Cycloaliphatic Quaternary Ammonium Functionalized Poly(oxindole biphenyl) Based Anion-Exchange Membranes for Water Electrolysis: Stability and Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
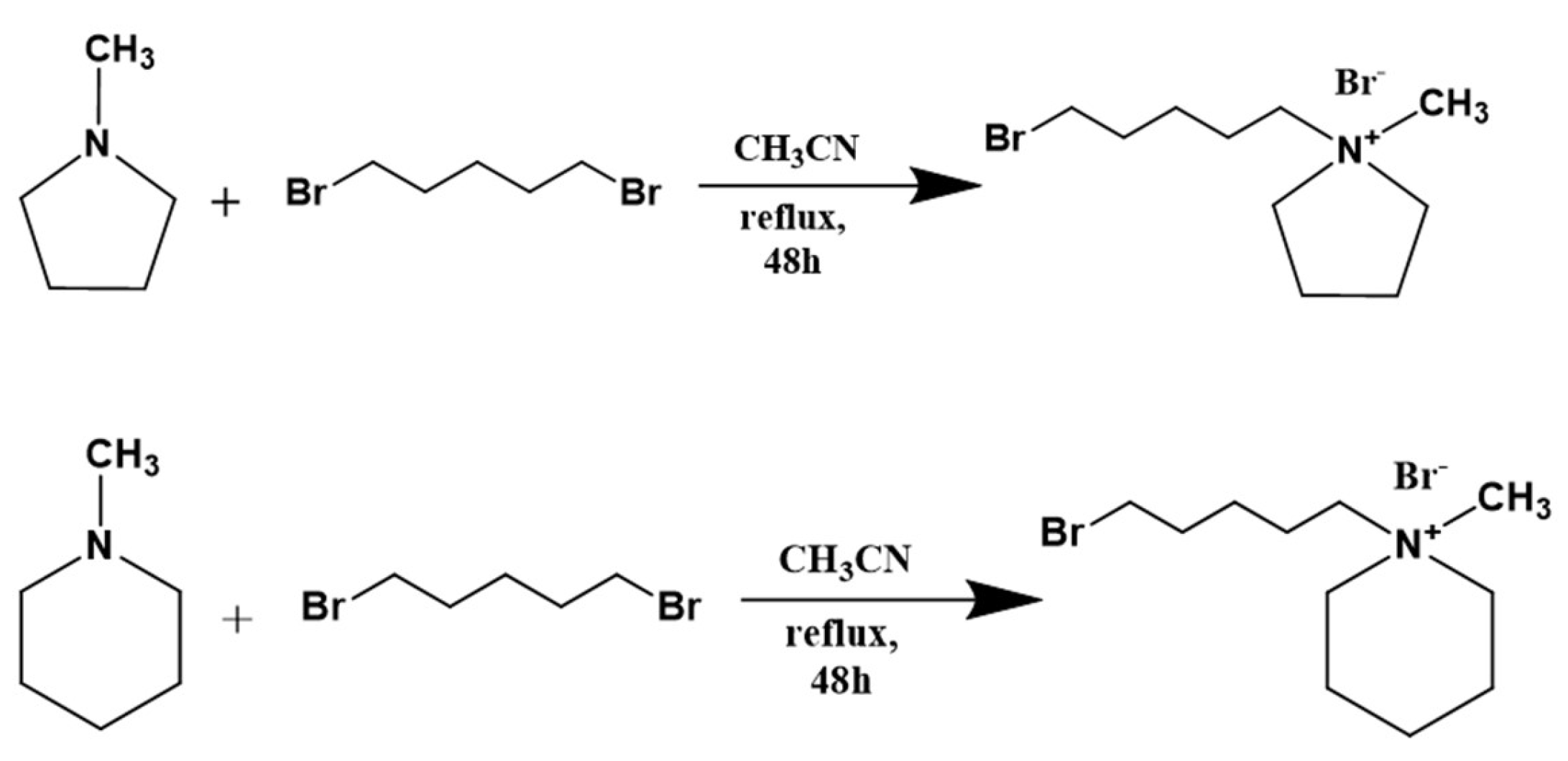
2.2. Preparation of 1-(5-Bromopentyl)-1-methylpyrrolidinium Bromide (BrPyr) and 1-(5-Bromopentyl)-1-methylpiperidinium Bromide (BrPip)
2.3. Synthesis of Poly(oxindole biphenyl-co-trifluoroacetophenone) Copolymers P(OBx-TFAP)
2.4. Synthesis of Pyrrolidinium-Functionalized Copolymers P(OB70pyr-TFAP) and Piperidinium-Functionalized Copolymers P(OB73pip-TFAP)
2.5. Membrane Preparation
2.6. Characterization
2.7. Ion-Exchange Capacity (IEC) of the AEMs
2.8. Water, Electrolyte Uptake and Swelling Ratio
2.9. Ionic Conductivity
2.10. Alkaline Stability
2.11. Alkaline Water Electrolysis Testing
3. Results and Discussion
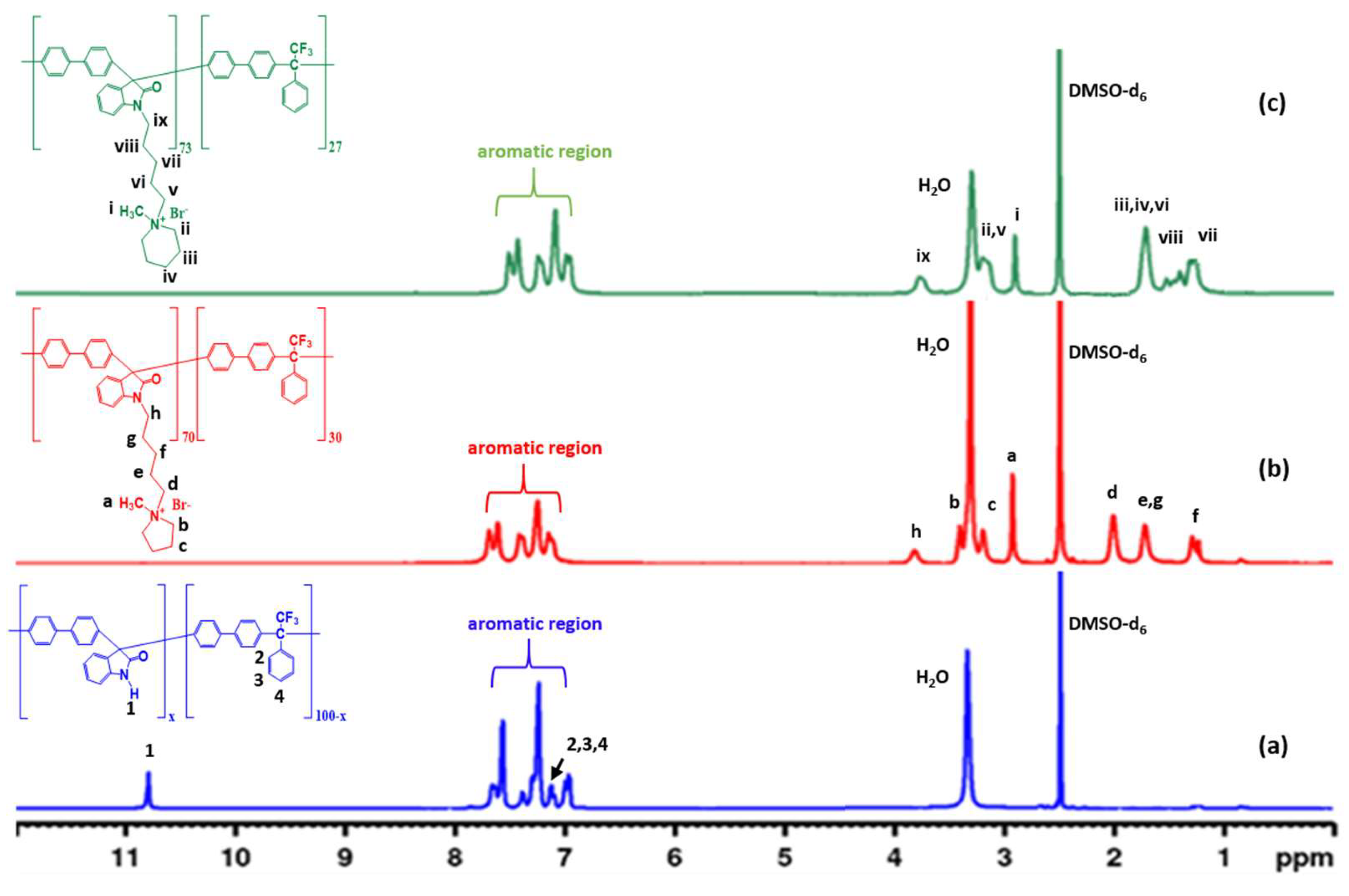
3.1. Synthesis and Characterization of Pyrrolidinium-Functionalized Copolymers P(OB70pyr-TFAP) and Piperidinium-Functionalized Copolymers P(OB73pip-TFAP)
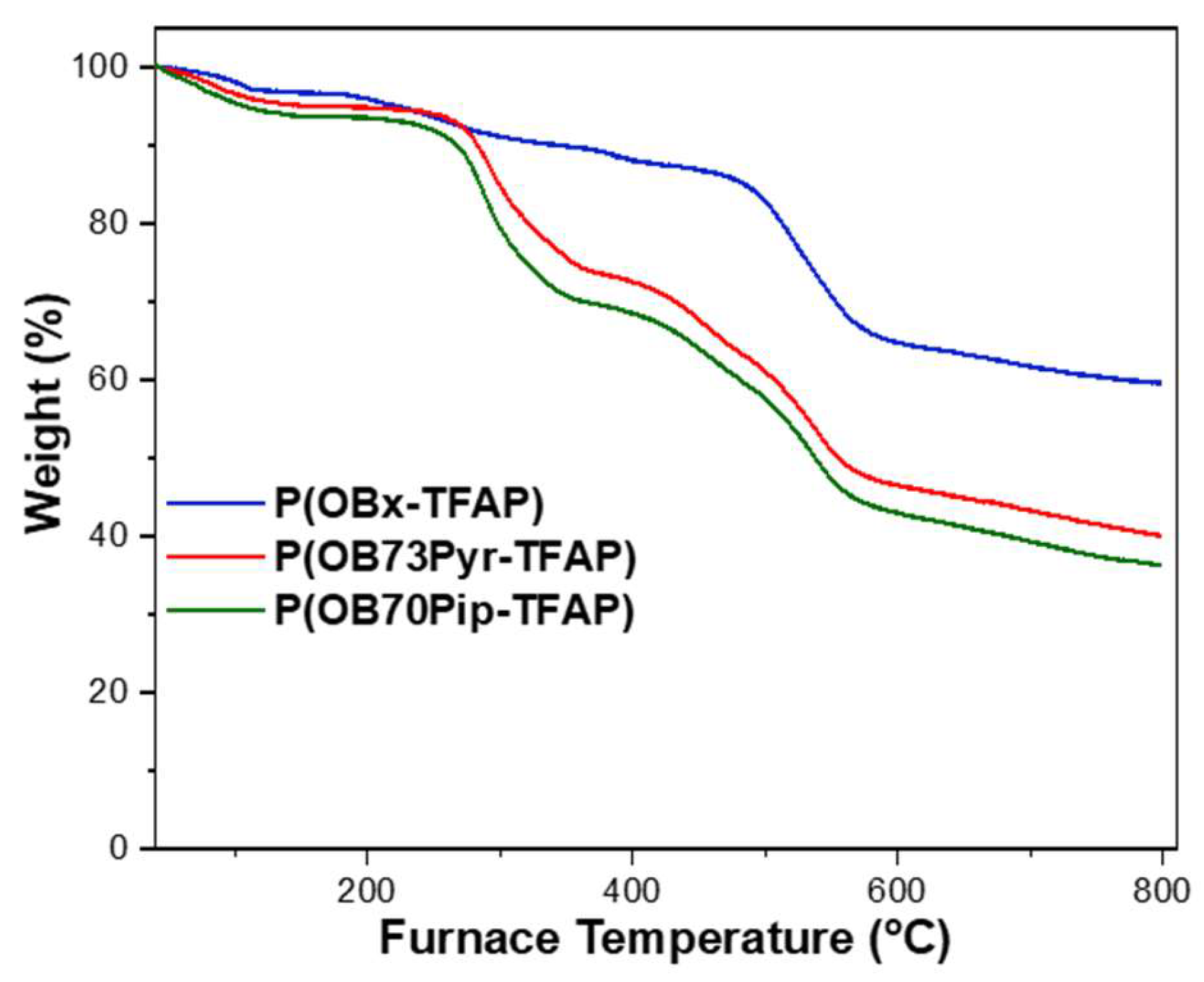
3.2. Thermal Stability
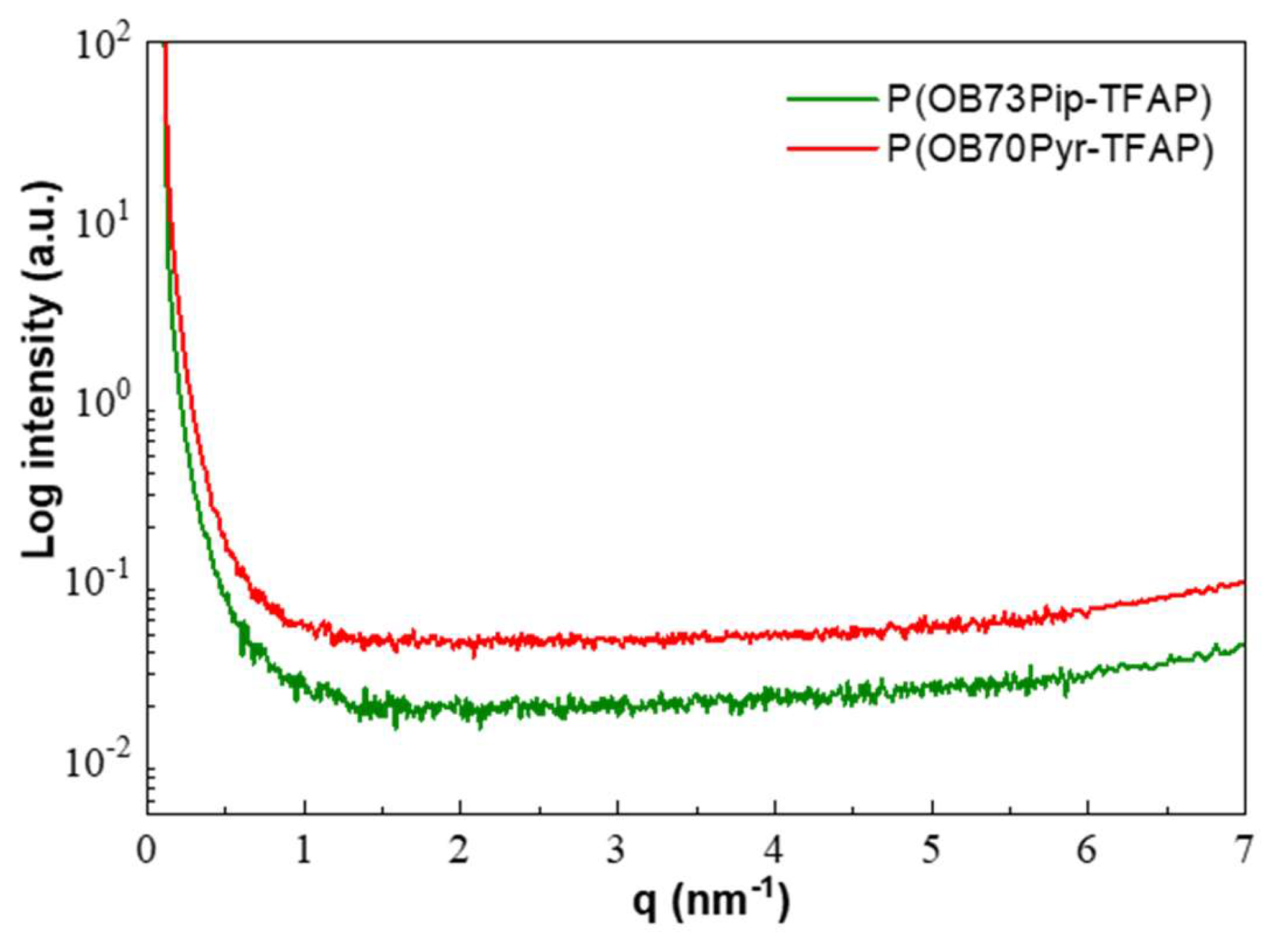
3.3. Morphology
3.4. Water and Electrolyte Uptake and Swelling
3.5. Ionic Conductivity
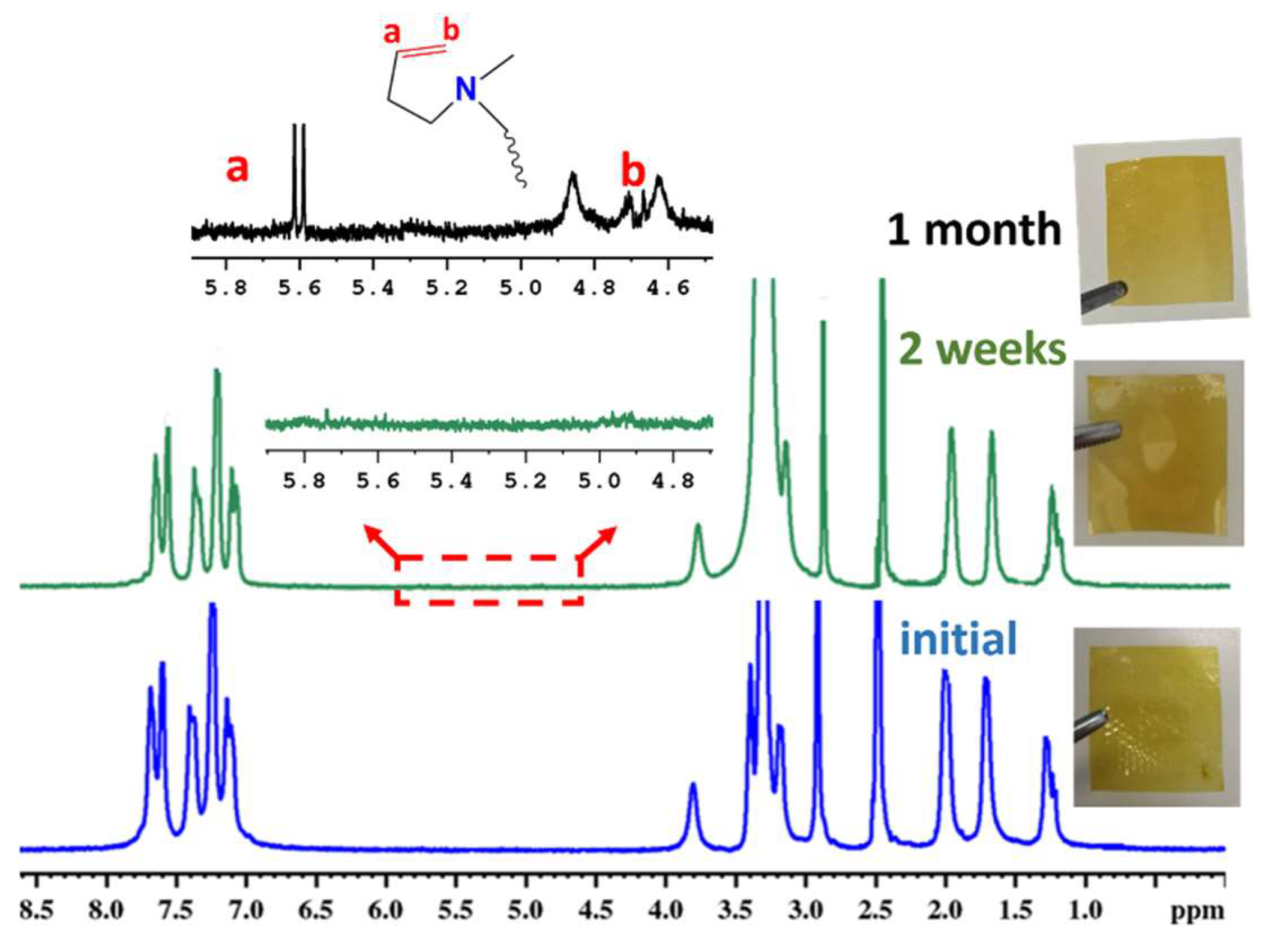
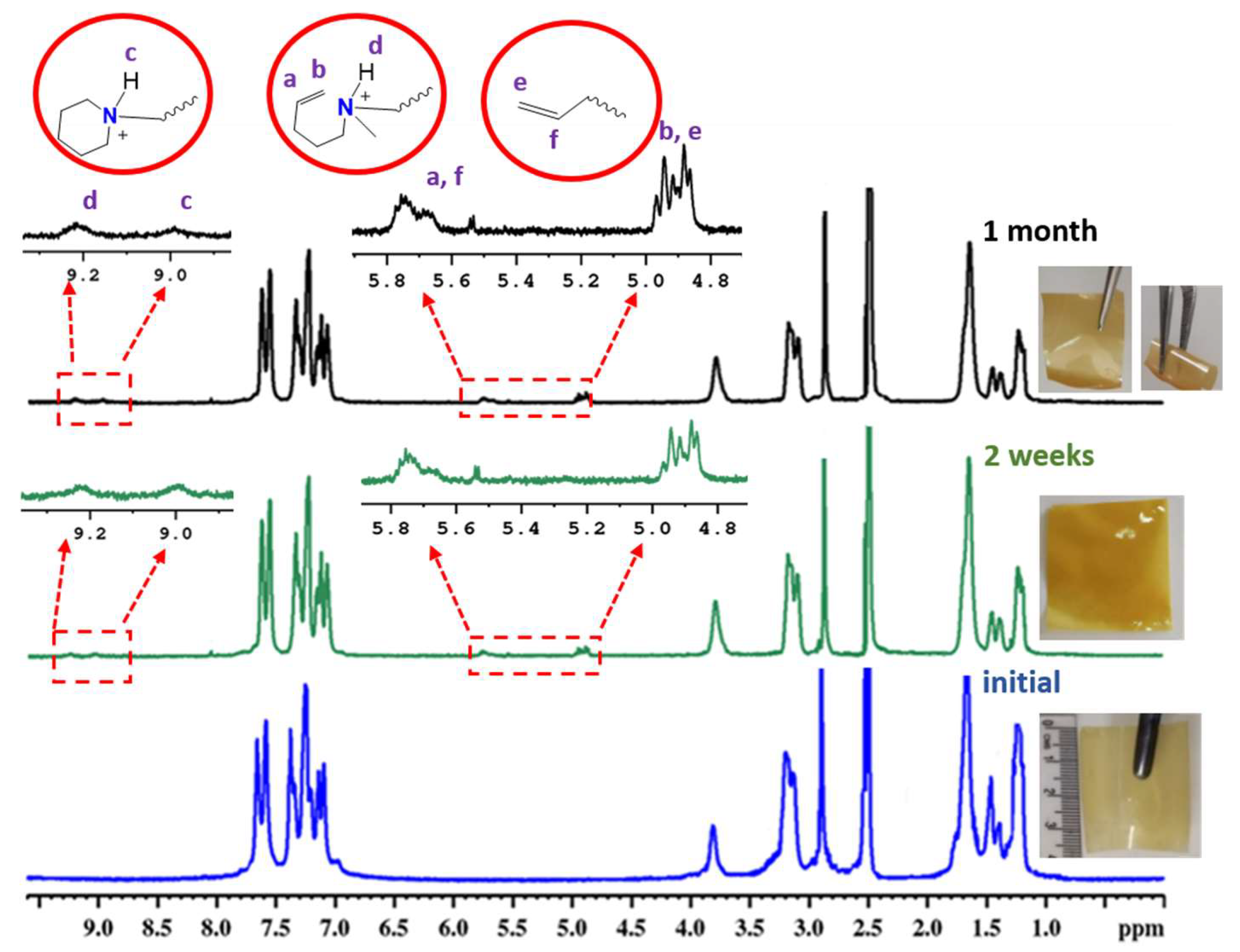
3.6. Alkaline Stability
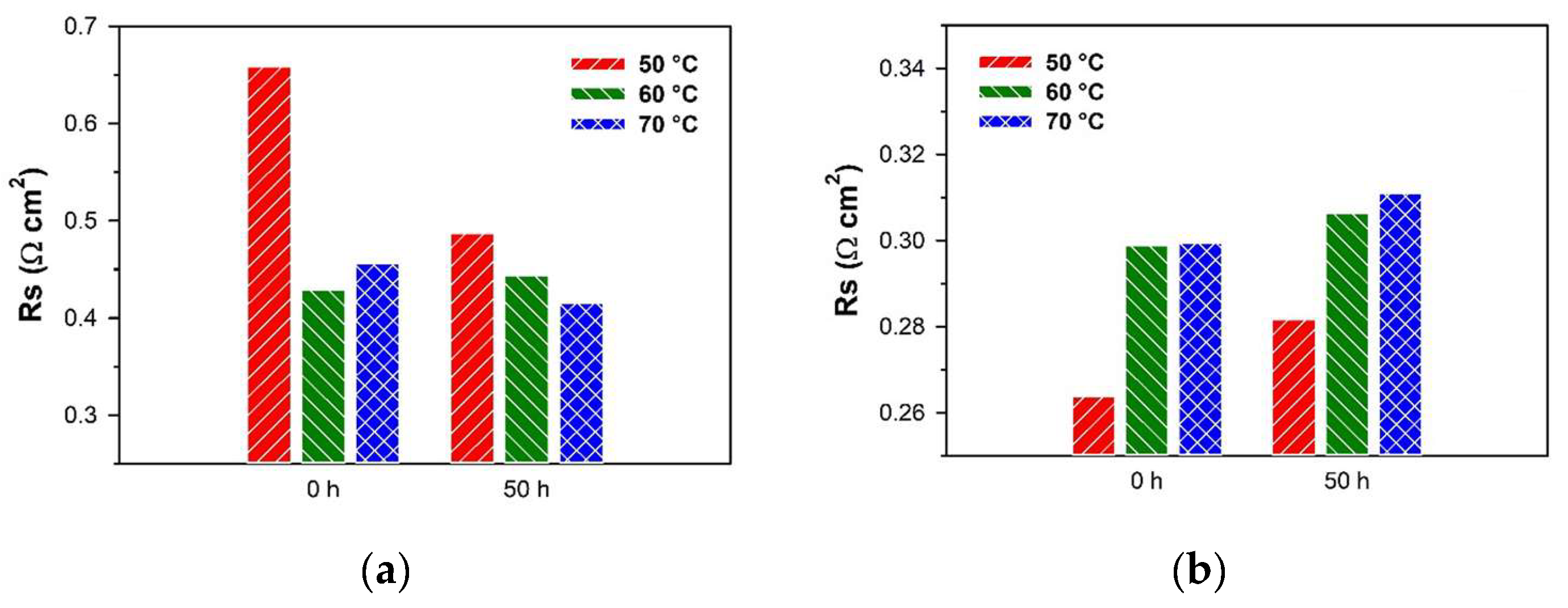
3.7. AEMWE Electrolysis Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pivovar, B.; Rustagi, N.; Satyapal, S. Hydrogen at Scale (H2@Scale): Key to a Clean, Economic, and Sustainable Energy System. Electrochem. Soc. Interface 2018, 27, 47–52. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Li, Q.; Molina Villarino, A.; Peltier, C.R.; Macbeth, A.J.; Yang, Y.; Kim, M.J.; Abruna, H.D. Anion Exchange Membrane Water Electrolysis: The Future of Green Hydrogen. J. Phys. Chem. 2023, 127, 7901–7912. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Park, E.J.; Arges, C.G.; Xu, H.; Kim, Y.S. Membrane strategies for water electrolysis. ACS Energy Lett. 2022, 7, 3447–3457. [Google Scholar] [CrossRef]
- Motz, A.R.; Li, D.; Keane, A.; Manriquez, L.D.; Park, E.J.; Maurya, S.; Chung, H.; Fujimoto, C.; Jeon, J.; Pagels, M.K.; et al. Performance and durability of anion exchange membrane water electrolyzers using down-selected polymer electrolytes. J. Mater. Chem. A 2021, 9, 22670–22683. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Zheng, X.; Sun, W.; Dou, S.X.; Ma, T.; Pan, H. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 2022, 51, 9620–9693. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-exchange membrane water electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef]
- Aili, D.; Kraglund, M.R.; Rajappan, S.C.; Serhiichuk, D.; Xia, Y.; Deimede, V.; Kallitsis, K.; Bae, C.; Jannasch, P.; Henkensmeier, D.; et al. Electrode Separators for the Next-Generation Alkaline Water Electrolyzers. ACS Energy Lett. 2023, 8, 1900–1910. [Google Scholar] [CrossRef]
- Razmjooei, F.; Morawietz, T.; Taghizadeh, E.; Hadjixenophontos, E.; Mues, L.; Gerle, M.; Wood, B.D.; Harms, C.; Gago, A.S.; Ansar, S.A.; et al. Increasing the performance of an anion-exchange membrane electrolyzer operating in pure water with a nickel-based microporous layer. Joule 2021, 5, 1776–1799. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-based electrocatalysts for cost-effective ultrahigh-current-density water splitting in anion exchange membrane electrolyzer cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef]
- Li, D.; Motz, A.R.; Bae, C.; Fujimoto, C.; Yang, G.; Zhang, F.Y.; Kim, Y.S. Durability of anion exchange membrane water electrolyzers. Energy Environ. Sci. 2022, 14, 3393–3419. [Google Scholar] [CrossRef]
- Wan, L.; Xu, Z.; Xu, Q.; Pang, M.; Lin, D.; Liu, J.; Wang, B. Key components and design strategy of the membrane electrode assembly for alkaline water electrolysis. Energy Environ. Sci. 2023, 16, 1384–1430. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Q.; Chen, P.; Zhou, J.; Li, S.; Xu, H.; An, Z. Block poly (arylene ether sulfone) copolymers tethering aromatic side-chain quaternary ammonium as anion exchange membranes. Polym. Chem. 2018, 9, 699–711. [Google Scholar] [CrossRef]
- Liu, L.; Chu, X.; Liao, J.; Huang, Y.; Li, Y.; Ge, Z.; Li, N. Tuning the properties of poly(2,6-dimethyl-1,4-phenylene oxide) anion exchange membranes and their performance in H2/O2 fuel cells. Energy Environ. Sci. 2018, 11, 435–446. [Google Scholar] [CrossRef]
- Liu, H.; Yang, S.; Wang, S.; Fang, J.; Jiang, L.; Sun, G. Preparation and characterization of radiation-grafted poly(tetrafluoroethylene-co-perfluoropropyl vinyl ether) membranes for alkaline anion-exchange membrane fuel cells. J. Membr. Sci. 2011, 369, 277–283. [Google Scholar] [CrossRef]
- Li, N.; Yan, T.; Li, Z.; Thurn-Albrecht, T.; Binder, W.H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 2012, 5, 7888–7892. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.S. Quaternized aryl ether-free polyaromatics for alkaline membrane fuel cells: Synthesis, properties, and performance—A topical review. J. Mater. Chem. 2018, 6, 15456–15477. [Google Scholar] [CrossRef]
- Akiyama, R.; Yokota, N.; Miyatake, K. Chemically stable, highly anion conductive polymers composed of quinquephenylene and pendant ammonium groups. Macromolecules 2019, 52, 2131–2138. [Google Scholar] [CrossRef]
- Bakvand, P.M.; Jannasch, P. Poly(arylene alkylene)s with pendent benzyl-tethered ammonium cations for anion exchange membranes. J. Membr. Sci. 2023, 668, 121229. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, E.J.; Han, J.; Shin, D.W.; Kim, Y.S.; Bae, C. Poly(terphenylene) anion exchange membranes: The effect of backbone structure on morphology and membrane property. ACS Macro Lett. 2017, 6, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Functionalizing Polystyrene with N-Alicyclic Piperidine-Based Cations via Friedel–Crafts Alkylation for Highly Alkali-Stable Anion-Exchange Membranes. Macromolecules 2020, 53, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.G.; Kreuer, K.D. Alkaline stability of quaternary ammonium cations for alkaline fuel cell membranes and ionic liquids. ChemSusChem. 2015, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.S.; Jannasch, P. Exploring different cationic alkyl side chain designs for enhanced alkaline stability and hydroxide ion conductivity of anion-exchange membranes. Macromolecules 2015, 48, 5742–5751. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Liang, X.; Yu, W.; Ge, X.; Shehzad, M.A.; Xu, T. Self-aggregating cationic-chains enable alkaline stable ion-conducting channels for anion-exchange membrane fuel cells. J. Mater. Chem. A. 2021, 9, 327–337. [Google Scholar] [CrossRef]
- Sriram, G.; Dhanabalan, K.; Ajeya, K.V.; Aruchamy, K.; Ching, Y.C.; Oh, T.H.; Kurkuri, M. Recent progress in anion exchange membranes (AEMs) in water electrolysis: Synthesis, physiochemical analysis, properties, and applications. J. Mater. Chem. A 2023, 11, 20886–21008. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Jin, C. Development of high-performance anion exchange membrane fuel cell using poly(isatin biphenylene) with flexible heterocycle quaternary ammonium cations. J. Mater. Chem. A. 2019, 7, 6883–6893. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, L.; Zhang, G.; Li, R.; Hu, X.; Chang, X.; Li, N. Rational design of comb-shaped poly (arylene indole piperidinium) to enhance hydroxide ion transport for H2/O2 fuel cell. J. Membr. Sci. 2021, 631, 119335. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.; Liu, L.; Ju, Q.; Zhou, X.; Qiao, X.; Li, N. Piperidinium functionalized aryl ether-free polyaromatics as anion exchange membrane for water electrolysers: Performance and durability. J. Membr. Sci. 2021, 621, 118964. [Google Scholar] [CrossRef]
- Song, W.; Peng, K.; Xu, W.; Liu, X.; Zhang, H.; Liang, X.; Xu, T. Upscaled production of an ultramicroporous anion-exchange membrane enables long-term operation in electrochemical energy devices. Nat. Commun. 2023, 14, 2732. [Google Scholar] [CrossRef]
- Liu, M.; Hu, X.; Hu, B.; Liu, L.; Li, N. Soluble poly(aryl piperidinium) with extended aromatic segments as anion exchange membranes for alkaline fuel cells and water electrolysis. J. Membr. Sci. 2022, 642, 119966. [Google Scholar] [CrossRef]
- Long, C.; Zhao, T.; Tian, L.; Liu, Q.; Wang, F.; Wang, Z.; Zhu, H. Highly stable and conductive multicationic poly(biphenyl indole) with extender side chains for anion exchange membrane fuel cells. ACS Appl. Energy Mater. 2021, 4, 6154–6165. [Google Scholar] [CrossRef]
- Cha, M.; Park, J.; Kim, S.; Han, S.; Shin, S.; Yang, S.; Kim, T.; Yu, D.; So, S.; Hong, Y.; et al. Poly(carbazole)-Based Anion-Conducting Materials with High Performance and Durability for Energy Conversion Devices. Energy Environ. Sci. 2020, 13, 3633–3645. [Google Scholar] [CrossRef]
- Chen, N.; Paek, S.; Lee, J.; Park, J.; Lee, S.; Lee, Y. High-Performance Anion Exchange Membrane Water Electrolyzers with a Current Density of 7.68 A cm−2 and Durability of 1000 h. Energy Environ. Sci. 2021, 14, 6338–6348. [Google Scholar] [CrossRef]
- Hu, X.; Hu, B.; Liu, M.; Tao, H.; Huang, Y.; Kang, S.; Li, N. An operationally broadened alkaline water electrolyser enabled by highly stable poly (oxindole biphenylene) ion-solvating membranes. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Makrygianni, M.; Aivali, S.; Xia, Y.; Kraglund, M.R.; Aili, D.; Deimede, V. Polyisatin derived ion-solvating blend membranes for alkaline water electrolysis. J. Membr. Sci. 2023, 669, 121331. [Google Scholar] [CrossRef]
- Hossain, I.; Husna, A.; Jeong, I.; Kim, T.H. Biphenyl(isatin-co-trifluoroacetophnone) based copolymers synthesized using the Friedel Crafts Reaction as mechanical robust membranes for Efficient CO2 separation. ACS Appl. Polym. Mater. 2022, 4, 3779–3790. [Google Scholar] [CrossRef]
- Nieto, D.R.; Fomine, S.; Zolotukhin, M.G.; Fomina, L.; Hernandez, M.C.G. Superelectrophilic Activation of N-Substituted Isatins: Implications for Polymer Synthesis, a Theoretical Study. Macromol. Theory Simul. 2009, 18, 138–144. [Google Scholar] [CrossRef]
- Hernandez, M.C.G.; Zolotukhin, M.G.; Fomine, S.; Cedillo, G.; Morales, S.L.; Fröhlich, N.; Ruiz-Trevino, A. Novel, metal-free, superacid-catalyzed “click” reactions of isatins with linear, nonactivated, multiring aromatic hydrocarbons. Macromolecules 2010, 43, 6968–6979. [Google Scholar] [CrossRef]
- Papakonstantinou, P.; Deimede, V. Self-cross-linked quaternary phosphonium based anion exchange membranes: Assessing the influence of quaternary phosphonium groups on alkaline stability. RSC Adv. 2016, 6, 114329–114343. [Google Scholar] [CrossRef]
- Tsagdi, A.; Dimitriou, M.; Druvari, D.; Deimede, V. Blend membranes based on N1-alkyl-substituted imidazolium functionalized polymers and aromatic polyethers: Influence of N1-alkyl substituent on properties and alkaline stability. Polym. Bull. 2022, 79, 1647–1668. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Liu, F.H.; Lin, C.X.; Hu, E.N.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Anion exchange membranes with well-developed conductive channels: Effect of the functional groups. J. Membr. Sci. 2018, 564, 298–307. [Google Scholar] [CrossRef]
- Xu, Z.; Delgado, S.; Atanasov, V.; Morawietz, T.; Gago, A.S.; Friedrich, K.A. Novel Pyrrolidinium-Functionalized Styrene-b-ethylene-b-butylene-b-styrene Copolymer Based Anion Exchange Membrane with Flexible Spacers for Water Electrolysis. Membranes 2023, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, L.; Liu, J.; Su, X.; Yan, X.; Dai, Y.; He, G. Comb-shaped ether-free poly(biphenyl indole) based alkaline membrane. J. Membr. Sci. 2019, 588, 117216. [Google Scholar] [CrossRef]
- Boström, O.; Choi, S.Y.; Xia, L.; Meital, S.; Lohmann-Richters, F.; Jannasch, P. Alkali-stable polybenzimidazole anion exchange membranes tethered with N,N dimethylpiperidinium cations for dilute aqueous KOH fed water electrolyzers. J. Mater. Chem. A 2023, 11, 21170. [Google Scholar] [CrossRef]
- Ponce Gonzalez, J.; Whelligan, D.K.; Wang, L.; Bance-Soualhi, R.; Wang, Y.; Peng, Y.; Peng, H.; Apperley, D.C.; Sarode, H.N.; Pandey, T.P.; et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 2016, 9, 3724–3735. [Google Scholar] [CrossRef]
- Dong, X.; Lv, D.; Zheng, J.; Xue, B.; Bi, W.; Li, S.; Zhang, S. Pyrrolidinium-functionalized poly(arylene ether sulfone)s for anion exchange membranes: Using densely concentrated ionic groups and block design to improve membrane performance. J. Membr. Sci. 2017, 535, 301–311. [Google Scholar] [CrossRef]
- Bai, L.; Ma, L.; Li, L.; Zhang, A.; Yan, X.; Zhang, F.; He, G. Branched, side-chain grafted polyarylpiperidine anion exchange membranes for fuel cell application. ACS Appl. Energy Mater. 2021, 4, 6957–6967. [Google Scholar] [CrossRef]
- Ren, R.; Zhang, S.; Miller, H.A.; Vizza, F.; Varcoe, J.R.; He, Q. Facile preparation of novel cardo Poly(oxindolebiphenylene) with pendent quaternary ammonium by superacid-catalysed polyhydroxyalkylation reaction for anion exchange membranes. J. Membr. Sci. 2019, 591, 117320. [Google Scholar] [CrossRef]
- Allushi, A.; Pham, T.H.; Olsson, J.S.; Jannasch, P. Ether-free polyfluorenes tethered with quinuclidinium cations as hydroxide exchange membranes. J. Mater. Chem. A 2019, 7, 27164–27174. [Google Scholar] [CrossRef]
- Lin, C.X.; Wang, X.Q.; Li, L.; Liu, F.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Triblock copolymer anion exchange membranes bearing alkyl-tethered cycloaliphatic quaternary ammonium-head-groups for fuel cells. J. Power Sources 2017, 365, 282–292. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Tignor, S.E.; Sturgeon, M.R.; Long, H.; Pivovar, B.S.; Bae, C. Thermochemical stability study of alkyl-tethered quaternary ammonium cations for anion exchange membrane fuel cells. J. Electrochem. Soc. 2017, 164, F1279–F1285. [Google Scholar] [CrossRef]






| Water | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Membranes | IEC (mmol g−1) | WU (%) | SRa (%) | SRt (%) | EU (%) a | σ (mS cm−1) a | |||||
| 1HNMR | Titration | 20 °C | 80 °C | 20 °C | 80 °C | 20 °C | 80 °C | 20 °C | 80 °C | 80 °C | |
| P(OB70pyr-TFAP) | 1.54 | 1.28 | 10 | 24 | 8 | 11 | 4.5 | 14 | 24 | 58 | 81.5 |
| P(OB73pip-TFAP) | 1.55 | 1.34 | 35 | 48 | 16.5 | 28 | 4.5 | 29 | 32 | 55 | 120.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gjoshi, S.; Loukopoulou, P.; Plevova, M.; Hnat, J.; Bouzek, K.; Deimede, V. Cycloaliphatic Quaternary Ammonium Functionalized Poly(oxindole biphenyl) Based Anion-Exchange Membranes for Water Electrolysis: Stability and Performance. Polymers 2024, 16, 99. https://doi.org/10.3390/polym16010099
Gjoshi S, Loukopoulou P, Plevova M, Hnat J, Bouzek K, Deimede V. Cycloaliphatic Quaternary Ammonium Functionalized Poly(oxindole biphenyl) Based Anion-Exchange Membranes for Water Electrolysis: Stability and Performance. Polymers. 2024; 16(1):99. https://doi.org/10.3390/polym16010099
Chicago/Turabian StyleGjoshi, Sara, Paraskevi Loukopoulou, Michaela Plevova, Jaromir Hnat, Karel Bouzek, and Valadoula Deimede. 2024. "Cycloaliphatic Quaternary Ammonium Functionalized Poly(oxindole biphenyl) Based Anion-Exchange Membranes for Water Electrolysis: Stability and Performance" Polymers 16, no. 1: 99. https://doi.org/10.3390/polym16010099
APA StyleGjoshi, S., Loukopoulou, P., Plevova, M., Hnat, J., Bouzek, K., & Deimede, V. (2024). Cycloaliphatic Quaternary Ammonium Functionalized Poly(oxindole biphenyl) Based Anion-Exchange Membranes for Water Electrolysis: Stability and Performance. Polymers, 16(1), 99. https://doi.org/10.3390/polym16010099












