Controlling the Synthesis of Polyurea Microcapsules and the Encapsulation of Active Diisocyanate Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
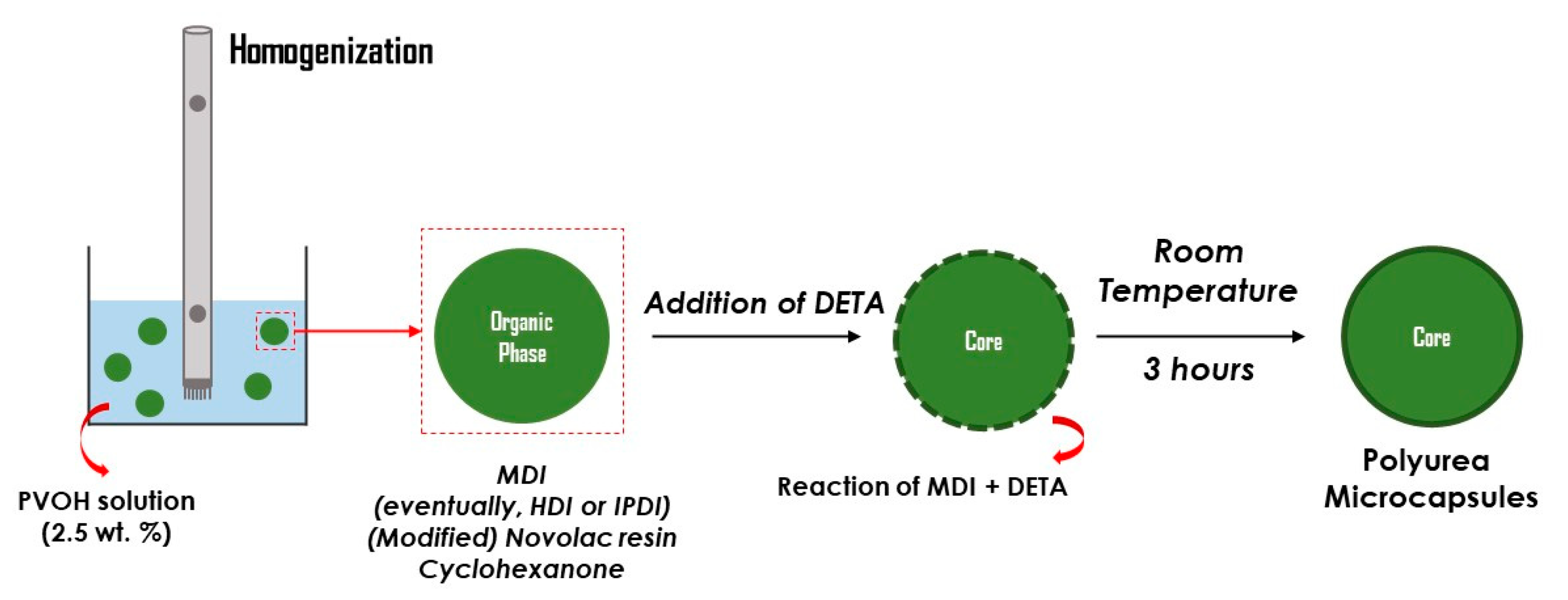
2.2. Syntheis of Polyurea Microcapsules (MCs)
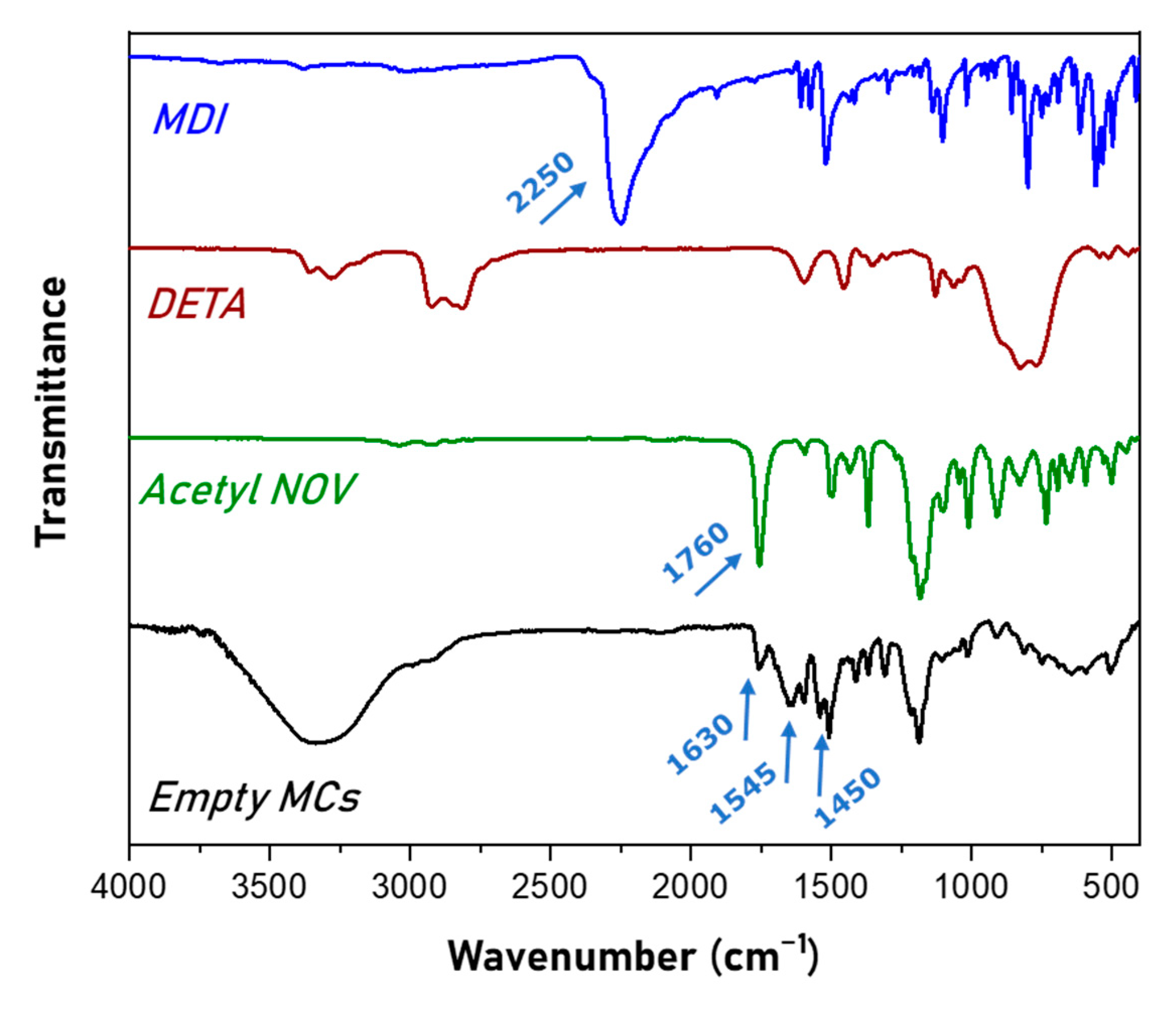
2.3. Characterization of MCs
2.4. Study of the Organic Phase
2.5. Characterization
3. Results
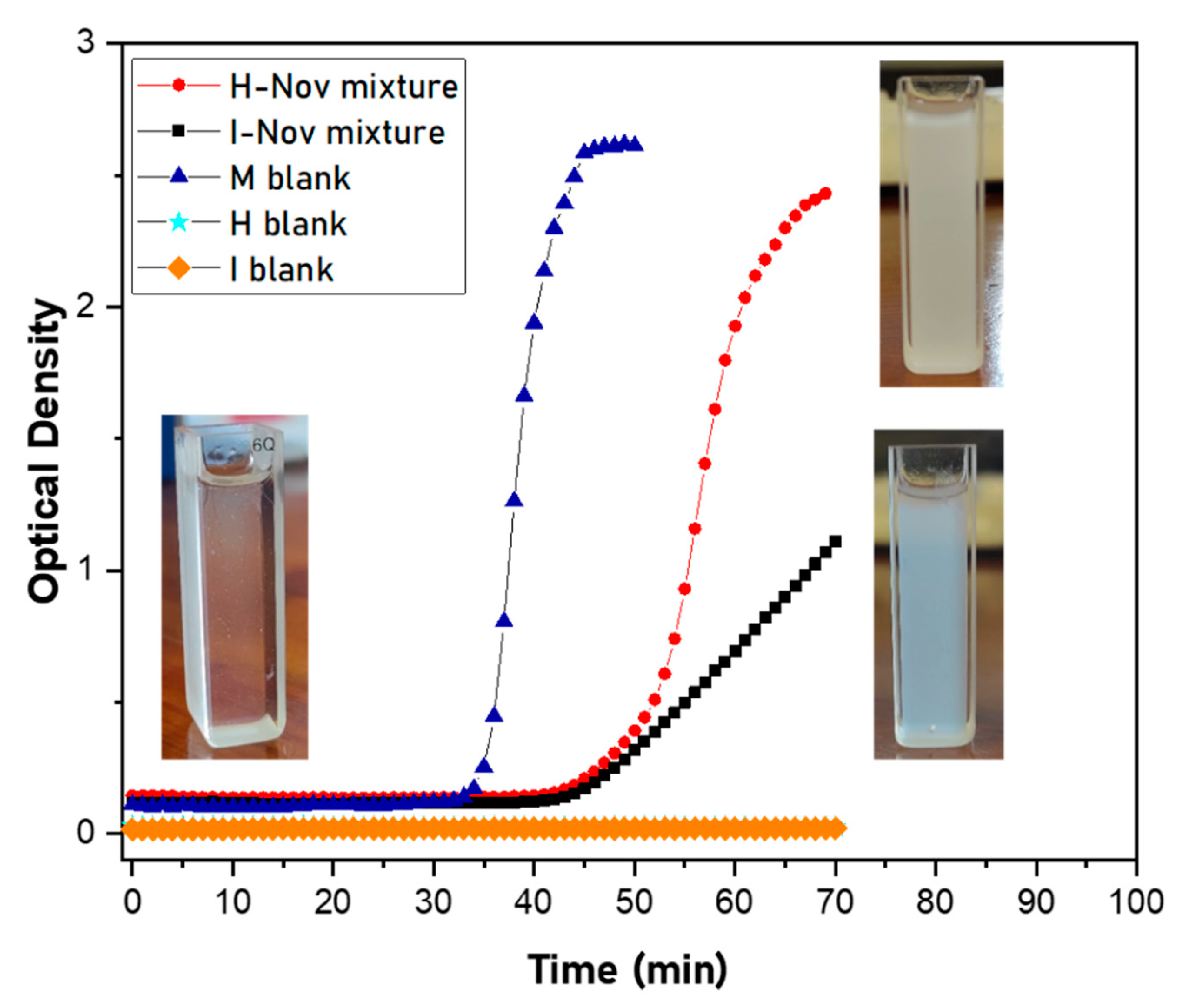
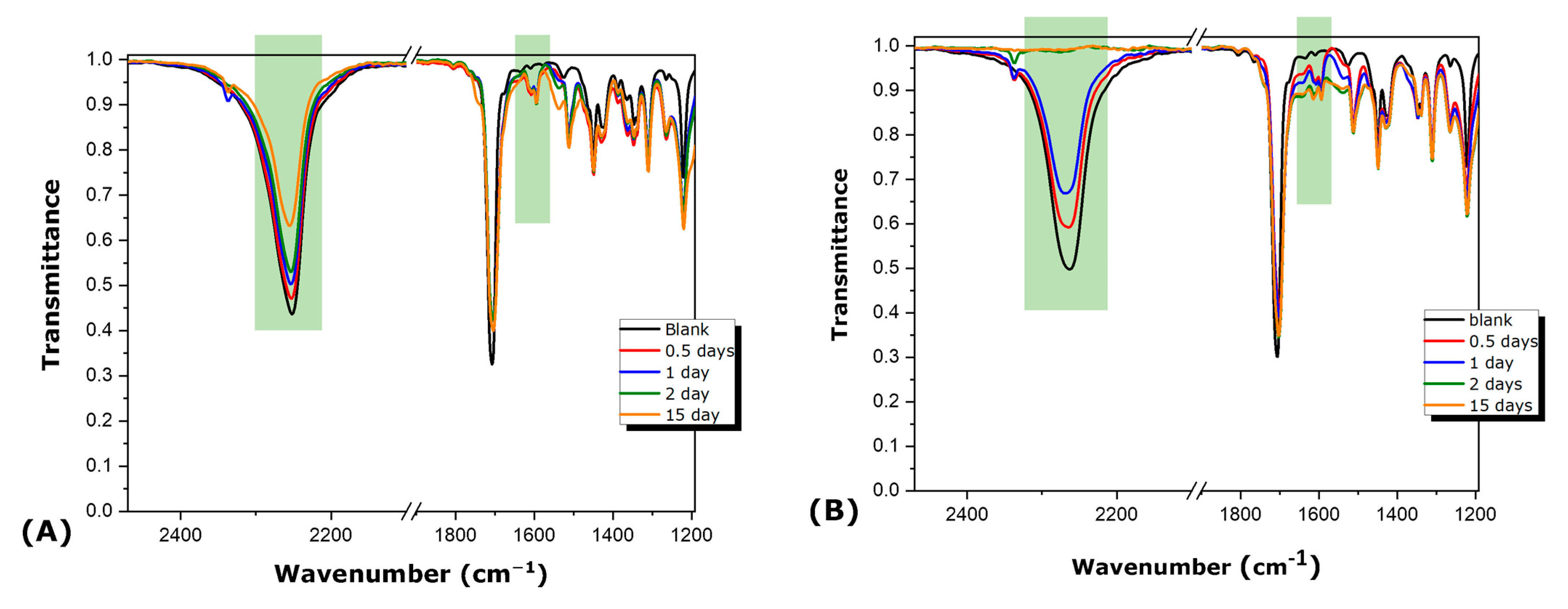
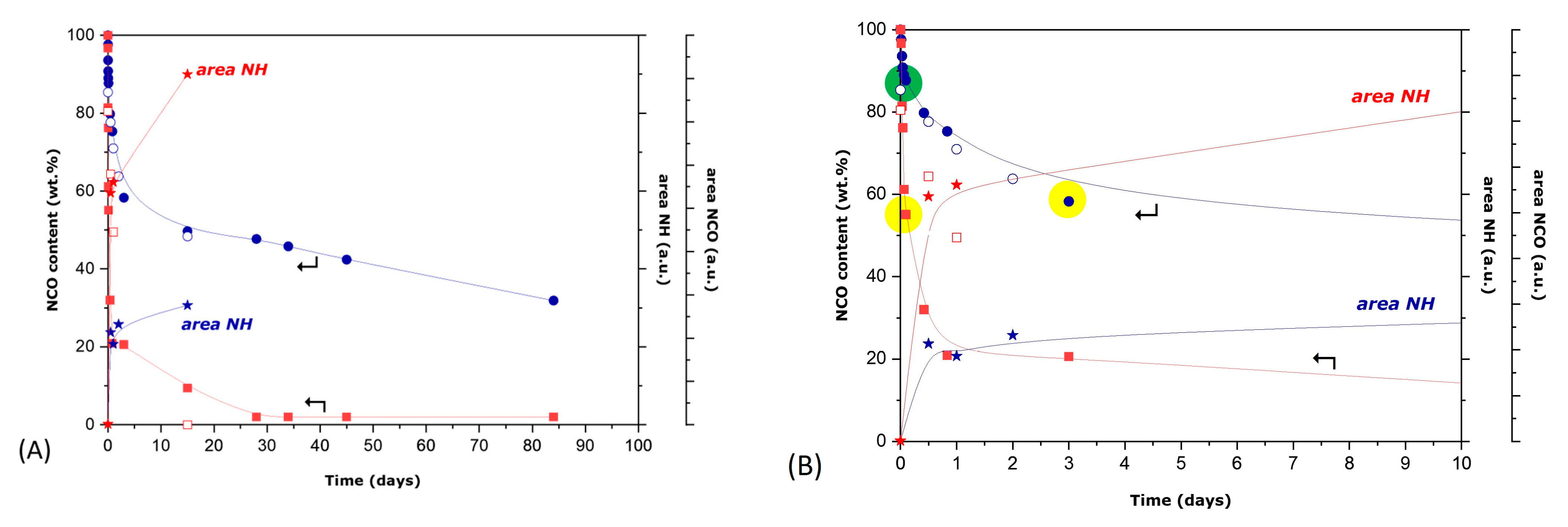
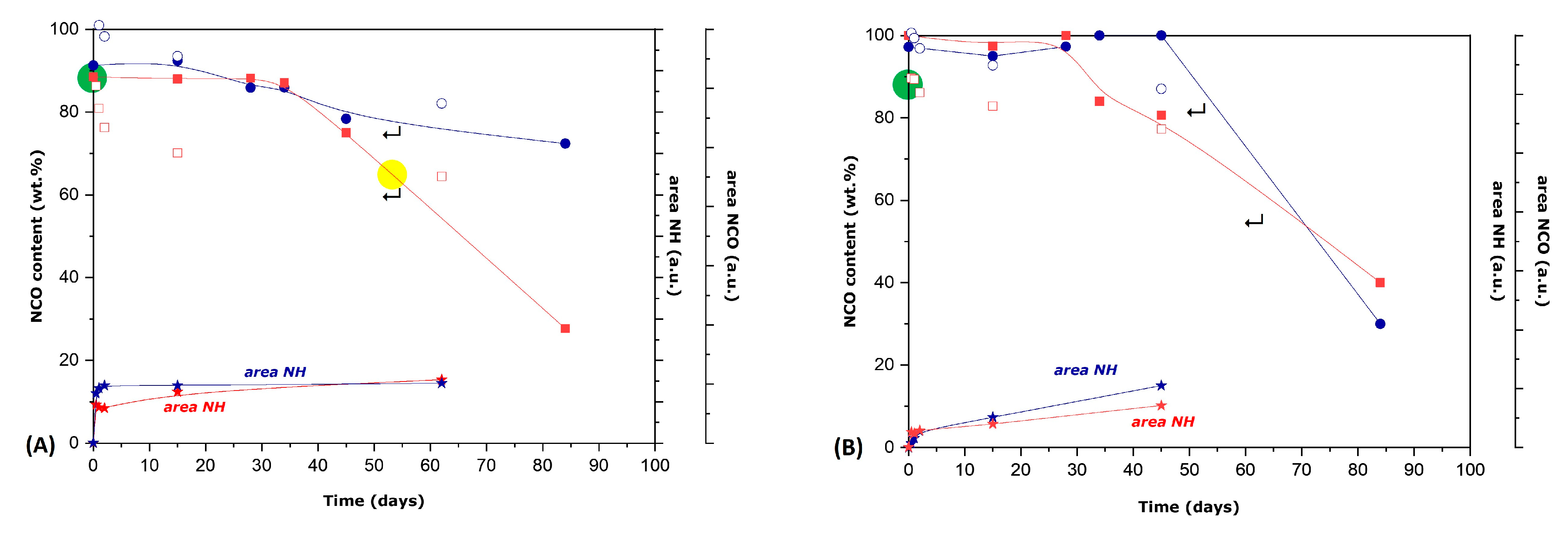
3.1. Synthesis and Characterization of Microcapsules
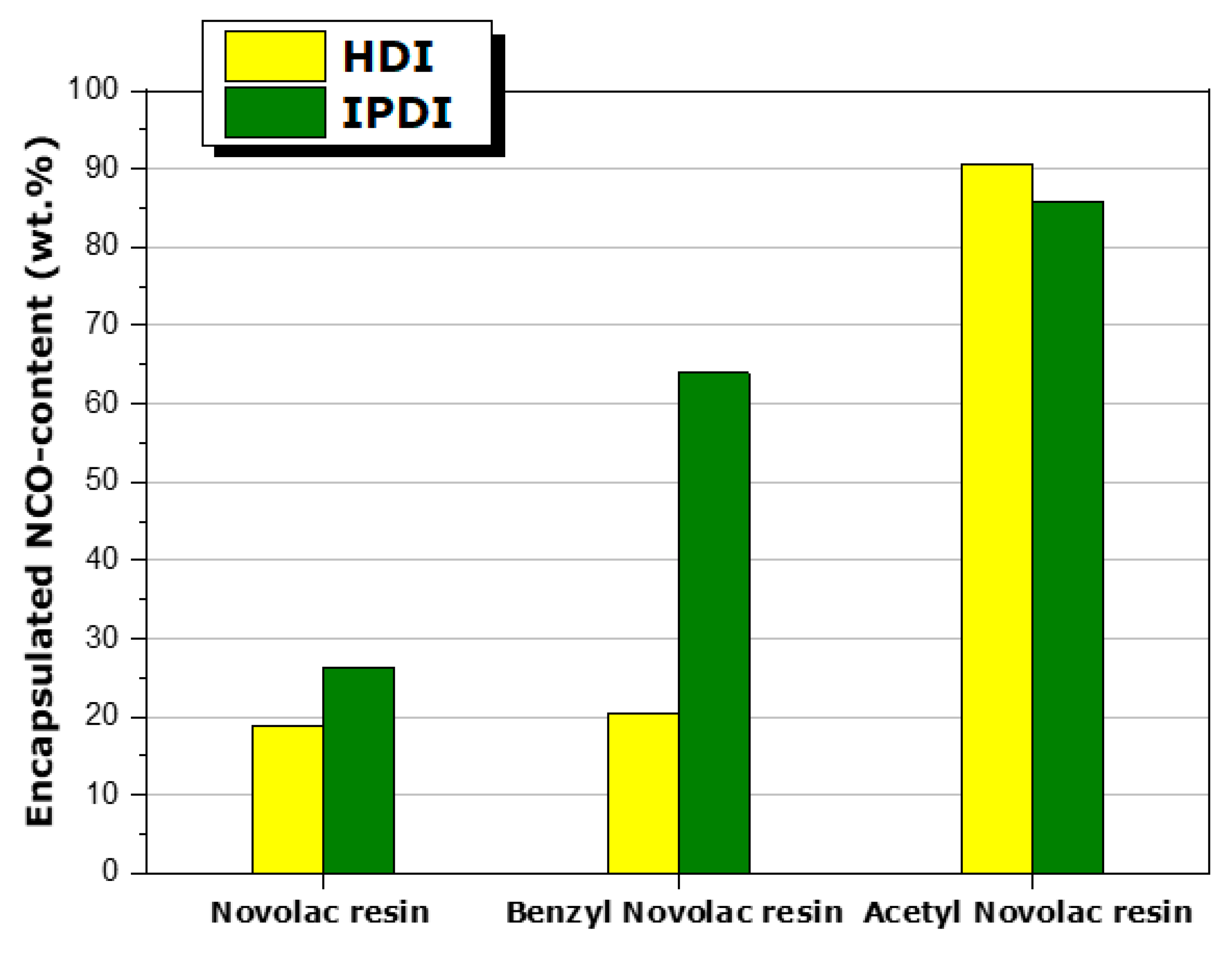
3.2. Study of HDI- or IPDI-Loaded Organic Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badulescu, R.; Vivod, V.; Jausovec, D.; Voncina, B. Treatment of cotton fabrics with ethyl cellulose microcapsules. In Medical and Healthcare Textiles; Woodhead Publishing: Sawston, UK, 2010; pp. 226–235. [Google Scholar]
- Henrique Rodrigues do Amaral, P.; Lopes Andrade, P.; Costa de Conto, L. Microencapsulation and its uses in food science and technology: A review. In Microencapsulation: Processes, Technologies and Industrial Applications; InTechOpen: London, UK, 2019. [Google Scholar]
- Dixit, V. Artificial cells in liver disease. In Artificial Cells, Cell Engineering and Therapy; Woodhead Publishing: Sawston, UK, 2007; pp. 222–235. [Google Scholar]
- Drug delivery systems. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 87–194.
- Mathis, R.; Mehling, A. Textiles with cosmetic effects. In Handbook of Medical Textiles; Woodhead Publishing: Sawston, UK, 2011; pp. 153–172. [Google Scholar]
- Huang, M.; Yang, J. Facile microencapsulation of HDI for self-healing anticorrosion coatings. J. Mater. Chem. 2011, 21, 11123–11130. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Wen, N.; Song, T.; Ji, Z.; Jiang, D.; Wu, Z.; Wang, Y.; Guo, Z. Recent advancements in self-healing materials: Mechanicals, performances and features. React. Funct. Polym. 2021, 168, 105041. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar]
- Cao, Q.; Zhang, L.; Zhang, F.; Wang, D.; Wu, D.; Huang, K. Self-healing polyurethane based on a substance supplement and dynamic chemistry: Repair mechanisms and applications. J. Appl. Polym. 2023, 55007. [Google Scholar] [CrossRef]
- He, Z.; Jiang, S.; Li, Q.; Wang, J.; Zhao, Y.; Kang, M. Facile and cost-effective synthesis of isocyanate microcapsules via polyvinyl alcohol-mediated interfacial polymerization and their application in self-healing materials. Compos. Sci. Technol. 2017, 138, 15–23. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Dong, X.; Liang, Y.; Lu, W.; He, Z.; Qi, G. Optimized synthesis of isocyanate microcapsules for self-healing application in epoxy composites. High Perform. Polym. 2020, 32, 669–680. [Google Scholar] [CrossRef]
- Ismail, N.A.; Khan, A.; Fayyad, E.; Kahraman, R.; Abdullah, A.M.; Shakoor, R.A. Self-Healing performance of smart polymeric coatings modified with tung oil and linalyl acetate. Polymers 2021, 13, 1609. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Li, X.; Li, Q.; Kang, M. Compatibility of high strength silica-modified microcapsules modified with polydopamine in elastomer substrates. Polym. Compos. 2023, 1–16. [Google Scholar] [CrossRef]
- Siroros, N.; Merfort, R.; Migliorini, F.; Lecouturier, S.; Leven, S.; Praster, M.; Hildebrand, F.; Escheweiler, J. Evaluation of an early-stage prototype polyurethane femoral head implant for hip arthroplasty. J. Orthop. 2024, 50, 49–57. [Google Scholar] [CrossRef]
- Lenzi, V.; Crema, A.; Pyrlin, S.; Marques, L. Current state and perspectives of simulation and modeling of aliphatic isocyanates and polyisocyanates. Polymers 2022, 14, 1642. [Google Scholar] [CrossRef] [PubMed]
- Willocq, B.; Odent, J.; Dubois, P.; Raquez, J.-M. Advances in intrinsic self-healing polyurethanes and related composites. RSC Adv. 2020, 10, 13766–13782. [Google Scholar] [CrossRef] [PubMed]
- Ardebili, H.; Pecht, M.G. Encapsulation technologies for electronic applications, plastic encapsulant materials. In Materials and Processes for Electronic Applications; Elsevier: Amsterdam, The Netherlands, 2009; Chapter 2; pp. 47–127. [Google Scholar]
- Qian, Y.; Zhou, Y.; Li, L.; Liu, W.; Yang, D.; Qiu, X. Facile preparation of active lignin capsules for developing self-healing and UV-blocking polyurea coatings. Prog. Org. Coat. 2020, 138, 105354. [Google Scholar] [CrossRef]
- Sun, D.; An, J.; Wu, G.; Yang, J. Double-layered reactive microcapsules with excellent thermal and non-polar solvent resistance for self-healing coatings. J. Mater. Chem. A 2015, 3, 4435–4444. [Google Scholar] [CrossRef]
- Yan, B.; Lu, H.; Li, M.; Wang, X.; Wang, Z.; Pi, M.; Cui, W.; Ran, R. Preparation of phase change microcapsules with high thermal storage and temperature sensitive for thermal management. J. Energy Storage 2023, 64, 107003. [Google Scholar] [CrossRef]
- Lu, W.; Meng, Q.; Qin, C.; Li, J.; Qi, G.; Kong, B.; He, Z. Facile and efficient isocyanate microencapsulation via SDBS/PVP synergetic emulsion. J. Appl. Polym. Sci. 2019, 136, 48045. [Google Scholar] [CrossRef]
- Tzoumani, I.; Iatridi, Z.; Fidelli, A.M.; Krassa, P.; Kallitsis, J.K.; Bokias, G. Room-temperature self-healable blends of waterborne polyurethanes with 2-hydroxyethyl methacrylate-based polymers. Int. J. Mol. Sci. 2023, 24, 2575. [Google Scholar] [CrossRef]
- Tzoumani, I.; Soto Beobide, A.; Iatridi, Z.; Voyiatzis, G.A.; Bokias, G.; Kallitsis, J.K. Glycidyl methacrylate-based copolymers as healing agents of waterborne polyurethanes. Int. J. Mol. Sci. 2022, 23, 8118. [Google Scholar] [CrossRef]
- Avdeliodi, E.; Soto Beobide, A.; Voyiatzis, G.A.; Bokias, G.; Kallitsis, J.K. Novolac-based microcapsules containing isocyanate reagents for self-healing applications. Prog. Org. Coat. 2022, 173, 107204. [Google Scholar] [CrossRef]
- Misin, V.M.; Maltseva, I.E.; Matsev, A.A.; Naumkin, A.V.; Kazakov, M.E. Anionic polymerization of para-diethynylbenzene: Synthesis of a strictly linear polymer. Polymers 2022, 14, 900. [Google Scholar] [CrossRef]
- Jaswal, S.; Thakur, T.; Gaur, B. Rosin-modified o-cresol novolac based vinyl ester thermosets containing methacrylated lignin model compounds: Synthesis, curing and thermo-mechanical analysis. J. Polym. Res. 2021, 28, 111. [Google Scholar] [CrossRef]
- Gong, P.; Li, Y.; Zhang, G. Enhancing anti-corrosion property of novolac vinyl ester coatings on mild steel through introduction of fluoric acrylic monomer and β-Si3N4 nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128075. [Google Scholar] [CrossRef]
- ASTM D2572 standard; Standard Test Method for Isocyanate Groups in Urethane Materials or Prepolymers. World Trade Organization: Geneva, Switzerland, 2019.
- Wang, G.; Li, K.; Zou, W.; Hu, A.; Hu, C.; Zhu, Y.; Chen, C.; Guo, G.; Yang, A.; Drumright, R.; et al. Synthesis of HDI/IPDI hybrid isocyanurate and its application in polyurethane coating. Prog. Org. Coat. 2015, 78, 225–233. [Google Scholar] [CrossRef]
- Luo, W.; Xu, R.; Liu, Y.; Hussain, I.; Lu, Q.; Tan, B. Emulsion-templated poly(acrylamide)s by using polyvinyl alcohol (PVA) stabilized CO2-in-water emulsions and their applications in tissue engineering scaffolds. RSC Adv. 2015, 5, 92017–92024. [Google Scholar] [CrossRef]
- Feczkó, T.; Tóth, J.; Gyenis, J. Comparison of the preparation of PLGA-BSA nano- and microparticles by PVA, poloxamer and PVP. Colloids Surf. A Physicochem. Eng. Asp. 2008, 319, 188–195. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, X.; Li, Q.; Wu, J.; Lin, J. Fabrication of a high-strength hydrogel with an interpenetrating network structure. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 91–98. [Google Scholar] [CrossRef]
- Mulia, K.; Safiera, A.; Pane, I.F.; Krisanti, E.A. effect of high speed homogenizer speed on particle size of polylactic acid. J. Phys. Conf. Ser. 2019, 1198, 062006. [Google Scholar] [CrossRef]
- Sfakianakis, P.; Topakas, E.; Tzia, C. Comparative Study on high-intensity ultrasound and pressure milk homogenization: Effect on the kinetics of yogurt fermentation process. Food Bioprocess Technol. 2015, 8, 548–557. [Google Scholar] [CrossRef]
- He, Z.; Jiang, S.; An, N.; Li, X.; Li, Q.; Wang, J.; Zhao, Y.; Kang, M. Self-healing isocyanate microcapsules for efficient restoration of fracture damage of polyurethane and epoxy resins. J. Mater. Sci. 2019, 54, 1–14. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Cui, Y.; Ma, Y.; Zheng, Z.; Qian, B.; Wang, H.; Semenov, A.; Shchukin, D. Polyurea/polyaniline hybrid shell microcapsules loaded with isophorone diisocyanate for synergetic self-healing coatings. Prog. Org. Coat. 2020, 145, 105684. [Google Scholar] [CrossRef]
- Avdeliodi, E.; Bokias, G.; Kallitsis, J.K. Effect of Microcapsule Stability on Self-Healing Ability of Polyurethane Dispersions. In Proceedings of the 10th ECCOMAS Thematic Conference on Smart Structures and Materials, Patras, Greece, 3–5 July 2023; pp. 1481–1490. [Google Scholar] [CrossRef]
- Loureiro, M.V.; Attaei, M.; Rocha, S.; Vale, M.; Bordado, J.C.; Simões, R.; Marques, A.C. The role played by different active hydrogen sources in the microencapsulation of a commercial oligomeric diisocyanate. J. Mater. Sci. 2019, 55, 4607–4623. [Google Scholar] [CrossRef]
- Loureiro, M.V.; Mariquito, A.; Vale, M.; Bordado, J.C.; Pinho, I.; Marques, A.C. Emulsion Stabilization Strategies for Tailored Isocyanate Microcapsules. Polymers 2023, 15, 403. [Google Scholar] [CrossRef] [PubMed]

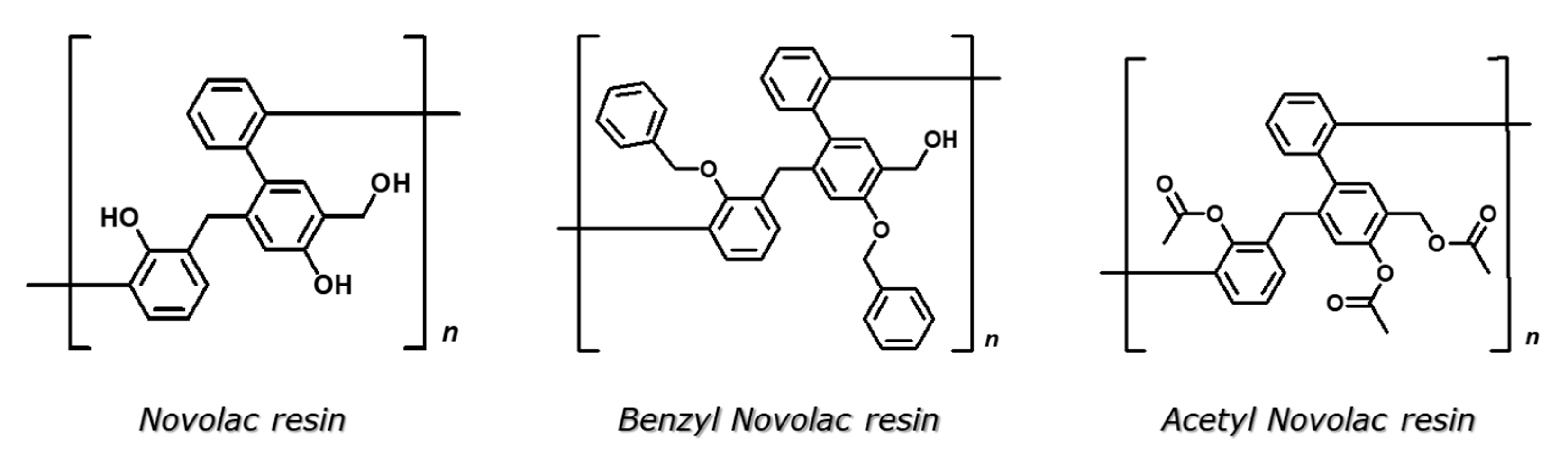



| Product | Number of Repeating Units | Mn | Total mol of OH | mol of Phenolic OH | mol of Aliphatic OH |
|---|---|---|---|---|---|
| Novolac resin | 4 | 921 | 12 | 8 | 4 |
| Benzyl Novolac resin | 4 | 1700 | 4 | - | 4 |
| Acetyl Novolac resin | 4 | 1300 | 0 | - | - |
| Type of MCs | MDI (g) | IPDI (g) | HDI (g) | Type of Resin | Resin (g) | Cyclohexanone (mL) | DETA (g) |
|---|---|---|---|---|---|---|---|
| Empty MCs | 3.7 | - | - | Acetyl Novolac | 5 | 15 | 1.02 |
| I-Nov MCs | 1 | 5 | - | Novolac | |||
| I-Bnz MCs | Benzyl Novolac | ||||||
| I-Ac MCs | Acetyl Novolac | ||||||
| H-Nov MCs | 1 | - | 3.36 | Novolac | |||
| H-Bnz MCs | Benzyl Novolac | ||||||
| H-Ac MCs | Acetyl Novolac |
| Mixture Code | Type of Resin | Resin | MDI (wt.%) (mmol NCO) | IPDI (wt.%) (mmol NCO) | HDI (wt.%) (mmol NCO) | |||
|---|---|---|---|---|---|---|---|---|
| (wt.%) | Total OH (mmol) | Phenolic OH (mmol) | Aliphatic OH (mmol) | |||||
| I-NOV | Novolac resin | 19.8 | 65 | 43 | 22 | 4.0 wt.% | 19.8 wt.% | - |
| I-BNZ | Benzyl Novolac resin | 11.8 | - | 11.8 | ||||
| I-AC | Acetyl Novolac resin | - | - | - | (8 mmol) | (44 mmol) | ||
| H-NOV | Novolac resin | 21.2 | 65 | 43 | 22 | 4.2 wt.% | - | 14.2 wt.% |
| H-BNZ | Benzyl Novolac resin | 11.8 | - | 11.8 | ||||
| H-AC | Acetyl Novolac resin | - | - | - | (8 mmol) | (40 mmol) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeliodi, E.; Tsioli, A.; Bokias, G.; Kallitsis, J.K. Controlling the Synthesis of Polyurea Microcapsules and the Encapsulation of Active Diisocyanate Compounds. Polymers 2024, 16, 270. https://doi.org/10.3390/polym16020270
Avdeliodi E, Tsioli A, Bokias G, Kallitsis JK. Controlling the Synthesis of Polyurea Microcapsules and the Encapsulation of Active Diisocyanate Compounds. Polymers. 2024; 16(2):270. https://doi.org/10.3390/polym16020270
Chicago/Turabian StyleAvdeliodi, Efterpi, Anastasia Tsioli, Georgios Bokias, and Joannis K. Kallitsis. 2024. "Controlling the Synthesis of Polyurea Microcapsules and the Encapsulation of Active Diisocyanate Compounds" Polymers 16, no. 2: 270. https://doi.org/10.3390/polym16020270
APA StyleAvdeliodi, E., Tsioli, A., Bokias, G., & Kallitsis, J. K. (2024). Controlling the Synthesis of Polyurea Microcapsules and the Encapsulation of Active Diisocyanate Compounds. Polymers, 16(2), 270. https://doi.org/10.3390/polym16020270








