A Spike-like Self-Assembly of Polyaspartamide Integrated with Functionalized Nanoparticles
Abstract
:1. Introduction
2. Experiments
2.1. Synthesis of Poly(2-hydroxyethyl aspartamide) Substituted with Octadecyl Chains and Menthyl Anthranilate (C18-M-PHEA)
2.2. Self-Assembly of M-PHEA and C18-M-PHEA in an Aqueous Solution
2.3. Self-Assembly of C18-M-PHEA Integrated with TiO2 NPs
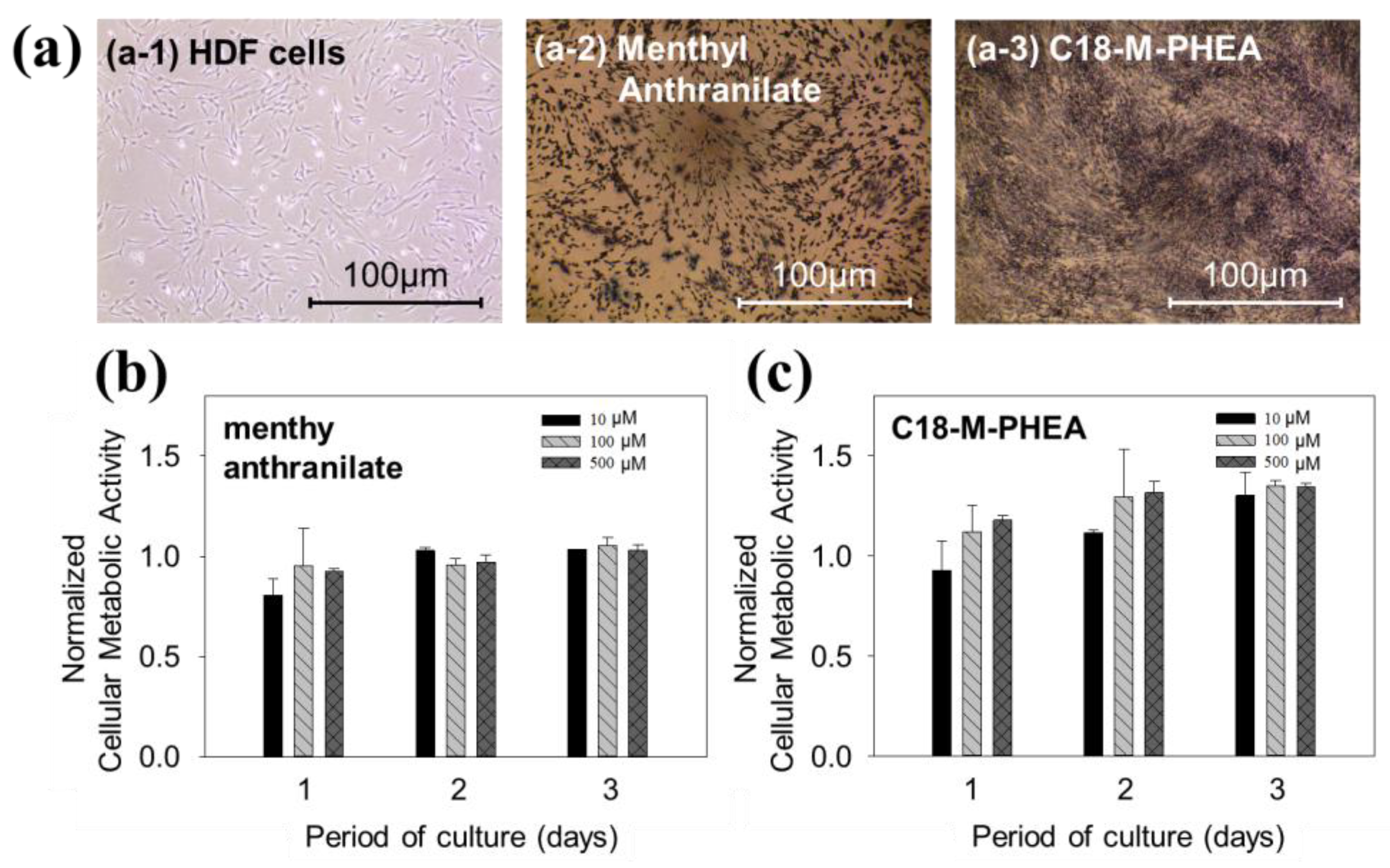
3. Results and Discussion
3.1. Synthesis and Self-Assembly of C18-M-PHEA
3.2. Spike-like Self-Assembly of C18-M-PHEA Integrated with TiO2 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, Y.; Nagatsuka, M.; Akino, K.; Yamauchi, N.; Nakashima, K.; Inose, T.; Nishidate, C.; Sato, K.; Gonda, K.; Kobayashi, Y. Development of methods for fabricating nanoparticles composed of magnetite, gold, and silica toward diagnostic imaging. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128773. [Google Scholar] [CrossRef]
- Hussain, S.; Faisal, K.S.; Clulow, A.J.; Albrecht, H.; Krasowska, M.; Blencowe, A. Influence of lyophilization and Cryoprotection on the stability and morphology of drug-loaded poly(ethylene glycol-b-caprolactone) micelles. Polymers 2023, 15, 1974. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Cha, C.; Kaczmarowski, A.; Haan, J.; Oh, S.; Kong, H. Polyaspartamide vesicle induced by metallic nanoparticles. Soft Matter 2012, 8, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Deng, J.; Hu, L.; Zhang, Z.; Jiang, H.; Li, Y.; Yi, Z.; Ngai, T. Investigation of the stability in Pickering emulsions preparation with commercial cosmetic ingredients. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125082. [Google Scholar] [CrossRef]
- Chen, X.W.; Sun, S.D.; Ma, C.G.; Yang, X.Q. Oil–water interfacial-directed spontaneous self-assembly of natural Quillaja saponin for controlling interface permeability in colloidal emulsions. J. Agric. Food Chem. 2020, 68, 13854–13862. [Google Scholar] [CrossRef] [PubMed]
- Xiudong, L.; Huofei, Z.; Weiting, Y.; Xin, X.; Rumen, K.; Xiaojun, M. Preparation of Cationic Amphiphilic Nanoparticles with Modified Chitosan Derivatives for Doxorubicin Delivery. Materials 2021, 14, 7010. [Google Scholar]
- Jeong, J.H.; Kang, H.S.; Yang, S.R.; Park, K.; Kim, J.-D. Biodegradable poly(asparagine) grafted with poly(caprolactone) and the effect of substitution on self-aggregation. Colloids Surf. A Physicochem. Eng. Asp. 2005, 264, 187–194. [Google Scholar] [CrossRef]
- Jahan, A.; Ahmad, I.Z.; Fatima, N.; Ansari, V.A.; Akhtar, J. Algal bioactive compounds in the cosmeceutical industry: A review. Phycologia 2017, 5, 410–422. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2017, 1, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Brandt, F.S.; Cazzaniga, A.; Hann, M. Cosmeceuticals: Current trends and market analysis. Semin. Cutan. Med. Surg. 2011, 30, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorganica Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Zaki, N.A.A.; Mahmud, S.; Omar, A.F. Ultraviolet protection properties of commercial sunscreens and sunscreens containing ZnO nanorods. J. Phys. Conf. Ser. 2018, 1083, 012012. [Google Scholar] [CrossRef]
- Xu, B.; Cai, Z. Trial-manufacture and UV-blocking property of ZnO nanorods on cotton fabrics. J. Appl. Polym. Sci. 2008, 108, 3781–3786. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Manaia, E.B.; Kaminski, R.C.K.; Correa, M.A.; Chiavacci, L.A. Inorganic UV filters. Braz. J. Pharm. Sci. 2013, 49, 201–209. [Google Scholar] [CrossRef]
- Gruijl, F.R. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000, 319, 359–366. [Google Scholar] [PubMed]
- Cho, S.W.; Kim, H.-J.; Cho, Y.N.; Jeong, J.H. Top-down synthesis of polyaspartamide morphogens to derive platinum nanoclusters. Mater. Lett. 2016, 168, 184–187. [Google Scholar] [CrossRef]
- Jeong, J.H.; Liang, Y.; Jang, M.; Cha, C.; Chu, C.; Lee, H.; Jung, W.; Kim, J.W.; Boppart, S.A.; Kong, H. Stiffness-modulated water retention and neovascularization of dermal fibroblast-encapsulating collagen gel. Tissue Eng. Part A 2013, 19, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Zhou, X.; Huang, P.; Sun, Z. Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint. PLoS ONE 2013, 8, e62087. [Google Scholar] [CrossRef] [PubMed]

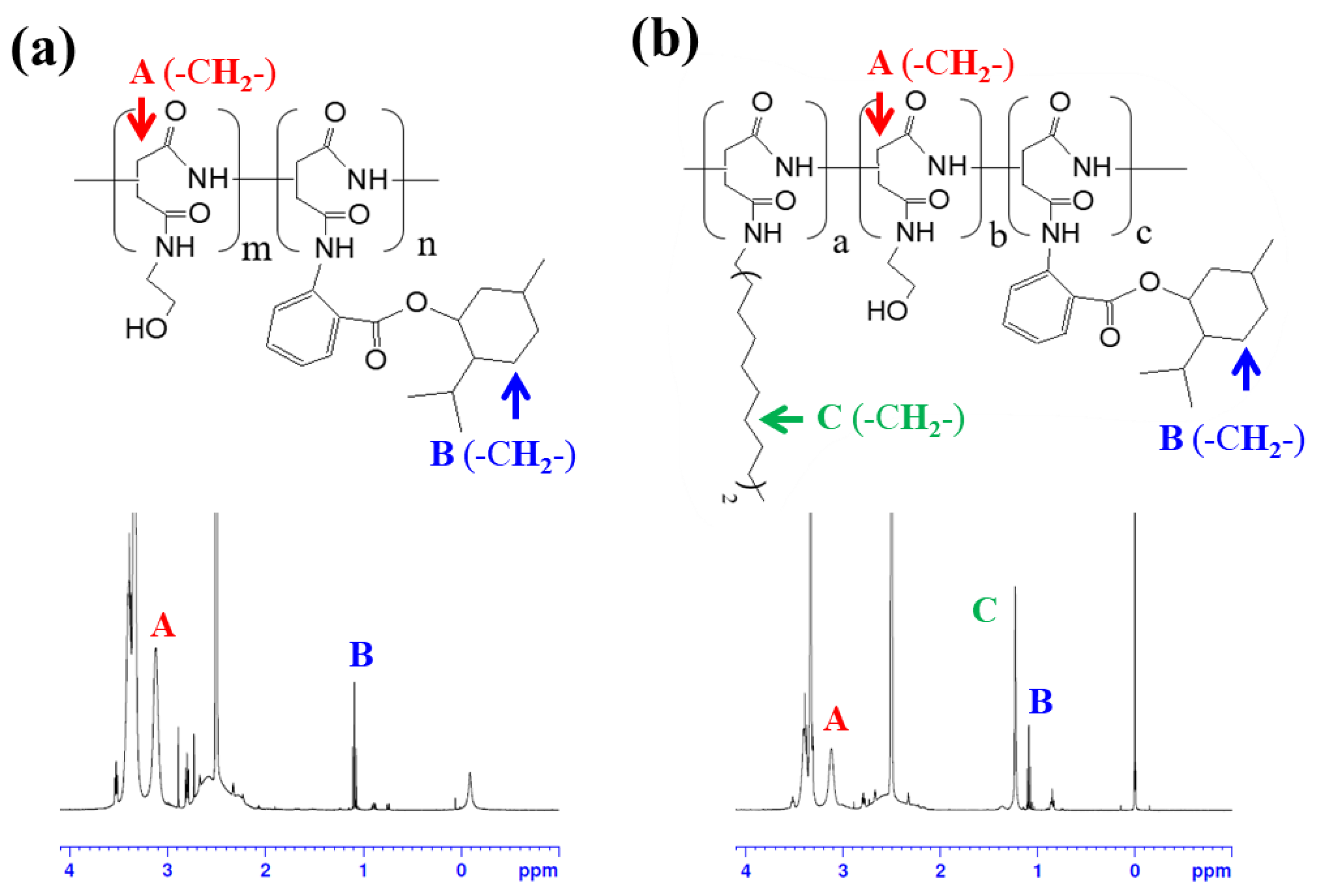
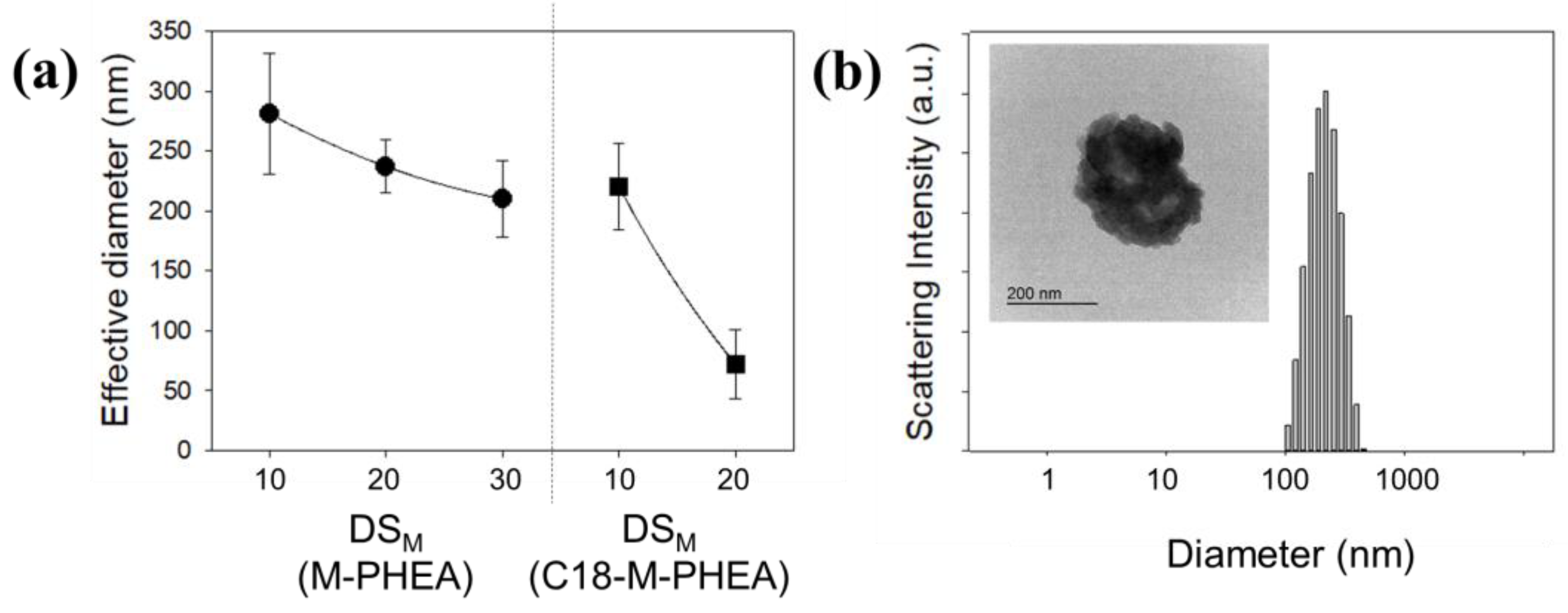

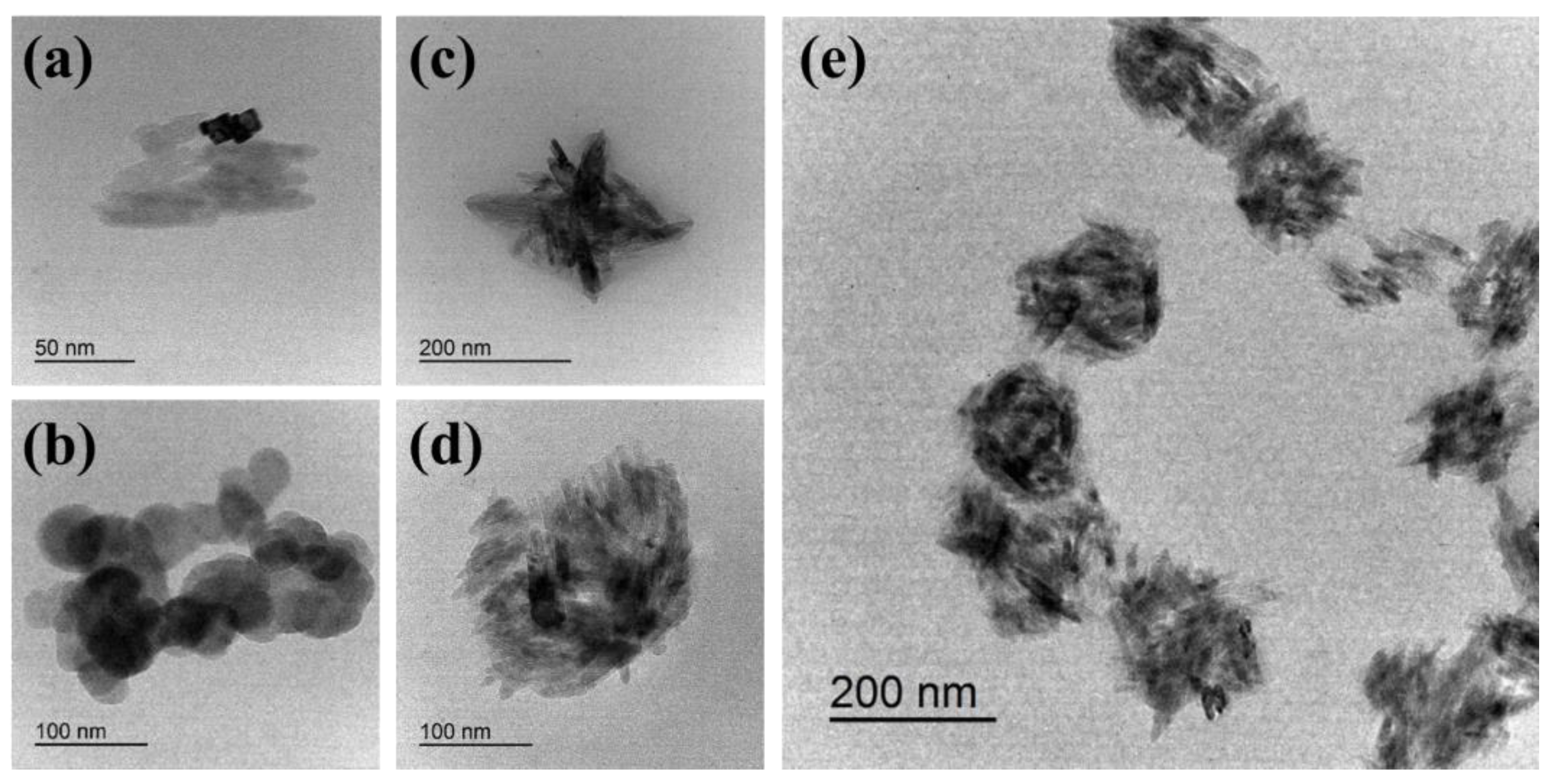
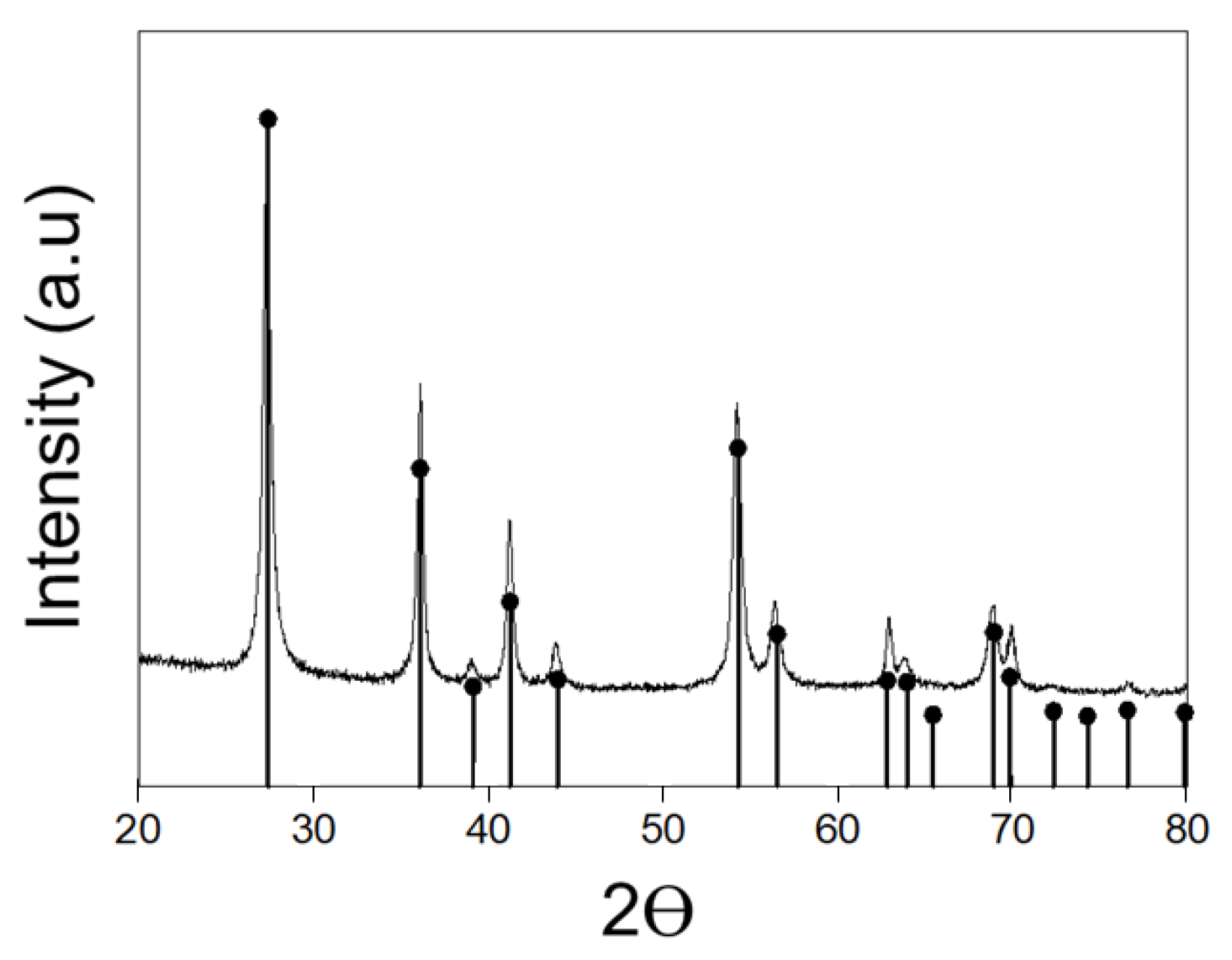

| Sample | Feed a | DSC18 b | DSM c | NumberC18 d | NumberM e | Diameter f |
|---|---|---|---|---|---|---|
| PHEA | 100/0 | - | - | - | - | Highly soluble |
| M10-PHEA | 90/10 | - | 4 | - | 24 | 281 |
| M20-PHEA | 80/20 | - | 8 | - | 47 | 237 |
| M30-PHEA | 70/30 | - | 15 | - | 88 | 210 |
| C18-M10-PHEA | 90/10 | 4 | 8 | 23 | 47 | 220 |
| C18-M20-PHEA | 80/20 | 5 | 13 | 29 | 76 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.R.; Ahn, Y.; Cho, S.; Jeong, H.; Ji, Y.; Jung, W.; Jeong, J.H. A Spike-like Self-Assembly of Polyaspartamide Integrated with Functionalized Nanoparticles. Polymers 2024, 16, 234. https://doi.org/10.3390/polym16020234
Han SR, Ahn Y, Cho S, Jeong H, Ji Y, Jung W, Jeong JH. A Spike-like Self-Assembly of Polyaspartamide Integrated with Functionalized Nanoparticles. Polymers. 2024; 16(2):234. https://doi.org/10.3390/polym16020234
Chicago/Turabian StyleHan, Sa Ra, Yujin Ahn, Sungwoo Cho, Hyewon Jeong, Yoonsook Ji, Woonggyu Jung, and Jae Hyun Jeong. 2024. "A Spike-like Self-Assembly of Polyaspartamide Integrated with Functionalized Nanoparticles" Polymers 16, no. 2: 234. https://doi.org/10.3390/polym16020234
APA StyleHan, S. R., Ahn, Y., Cho, S., Jeong, H., Ji, Y., Jung, W., & Jeong, J. H. (2024). A Spike-like Self-Assembly of Polyaspartamide Integrated with Functionalized Nanoparticles. Polymers, 16(2), 234. https://doi.org/10.3390/polym16020234







