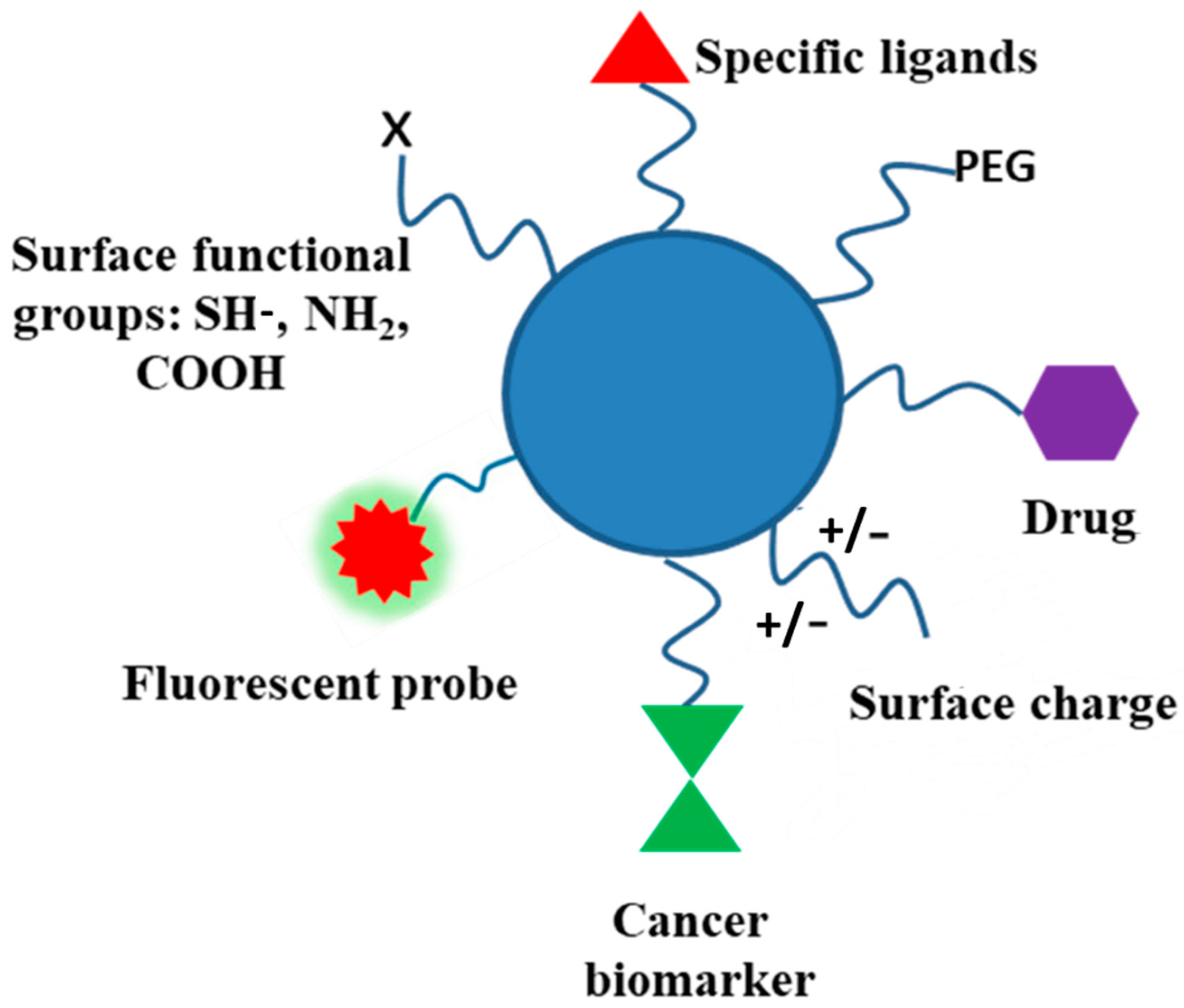
Biodegradable polymers, and polysaccharides in particular, contain several functional groups and can be modified through covalent attachment of hydrophobic or hydrophilic substances. This renders interesting properties to them for biomedical applications. Chemical modification of polysaccharides (i.e., introducing hydrophobic, acidic, basic, or other functionality into polysaccharide molecules) can change their physical properties and solution behavior which can be employed for desired applications. Several strategies have been adopted for such chemical modification of polysaccharides. PEGylation, grafting of polymeric chains through polymerization, formation of polysaccharide-drug conjugates, etc., are the important examples of chemical modification of polysaccharides. The schematic representation of the surface functionalization of NPs is illustrated in Figure 7.
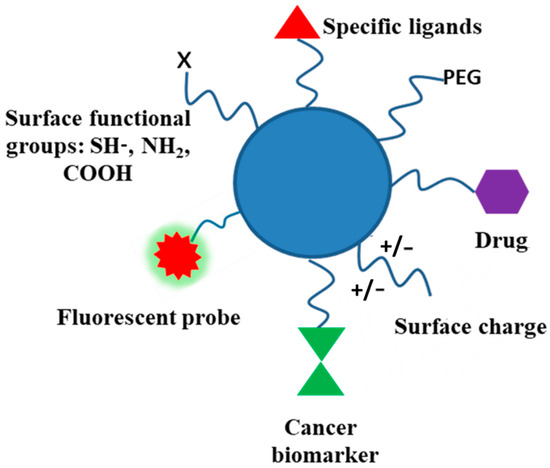
Figure 7.
Schematic representation of functionalized NPs using different approaches.
Several polysaccharides are water insoluble. Here, ionic liquids are used as appropriate solvents. Charged polysaccharides like chitosan or alginic acids can show marked solubility in water by altering the pH of the solution. Several inefficient methods have been developed for the modification of polysaccharides, as they produce waste products and may degrade the polysaccharide molecule. The presence of several hydroxyl functional groups along the polysaccharide backbone may use the saccharide oxygen as a nucleophile and may be etherified (with alcohols) or esterified (with acids, organic, or inorganic) at controlled degree/regioselectivity. Alkyl/benzyl/hydroxyalkyl/carboxyalkyl ethers, carboxylate/sulfonate, and other esters can be conveniently prepared. Saccharide carbon, as an electrophile, can replace polysaccharide oxygen by a heteroatomic nucleophile in a nucleophilic substitution reaction. These chemical reactions of polysaccharides may produce a variety of derivatives with better physical, chemical, and biological properties.
The introduction of reactive functional groups by alkylation and acylation, sulfation, phosphorylation, etc., can also have a profound effect on their intrinsic properties. The glycosidic bond can be destroyed by physical means such as ultrasound, photo, or microwave exposure, or can be hydrolyzed by the enzymes. The hydroxyl groups can be replaced by reactive azide, thiol, diol, and aldehyde groups. In the past decades, many enzyme-catalyzed modifications have been developed. Typical reactions include enzymatic oxidation, ester formation, amidation, glycosylation, and molecular weight reduction [5,10,12,332,333,334,335,336,337].
3.5. Polyelectrolytes (or Polyion) Complexes
Polyelectrolytes are water-soluble charged polymers. In aqueous solutions, they dissociate to form a macroion and counterion. The counter ion gets condensed on to the macroion or remains hydrated in the bulk solution. The solution behavior of polyelectrolytes is greatly influenced in the presence of salts and also by pH and temperature. Polyelectrolytes (both natural and synthetic) are highly useful in industries. Charged polymers can interact with oppositely charged species such as dyes, surfactants, drugs, and polymers, and form soluble polyion complexes or insoluble coacervates which are biologically important. For example, polyion complexes formed between oppositely charged polysaccharides such as chitosan and alginates, and those with charged proteins/polysaccharides with oppositely charged small molecules or polymers. Dilute solutions of a polyelectrolyte (e.g., charged polyelectrolyte or protein) when mixed with an oppositely charged low molecular weight or polymeric substance, led to a new phase which is formed by the spontaneous complexation through strong electrostatic interaction. However, a deeper understanding of the physical state (solid-like or liquid-like) is required. The effect of supporting electrolytes greatly influences the formation and properties of such complexes [338,339,340,341,342,343,344,345].
3.6. Block Copolymers
Biodegradable block copolymers have gained a medical and pharmaceutical research interest due to their tunable biocompatibility, biodegradability, and self-assembly properties. These polymers can be considered as suitable vehicles for drug delivery. The drug-loaded nanoparticles of these polymers get degraded in the biological environment and eliminated through the renal route. Block copolymers from degradable polymers are well-defined polymeric materials that can self-assemble to polymer micelles or polymersomes. These core–shell nanoscale structures are highly useful in biomedical applications. In particular, advances in polymerization techniques in the last two decades; such as controlled polymerization ATRP and RAFT processes, as well as the modern techniques available for characterization of their nanoaggregates; have led to an immense interest in biomedical application [345,346,347,348,349].
3.7. Graft Copolymers
Grafting offers a convenient approach to improve and increase the compatibility between synthetic and natural polymers. It is an effective and accessible method of chemical modification in polysaccharides. Several polysaccharides such as starch, chitosan, hyaluronic acid, cellulose, etc., have been used to achieve fruitful results of grafting. Hydrophilic or hydrophobic polymeric moiety can be grafted on the polysaccharide backbone by reactions with functional groups or polymerizing a monomer by creating active centers of polysaccharide chain via ‘grafting through’, ‘grafting on’, and ‘grafting from’ processes. Amongst these, the former approach is the most extensively studied. Grafting can be achieved by microwave or enzymatic synthesis. The microwave irradiation approach in grafting reduces the use of toxic solvents as well as cutting short the reaction time and increasing the grafting yield. Consequently, the microwave-grafted polymer exhibits superior properties as compared to polymer grafted by the conventional method.
In this way, polysaccharide-based graft copolymers show some interesting and improved properties such as water repellence, thermal stability, flame resistance, dye-ability, and resistance toward acid-base attack and abrasion. However, such behavior may be easily influenced by the presence of polar functional groups, high molecular weight, and relatively rigid backbone. The grafted copolymers possess many useful properties as compared to individual backbone or graft sequences. For example, grafting of a variety of vinyl monomers onto the backbone of different polysaccharides under different conditions of polymerization leads to the synthesis of new and interesting amphiphilic copolymers. However, it is difficult to precisely control and quantify the number and size of grafts, which hampers the reproducibility in the polymer. Hydrophobic grafts make the polysaccharide amphiphilic and thus can form core–shell polymer micelles. Grafting of synthetic polymers onto polysaccharides has been of interest to researchers because of their applications to the biomedical field as biomaterials or vehicles for drug delivery systems [350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368].
4. Mechanism of Polymer Degradation
Polymer degradation has often been regarded as a negative property but it has turned out to be quite beneficial in recent years particularly in connection with their biomedical applications. Often, it involves hydrolysis and oxidation and other physical/chemical/biological stresses. Hydrolysis refers to reactions with water that lead to cleavage of weak hydrolysable chemical bonds within a material. It has been studied extensively, especially for biodegradable polymers like polyorthoesters, polyanhydrides, polycarbonates, and polyamides. Hydrophilic polymers are very susceptible to hydrolysis and fast biodegradation of certain polymers occurs when the rate of diffusion and permeability are high. Also, the degradation process of polymeric products depends on the chemical structure and the degrading environmental conditions of polymers. The degradation is thus dependent on the polymer’s degree of crystallinity, cross-linking, porosity, mechanical stress, etc., and can be accelerated in the presence of a catalyst. The degradation leads to surface or bulk erosion involving the loss of material from the polymer bulk and changes in morphology, and crystallinity. Polymer biodegradation involves biodeterioration, bio fragmentation, and assimilation without neglecting the participation of abiotic factors. The knowledge of the degradation mechanism and kinetics of degradable polymers for biomedical applications are a prerequisite for their functioning.
Oxidative degradation of biomedical polymers such as polyurethane is yet to be fully understood. The oxidation mechanism involves biological defense action where the inflammatory cells generate oxidative agents that diffuse into polymeric implants and degrade them. Enzymatic degradation, which involves biodegradation of collagens, polysaccharides such as hyaluronic acids, some polyesters like polyhydroxyalkanoate, synthetic polycarbonates, and proteins, is also due to a defensive action. Several experimental studies on polymer degradation involving the mechanism, kinetics, and identification/analysis, as well as theoretical modeling, have been undertaken in the past and are not discussed here [369,370,371,372,373].
5. Preparation and Characterization of Polymeric Nanoparticles
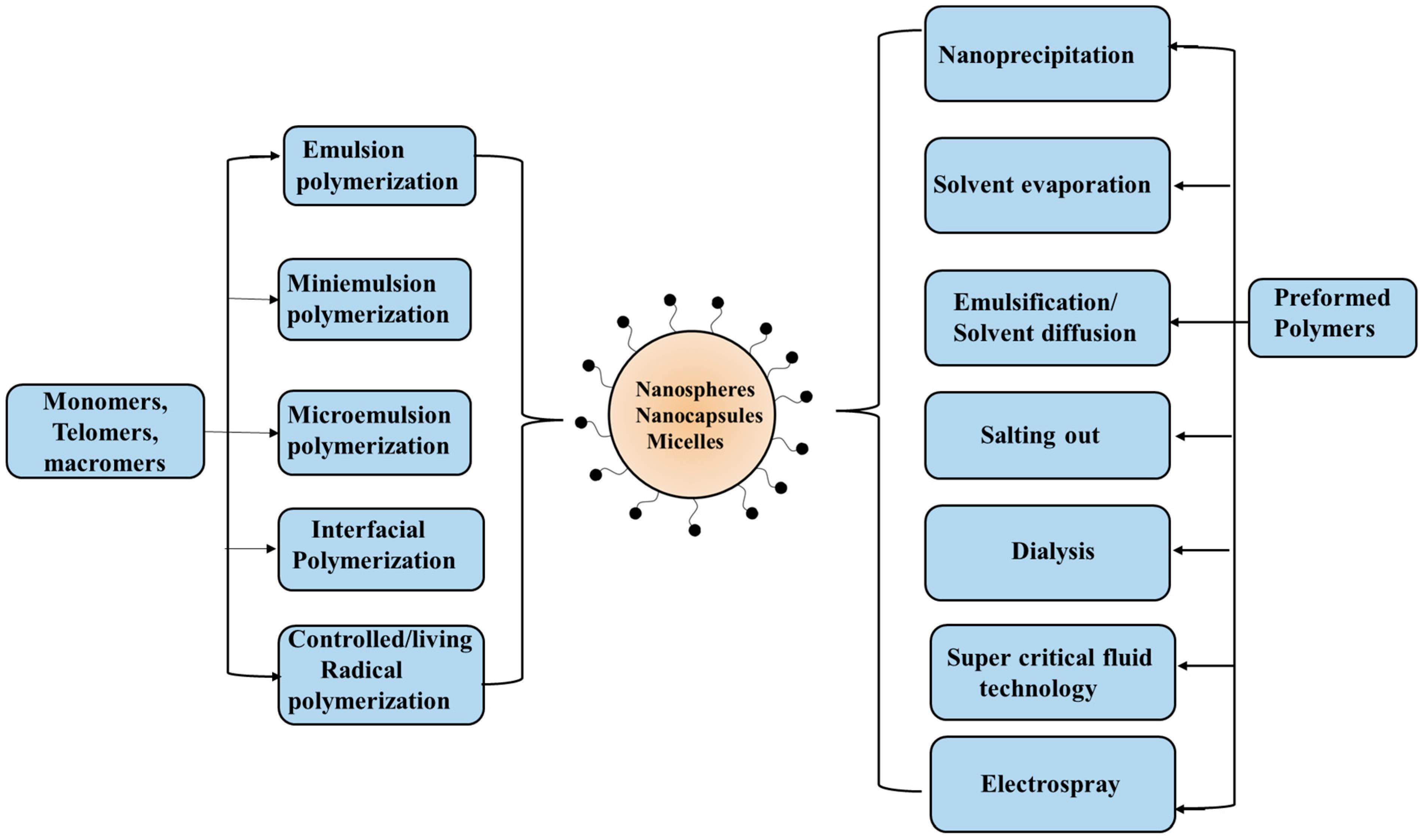
Polymeric nanoparticles have become quite promising in recent years. Such entities made from biopolymers (proteins and polysaccharides) and synthetic polymers (including soft aggregates viz. polymer micelles and polymersomes from self-assembly of amphiphilic block/graft copolymers, and polyion complex micelles) are of great research interest. Fabrication of polymeric nanoparticles can be made possible by tuning some important parameters such as size, shape, and surface charge, along with high encapsulation efficiency for bioactive substances and their release profile. In fact, the size is very crucial. The nanoparticles from these biodegradable polymers and other materials based on them can be prepared using different procedures, as illustrated in Figure 8.
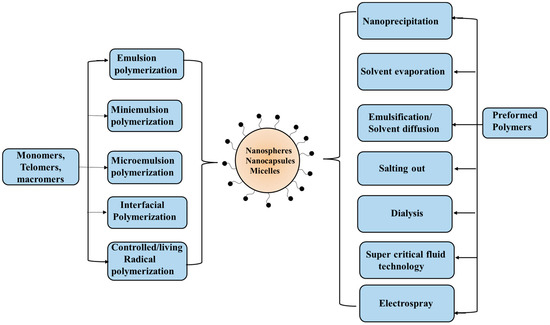
Figure 8.
Preparation techniques specific for synthetic monomers and polymers.
The most common preparation method of nanoparticles involves processes such as desolvation, coacervation, emulsification, and electrodynamic atomization. The desolvation process is a thermodynamically driven process that is induced by the addition of desolvating agents to produce nanoparticles. Here, the size is controlled by nucleation, condensation, and coagulation. The desired experimental conditions, such as amount of desolvating agent, solution pH, ionic strength, and nature/concentration of cross-linking agent, can be optimized for rapid nucleation and slow growth of the particle size that has control over the size/polydispersity. The emulsification phenomenon involves the preparation of liquid/liquid systems spontaneously formed using an aqueous and a non-aqueous (often non-toxic such as plant oils and surfactants) medium. The oil–water emulsion provides the stable nanoparticle dispersion with higher yield. Nanoparticles so prepared possess high loading and entrapment efficiencies. However, the use of toxic organic solvents and the large volume of water required are the drawbacks of this process. The coacervation approach is a phase separation technique that involves the two-phase separation of a molecularly dissolved polymer solution into a dense polymer-rich phase (coacervates), and another water-rich phase with low polymer content. Such coacervates can be produced by the addition of electrolytes (with salting out effect) and non-solvents, along with temperature changes (in case of thermo-responsive polymers). The coacervates are stabilized and further hardened by using chemical cross-linkers such as glutaraldehyde or formaldehyde, or by heat treatment. The mixing of aqueous solutions of two oppositely charged polymers (a polyanion like hyaluronic acid and a polycation like chitosan) also forms coacervates as a result of strong electrostatic interaction. The formation of soluble complexes or coacervates, however, depends on the relative amount of the two oppositely charged polymers. Electrohydrodynamic atomization or electrospray deposition involves the generation of a cloud of charge droplets using liquid atomization by high voltage electrical forces. Here, the nozzle tip sprays the charged particles, which further on during the process reach the substrate and produce nanoparticles. The particle size, charge, and droplet speed are regulated by varying the high voltage power supply. This approach is advantageous as it does not use any surfactant or other additives such as salt, oil, etc.
The information on the chemical composition, molecular structure, and purity of the polysaccharide is needed for cross-linking/blending with other polymers or inorganic nanoparticles, and more importantly, ligand attachment on chemical modification. Likewise, comprehensive characterization of such polymeric entities in terms of size/shape/charge, surface characteristics, and long-term stability/degradation on prolonged storage and rheological behavior is also necessary for their further evaluation for toxicity, drug loading, and targeting capacity. Various spectroscopic (FT-IR, Raman, NMR, UV/Vis, fluorescence) methods, in dispersed/dry medium, yield information on chemical composition and the functional group associated with the nanoparticles. The size/shape, polydispersity, and surface charge (as zeta potential) can be routinely measured by dynamic light scattering (DLS) (also called quasi-elastic light scattering (QELS) or photon correlation spectroscopy (PCS)). Here, this study provides the size/polydispersity of nanoparticles by measuring the diffusion coefficient and then using Stokes–Einstein equation. The monochromatic laser is used as a light source and the scattered beam is recorded effectively by two detectors mounted for direct (90°) or backscattered beams (173°). Besides the information on size and polydispersity from DLS, another important parameter in the characterization of the nanoparticle is the charge on its surface. It is often determined in terms of zeta potential. Several particle size analyzer instruments can be successfully employed to quickly determine zeta potential. Usually, a high zeta potential value on colloidal particles results the higher its dispersion stability. The presence of additives, like salts, and pH affect the zeta potential value.
Small-angle X-ray (SAXS) and neutron scattering (SANS) can be complementary techniques that provide accurate information on shape/core–shell morphology in self-assembled systems. Scanning electron microscopy (SEM) is a high resolution imaging technique based on a focused beam of a high energy electron beam. The secondary electron emitted during this interaction provides information on the geometry, topography, and composition of nanomaterials. Here, the image corresponding to the intensity of secondary electrons emitted at each interface is developed on the screen of a cathode ray tube. SEM is disadvantageous as the specimen or sample preparation is very tedious and requires extreme vacuum conditions and pre-sonication to avoid agglomeration. Likewise, Transmission electron microscopy (TEM) is based on interaction with a high energy electron beam similar to SEM. Here, a transmitted beam of electrons provides the final image. Here, the sample preparation is long and complex due to preparation of ultra-thin sections (≤100 nm) for smooth electron transmittance. Further advances in EM techniques like high resolution transmission electron microscopy (HR-TEM), cryo-EM for direct imaging, and tomography are becoming of great interest. Atomic force microscopy (AFM) is a non-destructive and non-invasive technique for biological and fragile polymeric specimens. It requires minimum sample preparation efforts with no necessary immobilization and even the non-conducting materials can be easily visualized without any prior surface treatment. AFM can be operated mainly in three modes, i.e., contact mode, non-contact mode, and tapping mode, each with useful information. It offers evidence to surface texture and roughness by providing high resolution 3D topographic images of the nanoparticles [374,375,376,377,378,379,380,381,382,383,384,385,386,387].
6. Drug Loading Efficiency and Release Profile
Drug loading and encapsulation efficiencies (DLE and DEE, respectively) are important parameters used for the characterization of a drug delivery system, and can be determined using the following equations:
The encapsulation efficiency of drugs within the polymeric nanoparticles, or solubilized in self-assembled nanoaggregates, and the drug release profile depend on several factors. These have to be optimized in order to obtain a controlled delivery of drugs to the target site and desired doses. Other parameters, like size/shape/charge onto the nanoparticle, surface- or stimuli-responsive groups, and polymer biodegradation kinetics, also play an important role. Several experimental studies, as well as theoretical modeling in search of an appropriate design of nanoparticles, have been carried out. Precise control of drug release has a broad potential in the field of clinical medicine that can be of great help in drug delivery systems. There cannot be a single material or designed particles for different applications and thus each individual system has to be modified independently.
An ideal nanoparticle-based delivery system should have a high loading capacity. This would reduce the amount of the carrier required for administration. Drug loading is achieved by incorporating the drug at the time of nanoparticle preparation or by diffusion of the drug after incubating nanoparticles in the drug solution. However, the amount of bound drug and its interaction with nanoparticles depends on the chemical structure of the drug as well as the polymer. The study of the adsorption isotherms can detect the type of binding and the binding rate. The unbound drug is separated by ultracentrifugation or gel filtration, and from the amount of drug bound, the drug encapsulation efficiency can be calculated.
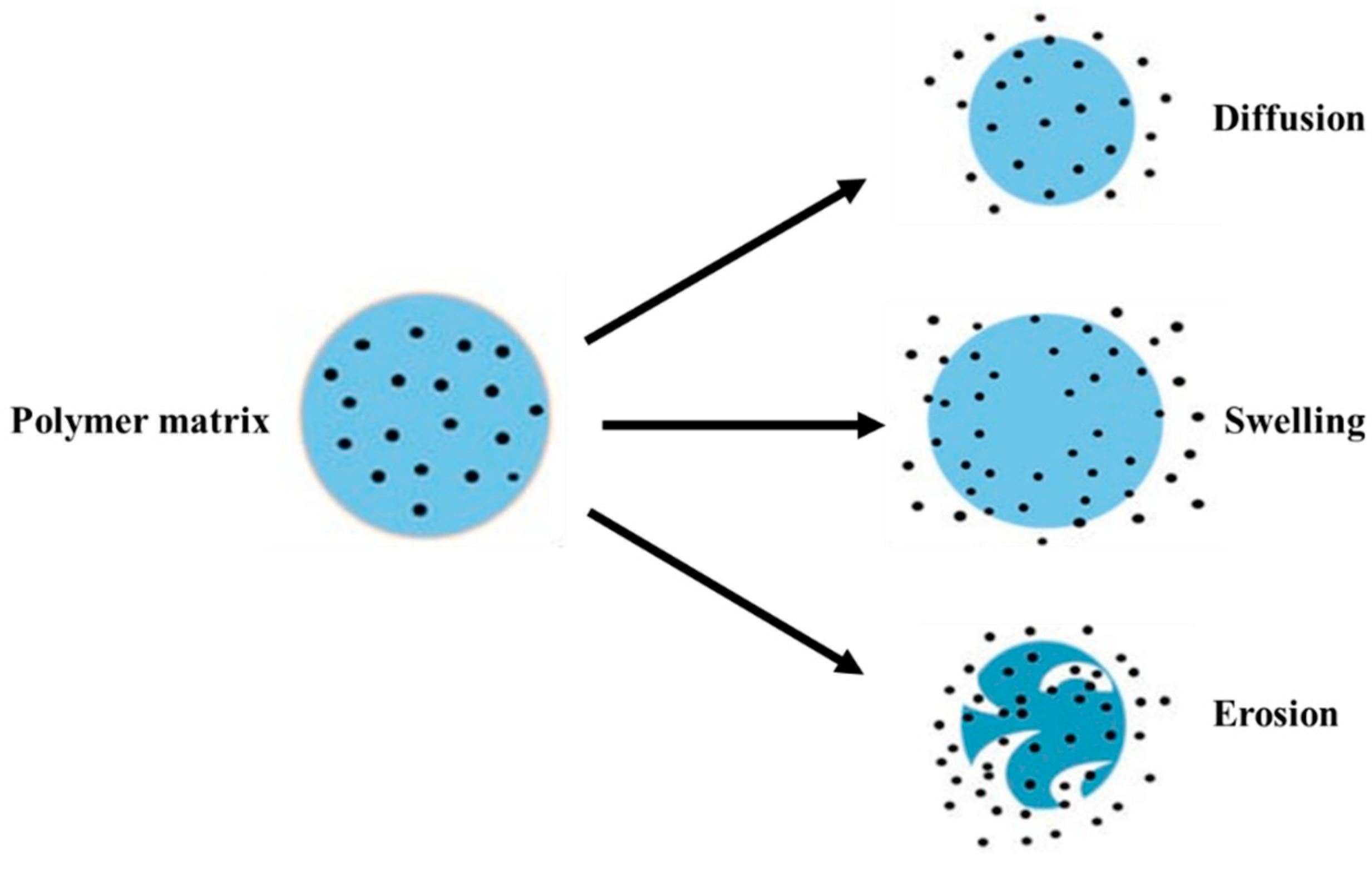
For the drug release mechanism, the rate and timing of the drug release from nanoparticles is required. The mechanisms of drug release can be made possible following one or more methods viz. desorption of drug bound to the nanoparticle, diffusion through the nanoparticle matrix or the polymer wall of nanocapsules, or by the erosion of the nanoparticle matrix [388]. The schematic representation is provided in Figure 9.
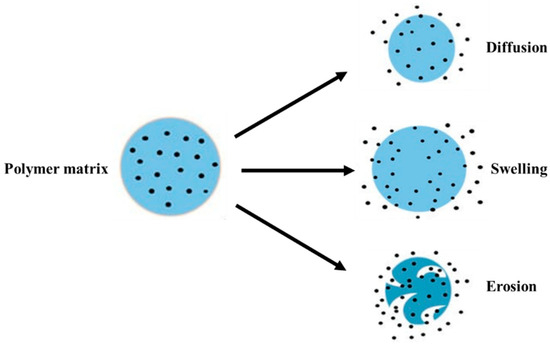
Figure 9.
Schematic representation of the possible mechanisms of drug release from a polymer matrix.
The transport of a molecule by diffusion is related to a concentration gradient. In order to achieve the diffusion of a drug molecule through a polymeric matrix, the drug must be in a mobile (dissolved) state. Diffusion can occur with or without the swelling of the polymeric matrix. The swelling occurs when the polymeric matrix increases its volume due to the absorption of water. Hydrogels have the highest swelling capacity. The presence of water can activate the release of a water-soluble drug but also can control the release kinetics. The drug release kinetics are also controlled by the hydrolytic or enzymatic degradation of the polymeric matrix. This erosion can start from the surface, the bulk, or a combination of both.
The kinetics of the drug release depend upon the solubility and diffusion/biodegradation of the matrix materials, and the loading efficiency of the drug. In the case of DDS based on biodegradable polymers, drug diffusion, swelling, erosion, or dissolution of the polymeric matrix can occur simultaneously and may lead to zero-order release kinetics. The size of nanoparticles also affects the release; large size particles have a shorter initial burst release than smaller particles [389].
7. Toxicological Concerns
As such, degradable polymers bear no toxicological problems, but their chemically modified materials and nanocomposites may be risky for biomedical applications. A critical examination associated with these problems is thus necessary before putting them into tissue engineering/regenerative medicine, drug delivery vehicles, and other biomedical applications.
Nanomaterials, from inorganic, organic, or hybrid materials have been of interest in drug/gene delivery, tissue engineering, and bioimaging. However, the risks associated are adverse effects on interaction with living cells/organs. It is therefore necessary to examine their toxic effect and the health risks associated with these materials. Though nanoparticles based on biodegradable polymers may be regarded safe, the procedure used in their fabrication involving certain chemical processes may render them with some toxicity-related problems. For example, chitosan has been extensively used in the biomedical field although it has been shown to cause membrane damage. PGA has low solubility and a high degradation rate, with formation of an acidic product that can also provoke an inflammatory reaction. PLGA exhibits minimal systemic toxicity. Synthetic degradable polymers also may generate toxicity during their synthesis. Further, the products of hydrolysis of biodegradable polymers (carboxylic acid and/or hydroxyl chain end) may be oxidized, producing short chain carboxylic acid that may change the pH, which will further trigger an inflammatory response [390,391].
8. Biomedical Applications
Uses of biodegradable (carbohydrate- and protein-based) nanoparticles are economically, socially, and environmentally sustainable and so are applied in wide areas. Also, a lot of research is ongoing in this direction. Their biocompatibility, biodegradability, and reduced immune response induction make them potential candidates for tissue engineering and in drug/gene delivery systems (Figure 10).
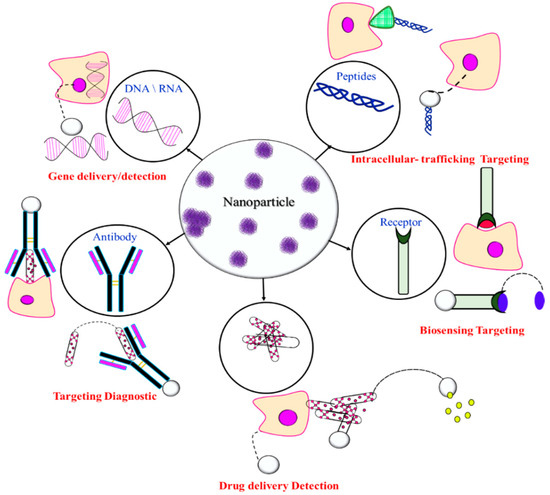
Figure 10.
Degradable polymer nanoparticles of biomedical interest.
Some typical examples of studies investigating the use of biodegradable polymers in different biomedical applications are succinctly provided in Table 1.

Table 1.
Recent examples of biomedical applications of biodegradable polymers.
8.1. Wound Dressing/Healing
Wound healing is a complex biochemical and cellular process that involves hemostasis, inflammatory, proliferative, and remodeling stages due to the physical disruption of tissue. Skin injuries constitute a gateway for pathogenic bacteria which produce virulence factors that impair tissue integrity. These pathogenic infections are usually associated with biofilm formation, where microorganisms interact to produce extracellular substances. A wide range of dressings are used to treat such severe wounds. The ideal dressing should have high absorption capacity, be comfortable, allow the visualization of the wound, and avoid pain in the removal, and should not provoke allergic reaction.
Polymers (both synthetic and natural) are used for the development of wound dressing foams, hydrogels, hydrocolloids, films, and membranes. Among these, polysaccharides such as alginate, cellulose, chitosan, and hyaluronic acid are versatile polymers for the development of formulations against skin infections. The efficacy of these biomacromolecules is related to their enhanced biocompatibility, biodegradability, non-toxic nature, chelation, presence of multifunctional groups, and ease of chemical modification [421,422,423,424,425,426,427,428,429,430,431,432,433].
8.2. Biosensors
Many diagnostic testing tools today include automated analyzers whose maintenance are very costly and time demanding. So, faster, smaller, and cheaper devices are much needed for laboratory testing. Nowadays, many medical conditions can be monitored by biosensors. A biosensor is a diagnostic device that detects specific biochemicals generated by the reaction between targeted analytes and the immobilized biomolecules, such as enzymes, antibodies, or receptors on the electrode. Biosensors show high specificity, fast response time, user convenience, and portability. They generally consist of biological elements that react with the target analytes and a transducer that transforms the signal that results from the reaction between the biomolecules to measurable or quantifiable values. They can be used for analysis of clinical samples or for ex vivo and in vivo monitoring of different physiological changes within the human body. Chitosan-based films have been applied to the cholesterol biosensor to improve its sensitivity [434,435,436,437,438].
8.3. Drug Delivery
The conventional and most convenient route of drug administration is through oral consumption. However, hydrophobic drugs show poor bioavailability, and those protein-based drugs are susceptible to enzymic degradation in the gastrointestinal tract. Hence, they have to be formulated carefully and must be administered via other routes such as infusions, injections, and implants. On administration of drugs though mouth or injection, the concentration is too high (resulting in toxicity) or too low for the drug to be effective. Thus, in order to circumvent this problem and maintain optimal drug concentration, novel drug delivery systems have been designed. These systems help in the release of bioactive substances at the precise rate and at the exact site while maintaining the optimum level of drug, leading to maximum efficacy and minimum associated side effects. In such controlled/targeted drug release formulations, one needs to encapsulate/solubilize the drug. Usually, nanosized materials such as solid lipid dispersions, liposomes, polymer micelles, polymersomes, dendrimers, inorganic nanoparticles, and mesoporous materials have been of great interest.
In this context, amphiphilic block (and graft, in particular), polymers are important. Their self-assembly to different size/shape nanostructures show high drug loading capacity and are versatile materials for the development of drug delivery systems. These nanoaggregates can optimize the drug release, protect it from degradation, and release rate/site-improving pharmacokinetics by prolonging drug circulation and capability of tissue targeting. Furthermore, the polymer micelles can be functionalized/cross-linked and used as mixed micelles and polyion complex micelles through chemical modification. Also, one or more blocks can be responsive to some stimuli, or biodegradable, and these additional features make these smart and intelligent materials good candidates for drug delivery systems.
Often thermo- and pH-responsive polymers are employed in targeted drug delivery systems. For example, the pH of cancerous cells is more acidic than the normal/healthier cells, and they also possess a higher concentration of glutathione (GSH) triphosphate than extracellular fluids, which are responsible for high redox potential in cytosol and nuclei. Degradable polymers have been promising materials for the design and development of new drug delivery systems. Due to degradation into biocompatible by-products, the biodegradable polymers formulated with drug constituents can be incorporated and used as drug delivery vehicles for the sustained release at the targeted site in desired concentration ranges within a therapeutic window [349,439,440,441,442,443,444,445,446,447]. In perspective, the main challenge is related to the preparation of multiple-functionalized DDS which will assure the on-demand drug release in a controllable manner under the influence of different types of external stimuli. The application of this type of smart DDS was very well reviewed in different papers [448,449,450,451].
8.4. Tissue Engineering and Regenerative Medicine
Tissue engineering is an interdisciplinary field that applies the principles of engineering and the life sciences to develop biological tissue substitutes that restore, maintain, or improve function. The new tissue is either grown inside a patient (in vivo) or outside a patient (in vitro) and then transplanted. The engineered tissue can also be used for diagnosis. Scaffolds play a crucial role in tissue engineering and biodegradable polymers. Both synthetic and natural polymers are important as scaffolding materials. An ideal porous scaffold is biocompatible, mechanically strong, biodegrades at the desired rate without producing any toxic substance, has a surface morphology favorable to interact with cells, acts as a template for the tissue, and supports cell attachment, proliferation, and differentiation.
The extracellular matrix (ECM) consists of a variety of proteins and polysaccharides that are assembled into an organized network that provides structural support to cells. Thus, protein- and polysaccharide-based materials have the potential advantage of supporting cell adhesion and function. Proteins such as collagen, gelatin, and silk; and polysaccharide such as alginate, hyaluronic acid, and chitosan are commonly used for scaffolds. However, complex structural features of proteins and polysaccharides, as well as immunogenicity and pathogen transmission, have generated interest toward synthetic polymers.
These polymers can have well-defined structural features, better processing flexibility, and no immunological concerns. Potentially useful synthetic polymers for tissue engineering are polyesters, polyanhydrides, polyphosphazenes, and polyurethanes.
Bone is complex living connective tissue that provides a mechanical chassis to tissues, anchor to muscles, and acts as a calcium storehouse. It is made of about 60% calcium phosphate minerals, 30% collagen-based matrix, and 10% of water. The minerals are composed of a mixture of tricalcium phosphate and hydroxyapatite, and provide rigidity and tensile strength (Young’s modulus 5–20 MPa). Bone defects caused by trauma, fractures, and tumors do not regenerate due to problems associated with regular autogenic and allogenic bone grafting methods. Thus, bone tissue engineering becomes important in this context.
Ceramic materials, polymers, and metals have been of interest as scaffolds which provide temporary support for cells, and to encourage cell separation and proliferation toward the formation of the desired new tissue. An ideal scaffold degrades when the support is no longer required. Also, its biocompatibility and pore size allow small biomolecules to pass to host tissue. Several inorganic materials such as steel and titanium and non-degradable porous composites have been used to heal the damaged bone by providing temporary support. Ceramics possess good osteoconductive and bone-bonding properties, but are brittle and show poor processing. These are, however, better as implants instead of their metal counterparts due to no distortion in magnetic resonance imaging, and reduce the cost and risk to the patient. Several degradable polymers and their composite materials have shown excellent mechanical and cell adhesion properties that suit orthopedic applications, implants, or devices. Magnesium-based composite materials possess good mechanical strength and are ductile and so have been found very useful in orthopedic uses. Besides use in scaffolds, this material can also be used as bone cement which degrades with time and promotes bone growth. Acrylate polymers polyblends starch/cellulose acetate have been found to be good materials as bone cements. Similarly, hydroxyalkanoate composites with silicate and borate with acrylic acid matrixes show enhanced mechanical properties [452,453,454,455,456,457,458,459,460,461,462,463,464].
8.5. Gene Delivery
Gene therapy combats inherited/acquired diseases like various genetic and neurological disorders, cancer, cardiovascular diseases, diabetes mellitus, etc. It involves therapeutic gene molecules (DNA and RNA) that can alter defective genes, modify missing genes, and silence mutated genes. However, the fragile nature of therapeutic genes and extracellular or intracellular biological barriers limit its use and therefore newer systems are being developed. Various types of non-viral vectors like cationic polyelectrolytes that form complexes with nucleic acids, and lipid-based nano-assemblies, as well as inorganic nanoparticles have been employed for gene-delivery applications, including nano-size cationic polyelectrolyte-siRNA/plasmid DNA/polyplexes. They are formed as a result of the electrostatic interaction taken up by cells through endocytosis mechanisms and thereafter polyplexes degrade, releasing the payloads. Polyelectrolytes such as polyethylene imines, poly(2-N-(dimethylaminoethyl) methacrylate), and poly(L-lysine) have been frequently investigated as polyplexes for gene delivery. The toxicity and degradability of polyelectrolytes need to be carefully ascertained and the molecular weight/chemical structure of the polymer needs to be carefully designed. Double hydrophilic block copolymers with one polycationic block and another neutral block may form complexes with nucleic acids and then undergo self-assembly to core-complex micelles which possess enhanced stability. Protein and polysaccharides are suitable vectors for their non-toxicity and biocompatibility.
Proteins have a 3D folded structure and are a major component of many natural tissues, are biocompatible, and biodegradable. Protein-based vectors, such as gelatin and albumin, have been widely employed for gene delivery. Cationic polysaccharides like chitosan are of interest as nano vectors due to their particular biological functions, such as cell signaling and immune recognition. However, due to its low pKa values (ca 6.3), chitosan has very limited solubility and low complexation ability to therapeutic genes in a physiological condition. Attempts have been made to chemically modify chitosan through functionalization or grafting [465,466,467].
8.6. Targeting Diagnostics
Targeted therapy is a type of treatment that uses nanoparticles functionalized with specific ligands in order to precisely identify particular receptors overexpressed in malignant cancer cells [405]. In order to prepare tumor-selective drug delivery systems, different types of ligands such as folic acid, peptides, transferrin, aptamers, and antibodies have been investigated, as illustrated in Figure 11.
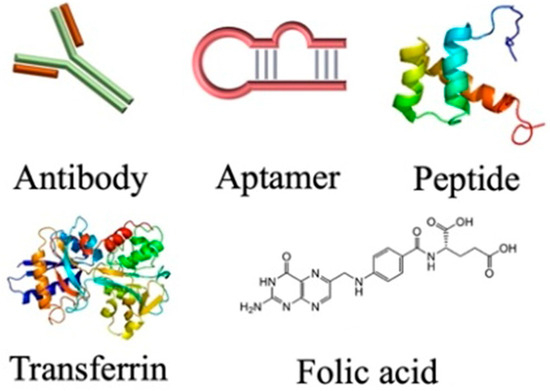
Figure 11.
Types of specific ligands Redrawn from reference [468].
Ligand-functionalized nanoparticles, obtained from biodegradable polymers, are very well suited for accomplishing anti-cancer targeting. The drug release from their nanoparticles directly to the tumor cells is facilitated by their high cellular uptake compared to non-functionalized particles. The immune cells are a critical target as the balance between an anti-cancer immune response and a tumor’s immunosuppressive microenvironment may show whether a tumor goes into remission or expands. Chitosan being a polybasic facilitates cellular uptake and adhesion to negatively charged mucosal surfaces, and is therefore well-suited for oral drug delivery [468,469,470,471,472,473,474,475].
8.7. Intracellular Trafficking
Cellular uptake and intracellular trafficking of nanocarriers are crucial in designing safe and efficient delivery systems. Intracellular trafficking can be optimized by tuning size/shape/charge and surface properties and is crucial for assessing their fate and toxicity [391,476,477,478].
9. Conclusions and Future Perspectives
Biodegradable polymers, both from natural and synthetic origin, and their derivatized products provide a plethora of materials with a great potential in biomedical applications. The cross-linking, interpenetrating polymer networks (IPNs), polyion complexes, polyblends, nanocomposites with organic and inorganic substances, and chemical modification to amphiphilic copolymers; and sometimes stimuli-responsive block and graft copolymers with interesting self-assembly; make these materials unique. These have been exploited in and as drug/protein delivery, gene transfection, bioimaging/diagnosis, tissue engineering, orthopedics biomedical devices, etc. The degradability, compatibility, non-toxicity, sterility, and stimuli-responsiveness show a wide scope of applications in the biomedical field. Interesting properties in these biodegradable polymers can be developed by physical or chemical modification such as cross-linking, making multiphase systems like polyblends and IPNs, and surface modification through functional groups, forming polyelectrolyte complexes through electrostatic interaction of charged biodegradable polymers with oppositely charged species producing amphiphilic or stimuli-responsive block and graft copolymers and their self-assembly in aqueous solutions. The present review focuses on the chemistry of degradable polymers and their derivatizing in different ways for potential biomedical applications such as and in devices, sensors, and tissue engineering with a focus on drug delivery applications. The advanced techniques in designing nanoparticles from degradable polymers and their chemical modification, coupled with their characterization for biomedical applications, inspires further improvements.
The use of these biodegradable polymers as DDS has immense potential, owing to their specific and superior features. However, there are some important challenges that must be overcome in the future. Until now, the majority of published studies focused only on the in vitro characterization of these systems. Therefore, at present, their practical applications and clinical translation are still quite scarce, with the exception of a few FDA-approved biodegradable polymers. Although there is clear progress in the synthesis of different types of DDS, their in vivo bioactivity, interaction with human cells, and long-term stability in a physiological medium should be investigated more profoundly in order to make them widely used in practical biomedical applications. Moreover, the multidisciplinary collaboration between polymer chemists, biologists, engineers, and physicians is indispensable for the synthesis of cost-effective and tailor-made DDS.
Author Contributions
Conceptualization, K.K., L.I.A., A.B., I.C.C. and P.B.; methodology, L.I.A. and P.B.; validation, L.I.A. and P.B.; investigation, K.K., L.I.A., A.B., I.C.C. and P.B.; resources, K.K. and L.I.A.; writing—original draft preparation, K.K., L.I.A., A.B., I.C.C. and P.B.; writing—review and editing, L.I.A. and P.B.; visualization, K.K., L.I.A. and P.B.; supervision, L.I.A. and P.B.; funding acquisition, L.I.A. and I.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Merlettini, A. Micro-Nanostructured Polymeric Materials with Specific Functionalities for Advanced Biomedical Applications. Ph.D. Thesis, Università di Bologna, Bologna, Italy, 2019. [Google Scholar]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191–109206. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Veeramachineni, A.K.; Owh, C.; Loh, X.J. Biodegradable polysaccharides for controlled drug delivery. ChemPlusChem 2016, 81, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; St. Pourçain, C.B.; Garnett, M.C. Novel functionalized biodegradable polymers for nanoparticle drug delivery systems. Biomacromolecules 2005, 6, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Dhanya, B.S.; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef]
- Thomas, C.M.; Lutz, J.F. Precision synthesis of biodegradable polymers. Angew. Chem. Int. Ed. 2011, 50, 9244–9246. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers Preparation functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Mallick, S.P.; Suman, D.K.; Singh, B.N.; Srivastava, P.; Siddiqui, N.; Yella, V.R. Strategies toward development of biodegradable hydrogels for biomedical applications. Polym. Plast. Technol. Eng. 2020, 59, 911–927. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.; Ma, P.X. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Stoppel, W.L.; Ghezzi, C.E.; McNamara, S.L.; Iii, L.D.B.; Kaplan, D.L. Clinical Applications of Naturally Derived Biopolymer-Based Scaffolds for Regenerative Medicine. Ann. Biomed. Eng. 2015, 43, 657–680. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sean, T.C.; Kudaiyappan, T.; Bisyarah, U.; Mirarmandi, A.; Faradjeva, E.; Abubakar, A.; Ali, H.H.; Angalene, J.L.; Kumar, S.S. Production characterization and application of nanocarriers made of polysaccharides proteins bio polyesters and other biopolymers A review. Int. J. Biol. Macromol. 2020, 165, 3088–3105. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Alibakhshi, A.; Yaghoobi, H.; Hashemi, M.; Hejazi, M.; Ramezani, M. Recent advances on biocompatible and biodegradable nanoparticles as gene carriers. Expert Opin. Biol. Ther. 2016, 16, 771–785. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; André, A.A.; Men, Y.; Srinivas, M.; Wilson, D.A. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 2017, 11, 1957–1963. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Chen, C.K.; Huang, P.K.; Law, W.C.; Chu, C.H.; Chen, N.T.; Lo, L.W. Biodegradable polymers for gene delivery applications. Int. J. Nanomed. 2020, 15, 2131–2150. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 21, 2–7. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24–32. [Google Scholar] [CrossRef]
- Liu, G.; Yang, L.; Chen, G.; Xu, F.; Yang, F.; Yu, H.; Li, L.; Dong, X.; Han, J.; Cao, C.; et al. A Review on drug delivery system for tumor therapy. Front. Pharmacol. 2021, 12, 735446. [Google Scholar] [CrossRef]
- Wajid, A.; Taimur, K.; Imran, B.; Javed, I. A Comprehensive Review on Targeted Drug Delivery System. Asian J. Pharm. Res. 2022, 12, 335–340. [Google Scholar] [CrossRef]
- Abasian, P.; Ghanavati, S.; Rahebi, S.; Khorasani, S.N.; Khalili, S. Polymeric nanocarriers in targeted drug delivery systems: A review. Polym. Adv. Technol. 2020, 31, 2939–2954. [Google Scholar] [CrossRef]
- Prakash, S. Nano-based drug delivery system for therapeutics: A comprehensive review. Biomed. Phys. Eng. Express 2023, 9, 052002. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Demina, N.B. Implants as Targeted Drug Delivery Systems (Review). Pharm. Chem. J. 2022, 56, 396–402. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Shah, T.V.; Vasava, D.V. A glimpse of biodegradable polymers and their biomedical applications. e-Polymers 2019, 19, 385–410. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Rashmi, K. Polyhydroxyalkanoates an overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Srivastava, A.K. Recent advances in microbial polyhydroxyalkanoates. Process Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Suriyamongkol, P.; Weselake, R.; Narine, S.; Moloney, M.; Shah, S. Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants A review. Biotechnol. Adv. 2007, 25, 148–175. [Google Scholar] [CrossRef]
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates the future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743. [Google Scholar] [CrossRef]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, J.; Chen, G.Q. Polyhydroxyalkanoates challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef]
- Li, Z.; Loh, X.J. Water soluble polyhydroxyalkanoates future materials for therapeutic applications. Chem. Soc. Rev. 2015, 44, 2865–2879. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Y.L.; Li, Z.; Loh, X.J. Recent progress in polyhydroxyalkanoates based copolymers for biomedical applications. Biotechnol. J. 2019, 14, 1900283–1900299. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Lin, K.Y.; Tsang, D.C.; Thakur, I.S. Bacterial polyhydroxyalkanoates Opportunities challenges and prospects. J. Clean. Prod. 2020, 263, 121500–121520. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Ponjavic, M.; Malagurski, I.; Lazic, J.; Jeremic, S.; Pavlovic, V.; Prlainovic, N.; Maksimovic, V.; Cosovic, V.; Atanase, L.I.; Freitas, F.; et al. Advancing PHBV Biomedical Potential with the Incorporation of Bacterial Biopigment Prodigiosin. Int. J. Mol. Sci. 2023, 24, 1906. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Furuhashi, Y.; Sogo, K.; Miura, S.; Kimura, Y. Stereoblock poly (lactic acid) synthesis via solid state polycondensation of a stereocomplexed mixture of poly (L-lactic acid) and poly (D-lactic acid). Macromol. Biosci. 2005, 5, 21–29. [Google Scholar] [CrossRef]
- Tan, B.H.; Muiruri, J.K.; Li, Z.; He, C. Recent progress in using stereocomplexation for enhancement of thermal and mechanical property of polylactide. ACS Sustain. Chem. Eng. 2016, 4, 5370–5391. [Google Scholar] [CrossRef]
- Singhvi, M.; Zinjarde, S.; Gokhale, D. Polylactic acid synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.; Deri, F.; Ko, Y. Properties and medical applications of polylactic acid A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Ghalia, M.A.; Dahman, Y. Biodegradable poly (lactic acid) based scaffolds synthesis and biomedical applications. J. Polym. Res. 2017, 24, 74. [Google Scholar] [CrossRef]
- Lasprilla, A.J.; Martinez, G.A.; Lunelli, B.H.; Jardini, A.L.; Maciel Filho, R. Poly lactic acid synthesis for application in biomedical devices A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Peesan, M.; Supaphol, P.; Rujiravani, T.R. Preparation and characterization of hexanoyl chitosan/polylactide blend films. Carbohydr. Polym. 2005, 60, 343–350. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M.; Darroudi, M. Silver/poly (lactic acid) nanocomposites preparation characterization and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic acid induced inflammation role of hydrolysis and resulting complement activation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Sharad, J. Glycolic acid peel therapy A current review. Clin. Cosmet. Investig. Dermatol. 2013, 6, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Glaied, O.; Riess, G. Crystallization kinetics of PCL tagged with well defined positional triazole defects generated by click chemistry. Polymer 2011, 52, 3074–3081. [Google Scholar] [CrossRef]
- Winninger, J.; Iurea, D.M.; Atanase, L.I.; Salhi, S.; Delaite, C.; Riess, G. Micellization of novel biocompatible thermo sensitive graft copolymers based on poly (ε-caprolactone) poly (N-vinylcaprolactam) and poly (N-vinylpyrrolidone). Eur. Polym. J. 2019, 119, 74–82. [Google Scholar] [CrossRef]
- Salhi, S.; Mahfoudh, J.; Abid, S.; Atanase, L.I.; Popa, M.; Delaite, C. Random poly (ε-caprolactone-l-alanine) by direct melt copolymerization. Polym. Int. 2020, 69, 1161–1168. [Google Scholar] [CrossRef]
- Atanase, L.I.; Salhi, S.; Cucoveica, O.; Ponjavic, M.; Nikodinovic Runic, J.; Delaite, C. Biodegradability Assessment of Polyester Copolymers Based on Poly (ethylene adipate) and Poly (ε-caprolactone). Polymers 2022, 14, 3736. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral drug loaded biocompatible polymeric nanoparticles obtained by non aqueous emulsion polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Menaszek, E.; Zagrajczuk, B.; Pawlik, J.; Cholewa Kowalska, K. New generation poly (ε-caprolactone)/gel derived bioactive glass composites for bone tissue engineering Part I Material properties. Mater. Sci. Eng. C 2015, 56, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Christen, M.O.; Vercesi, F. Polycaprolactone how a well known and futuristic polymer has become an innovative collagen stimulator in esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Gull, N.; Islam, A.; Dilshad, M.R.; Atanase, L.I.; Delaite, C. Needleless electrospinning of poly (ε-caprolactone) nanofibers deposited on gelatin film for controlled release of Ibuprofen. Chem. Pap. 2023, 77, 2657–2669. [Google Scholar] [CrossRef]
- Goonoo, N.; Jeetah, R.; Bhaw Luximon, A.; Jhurry, D. Polydioxanone based bio materials for tissue engineering and drug/gene delivery applications. Eur. J. Pharm. Biopharm. 2015, 97, 371–391. [Google Scholar] [CrossRef]
- Bezrouk, A.; Hosszu, T.; Hromadko, L.; Olmrova Zmrhalova, Z.; Kopecek, M.; Smutny, M.; Selke Krulichova, I.; Macak, J.M.; Kremlacek, J. Mechanical properties of a biodegradable self-expandable polydioxanone monofilament stent In vitro force relaxation and its clinical relevance. PLoS ONE 2020, 15, 0235842–0235864. [Google Scholar] [CrossRef]
- Boland, E.D.; Coleman, B.D.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005, 1, 115–123. [Google Scholar] [CrossRef]
- Ahlinder, A.; Fuoco, T.; Finne Wistrand, A. Medical grade polylactide copolyesters and polydioxanone Rheological properties and melt stability. Polym. Test. 2018, 72, 214–222. [Google Scholar] [CrossRef]
- Elzeny, H.; Zhang, F.; Ali, E.N.; Fathi, H.A.; Zhang, S.; Li, R.; El Mokhtar, M.A.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Polyphosphoester nanoparticles as biodegradable platform for delivery of multiple drugs and siRNA. Drug Des. Devel Ther. 2017, 11, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Z.E.; Jérôme, C. Polyphosphoesters New trends in synthesis and drug delivery applications. Macromol. Biosci. 2016, 16, 1745–1761. [Google Scholar] [CrossRef]
- Iwasaki, Y. Intelligent Polyphosphoester Based Biomaterials. Kobunshi Ronbunshu 2017, 74, 172–181. [Google Scholar] [CrossRef]
- Riva, R.; Shah, U.; Thomassin, J.M.; Yilmaz, Z.; Lecat, A.; Colige, A.; Jérôme, C. Design of degradable polyphosphoester networks with tailor made stiffness and hydrophilicity as scaffolds for tissue engineering. Biomacromolecules 2019, 21, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Fonte, P.; Cruz, A.; Kennedy, P.J.; Pinto, I.M.; Sarmento, B. Polyester based nanoparticles for the encapsulation of monoclonal antibodies Recombinant Glycoprotein Production. Methods Protoc. Biol. 2018, 1674, 239–253. [Google Scholar] [CrossRef]
- Niza, E.; Ocaña, A.; Castro Osma, J.A.; Bravo, I.; Alonso Moreno, C. Polyester polymeric nanoparticles as platforms in the development of novel nanomedicines for cancer treatment. Cancers 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Molavi, F.; Barzegar Jalali, M.; Hamishehkar, H. Polyester based polymeric nano and microparticles for pharmaceutical purposes A review on formulation approaches. J. Control. Release 2020, 320, 265–282. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M.L. PLA nano and microparticles for drug delivery an overview of the methods of preparation. Macromol. Biosci. 2007, 7, 767–783. [Google Scholar] [CrossRef]
- Ruiz Ruiz, F.; Mancera Andrade, E.I.; Parra Saldivar, R.; Keshavarz, T.; Iqbal, H. Drug delivery and cosmeceutical applications of poly lactic acid based novel constructs A review. Curr. Drug Metab. 2017, 18, 914–925. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A perspective on polylactic acid based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259–274. [Google Scholar] [CrossRef]
- Blasi, P. Poly(lactic acid)/poly(lactic co glycolic acid) based microparticles an overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly (lactic acid) based microparticles for drug delivery applications An overview of recent advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Ventura, C.A.; Giannone, I.; Ruozi, B.; Montenegro, L.; Pignatello, R.; Puglisi, G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int. J. Pharm. 2006, 325, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K. Polylactide (PLA) based amphiphilic block copolymers synthesis self assembly and biomedical applications. Soft Matter 2011, 7, 5096–5108. [Google Scholar] [CrossRef]
- Bawa, K.K.; Oh, J.K. Stimulus responsive degradable polylactide based block copolymer nanoassemblies for controlled/enhanced drug delivery. Mol. Pharm. 2017, 14, 2460–2474. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, K.; Masek, A. Green Copolymers Based on Poly (Lactic Acid) Short Review. Materials 2021, 14, 5254. [Google Scholar] [CrossRef]
- Zhang, B.; Sai Lung, P.; Zhao, S.; Chu, Z.; Chrzanowski, W.; Li, Q. Shape dependent cytotoxicity of PLGA PEG nanoparticles on human cells. Sci. Rep. 2017, 7, 7315–7322. [Google Scholar] [CrossRef]
- Wilkosz, N.; Lazarski, G.; Kovacik, L.; Gargas, P.; Nowakowska, M.; Jamroz, D.; Kepczynski, M. Molecular insight into drug loading capacity of PEG PLGA nanoparticles for itraconazole. J. Phys. Chem. B 2018, 122, 7080–7090. [Google Scholar] [CrossRef]
- Dodda, J.M.; Remiš, T.; Rotimi, S.; Yeh, Y.C. Progress in the drug encapsulation of poly (lactic co glycolic acid) and folate decorated poly (ethylene glycol) poly (lactic co glycolic acid) conjugates for selective cancer treatment. J. Mater. Chem. B 2022, 10, 4127–4141. [Google Scholar] [CrossRef]
- Mundel, R.; Thakur, T.; Chatterjee, M. Emerging uses of PLA PEG copolymer in cancer drug delivery. 3 Biotech 2022, 12, 41–52. [Google Scholar] [CrossRef]
- Kannapiran, S.; Vitta, S.; Ranganathan, B.; Rajagopal, V.; Gimbun, J.; Ballamurugan, A. PEG PLA Nanoformulation for Breast Cancer Therapy. Trends Biomater. Artif. Organs. 2022, 36, 76–82. [Google Scholar]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly (lactic co glycolic acid) based drug delivery systems A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sabliov, C. PLA/PLGA nanoparticles for delivery of drugs across the blood brain barrier. Nano. Rev. 2013, 2, 241–257. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA based nanoparticles A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic co glycolic) acid (PLGA) based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly (lactic co glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X. Microfluidics for producing poly (lactic co glycolic acid) based pharmaceutical nanoparticles. Adv. Drug Deliv. Rev. 2018, 128, 101–114. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Li, H.; Ma, G. Recent research and development of PLGA/PLA microspheres/nanoparticles A review in scientific and industrial aspects. Front. Chem. Sci. Eng. 2019, 13, 14–27. [Google Scholar] [CrossRef]
- Gangapurwala, G.; Vollrath, A.; De San Luis, A.; Schubert, U.S. PLA/PLGA based drug delivery systems produced with supercritical CO2 A green future for particle formulation. Pharmaceutics 2020, 12, 1118. [Google Scholar] [CrossRef]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA based nanoparticles for the treatment of cancer current strategies and perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Arpagaus, C. PLA/PLGA nanoparticles prepared by nano spray drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef]
- Nouvel, C.; Dubois, P.; Dellacherie, E.; Six, J.L. Controlled synthesis of amphiphilic biodegradable polylactide grafted dextran copolymers. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2577–2588. [Google Scholar] [CrossRef]
- Liu, L.; Shi, A.; Guo, S.; Chen, S.; Li, J. Preparation of chitosan g polylactide graft copolymers via self catalysis of phthaloylchitosan and their complexation with DNA. React. Funct. Polym. 2010, 70, 301–305. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shu, X.; Shen, Z.; Sun, R.C. Self assembly and paclitaxel loading capacity of cellulose graft poly (lactide) nanomicelles. J. Agric. Food Chem. 2012, 60, 3900–3908. [Google Scholar] [CrossRef]
- Lu, H.W.; He, H.; Zhang, B.; Liu, G.Q.; Li, M.Y.; Nie, Q.L. Facile synthesis of amphiphilic chitosan g poly (lactic acid) derivatives and the study of their controlled drug release. J. Appl. Polym. Sci. 2013, 130, 908–915. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.K.; Law, W.C.; Weinheimer, E.; Sengupta, S.; Prasad, P.N.; Cheng, C. Polylactide graft doxorubicin nanoparticles with precisely controlled drug loading for pH triggered drug delivery. Biomacromolecules 2014, 15, 524–532. [Google Scholar] [CrossRef]
- Maharana, T.; Pattanaik, S.; Routaray, A.; Nath, N.; Sutar, A.K. Synthesis and characterization of poly (lactic acid) based graft copolymers. React. Funct. Polym. 2015, 93, 47–67. [Google Scholar] [CrossRef]
- Coudane, J.; Van Den Berghe, H.; Mouton, J.; Garric, X.; Nottelet, B. Poly (lactic acid) based graft copolymers Syntheses strategies and improvement of properties for biomedical and environmentally friendly applications A review. Molecules 2022, 27, 4135. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Biodegradable polylactide and its nanocomposites opening a new dimension for plastics and composites. Macromol. Rapid Commun. 2003, 24, 815–840. [Google Scholar] [CrossRef]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic acid based nanocomposites Promising safe and biodegradable materials in biomedical field. Int. J. Polym. Sci. 2016, 9, 6869154. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Zhang, R. Composite poly (lactic acid)/chitosan nanofibrous scaffolds for cardiac tissue engineering. Int. J. Biol. Macromol. 2017, 103, 1130–1137. [Google Scholar] [CrossRef]
- Zeng, J.B.; Li, K.A.; Du, A.K. Compatibilization strategies in poly (lactic acid) based blends. RSC Adv. 2015, 5, 32546–32565. [Google Scholar] [CrossRef]
- González Aramundiz, J.V.; Lozano, M.V.; Sousa Herves, A.; Fernandez Megia, E.; Csaba, N. Polypeptides and polyaminoacids in drug delivery. Expert. Opin. Drug Deliv. 2012, 9, 183–201. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, H.; Fang, L.; Tan, T. Superabsorbent hydrogels from poly (aspartic acid) with salt temperature and pH responsiveness properties. Polymer 2005, 46, 5368–5376. [Google Scholar] [CrossRef]
- Robla, S.; Alonso, M.J.; Csaba, N.S. Polyaminoacid-based nanocarriers: A review of the latest candidates for oral drug delivery. Expert. Opin. Drug Deliv. 2020, 17, 1081–1092. [Google Scholar] [CrossRef]
- Teng, W.; Jia, F.; Han, H.; Qin, Z.; Jin, Q.; Ji, J. Polyamino acid-based gemcitabine nanocarriers for targeted intracellular drug delivery. Polym. Chem. 2017, 8, 2490–2498. [Google Scholar] [CrossRef]
- Boddu, S.H.S.; Bhagav, P.; Karla, P.K.; Jacob, S.; Adatiya, M.D.; Dhameliya, T.M.; Ranch, K.M.; Tiwari, A.K. Polyamide/poly (amino acid) polymers for drug delivery. J. Funct. Biomater. 2021, 12, 58. [Google Scholar] [CrossRef]
- Zavradashvili, N.; Puiggali, J.; Katsarava, R. Artificial polymers made of α-amino acids-poly (amino acid) s, pseudo-poly (amino acid) s, poly (depsipeptide) s, and pseudo-proteins. Curr. Pharm. Des. 2020, 26, 566–593. [Google Scholar] [CrossRef]
- Lu, C.; Wang, X.; Wu, G.; Wang, J.; Wang, Y.; Gao, H.; Ma, J. An injectable and biodegradable hydrogel based on poly (α, β-aspartic acid) derivatives for localized drug delivery. J. Biomed. Mater. Res. A 2014, 102, 628–638. [Google Scholar] [CrossRef]
- Gong, C.; Lu, C.; Li, B.; Shan, M.; Wu, G. Injectable dopamine-modified poly (α, β-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system. J. Biomed. Mater. Res. A 2017, 105, 1000–1008. [Google Scholar] [CrossRef]
- Brenza, T.M.; Schlichtmann, B.W.; Bhargavan, B.; Vela Ramirez, J.E.; Nelson, R.D.; Panthani, M.G.; McMillan, J.M.; Kalyanaraman, B.; Gendelman, H.E.; Anantharam, V.; et al. Biodegradable polyanhydride-based nanomedicines for blood to brain drug delivery. J. Biomed. Mater. Res. A 2018, 106, 2881–2890. [Google Scholar] [CrossRef]
- Poetz, K.L.; Shipp, D.A. Polyanhydrides: Synthesis, properties, and applications. Aust. J. Chem. 2016, 69, 1223–1239. [Google Scholar] [CrossRef]
- Snyder, B.L.; Mohammed, H.S.; Samways, D.S.; Shipp, D.A. Drug delivery and drug efficacy from amorphous poly (thioether anhydrides). Macromol. Biosci. 2020, 20, 1900377–1900385. [Google Scholar] [CrossRef]
- Niewolik, D.; Dzido, G.; Jaszcz, K. Studies on the Preparation of Nanoparticles from Betulin-Based Polyanhydrides. Eng. Proc. 2021, 11, 10. [Google Scholar] [CrossRef]
- Hsu, W.H.; Csaba, N.; Alexander, C.; Garcia-Fuentes, M. Polyphosphazenes for the delivery of biopharmaceuticals. J. Appl. Polym. Sci. 2020, 137, 48688–48699. [Google Scholar] [CrossRef]
- Andrianov, K. Polyphosphazenes for Biomedical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0-470-19343-3. [Google Scholar]
- Khalid, Z.; Ali, S.; Akram, M. Review on polyphosphazenes-based materials for bone and skeleton tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 693–701. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Allcock, H.R. Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis; American Chemical Society: Washington, DC, USA, 2018; ISBN 9780841233607. [Google Scholar]
- Hajfathalian, M.; Bouche, M.; Cormode, D.P. Polyphosphazene-Based Nanoparticles as Contrast Agents. In Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis; American Chemical Society: Washington, DC, USA, 2018; pp. 77–100. [Google Scholar] [CrossRef]
- Jin, G.-W.; Rejinold, N.S.; Choy, J.-H. Polyphosphazene-Based Biomaterials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 15993. [Google Scholar] [CrossRef]
- Ilayaperumal, P.; Chelladurai, P.; Vairan, K.; Anilkumar, P.; Balagurusamy, B. Polyphosphazenes—A Promising Candidate for Drug Delivery, Bioimaging, and Tissue Engineering: A Review. Macromol. Mater. Eng. 2023, 308, 2200553–2200581. [Google Scholar] [CrossRef]
- Ni, Z.; Yu, H.; Wang, L.; Shen, D.; Elshaarani, T.; Fahad, S.; Khan, A.; Haq, F.; Teng, L. Recent research progress on polyphosphazene-based drug delivery systems. J. Mater. Chem. B 2020, 8, 1555–1575. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R.; Felton, G. Biodegradable polyurethanes: Design, synthesis, properties and potential applications. In Biodegradable Polymers: Processing, Degradation Applications; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 431–470. Available online: https://www.researchgate.net/publication/234027642 (accessed on 28 November 2023).
- Sartori, S.; Chiono, V.; Tonda-Turo, C.; Mattu, C.; Gianluca, C. Biomimetic polyurethanes in nano and regenerative medicine. J. Mater. Chem. B 2014, 2, 5128–5144. [Google Scholar] [CrossRef]
- Gostev, A.A.; Karpenko, A.A.; Laktionov, P.P. Polyurethanes in cardiovascular prosthetics. Polym. Bull. 2018, 75, 4311–4325. [Google Scholar] [CrossRef]
- Sobczak, M.; Kędra, K. Biomedical polyurethanes for anti-cancer drug delivery systems: A brief, comprehensive review. Int. J. Mol. Sci. 2022, 23, 8181. [Google Scholar] [CrossRef]
- Eftekhari, B.S.; Karkhaneh, A.; Alizadeh, A. Physically targeted intravenous polyurethane nanoparticles for controlled release of atorvastatin calcium. Iran. Biomed. J. 2017, 21, 369–379. [Google Scholar] [CrossRef]
- Lowinger, M.B.; Barrett, S.E.; Zhang, F.; Williams, R.O., III. Sustained release drug delivery applications of polyurethanes. Pharmaceutics 2018, 10, 55. [Google Scholar] [CrossRef]
- Morral-Ruíz, G.; Melgar-Lesmes, P.; Solans, C.; García-Celma, M.J. Polyurethane nanoparticles, a new tool for biomedical applications? In Advances in Polyurethane Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 195–216. [Google Scholar] [CrossRef]
- Atanase, L.I.; Desbrieres, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Atanase, L.I. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- Liu, W.; Liu, R.; Li, Y.; Kang, H.; Shen, D.; Wu, M.; Huang, Y. Self-assembly of ethyl cellulose-graft-polystyrene copolymers in acetone. Polymer 2009, 50, 211–217. [Google Scholar] [CrossRef]
- Carlmark, A.; Malmström, E. ATRP grafting from cellulose fibers to create block-copolymer grafts. Biomacromolecules 2003, 4, 1740–1745. [Google Scholar] [CrossRef]
- Wang, D.; Tan, J.; Kang, H.; Ma, L.; Jin, X.; Liu, R.; Huang, Y. Synthesis, self-assembly and drug release behaviors of pH-responsive copolymers ethyl cellulose-graft-PDEAEMA through ATRP. Carbohydr. Polym. 2011, 84, 195–202. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Parın, F.N.; Sıcak, Y. Lignin composites for biomedical applications: Status, challenges and perspectives. In Lignin: Biosynthesis Transformation for Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 253–273. [Google Scholar] [CrossRef]
- Iravani, S. Biomedical applications of lignin-based nanoparticles. In Nanoparticles their Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 217–224. [Google Scholar] [CrossRef]
- Sakeer, K.; Ispas-Szabo, P.; Benyerbah, N.; Mateescu, M.A. Ampholytic starch excipients for high loaded drug formulations: Mechanistic insights. Int. J. Pharm. 2018, 535, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Singh, J.; Liu, Q. Starch—A potential biomaterial for biomedical applications. In Nanomaterials Nanosystems for Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2007; pp. 83–98. [Google Scholar] [CrossRef]
- Lu, D.; Xiao, C.; Xu, S. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, T.; Lin, Q.; Liu, G.-Q.; Zhang, L.; Yu, F.; Chen, Y. Acetylated starch nanocrystals: Preparation and antitumor drug delivery study. Int. J. Biol. Macromol. 2016, 89, 456–464. [Google Scholar] [CrossRef]
- Pokusaeva, B.G.; Karlova, S.P.; Nekrasova, D.A.; Zakharova, N.S.; Khramtsova, D.P.; Reznika, V.V.; Vyazminb, A.V.; Shumovab, N.V. Agarose Gels with Bioresorbable Additives: The Kinetics of the Formation, Structure, Some Properties. Chem. Eng. Trans. 2019, 74, 1171–1176. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-based hydrogels as suitable bioprinting materials for tissue engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B. Continuous production of β-cyclodextrin by cyclodextrin glycosyltransferase immobilized in mixed gel beads: Comparative study in continuous stirred tank reactor and packed bed reactor. Biochem. Eng. J. 2016, 105, 107–113. [Google Scholar] [CrossRef]
- Mejia-Ariza, R.; Graña-Suárez, L.; Verboom, W.; Huskens, J. Cyclodextrin-based supramolecular nanoparticles for biomedical applications. J. Mater. Chem. B 2017, 5, 36–52. [Google Scholar] [CrossRef]
- Dodi, G.; Pala, A.; Barbu, E.; Peptanariu, D.; Hritcu, D.; Popa, M.I.; Tamba, B.I. Carboxymethyl guar gum nanoparticles for drug delivery applications: Preparation and preliminary in-vitro investigations. Mater. Sci. Eng. C 2016, 63, 628–636. [Google Scholar] [CrossRef]
- Singh, V.K.; Banerjee, I.; Agarwal, T.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Guar gum and sesame oil based novel bigels for controlled drug delivery. Colloids Surf. B Biointerfaces 2014, 123, 582–592. [Google Scholar] [CrossRef]
- Prabaharan, M. Prospective of guar gum and its derivatives as controlled drug delivery systems. Int. J. Biol. Macromol. 2011, 49, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Subuddhi, U. pH-Responsive guar gum hydrogels for controlled delivery of dexamethasone to the intestine. Int. J. Biol. Macromol. 2015, 79, 856–863. [Google Scholar] [CrossRef]
- Seeli, D.S.; Prabaharan, M. Guar gum oleate-graft-poly (methacrylic acid) hydrogel as a colon-specific controlled drug delivery carrier. Carbohydr. Polym. 2017, 158, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, Y.; He, J.; Bu, T.; He, X.; He, K.; Xiang, C.; Yu, Z.; Wu, H. The gelation of hydroxypropyl guar gum by nano-ZrO2. Polym. Adv. Technol. 2018, 29, 587–593. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Guar gum: Versatile natural polymer for drug delivery applications. Eur. Polym. J. 2019, 112, 722–735. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef]
- Lapcık, L., Jr.; Lapcık, L.; De Smedt, S.; Demeester, J.; Chabrecek, P. Hyaluronan: Preparation, structure, properties, and applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on hyaluronan behavior in aqueous solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B.J. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Šmejkalová, D.; Muthný, T.; Nešporová, K.; Hermannová, M.; Achbergerová, E.; Huerta-Angeles, G.; Svoboda, M.; Čepa, M.; Machalová, V.; Luptáková, D.; et al. Hyaluronan polymeric micelles for topical drug delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, Z.; Li, W.; Liu, R.; Chen, Y.; Song, Y.; Liu, G.; Song, Z.; Liu, Z.; Lu, C.; et al. A review on the wide range applications of hyaluronic acid as a promising rejuvenating biomacromolecule in the treatments of bone related diseases. Int. J. Biol. Macromol. 2020, 165, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Khanlari, A.; Schulteis, J.E.; Suekama, T.C.; Detamore, M.S.; Gehrke, S.H. Designing crosslinked hyaluronic acid hydrogels with tunable mechanical properties for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 42009. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Merlusca, I.P.; Ibanescu, C.; Tuchilus, C.; Danu, M.; Atanase, L.I.; Popa, I.M. Characterization of neomycin-loaded xanthan-chitosan hydrogels for topical applications. Cellul. Chem. Technol. 2019, 53, 709–719. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Popa, M.; Atanase, L.I.; Daraba, O.M.; Popescu, I.; Romila, L.E.; Ichim, D.L. Biocomposite Hydrogels for the Treatment of Bacterial Infections: Physicochemical Characterization and In Vitro Assessment. Pharmaceutics 2021, 13, 2079. [Google Scholar] [CrossRef] [PubMed]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.; Junginger, H. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50, S91–S101. [Google Scholar] [CrossRef]
- Paul, P.; Kolesinska, B.; Sujka, W. Chitosan and its derivatives-biomaterials with diverse biological activity for manifold applications. Mini Rev. Med. Chem. 2019, 19, 737–750. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Sonia, T.; Sharma, C.P. Chitosan and its derivatives for drug delivery perspective. In Chitosan for Biomaterials; Springer: Berlin/Heidelberg, Germany, 2011; pp. 23–53. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-based drug nanocarriers: Where do we stand? J. Control. Release 2012, 161, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Ravikumar, M.; Dutta, J. Chitin and chitosan for versatile applications. J. Macromol. Sci. Polym. Rev. 2002, 42, 307–354. [Google Scholar] [CrossRef]
- Tapola, N.S.; Lyyra, M.L.; Kolehmainen, R.M.; Sarkkinen, E.S.; Schauss, A.G. Safety aspects and cholesterol-lowering efficacy of chitosan tablets. J. Am. Coll. Nutr. 2008, 27, 22–30. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sarei, F.; Dounighi, N.M.; Zolfagharian, H.; Khaki, P.; Bidhendi, S.M. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian. J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar] [CrossRef]
- Venkatesan, J.; Nithya, R.; Sudha, P.N.; Kim, S.-K. Role of alginate in bone tissue engineering. Adv. Food Nutr. Res. 2014, 73, 45–57. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Sosnik, A. Alginate particles as platform for drug delivery by the oral route: State-of-the-art. Int. Sch. Res. Not. 2014, 2014, 926157. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Oliveira, D.; Chen, M.; Souto, E.B. Advanced applications of alginates in biomedical. In Applications of Advanced Green Materials; Woodhead Publishing: Sawston, UK, 2021; pp. 321–337. [Google Scholar] [CrossRef]
- Mollah, M.Z.I.; Zahid, H.M.; Mahal, Z.; Mohammad, R.I.F.; Khandaker, M.U. The usages and potential uses of alginate for healthcare applications. Front. Mol. Biosci. 2021, 8, 719972. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A wonder polymer from marine algae for potential drug delivery applications. Curr. Pharm. Des. 2019, 25, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.d.S.; de Carvalho, R.G. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.-N.; Letourneur, D.; Chaubet, F. Fucoidans in nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef]
- Etman, S.M.; Elnaggar, Y.S.; Abdallah, O.Y. Fucoidan, a natural biopolymer in cancer combating: From edible algae to nanocarrier tailoring. Int. J. Biol. Macromol. 2020, 147, 799–808. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Hwang, P.-A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Yang, X.; Du, X.; Yang, H.; Liu, Y.; Khan, A.Q.; Zhai, G. Chondroitin sulfate derived theranostic and therapeutic nanocarriers for tumor-targeted drug delivery. Carbohydr. Polym. 2020, 233, 115837–115894. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef]
- Varghese, O.P.; Liu, J.; Sundaram, K.; Hilborn, J.; Oommen, O.P. Chondroitin sulfate derived theranostic nanoparticles for targeted drug delivery. Biomater. Sci. 2016, 4, 1310–1313. [Google Scholar] [CrossRef]
- Kwon, H.J.; Han, Y. Chondroitin sulfate-based biomaterials for tissue engineering. Turk. J. Biol. 2016, 40, 290–299. [Google Scholar] [CrossRef]
- Pal, D.; Saha, S. Chondroitin: A natural biomarker with immense biomedical applications. RSC Adv. 2019, 9, 28061–28077. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Rao, S.; Bhatnagar, I.; Kim, S.-K. Sulfated polysaccharides from macroalgae for bone tissue regeneration. Curr. Pharm. Des. 2019, 25, 1200–1209. [Google Scholar] [CrossRef]
- Rekha, M.; Sharma, C.P. Pullulan as a promising biomaterial for biomedical applications: A perspective. Trends Biomater. Artif. Organs. 2007, 20, 116–121. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Pullulan: A novel molecule for biomedical applications. Carbohydr. Polym. 2017, 171, 102–121. [Google Scholar] [CrossRef]
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, P. Derivatization approaches and applications of pullulan. Adv. Colloid. Interface Sci. 2019, 269, 296–308. [Google Scholar] [CrossRef]
- Raychaudhuri, R.; Naik, S.; Shreya, A.B.; Kandpal, N.; Pandey, A.; Kalthur GSrinivash, M. Pullulan based stimuli responsive and sub cellular targeted nanoplatforms for biomedical application: Synthesis, nanoformulations and toxicological perspective. Int. J. Biol. Macro. Mol. 2020, 161, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, F.; Morelli, A. Ulvan: A versatile platform of biomaterials from renewable resources. In Biomaterials—Physics Chemistry; IntechOpen: London, UK, 2011; pp. 75–98. [Google Scholar] [CrossRef]
- Cindana Mo’o, F.R.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a polysaccharide from macroalga Ulva sp.: A review of chemistry, biological activities and potential for food and biomedical applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Huang, H. Preparation and application of dextran and its derivatives as carriers. Int. J. Biol. Macromol. 2020, 145, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Chenglong, W.; Shuhan, X.; Jiayi, Y.; Wencai, G.; Guoxiong, X.; Hongjing, D. Dextran-based coacervate nanodroplets as potential gene carriers for efficient cancer therapy. Carbohydr. Polym. 2020, 231, 115687–115711. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, G. Design and application of dextran carrier. J. Drug Deliv. Sci. Technol. 2020, 55, 101392–101416. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of natural polymer gum arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Alkarib, S.Y.; MohamedElhassan, D.E.; Nur, A.O. Evaluation of gum Arabic as a film coating former for immediate release oral tablet formulation. World J. Pharm. Pharmaceut Sci. 2016, 5, 32–41. [Google Scholar]
- Butstraen, C.; Salaün, F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 2014, 99, 608–616. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Li, X.; Zhu, H.; Xu, Z.; Liu, L.; Ma, J.; Zhang, M. A bioinspired alginate-gum arabic hydrogel with micro-/nanoscale structures for controlled drug release in chronic wound healing. Appl. Mater. Interfaces 2017, 9, 22160–22175. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; Moustafa, D.; El-Daly, S.M.; El-Hussieny, E.A.; Saleh, S.; Khoobchandani, M.; Bacon, K.L.; Gupta, S.; Katti, K.; Shukla, R.; et al. Photothermal therapy mediated by gum Arabic-conjugated gold nanoparticles suppresses liver preneoplastic lesions in mice. J. Photochem. Photobiol. B Biol. 2016, 163, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.; James, N.R.; Kumar, P.A.; Raj, D.K.; Kumary, T. Gum arabic-curcumin conjugate micelles with enhanced loading for curcumin delivery to hepatocarcinoma cells. Carbohydr. Polym. 2015, 134, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.; Cinthya, K.; Jayakrishnan, A.; Anilkumar, P.; James, N.R. Modified gum arabic cross-linked gelatin scaffold for biomedical applications. Mater. Sci. Eng. 2014, 43, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Jayakrishnan, A. Self-gelling primaquine gum arabic conjugate: An injectable controlled delivery system for primaquine. Biomacromolecules 2007, 8, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Gerola, A.P.; Rubira, A.F.; Muniz, E.C.; Valente, A.J. Gum Arabic: A Remarkable Biopolymer for Food and Biomedical Applications. In Engineering Technology and Industrial Chemistry with Applications; Apple Academic Press: New York, NY, USA, 2018; pp. 281–312. ISBN 9781315100449. [Google Scholar]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc, C.-E.; Savin, A.; Atanase, L.I.; Martin, P.; Popa, M. Physico-chemical characteristics and fermentative activity of the hydrogel particles based on polysaccharides mixture with yeast cells immobilized, obtained by ionotropic gelation. Food Bioprod. Process. 2017, 104, 104–123. [Google Scholar] [CrossRef]
- Peptu, C.; Savin, A.; Atanase, L.-I.; Souidi, K.; Mackenzie, G.; Martin, P.; Riess, G.; Popa, M. Microencapsulation of Baker’s yeast in gellan gum beads used in repeated cycles of glucose fermentation. Int. J. Polym. Sci. 2017, 2017, 7610420. [Google Scholar] [CrossRef]
- Iurciuc, C.-E.; Savin, A.; Atanase, L.I.; Danu, M.; Martin, P.; Popa, M. Encapsulation of Saccharomyces cerevisiae in hydrogel particles based gellan ionically cross-linked with zinc acetate. Powder Technol. 2018, 325, 476–489. [Google Scholar] [CrossRef]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef]
- Aiyelabegan, H.T.; Zaidi, S.S.; Fanuel, S.; Eatemadi, A.; Ebadi, M.T.; Sadroddiny, E. Albumin-based biomaterial for lung tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 853–861. [Google Scholar] [CrossRef]
- Tao, C.; Chuah, Y.J.; Xu, C.; Wang, D.-A. Albumin conjugates and assemblies as versatile bio-functional additives and carriers for biomedical applications. J. Mater. Chem. B 2019, 7, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Chung, Y.-I.; Kim, Y.H.; Tae, G.; Kundu, S. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Wongpinyochit, T.; Uhlmann, P.; Urquhart, A.J.; Seib, F.P. PEGylated silk nanoparticles for anticancer drug delivery. Biomacromolecules 2015, 16, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-Y.; Kim, S.-G.; Kwon, K.-J.; Kweon, H.; Chae, W.-S.; Yang, W.G.; Lee, E.Y.; Seok, H. Silk fibroin-alginate-hydroxyapatite composite particles in bone tissue engineering applications in vivo. Int. J. Mol. Sci. 2017, 18, 858. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef]
- Seib, F.P.; Kaplan, D.L. Silk for drug delivery applications: Opportunities and challenges. J. Chem. 2013, 53, 756–766. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.V.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk fibroin-based biomaterials for biomedical applications: A review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, Q.; Li, R.; Wang, J.; Zhen, X.; Yue, G.; Wang, H.; Cui, F.; Wu, F.; Yang, M.; et al. Facile preparation of paclitaxel loaded silk fibroin nanoparticles for enhanced antitumor efficacy by locoregional drug delivery. Appl. Mater. Interfaces 2013, 5, 12638–12645. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; Catanzano, O.; d’Angelo, I.; Diaz-Gomez, L.; Concheiro, A.; Miro, A.; Alvarez-Lorenzo, C.; Quaglia, F. Microparticle-embedded fibroin/alginate beads for prolonged local release of simvastatin hydroxyacid to mesenchymal stem cells. Carbohydr. Polym. 2017, 175, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Gianak, O.; Kyzas, G.Z.; Samanidou, V.F.; Deliyanni, E.A. A review for the synthesis of silk fibroin nanoparticles with different techniques and their ability to be used for drug delivery. Curr. Anal. Chem. 2019, 15, 339–348. [Google Scholar] [CrossRef]
- Gou, S.; Huang, Y.; Sung, J.; Xiao, B.; Merlin, D. Silk fibroin-based nanotherapeutics: Application in the treatment of colonic diseases. Nanomedicine 2019, 14, 2373–2378. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, X.; Chen, X.; Shao, Z.; Yang, W. Doxorubicin-loaded magnetic silk fibroin nanoparticles for targeted therapy of multidrug-resistant cancer. Adv. Mater. 2014, 26, 7393–7398. [Google Scholar] [CrossRef]
- Subia, B.; Chandra, S.; Talukdar, S.; Kundu, S.C. Folate conjugated silk fibroin nanocarriers for targeted drug delivery. Integr. Biol. 2014, 6, 203–214. [Google Scholar] [CrossRef]
- Pham, D.T.; Saelim, N.; Tiyaboonchai, W.J. Paclitaxel loaded EDC-crosslinked fibroin nanoparticles: A potential approach for colon cancer treatment. Drug Deliv. Transl. Res. 2020, 10, 413–424. [Google Scholar] [CrossRef]
- Totten, J.D.; Wongpinyochit, T.; Carrola, J.; Duarte, I.F.; Seib, F.P. PEGylation-dependent metabolic rewiring of macrophages with silk fibroin nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 14515–14525. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, L.; Wang, Y.; Ye, B.; Deng, S.; Ding, A.; Teng, L.; Qiu, L.; Chen, J. Silk fibroin for CpG oligodeoxynucleotide delivery. ACS Biomater. Sci. Eng. 2019, 5, 6082–6088. [Google Scholar] [CrossRef]
- Crivelli, B.; Bari, E.; Perteghella, S.; Catenacci, L.; Sorrenti, M.; Mocchi, M.; Faragò, S.; Tripodo, G.; Prina-Mello, A.; Torre, M.L. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur. J. Pharm. Biopharm. 2019, 137, 37–45. [Google Scholar] [CrossRef]
- Babaei, Z.; Jahanshahi, M.; Sanati, M. Fabrication and evaluation of gelatin nanoparticles for delivering of anti-cancer drug. Int. J. Nanosci. Nanotechnol. 2008, 4, 23–30. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R. Gelatin based scaffolds for tissue engineering—A review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Saraogi, G.K.; Gupta, P.; Gupta, U.; Jain, N.; Agrawal, G. Gelatin nanocarriers as potential vectors for effective management of tuberculosis. Int. J. Pharm. 2010, 385, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Almine, J.F.; Bax, D.V.; Mithieux, S.M.; Nivison-Smith, L.; Rnjak, J.; Waterhouse, A.; Wise, S.G.; Weiss, A.S. Elastin-based materials. Chem. Soc. Rev. 2010, 39, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Machado, R.; Nürnberger, S.; Dopler, D.; Banerjee, A.; Cunha, A.M.; Rodríguez-Cabello, J.C.; Redl, H.; van Griensven, M.; Reis, R.L.; et al. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J. Control. Release 2010, 142, 312–318. [Google Scholar] [CrossRef]
- Daamen, W.F.; Veerkamp, J.; Van Hest, J.; Van Kuppevelt, T. Elastin as a biomaterial for tissue engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef]
- Miranda-Nieves, D.; Chaikof, E.L. Collagen and elastin biomaterials for the fabrication of engineered living tissues. ACS Biomater. Sci. Eng. 2017, 3, 694–711. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Chilkoti, A. Applications of elastin-like polypeptides in drug delivery. J. Control. Release 2014, 190, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Hnatuszko-Konka, K.; Gerszberg, A.; Kononowicz, A.K. Elastin-like polypeptides as a promising family of genetically-engineered protein based polymers. World J. Microbiol. Biotechnol. 2014, 30, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, I.C.; Milligan, J.J.; Chilkoti, A. Genetically Encoded Elastin-Like Polypeptides for Drug Delivery. Adv. Healthc. Mater. 2021, 10, 2100209–2100227. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, D.; Liu, G.; Zhang, H.; Wang, X.; Wu, Z.; Wu, Y.; Yu, F.; Xu, Q. Application of Elastin-like Polypeptide in Tumor Therapy. Cancers 2022, 14, 3683. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian. Soc. Periodontol. 2013, 17, 539–542. [Google Scholar] [CrossRef]
- Sahiner, M.; Alpaslan, D.; Bitlisli, B.O. Collagen-based hydrogel films as drug-delivery devices with antimicrobial properties. Polym. Bull. 2014, 71, 3017–3033. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Ulasan, M.; Yavuz, E.; Bagriacik, E.U.; Cengeloglu, Y.; Yavuz, M.S. Biocompatible thermoresponsive PEGMA nanoparticles crosslinked with cleavable disulfide-based crosslinker for dual drug release. J. Biomed. Mater. Res. A 2015, 103, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Lin, M.; Dai, Y.; Xia, F.; Zhang, X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater. Sci. Eng. C 2021, 119, 111626–111635. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef]
- Van Bochove, B.; Grijpma, D.W. Photo-crosslinked synthetic biodegradable polymer networks for biomedical applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Tincu, C.E.; Bouhadiba, B.; Atanase, L.I.; Stan, C.S.; Popa, M.; Ochiuz, L. An Accessible Method to Improve the Stability and Reusability of Porcine Pancreatic α-Amylase via Immobilization in Gellan-Based Hydrogel Particles Obtained by Ionic Cross-Linking with Mg2+ Ions. Molecules 2023, 28, 4695. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Ochiuz, L.; Jérôm, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual Cross-Linked Chitosan/PVA Hydrogels Containing Silver Nanoparticles with Antimicrobial Properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef]
- Dellali, K.Z.; Dellali, M.; Raţă, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Spataru, M.-C.; Solcan, C. Assessment of Physicochemical and In Vivo Biological Properties of Polymeric Nanocapsules Based on Chitosan and Poly(N-vinyl pyrrolidone-alt-itaconic anhydride). Polymers 2022, 14, 1811. [Google Scholar] [CrossRef]
- Talelli, M.; Barz, M.; Rijcken, C.J.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrum, C.F. Covalently cross-linked amphiphilic block copolymer micelles. Soft Matter 2011, 7, 3246–3259. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, L.; Li, Y.; Wu, H. Recent progress of crosslinking strategies for polymeric micelles with enhanced drug delivery in cancer therapy. Curr. Med. Chem. 2019, 26, 2356–2376. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Wei, J.; Liu, S.; Jing, X. Core crosslinking of biodegradable block copolymer micelles based on poly (ester carbonate). Macromol. Biosci. 2009, 9, 456–463. [Google Scholar] [CrossRef]
- Shariatinia, Z. Biopolymeric nanocomposites in drug delivery. In Advanced Biopolymeric Systems for Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 233–290. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ko, Y.G.; Deri, F. Biodegradable polymer blends and composites: An overview. Polym. Sci. Ser. A 2014, 56, 812–829. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Jhurry, D. Biodegradable polymer blends: Miscibility, physicochemical properties and biological response of scaffolds. Polym. Int. 2015, 64, 1289–1302. [Google Scholar] [CrossRef]
- Girdhar, M.; Mohan, A.; Sharma, A. Blending strategies of natural polymers: A review. Trends Biomater. Artif. Organs. 2016, 30, 61–76. [Google Scholar] [CrossRef]
- Ghadi, R.; Muntimadugu, E.; Domb, A.; Khan, W.; Zhang, X. Synthetic biodegradable medical polymer: Polyanhydrides. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Woodhead Publishing: Sawston, UK, 2017; pp. 153–188. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.R. Poly (lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly (hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Misra, M.A.; Hinrichsen, G. Biofibres biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276, 1–24. [Google Scholar] [CrossRef]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide based bionanocomposites, properties and applications: A review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Armentano, I.; Puglia, D.; Luzi, F.; Arciola, C.; Morena, F.; Martino, S.; Torre, L. Nanocomposites based on biodegradable polymers. Materials 2018, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Teuwen, J.; Dransfeld, C. Toughening of epoxy systems with interpenetrating polymer network (IPN): A review. Polymers 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, Y.S.; Alekseeva, T.T. Phase-Separated Interpenetrating Polymer Networks; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 3540730710. [Google Scholar]
- Kumari, K.; Kundu, P.P. Studies on in vitro release of CPM from semi-interpenetrating polymer network (IPN) composed of chitosan and glutamic acid. Bull. Mater. Sci. 2008, 31, 159–167. [Google Scholar] [CrossRef]
- Pescosolido, L.; Vermonden, T.; Malda, J.; Censi, R.; Dhert, W.J.; Alhaique, F.; Hennink, W.E.; Matricardi, P. In situ forming IPN hydrogels of calcium alginate and dextran-HEMA for biomedical applications. Acta Biomater. 2011, 7, 1627–1633. [Google Scholar] [CrossRef]
- Boppana, R.; Kulkarni, R.V.; Mutalik, S.S.; Setty, C.M.; Sa, B. Interpenetrating network hydrogel beads of carboxymethylcellulose and egg albumin for controlled release of lipid lowering drug. J. Microencapsul. 2010, 27, 337–344. [Google Scholar] [CrossRef]
- Kumar, G.A.; Wadood, S.A.; Maurya, S.D.; Ramchand, D. Interpenetrating polymeric network hydrogel for stomach-specific drug delivery of clarithromycin: Preparation and evaluation. Asian J. Pharm. 2010, 4, 179–184. [Google Scholar] [CrossRef]
- Shivashankar, M.; Mandal, B.K. A review on interpenetrating polymer network. Int. J. Phram. Phram. Sci. 2012, 4, 1–7. [Google Scholar]
- Kaity, S.; Isaac, J.; Ghosh, A. Interpenetrating polymer network of locust bean gum-poly (vinyl alcohol) for controlled release drug delivery. Carbohydr. Polym. 2013, 94, 456–467. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Vuković-Kwiatkowska, I. Preparation and characterization of interpenetrating networks based on polyacrylates and poly (lactic acid). Express Polym. Lett. 2012, 6, 78–94. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Lohani, A.; Singh, G.; Bhattacharya, S.S.; Verma, A. Interpenetrating polymer networks as innovative drug delivery systems. J. Drug Deliv. 2014, 2014, 583612. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Nadagouda, M.; More, U.A.; Joshi, S.D.; Kulkarni, V.H.; Noolvi, M.N.; Noolvi, M.N.; Kulkarni, P.V. Controlled release of therapeutics using interpenetrating polymeric networks. Expert. Opin. Drug Deliv. 2015, 12, 669–688. [Google Scholar] [CrossRef]
- Zoratto, N.; Matricardi, P. Semi-IPNs and IPN-based hydrogels. Polym. Gels 2018, 4, 91–124. [Google Scholar] [CrossRef]
- Sperling, L.H.; Hu, R. Interpenetrating polymer networks. In Polymer Blends Handbook; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2, pp. 677–724. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.S.; Lee, Y.M. Interpenetrating polymer network hydrogels based on poly (ethylene glycol) macromer and chitosan. Carbohydr. Polym. 2000, 41, 197–205. [Google Scholar] [CrossRef]
- Roland, C. Interpenetrating Polymer Networks (IPN Q1): Structure and Mechanical Behavior. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Ganguly, S.; Maity, P.P.; Mondal, S.; Das, P.; Bhawal, P.; Dhara, S.; Das, N.C. Polysaccharide and poly (methacrylic acid) based biodegradable elastomeric biocompatible semi-IPN hydrogel for controlled drug delivery. Mater. Sci. Eng. C 2018, 92, 34–51. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef] [PubMed]
- Cumpstey, I. Chemical modification of polysaccharides. Int. Sch. Res. Not. 2013, 2013, 417672. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Gu, Q.-M. Enzyme-catalyzed modifications of polysaccharides and poly (ethylene glycol). Polymers 2012, 4, 1311–1330. [Google Scholar] [CrossRef]
- Rossi, F.; Van Griensven, M. Polymer functionalization as a powerful tool to improve scaffold performances. Tissue Eng. Part A 2014, 20, 15–16. [Google Scholar] [CrossRef]
- Xiao, Y.; Chinoy, Z.S.; Pecastaings, G.; Bathany, K.; Garanger, E.; Lecommandoux, S. Design of polysaccharide-b-elastin-like polypeptide bioconjugates and their thermoresponsive self-assembly. Biomacromolecules 2019, 21, 114–125. [Google Scholar] [CrossRef]
- Thakor, P.; Bhavana, V.; Sharma, R.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Polymer–drug conjugates: Recent advances and future perspectives. Drug Discov. Today 2020, 25, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, Y.; Li, X.; Liu, X.; Yeung, K.W.; Wu, S.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Surface functionalization of biomaterials by radical polymerization. Prog. Mater. Sci. 2016, 83, 191–235. [Google Scholar] [CrossRef]
- Schatz, C.; Lecommandoux, S. Polysaccharide-containing block copolymers: Synthesis, properties and applications of an emerging family of glycoconjugates. Macromol. Rapid Commun. 2010, 31, 1664–1684. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Costa, R.R.; Mano, J.F. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem. Soc. Rev. 2014, 43, 3453–3479. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte complexes: Mechanisms, critical experimental aspects, and applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte complexes of natural polymers and their biomedical applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-based colloidal polyelectrolyte complexes for drug delivery: A review. Carbohydr. Polym. 2020, 238, 116126–116129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-based platforms for the delivery of peptides and proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Z. Polyion complexes via electrostatic interaction of oppositely charged block copolymers. Macromolecules 2020, 53, 8737–8740. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Kumar, N.; Ravikumar, M.N.; Domb, A. Biodegradable block copolymers. Adv. Drug Deliv. Rev. 2001, 53, 23–44. [Google Scholar] [CrossRef]
- Zhang, Q.; Ko, N.R.; Oh, J.K. Recent advances in stimuli-responsive degradable block copolymer micelles: Synthesis and controlled drug delivery applications. Chem. Commun. 2012, 48, 7542–7552. [Google Scholar] [CrossRef]
- Soni, V.; Pandey, V.; Asati, S.; Gour, V.; Tekade, R.K. Biodegradable block copolymers and their applications for drug delivery. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2019; pp. 401–447. [Google Scholar] [CrossRef]
- Thakur, V.K. Biopolymer Grafting: Synthesis and Properties; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128104613. [Google Scholar]
- Hu, Y.; Li, Y.; Xu, F.-J. Versatile functionalization of polysaccharides via polymer grafts: From design to biomedical applications. Acc. Chem. Res. 2017, 50, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Shishi, S.; Sasaki, Y.; Akiyoshi, K. Thermoresponsive polysaccharide graft polymer vesicles with tunable size and structural memory. J. Am. Chem. Soc. 2020, 142, 11784–11790. [Google Scholar] [CrossRef]
- Yao, H.; Wei, D.; Che, X.; Cai, L.; Tao, L.; Liu, L.; Wu, L.; Chen, G.Q. Comb-like temperature-responsive polyhydroxyalkanoate-graft-poly (2-dimethylamino-ethylmethacrylate) for controllable protein adsorption. Polym. Chem. 2016, 7, 5957–5965. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Tabasum, S.; Khalid, S.; Shareef, R. A review on grafting of hydroxyethylcellulose for versatile applications. Int. J. Biol. Macromol. 2020, 150, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.T.; Moraes, R.M.; Alves, G.M.; Lacerda, T.M.; Santos, J.C.; Santos, A.M.; Medeiros, S.F. Synthesis of amphiphilic pullulan-graft-poly (ε-caprolactone) via click chemistry. Int. J. Biol. Macromol. 2020, 145, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; El-Bisi, M.K. Grafting of polysaccharides: Recent advances. In Biopolymer Grafting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 469–519. [Google Scholar] [CrossRef]
- Atanase, L.I.; Winninger, J.; Delaite, C.; Riess, G. Reversible addition-fragmentation chain transfer synthesis and micellar characteristics of biocompatible amphiphilic poly(vinyl acetate)-graft-poly(N-vinyl-2-pyrrolidone) copolymers. Eur. Poly. J. 2014, 53, 109–117. [Google Scholar] [CrossRef]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yong, H.; Liu, Y.; Bai, R. Recent advances in the preparation, structural characteristics, biological properties and applications of gallic acid grafted polysaccharides. Int. J. Biol. Macromol. 2020, 156, 1539–1555. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Misra, B. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft modification of natural polysaccharides via reversible deactivation radical polymerization. Prog. Polym. Sci. 2018, 76, 151–173. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, J.; Raj, V.; Kumar, P. A review on the modification of polysaccharide through graft copolymerization for various potential applications. Open J. Med. Chem. 2017, 11, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kumar, P.; Sanghi, R. Use of microwave irradiation in the grafting modification of the polysaccharides—A review. Prog. Polym. Sci. 2012, 37, 340–364. [Google Scholar] [CrossRef]
- Kaneko, Y.; Matsuda, S.-I.; Kadokawa, J.-I. Chemoenzymatic syntheses of amylose-grafted chitin and chitosan. Biomacromolecules 2007, 8, 3959–3964. [Google Scholar] [CrossRef] [PubMed]
- Omagari, Y.; Kaneko, Y.; Kadokawa, J.-I. Chemoenzymatic synthesis of amylose-grafted alginate and its formation of enzymatic disintegratable beads. Carbohydr. Polym. 2010, 82, 394–400. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Arimura, T.; Takemoto, Y.; Yamamoto, K. Self-assembly of amylose-grafted carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.M.; Silva, H.V.; Curti, P.S.; Balaban, R.C. Study of the reaction of grafting acrylamide onto xanthan gum. Carbohydr. Polym. 2012, 90, 778–783. [Google Scholar] [CrossRef]
- Chhatbar, M.U.; Meena, R.; Prasad, K.; Siddhanta, A. Agar/sodium alginate-graft-polyacrylonitrile, a stable hydrogel system. NIScPR 2009, 48A, 1085–1090. Available online: http://nopr.niscpr.res.in/handle/123456789/5809 (accessed on 28 November 2023).
- Lyu, S.; Untereker, D. Degradability of polymers for implantable biomedical devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer biodegradation: Mechanisms and estimation techniques–A review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Banerjee, A.; Chatterjee, K.; Madras, G.J. Enzymatic degradation of polymers: A brief review. Mater. Sci. Technol. 2014, 30, 567–573. [Google Scholar] [CrossRef]
- Luten, J.; van Nostrum, C.F.; De Smedt, S.C.; Hennink, W.E. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J. Control. Release 2008, 126, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomas, H. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef] [PubMed]
- Koifman, N.; Talmon, Y. Cryogenic electron microscopy methodologies as analytical tools for the study of self-assembled pharmaceutics. Pharmaceutics 2021, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Issman, L.; Brenner, B.; Talmon, Y.; Aharon, A. Cryogenic transmission electron microscopy nanostructural study of shed microparticles. PLoS ONE 2013, 8, e83680. [Google Scholar] [CrossRef] [PubMed]
- Danino, D. Cryo-TEM of soft molecular assemblies. Curr. Opin. Colloid. Interface Sci. 2012, 17, 316–329. [Google Scholar] [CrossRef]
- Chu, B.; Liu, T. Characterization of nanoparticles by scattering techniques. J. Nanopart Res. 2000, 2, 29–41. [Google Scholar] [CrossRef]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of light scattering techniques to nanoparticle characterization and development. Front. Chem. 2018, 6, 237–254. [Google Scholar] [CrossRef]
- Dreiss, C. Wormlike micelles: Where do we stand? Recent developments, linear rheology and scattering techniques. Soft Matter 2007, 3, 956–970. [Google Scholar] [CrossRef]
- Adawy, A.; Groves, M.R. The use of size exclusion chromatography to monitor protein self-assembly. Crystals 2017, 7, 331. [Google Scholar] [CrossRef]
- Nguyen, A.B.; Hadjichristidis, N.; Fetters, L.J. Static light scattering study of high-molecular weight 18-arm star block copolymers. Macromolecules 1986, 19, 768–773. [Google Scholar] [CrossRef]
- Langridge, T.D.; Gemeinhart, R.A. Toward understanding polymer micelle stability: Density ultracentrifugation offers insight into polymer micelle stability in human fluids. J. Control. Release 2020, 319, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.J. Time-resolved fluorescence on self-assembly membranes. Curr. Opin. Colloid. Interface Sci. 1997, 2, 27–36. [Google Scholar] [CrossRef]
- Huang, C.-B.; Xu, L.; Zhu, J.-L.; Wang, Y.-X.; Sun, B.; Li, X.; Yang, H.B. Real-time monitoring the dynamics of coordination-driven self-assembly by fluorescence-resonance energy transfer. J. Am. Chem. Soc. 2017, 139, 9459–9462. [Google Scholar] [CrossRef] [PubMed]
- Jelinski, L.W. Solid state deuterium NMR studies of polymer chain dynamics. Annu. Rev. Mater. Sci. 1985, 15, 359–377. [Google Scholar] [CrossRef]
- Abdel-Azim, A.-A.A.; Atta, A.M.; Farahat, M.S.; Boutros, W.Y. Determination of intrinsic viscosity of polymeric compounds through a single specific viscosity measurement. Polymer 1998, 9, 6827–6833. [Google Scholar] [CrossRef]
- Yasunaga, T.; Takeda, K.; Harada, S. Kinetic study of Sodium Dodecyl Sulfate micelle dissociation by a stopped-flow method. J. Colloid. Interface Sci. 1973, 42, 457–463. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Loh, J.W.; Yeoh, G.; Saunders, M.; Lim, L.-Y. Uptake and cytotoxicity of chitosan nanoparticles in human liver cells. Toxicol. Appl. Pharmacol. 2010, 249, 148–157. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- Nakipoglu, M.; Özkabadayı, Y.; Karahan, S.; Tezcaner, A. Bilayer wound dressing composed of asymmetric polycaprolactone membrane and chitosan-carrageenan hydrogel incorporating storax balsam. Int. J. Biol. Macromol. 2024, 254, 128020. [Google Scholar] [CrossRef] [PubMed]
- Coban, S.N.; Polatoglu, I.; Eroglu, E. Methyl cellulose/okra mucilage composite films, functionalized with Hypericum perforatum oil and gentamicin, as a potential wound dressing. Int. J. Biol. Macromol. 2024, 254, 127757. [Google Scholar] [CrossRef]
- Amir, F.; Niazi, M.B.K.; Malik, U.S.; Jahan, Z.; Andleeb, S.; Ahmad, T.; Mustansar, Z. A multifunctional vanillin-infused chitosan-PVA hydrogel reinforced by nanocellulose and CuO-Ag nanoparticles as antibacterial wound dressing. Int. J. Biol. Macromol. 2023, 258, 128831. [Google Scholar] [CrossRef] [PubMed]
- Pitpisutkul, V.; Prachayawarakorn, J. Porous antimicrobial crosslinked film of hydroxypropyl methylcellulose/carboxymethyl starch incorporating gallic acid for wound dressing application. Int. J. Biol. Macromol. 2024, 256, 128231. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; Helmy, N.M.; Kamel, S. Dual-adhesive and self-healing alginate-based hydrogel for wound healing. Chem. Pap. 2023, 1–11. [Google Scholar] [CrossRef]
- Raza, H.; Ashraf, A.; Shamim, R. Synthesis and characterization of Hyaluronic Acid (HA) modified polymeric composite for effective treatment of wound healing by transdermal drug delivery system (TDDS). Sci. Rep. 2023, 13, 13425. [Google Scholar] [CrossRef]
- Khalid, A.; Peng, L.; Arman, A.; Warren-Smith, S.C.; Schartner, E.P.; Sylvia, G.M.; Hutchinson, M.R.; Ebendorff-Heidepriem, H.; McLaughlin, R.A.; Gibson, B.C.; et al. Silk: A bio-derived coating for optical fiber sensing applications. Sens. Actuators B Chem. 2020, 311, 127864. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Q.; Da, G.; Yao, J.; Shao, Z.; Chen, X. A highly stretchable and anti-freezing silk-based conductive hydrogel for application as a self-adhesive and transparent ionotronic skin. J. Mater. Chem. C 2021, 9, 8955–8965. [Google Scholar] [CrossRef]
- Neubauerova, K.; Carneiro, M.C.; Rodrigues, L.R.; Moreira, F.T.; Sales, M.G.F. Nanocellulose-based biosensor for colorimetric detection of glucose. Sens. Bio-Sens. Res. 2020, 29, 100368. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Wang, K.; Jiang, K.; Shen, G. Biocompatible MXene/chitosan-based flexible bimodal devices for real-time pulse and respiratory rate monitoring. ACS Mater. Lett. 2021, 3, 921–929. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, K.; Liu, Y.; Zhan, X.; Savari, R. Polymeric nanocomposite electrode for enhanced electrochemical detection of α-lipoic acid: Application in neuroinflammation prevention and clinical analysis. Environ. Res. 2023, 245, 117369. [Google Scholar] [CrossRef]
- Chen, L.; Deng, X.; Tian, L.; Xie, J.; Xiang, Y.; Liang, X.; Jiang, L.; Jiang, L. Preparation and properties of chitosan/dialdehyde sodium alginate/dopamine magnetic drug-delivery hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132739. [Google Scholar] [CrossRef]
- Rabeh, M.E.; Vora, L.K.; Moore, J.V.; Bayan, M.F.; McCoy, C.P.; Wylie, M.P. Dual stimuli-responsive delivery system for self-regulated colon-targeted delivery of poorly water-soluble drugs. Biomater. Adv. 2023, 157, 213735. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Zhang, Z.; Zou, X.; Song, H.; Zheng, W. Preparation and evaluation of luteolin-loaded PLA-based shape memory gastroretentive drug delivery systems. Int. J. Pharm. 2023, 650, 123670. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Wang, H.; Wu, T.; Chen, X.; Zhao, S.; Yan, H.; Lin, Q. Development and evaluation of redox-responsive alginate–SS–ibuprofen derivative based anti-tumor target drug delivery system for the controlled release of doxorubicin. J. Drug Deliv. Sci. Technol. 2023, 91, 105208. [Google Scholar] [CrossRef]
- Ghawanmeh, A.A.; Tan, L.L.; Ali, G.A.; Assiri, M.A.; Chong, K.F. Optimization of carboxymethyl cellulose-gum Arab-based hydrogel beads for anticancer drugs delivery. J. Mol. Liq. 2024, 393, 123631. [Google Scholar] [CrossRef]
- Rahatuzzaman, M.; Mahmud, M.; Rahman, S.; Hoque, M.E. Design, fabrication, and characterization of 3D-printed ABS and PLA scaffolds potentially for tissue engineering. Results Eng. 2023, 21, 101685. [Google Scholar] [CrossRef]
- Webb, C.B.; D’Costa, K.; Tawagi, E.; Antonyshyn, J.A.; Hofer, O.S.; Santerre, J.P. Electrospun methacrylated natural/synthetic composite membranes for gingival tissue engineering. Acta Biomater. 2024, 173, 336–350. [Google Scholar] [CrossRef]
- Pan, P.; Wang, J.; Wang, X.; Kang, Y.; Yu, X.; Chen, T.; Hao, Y.; Liu, W. Physically cross-linked chitosan gel with tunable mechanics and biodegradability for tissue engineering scaffold. Int. J. Biol. Macromol. 2023, 257, 128682. [Google Scholar] [CrossRef]
- Mostajeran, H.; Baheiraei, N.; Bagheri, H. Effects of cerium-doped bioactive glass incorporation on an alginate/gelatin scaffold for bone tissue engineering: In vitro characterizations. Int. J. Biol. Macromol. 2024, 255, 128094. [Google Scholar] [CrossRef]
- Shaz, N.; Maran, S.; Genasan, K.; Choudhary, R.; Alias, R.; Swamiappan, S.; Kamarul, T.; Raghavendran, H.R.B. Functionalization of poly (lactic-co-glycolic acid) nano calcium sulphate and fucoidan 3D scaffold using human bone marrow mesenchymal stromal cells for bone tissue engineering application. Int. J. Biol. Macromol. 2024, 256, 128059. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, Y.; Zhao, Z.; Wu, Y.; Kong, Q.; Wang, Y. Regulating inflammation and apoptosis: A smart microgel gene delivery system for repairing degenerative nucleus pulposus. J. Control. Release 2024, 365, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, A.; Vakili-Azghandi, M.; Abnous, K.; Taghdisi, S.M.; Babaei, M.; Ramezani, M.; Alibolandi, M. Amphiphilic polylactic acid-b-poly (N-(3-aminopropyl) methacrylamide) copolymers: Self-assembly to polymeric micelles for gene delivery. J. Drug Deliv. Sci. Technol. 2023, 91, 105236. [Google Scholar] [CrossRef]
- Pontes, A.P.; van der Wal, S.; Roelofs, K.; Grobbink, A.; Creemers, L.B.; Engbersen, J.F.; Rip, J. A poly (amidoamine)-based polymeric nanoparticle platform for efficient in vivo delivery of mRNA. Biomater. Adv. 2023, 156, 213713. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.D.; Nikapitiya, C.; De Zoysa, M. Chitosan nanoparticles-based in vivo delivery of miR-155 modulates the Viral haemorrhagic septicaemia virus-induced antiviral immune responses in zebrafish (Danio rerio). Fish Shellfish. Immunol. 2024, 144, 109234. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, X.; Zhou, C.; Zhou, Z.; Sun, M. Natural polysaccharide-based smart CXCR4-targeted nano-system for magnified liver fibrosis therapy. Chin. Chem. Lett. 2024, 35, 108803. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Liu, Y.; Lin, J.; Ding, L.; Wu, S.; Gong, J. Fabrication of targeted and pH responsive lysozyme-hyaluronan nanoparticles for 5-fluorouracil and curcumin co-delivery in colorectal cancer therapy. Int. J. Biol. Macromol. 2024, 254, 127836. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, X.; Shen, Y. Folate-modified carboxymethyl chitosan-based drug delivery system for breast cancer specific combination therapy via regulating mitochondrial calcium concentration. Carbohydr. Polym. 2024, 323, 121434. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, X.; Li, S.; Wu, Y.; Wu, J.; Duan, Z.; Luo, M.; Tang, J. Engineering of surface-altered polydopamine nanocomposites for successive drug release and in vivo antitumor effects in cervical cancer therapy: Investigation of antiproliferative effects and apoptosis. J. Drug Deliv. Sci. Technol. 2024, 91, 105189. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert. Rev. Anti Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent progress of polysaccharide-based hydrogel interfaces for wound healing and tissue engineering. Adv. Mater. Interfaces 2019, 6, 1900761–1900782. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Ribeiro, D.M.L.; Carvalho Junior, A.R.; Vale de Macedo, G.H.R.; Chagas, V.L.; Silva, L.d.S.; Cutrim, B.d.S. Polysaccharide-based formulations for healing of skin-related wound infections: Lessons from animal models and clinical trials. Biomol. Ther. 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated polysaccharides from marine algae as a basis of modern biotechnologies for creating wound dressings: Current achievements and future prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef]
- Debele, T.A.; Su, W.-P. Polysaccharide and protein-based functional wound dressing materials and applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 87–108. [Google Scholar] [CrossRef]
- Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 2023, 16, 5459. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Mahalakshmi, P.; Reshma, G.; Arthi, C.; Másson, M.; Rangasamy, J. Biodegradable polymeric scaffolds and hydrogels in the treatment of chronic and infectious wound healing. Eur. Polym. J. 2023, 198, 112390–112405. [Google Scholar] [CrossRef]
- Prete, S.; Dattilo, M.; Patitucci, F.; Pezzi, G.; Parisi, O.I.; Puoci, F. Natural and Synthetic Polymeric Biomaterials for Application in Wound Management. J. Funct. Biomater. 2023, 14, 455. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, A.T.; Le, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Gerhard, E.; Zhang, C.; Tierney, J.; Xie, D.; Liu, Z.; Yang, J. Polymeric biomaterials for biophotonic applications. Bioact. Mater. 2018, 3, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 16, 108293–108361. [Google Scholar] [CrossRef]
- Gamboa, J.; Paulo-Mirasol, S.; Estrany, F.; Torras, J. Recent Progress in Biomedical Sensors Based on Conducting Polymer Hydrogels. ACS Appl. Bio Mater. 2023, 15, 1720–1741. [Google Scholar] [CrossRef]
- Jadoun, S.; Rathore, D.S. Polymer-based biosensors for medical applications. In Handbook of Polymers in Medicine; Woodhead Publishing: Sawston, UK, 2023; pp. 635–652. [Google Scholar] [CrossRef]
- Chasin, M. Biodegradable Polymers as Drug Delivery Systems; Informa Health Care: London, UK, 1990. [Google Scholar]
- Edlund, U.; Albertsson, A.-C. Degradable polymer microspheres for controlled drug delivery. In Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002; pp. 67–112. [Google Scholar] [CrossRef]
- Ha, C.-S.; Gardella, J.A. Surface chemistry of biodegradable polymers for drug delivery systems. Chem. Rev. 2005, 105, 4205–4232. [Google Scholar] [CrossRef]
- Binauld, S.; Stenzel, M.H. Acid-degradable polymers for drug delivery: A decade of innovation. Chem. Commun. 2013, 49, 2082–2102. [Google Scholar] [CrossRef]
- Janssen, M.; Mihov, G.; Welting, T.; Thies, J.; Emans, P. Drugs and polymers for delivery systems in OA joints: Clinical needs and opportunities. Polymers 2014, 6, 799–819. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Domb, A.J.; Khan, W. Biodegradable polymers for targeted delivery of anti-cancer drugs. Expert. Opin. Drug Deliv. 2016, 13, 891–909. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Ricci, M.; Fujita, Y.; Van Zundert, I.; Masuhara, A.; Fron, E.; Mizuno, H.; Latterini, L.; Rocha, S.; et al. Polymeric engineering of nanoparticles for highly efficient multifunctional drug delivery systems. Sci. Rep. 2019, 9, 2666–2678. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Patel, D.; Atanas, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Nayak, A.K.; Kundu, A.; Das, B.; Samanta, A. Gum arabic-based nanomaterials in drug delivery and biomedical applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 165–182. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Bratek-Skicki, A. Towards a new class of stimuli-responsive polymer-based materials–Recent advances and challenges. Appl. Surf. Sci. Adv. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef]
- Zhu, M.; Whittaker, A.K.; Han, F.Y.; Smith, M.T. Journey to the Market: The Evolution of Biodegradable Drug Delivery Systems. Appl. Sci. 2022, 12, 935. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef]
- Deepthi, S.; Venkatesan, J.; Kim, S.-K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef]
- Patel, H.; Bonde, M.; Srinivasan, G. Biodegradable polymer scaffold for tissue engineering. Trends Biomater. Artif. 2011, 25, 20–29. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Biopolymer based three-dimensional biomimetic micro/nanofibers scaffolds with porous structures via tailored charge repulsions for skin tissue regeneration. Polym. Adv. Technol. 2021, 32, 3535–3548. [Google Scholar] [CrossRef]
- Samiei, M.; Fathi, M.; Barar, J.; Fathi, N.; Amiryaghoubi, N.; Omidi, Y. Bioactive hydrogel-based scaffolds for the regeneration of dental pulp tissue. J. Drug. Deliv. Sci. Technol. 2021, 64, 102600–102611. [Google Scholar] [CrossRef]
- Gathani, K.M.; Raghavendra, S.S. Scaffolds in regenerative endodontics: A review. J. Dent. Res. 2016, 13, 379–386. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature-and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862–110873. [Google Scholar] [CrossRef]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658–587672. [Google Scholar] [CrossRef]
- Rahmani, F.; Larbi Bouamrane, O.; Ben Bouabdallah, A.; Atanase, L.I.; Hellal, A.; Apintiliesei, A.N. Biomimetic Hydroxyapatite Crystals Growth on Phosphorylated Chitosan Films by In Vitro Mineralization Used as Dental Substitute Materials. Polymers 2023, 15, 2470. [Google Scholar] [CrossRef]
- Lim, J.; You, M.; Li, J.; Li, Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater. Sci. Eng. C 2017, 79, 917–929. [Google Scholar] [CrossRef]
- Revia, R.A.; Stephen, Z.R.; Zhang, M. Theranostic nanoparticles for RNA-based cancer treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, A.; Miyata, K.; Kataoka, K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv. Drug Deliv. Rev. 2016, 104, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Chen, W.C.; Heo, Y.; Wang, Y. Polycations and their biomedical applications. Prog. Polym. Sci. 2016, 60, 18–50. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.-M.; Kim, D.-H.; Kim, G.L.; Seo, S.H.; Park, J.W.; Jeong, S.H.; et al. Functional ligands for improving anticancer drug therapy: Current status and applications to drug delivery systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid. State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Wu, H.-Q.; Wang, C.-C. Biodegradable smart nanogels: A new platform for targeting drug delivery and biomedical diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Briceño, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, Y.; Jain, S.K. Perspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colon. J. Pharm. Pharm. Sci. 2007, 10, 86–128. [Google Scholar]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.-M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828–109838. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.-T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil-An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C 2021, 119, 111591–111602. [Google Scholar] [CrossRef]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).