Durable Surface Modification of Low-Density Polyethylene/Nano-Silica Composite Films with Bacterial Antifouling and Liquid-Repelling Properties for Food Hygiene and Safety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SRS
2.2. Fabrication of SRS-Embedded LDPE Films
2.3. Physical and Chemical Surface Characterization of the LDPE Films
2.4. Bacteria Cultivation
2.5. Inoculation of Culture Media on the LDPE Film Surfaces
2.6. Bacterial Adhesion Assay on the LDPE Film Surfaces
2.7. Evaluation of Antifouling Properties of the LDPE Film Surfaces against Contaminants
2.8. Mechanical Durability Test of the LDPE Films
2.9. Statistical Analysis for Analyzing Bacterial Enumeration
3. Results and Discussion
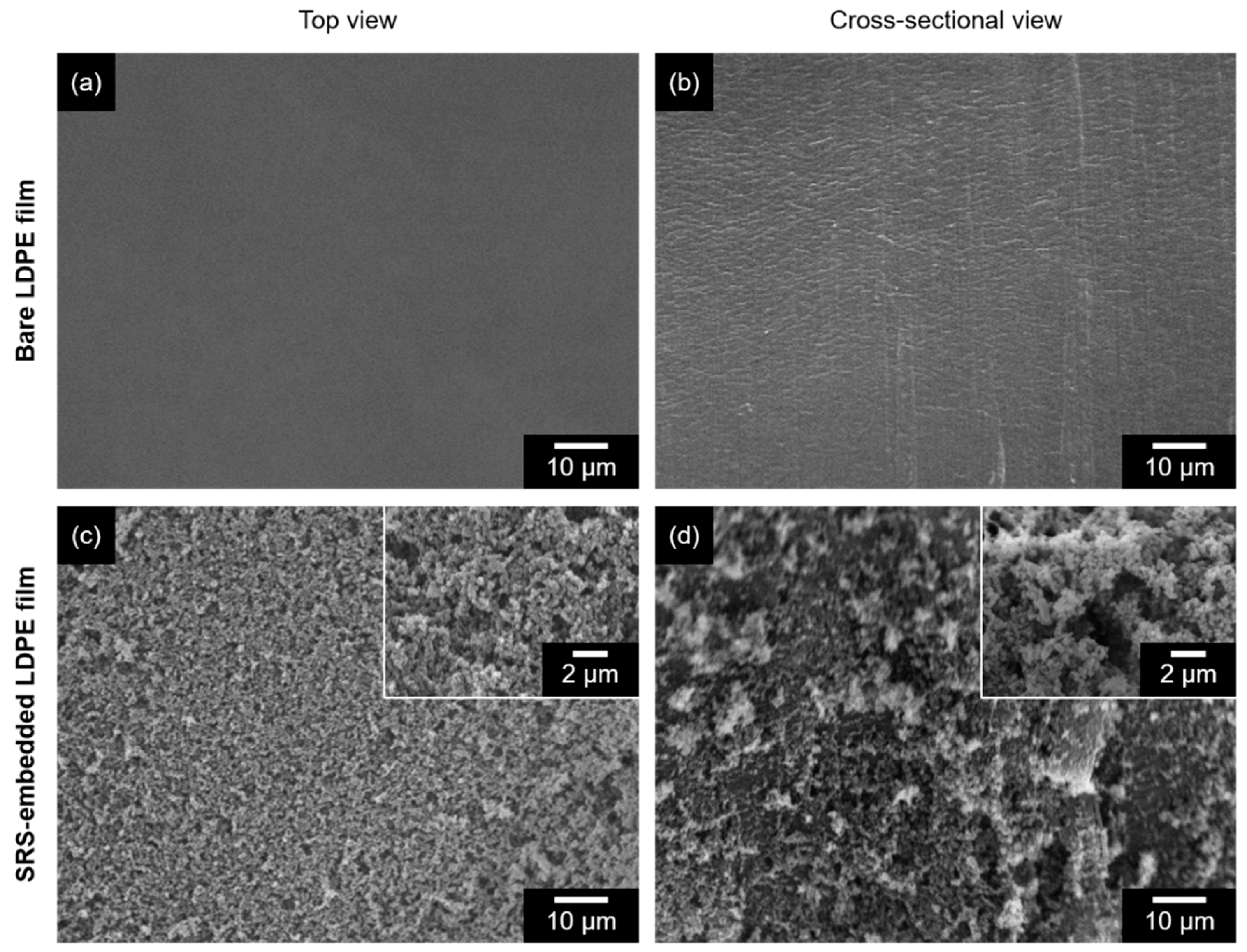
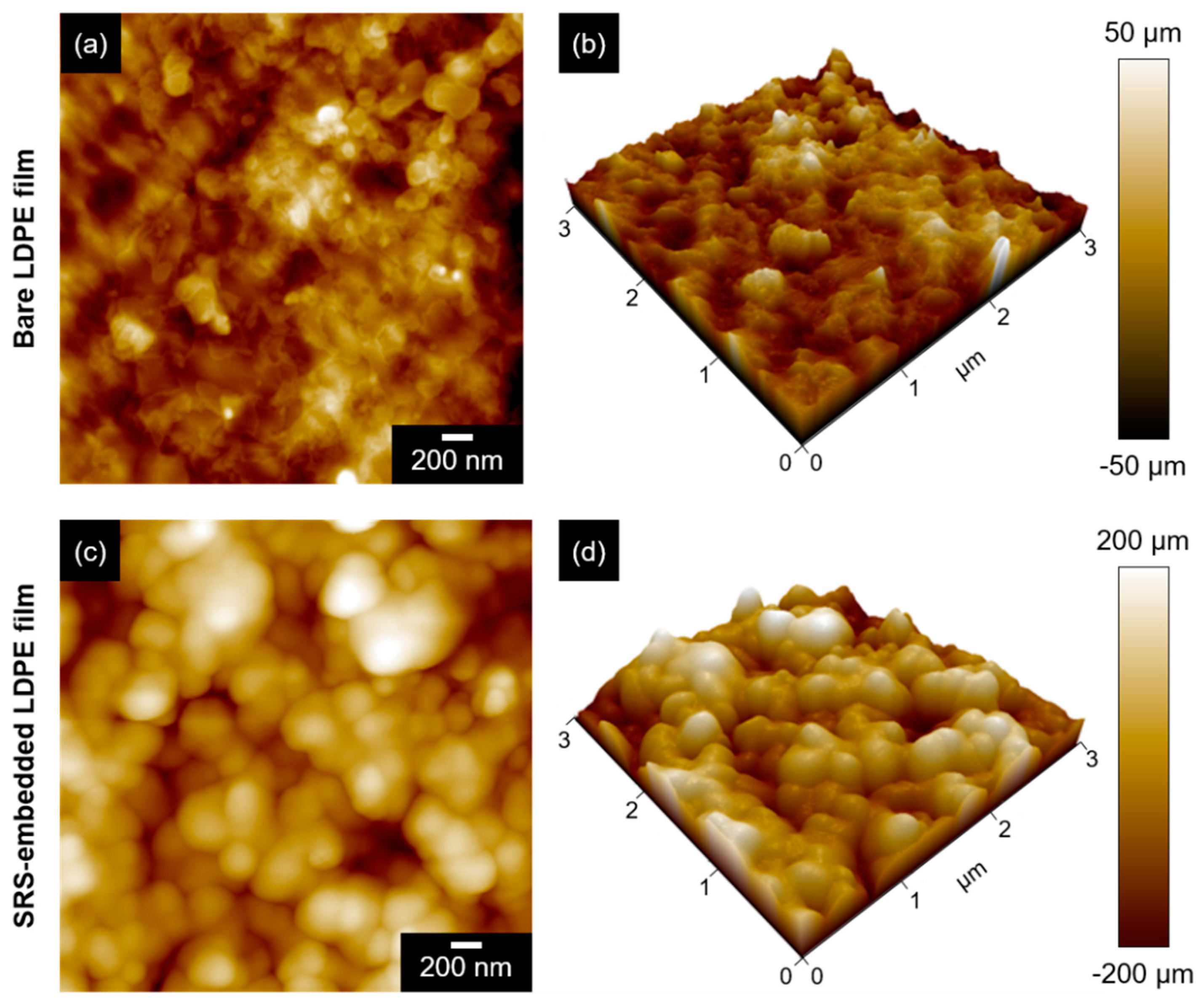
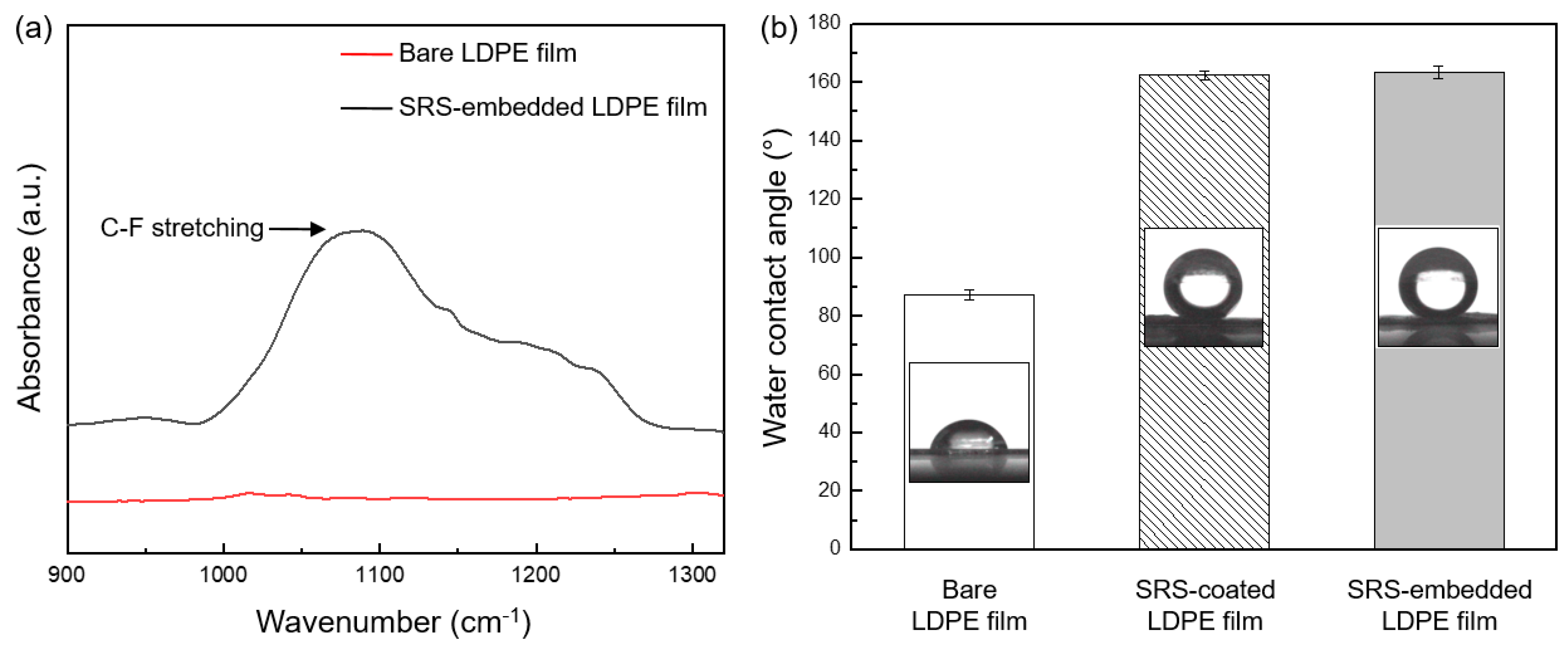
3.1. Characterization of SRS-Embedded LDPE Films
3.2. Bacterial Attachment to SRS-Embedded LDPE Films Characterized via Agar Plate Counting
3.3. Liquid-Repelling Properties of SRS-Embedded LDPE Films
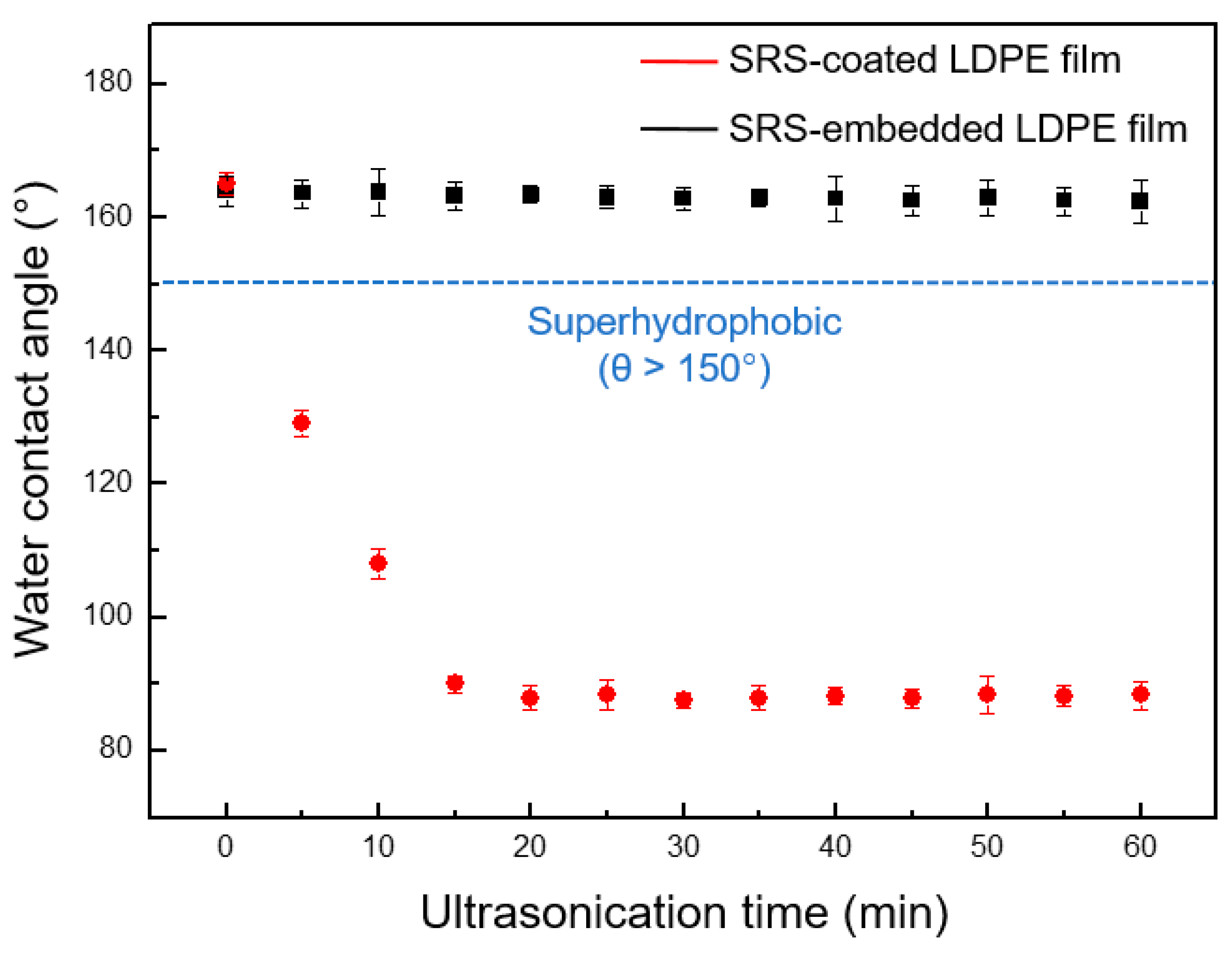
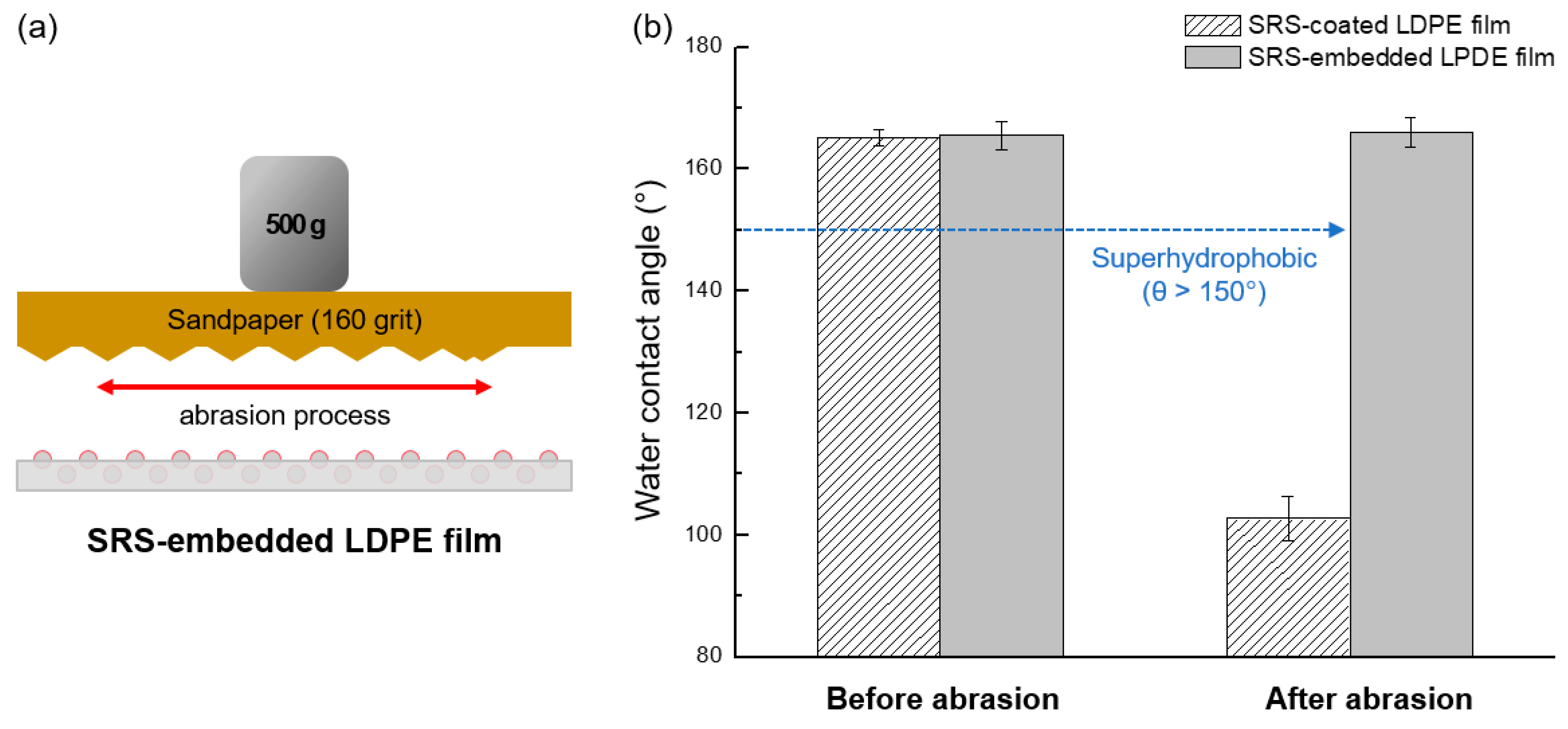
3.4. Mechanical Durability of SRS-Embedded LDPE Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFlorio, W.; Liu, S.; White, A.R.; Taylor, T.M.; Cisneros-Zevallos, L.; Min, Y.; Scholar, E.M.A. Recent developments in antimicrobial and antifouling coatings to reduce or prevent contamination and cross-contamination of food contact surfaces by bacteria. Compr. Rev. Food. Sci. Food Saf. 2021, 20, 3093–3134. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Sarker, M.R.; Hossain, A. Microbiological food safety: A dilemma of developing societies. Crit. Rev. Microbiol. 2014, 40, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.V. Foodborne diseases–disease burden. In Food Safety in the 21st Century, 1st ed.; Gupta, R.K., Dudeja, P., Minhas, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–10. [Google Scholar]
- Fleetwood, J.; Rahman, S.; Holland, D.; Millson, D.; Thomson, L.; Poppy, G. As clean as they look? Food hygiene inspection scores, microbiological contamination, and foodborne illness. Food Control 2019, 96, 76–86. [Google Scholar] [CrossRef]
- Gourama, H. Foodborne Pathogens. In Food Safety Engineering, 1st ed.; Demirci, A., Feng, H., Krishnamurthy, K., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 25–49. [Google Scholar]
- Thakali, A.; MacRae, J.D. A review of chemical and microbial contamination in food: What are the threats to a circular food system? Environ. Res. 2023, 136, 108305. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (bio)control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef]
- Hannon, J.C.; Kerry, J.; Cruz-Romero, M.; Morris, M.; Cummins, E. Advances and challenges for the use of engineered nanoparticles in food contact materials. Trends Food Sci. Technol. 2015, 43, 43–62. [Google Scholar] [CrossRef]
- Precedence Research. Available online: https://www.precedenceresearch.com/food-packaging-market (accessed on 10 November 2023).
- Fortune Business Insights. Available online: https://www.fortunebusinessinsights.com/food-contact-paper-market-104905 (accessed on 12 January 2024).
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 2015, 3, 54. [Google Scholar] [CrossRef]
- Bruna, J.E.; Galotto, M.J.; Guarda, A.; Rodríguez, F. A novel polymer based on MtCu2+/cellulose acetate with antimicrobial activity. Carbohydr. Polym. 2014, 102, 317–323. [Google Scholar] [CrossRef]
- Lopes, J.A.; Tsochatzis, E.D. Poly(ethylene terephthalate), poly(butylene terephthalate), and polystyrene oligomers: Occurrence and analysis in food contact materials and food. J. Agric. Food Chem. 2023, 71, 2244–2258. [Google Scholar] [CrossRef]
- Sharma, S.; Li, B.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Food contact surfaces: Challenges, legislation and solutions. Food Rev. Int. 2023, 39, 1086–1109. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.-M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Habib, S.; Lehocky, M.; Vesela, D.; Humpolíček, P.; Krupa, I.; Popelka, A. Preparation of progressive antibacterial LDPE surface via active biomolecule deposition approach. Polymers 2019, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, D.; Kim, J.; Oh, J.K.; Castaneda, H.; Kim, J.H. Antimicrobial activities of thermoplastic polyurethane/clay nanocomposites against pathogenic bacteria. ACS Appl. Bio Mater. 2020, 3, 6672–6679. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, X.; Zhou, N.; Li, J.; Liu, J.; Yue, J.; Hao, X.; Gan, M.; Lin, P.; Shang, X. Chemical characterization of the polar antibacterial fraction of the ethanol extract from Rosmarinus officinalis. Food Chem. 2021, 344, 128674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Zhang, L.-F.; Hu, Q.-P.; Hao, D.-L.; Xu, J.-G. Chemical composition, antibacterial activity of Cyperus rotundus rhizomes essential oil against Staphylococcus aureus via membrane disruption and apoptosis pathway. Food Control 2017, 80, 290–296. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus Var. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- He, F.; Wang, W.; Wu, M.; Fang, Y.; Wang, S.; Yang, Y.; Yang, Y.; Ye, C.; Xiang, F. Antioxidant and antibacterial activities of essential oil from Atractylodes lancea rhizomes. Ind. Crops Prod. 2020, 153, 112552. [Google Scholar] [CrossRef]
- Söz, C.K.; Yilgör, E.; Yilgör, I. Simple processes for the preparation of superhydrophobic polymer surfaces. Polymer 2016, 99, 580–593. [Google Scholar] [CrossRef]
- Lupoi, R.; Stenson, C.; McDonnell, K.A.; Dowling, D.P.; Ahearne, E. Antifouling coatings made with cold spray onto polymers: Process characterization. CIRP Ann.-Manuf. Technol. 2016, 65, 545–548. [Google Scholar] [CrossRef]
- Geissler, A.; Chen, L.; Zhang, K.; Bonaccurso, E.; Biesalski, M. Superhydrophobic surfaces fabricated from nano- and microstructured cellulose stearoyl esters. Chem. Commun. 2013, 49, 4962–4964. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Mérian, T.; Goddard, J.M. Advances in nonfouling materials: Perspectives for the food industry. J. Agric. Food Chem. 2012, 60, 2943–2957. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Rapisand, W.; Zhang, M.; Yegin, Y.; Min, Y.; Castillo, A.; Cisneros-Zevallos, L.; Akbulut, M. Surface modification of food processing and handling gloves for enhanced food safety and hygiene. J. Food Eng. 2016, 187, 82–91. [Google Scholar] [CrossRef]
- Jiang, H.; Wei, Y.; Cheng, Q.; Zhu, Z. Scratch behavior of low density polyethylene film: Effects of pre-stretch and aging. Mater. Des. 2018, 157, 235–243. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, J.; Hao, L.; Yegin, Y.; Bae, M.; Ulugun, B.; Taylor, T.M.; Scholar, E.A.; Cisneros-Zevallos, L.; Oh, J.K.; et al. Dual-functional, superhydrophobic coatings with bacterial anticontact and antimicrobial characteristics. ACS Appl. Mater. Interfaces 2020, 12, 21311–21321. [Google Scholar] [CrossRef]
- Lakhotia, S.R.; Mukhopadhyay, M.; Kumari, P. Cerium oxide nanoparticles embedded thin-film nanocomposite nanofiltration membrane for water treatment. Sci. Rep. 2018, 8, 4976. [Google Scholar] [CrossRef]
- Polisetti, V.; Ray, P. Nano SiO2 and TiO2 embedded polyacrylonitrile/polyvinylidene fluoride ultrafiltration membranes: Improvement in flux and antifouling properties. J. Appl. Polym. Sci. 2021, 138, 49606. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar]
- Kansara, A.M.; Prajapati, P.K.; Aswal, V.K.; Singh, P.S. Structure-property interplay of asymmetric membranes comprising of soft polydimethylsiloxane chains and hard silica nanomaterials. Polymer 2019, 160, 30–42. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, S.; DeFlorio, W.; Song, S.H.; Choi, H.; Cisneros-Zevallos, L.; Oh, J.K.; Akbulut, M.E.S. Nanostructured antifouling coatings for galvanized steel food storage and container surfaces to enhance hygiene and corrosion resistance against bacterial, fungal, and mud contamination. J. Food Eng. 2024, 363, 111784. [Google Scholar] [CrossRef]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 234. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Ávila-Sierra, A.; Zhang, Z.J.; Fryer, P.J. Effect of surface roughness and temperature on stainless steel—Whey protein interfacial interactions under pasteurisation conditions. J. Food Eng. 2021, 301, 110542. [Google Scholar] [CrossRef]
- Iacono, S.T.; Jennings, A.R. Recent studies on fluorinated silica nanometer-sized particles. Nanomaterials 2019, 9, 684. [Google Scholar] [CrossRef]
- Grainger, D.W.; Stewart, C.W. Fluorinated coatings and films: Motivation and significance. In Fluorinated Surfaces, Coatings, and Films, 1st ed.; Castner, D.G., Grainger, D.W., Eds.; American Chemical Society: Washington, DC, USA, 2001; pp. 1–14. [Google Scholar]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Zeitler, V.A.; Brown, C.A. The infrared spectra of some Ti-O-Si, Ti-O-Ti and Si-O-Si compounds. J. Phys. Chem. 1957, 61, 1174–1177. [Google Scholar] [CrossRef]
- DeMeester, K.E.; Liang, H.; Jensen, M.R.; Jones, Z.S.; D’Ambrosio, E.A.; Scinto, S.L.; Zhou, J.; Grimes, C.L. Synthesis of functionalized N-acetyl muramic acids to probe bacterial cell wall recycling and biosynthesis. J. Am. Chem. Soc. 2018, 140, 9458–9465. [Google Scholar] [CrossRef] [PubMed]
- Reith, J.; Mayer, C. Characterization of a glucosamine/glucosaminide N-acetyltransferase of Clostridium acetobutylicum. J. Bacteriol. 2011, 193, 5393–5399. [Google Scholar] [CrossRef] [PubMed]
- Law, K.-Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.S.; Koohmaraie, M. Cell surface charge characteristics and their relationship to bacterial attachment to meat surfaces. Appl. Environ. Microbiol. 1989, 55, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Lu, X.; Min, Y.; Cisneros-Zevallos, L.; Akbulut, M. Bacterially antiadhesive, optically transparent surfaces inspired from rice leaves. ACS Appl. Mater. Interfaces 2015, 7, 19274–19281. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, G.; Wang, S. Air-grid surface patterning provided by superhydrophobic surfaces. Small 2012, 8, 962–965. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. Apmis 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef]
- Kyzer, J.L.; Martens, M. Metabolism and toxicity of fluorine compounds. Chem. Res. Toxicol. 2021, 34, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, S.A.; Parale, V.; Vhatkara, R.S.; Mahadik, D.B.; Kavale, M.S.; Wagh, P.B.; Gupta, S.C.; Gurav, J.L. Superhydrophobic silica coating by dip coating method. Appl. Surf. Sci. 2013, 277, 62–72. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.H.; Bae, M.; Oh, J.K. Durable Surface Modification of Low-Density Polyethylene/Nano-Silica Composite Films with Bacterial Antifouling and Liquid-Repelling Properties for Food Hygiene and Safety. Polymers 2024, 16, 292. https://doi.org/10.3390/polym16020292
Song SH, Bae M, Oh JK. Durable Surface Modification of Low-Density Polyethylene/Nano-Silica Composite Films with Bacterial Antifouling and Liquid-Repelling Properties for Food Hygiene and Safety. Polymers. 2024; 16(2):292. https://doi.org/10.3390/polym16020292
Chicago/Turabian StyleSong, Sang Ha, Michael Bae, and Jun Kyun Oh. 2024. "Durable Surface Modification of Low-Density Polyethylene/Nano-Silica Composite Films with Bacterial Antifouling and Liquid-Repelling Properties for Food Hygiene and Safety" Polymers 16, no. 2: 292. https://doi.org/10.3390/polym16020292
APA StyleSong, S. H., Bae, M., & Oh, J. K. (2024). Durable Surface Modification of Low-Density Polyethylene/Nano-Silica Composite Films with Bacterial Antifouling and Liquid-Repelling Properties for Food Hygiene and Safety. Polymers, 16(2), 292. https://doi.org/10.3390/polym16020292





