Advancements in Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering
Abstract
:1. Introduction
| Section | Subsections |
| The Corneal Endothelium | - Structure and Function - Primary Endothelial Disorders - Secondary Endothelial Disorders |
| Corneal Transplantation Techniques | - Penetrating Keratoplasty (PK) - Endothelial Keratoplasty (EK) - DSAEK and DMEK - Bioengineered Corneas |
| Essential Properties of Polymer Biomaterials for Corneal Endothelial Implants | - Descemet’s Membrane (DM) Characteristics - Key Scaffold Properties |
| Polymer Biomaterials for Corneal Endothelium Tissue Engineering | - Natural Tissue Substrates - Natural and Semi-Synthetic Polymers - Synthetic Polymers |
| Micro- and Nano-Topological Morphologies | - Importance of Surface Morphology - Techniques for Creating Structures |
| Emerging Technologies and Innovation | - Peptide and Electroconductive Hydrogels - 3D and 4D Bioprinting - Scaffolds with Drug Delivery Systems |
| Challenges and Future Directions | - Current Limitations - Areas for Further Research - Need for Clinical Translation |
2. The Corneal Endothelium
Bioengineered Corneas: An Urgent Solution to the Donor Shortage
3. Essential Properties of Polymer Biomaterials for Corneal Endothelial Implants
3.1. Optical Clarity and Transparency
3.2. Biocompatibility and Biodegradibility
3.3. Mechanical Properties and Stability
3.4. Permeability and Nutrient Transport
3.5. Surface Wettability and Hydrophilic/Hydrophobic Nature
3.6. Ability of the Scaffold to Maintain the Differentiated State of CECs
4. Polymer Biomaterials for Corneal Endothelium Tissue Engineering
4.1. Natural Tissue Substrates
4.2. Polymer Substrates
4.3. Natural and Semi-Synthetic Polymers
4.4. Synthetic Polymers
| Material | Polymer Type | Study Type | Results | Year | Reference |
|---|---|---|---|---|---|
| Curcumin-loaded lipid-PLGA hybrid microparticles/gelatin (Cur@MP/gelatin) | Semi-synthetic | In vitro culture of rabbit CECs | Cur@MP demonstrated anti-inflammatory, anti-oxidative, and anti-angiogenic properties. Cur@MP/gelatin scaffold supported formation of CEC monolayer [103,104]. | 2021 | [73] |
| Non-mulberry silk fibroin | Natural | In vitro culture of human CECs | Good tensile strength, >95 light transmittance and refractive index. Better cell adhesion and monolayer formation on non-mulberry variations. | 2020 | [80] |
| Curcumin-enhanced silk fibroin | Natural | In vitro culture of rabbit CECs | Rough surface of the scaffold promoted cell adhesion and proliferation. Good hydrophilicity and transparency. | 2021 | [81] |
| Injectable magnetic hyaluronic acid gel | Natural | In vitro and in vivo assessment in a rabbit model | Precise cell delivery and retention in vivo. | 2024 | [86] |
| Chitosan and polycaprolactone scaffold with chitosan nanoparticles | Semi-synthetic | In vitro culture of human CECs | Improved biocompatibility and surface properties with maintained transparency. | 2021 | [90] |
| Agarose modified with GRGD, lysine, poly lysine, and fish-derived gelatin | Natural | In vitro testing with human and rabbit CECs | Fish-derived gelatin scaffolds had the best results with viable rabbit CECs for 4 weeks, better cell attachment, and >96% transparency. | 2019 | [91] |
| Alginate hydrogel with human fibroblast-derived ECM | Natural | In vitro transplantation of human CECs into a decellularized porcine cornea | Suitable microenvironment for cell attachment and growth. | 2024 | [92] |
| Polycaprolactone (PCL), PCL/collagen, PCL/gelatin, and PCL/chitosan | Synthetic and semi-synthetic | In vitro testing with human CECs | PCL/collagen and PCL/gelatin yielded the best cell viability. | 2021 | [100] |
| Poly-ε-lysine hydrogels | Synthetic | In vitro cell culture of human and porcine CECs | Good attachment and growth of human CECs but detachment of porcine CECs. | 2019 | [101] |
5. Micro- and Nano-Topological Morphologies
5.1. Importance of Surface Morphology in Cell Behavior
| Material | Topographical Property Features | Cell Type Affected | Effect of Added Topographical Feature | Key Applications | Fabrication Technique Used | Key | Reference |
|---|---|---|---|---|---|---|---|
| Silicone and collagen | Hexagonal structures, 1.52 to 2.02 µm depth, 10–20 µm width | Human mesenchymal stem cells (hMSCs) | Differentiation of hMSCs into corneal endothelial-like cells | Corneal endothelium tissue engineering, potential autologous stem cell therapy | Two-photon lithography | 2019 | [107] |
| Tissue culture polystyrene (TCPS) | 1 μm pillars, 1 μm wells, and 250 nm pillars; FNC coating containing fibronectin, collagen I, and albumin | Human corneal endothelial cells (HCECs) | Enhanced proliferation, maintenance of functional markers like ZO-1 and Na+/K+-ATPase | Corneal endothelium tissue engineering, cell therapy, and drug screening | Heat embossing | 2015 | [109] |
| PDMS (polydimethylsiloxane) | 1 μm pillars, 1 μm wells, 250 nm pillars, and 250 nm wells | Bovine corneal endothelial cells (BCECs) | Enhanced cell density on pillars, maintenance of functional markers like Na+/K+-ATPase | Corneal endothelium tissue engineering and drug screening | Soft lithography | 2012 | [110] |
| PDMS (polydimethylsiloxane) | Nanopillars: 250 nm diameter, 250 nm height; micropillars: 1 µm diameter, 1 µm height; FNC coating containing fibronectin, collagen I, laminin, and chondroitin sulfate | Human corneal endothelial cell line B4G12 (HCEC-B4G12) | Increased cell proliferation; improved Na+/K+-ATPase and ZO-1 expression | Corneal endothelium tissue engineering | Soft lithography | 2014 | [111] |
| Gelatin methacrylate (GelMA) | 1 μm pillars with 6 μm spacing (1 × 6 μmpS pillars); 250 nm pillars | Human corneal endothelial cells (HCECs) | Enhanced cell adhesion and mechanical strength, customizable degradation rates, increased amount of ZO-1 expression for 1 × 6 μmpS pillars | Corneal endothelium tissue engineering | -Hybrid crosslinking method (which combines physical crosslinking followed by UV crosslinking to improve the material’s mechanical strength) -PET stamp-based nano-molding for high-resolution patterns | 2017 | [112] |
| Silk nanofibrils (SNF) and gelatin methacryloyl (GelMA) | Nanoscale fibrillar structures with 30/70 volume ratio of SNF/GelMA | Human corneal stromal cells | Enhanced transparency, mechanical strength, cell attachment, spreading, and proliferation with customizable degradation rates | Cornea regeneration | UV crosslinking for GelMA; calcium chloride–formic acid dissolution and stirring for nanofibril formation. Casting followed by UV crosslinking for fabrication of SNF/GelMA hybrid films. | 2021 | [75] |
| Acrylated star-shaped poly(ethylene oxide-stat-propylene oxide) (Acr-sP(EO-stat-PO)) hydrogels | Micrometer-sized surface patterns (posts and grooves). Groove width: 10 μm, depth: 5 μm (grooves separated by 20 μm space). Wider pattern: 25 μm wide grooves, 10 μm depth (separated by 25 μm spaces) | Mouse fibroblast cell line (L929) and human mesenchymal stem cells (hMSC) | Induced vitronectin (VN) adsorption and strong cell adhesion, alignment, and spreading | Use topographic patterning to promote cell adhesion even on non-adhesive materials without additional surface chemistry modifications | UV-based imprinting | 2011 | [122] |
| Star-shaped poly (ethylene glycol) | Posts: 3 µm diameter, 3 µm height; lines: 5–50 µm spacing, 5–50 µm width, 5 µm depth | Mouse fibroblast (L929) | Enhanced cell adhesion and spreading. Posts: cells spread, wrapped around posts. Lines: cells aligned along grooves, best alignment in 5–10 µm grooves. | Use topographic patterning to promote cell adhesion even on non-adhesive materials without additional surface chemistry modifications | Nanoimprinting and replica molding | 2009 | [123] |
| Poly(vinyl alcohol) (PVA) hydrogel | Gratings, pillars, convex lens, concave lens (gratings: 250 nm, 10 μm, 2 μm; pillars: 10 μm, 2 μm; convex lens: 10 μm, 2 μm, 1.8 μm; concave lens: 1.8 μm) | Human endothelial cells (EA.hy926) | The 2 μm gratings on PVA hydrogels were found to increase hydrophobicity and were the most effective in promoting endothelial cell adhesion and density. Convex and concave lenses also performed well but were slightly less effective than gratings. Pillars were moderately effective and were the least optimal. | Corneal endothelium tissue engineering | Casting method (for the creation of micro-sized features), nanoimprint lithography (for the creation of nano-sized features), and nitrogen plasma modification | 2016 | [113] |
| PHEMA hydrogels | Lotus leaf topography (3 ± 1 µm height, 9 ± 2 µm width) | Human corneal epithelial cells (HCE-T) | Enhanced cell adhesion and proliferation, increased hydrophobicity with static contact angle of 86 ± 2°, and the presence of trapped air pockets. | Tissue engineering, especially in applications requiring enhanced cell adhesion and hydrophobic surfaces | Polymerization in mold-nanoimprint lithography (PIM-NIL), which involves the use of Teflon AF molds to capture the hierarchical structure of the lotus leaf, both at the micro- and nanoscale, and then polymerizing PHEMA within the mold to create a structured hydrogel. | 2016 | [142] |
| PHEMA (poly(hydroxyethyl methacrylate)) | Pillar structures (aspect ratio up to 100:1, 30 µm diameter, 1 mm height, 50–500 µm spacing) | Not specified | Microstructured surfaces showed higher contact angles compared to smooth surfaces, indicating increased hydrophobicity | Biomimetic surfaces and hydrophobic surface design | Combination of molding and radical polymerization | 2005 | [143] |
| Collagen films | Groove widths of 25 µm, 50 µm, and 100 µm, with a depth of 50 µm and a ridge width of 200 µm | Rabbit corneal epithelial cells (CECs) and keratocytes | Swelling capacity and optical clarity comparable to natural cornea, similar degradation rate to unpatterned films (14 h), significant cell alignment along grooves (alignment index 20% to 60%), normal exponential cell growth (slightly slower on wider grooves), accelerated wound healing with narrower grooves, and inhibition of keratocyte transformation into myofibroblasts (reduced CTGF, aSMA, COL1A1 gene expression). | Design surfaces that promote cell alignment, guide direction migration of cells, accelerate wound healing, and inhibit fibrosis. Epithelialization of corneal epithelial cells. | Combination of soft lithography and solvent casting | 2019 | [114] |
| Polydimethylsiloxane (PDMS) | Rose-petal-topography-mimicked surface. Microgroove mean depths of 12.9 µm (red rose) and 6.6 µm (white rose). White rose petal patterned PDMS exhibited hexagonal patterns similar to CEC. | Bovine corneal endothelial cells (BCE C/D-1b) | Collagen IV-functionalized PDMS (PDMS-C4) significantly enhanced CEC proliferation, but white-rose-patterned PDMS-C4 (PDMS-C4-R) provided the highest proliferation rate and cell density. PDMS-C4-R also maintained CEC-specific phenotype. | Corneal endothelium tissue engineering | Soft lithography, followed by functionalization with collagen IV and hyaluronic acid to enhance cell attachment and proliferation. | 2021 | [108] |
| Gold with SAMs of oligo(ethylene oxide) | Self-assembled monolayers (SAMs) with variable chain lengths | Proteins (e.g., fibrinogen, lysozyme) | Prevention of nonspecific protein adsorption | Design of non-fouling surfaces for biomedical applications | Adsorption of alkanethiols onto gold surfaces using ethanol solutions, leading to the creation of monolayers with oligo(ethylene oxide) chains | 1993 | [118] |
| Glass, silicon, and titanium panes | Ultrathin film (30 +/- 5 nm) of reactive star-shaped poly(ethylene glycol) prepolymers (star PEG). | Human dermal fibroblasts (HDFs), sarcoma osteogenic cells (SaOS-2), human mesenchymal stem cells (hMSCs) | Prevention of unspecific protein adsorption. Promotion of specific cell adhesion and proliferation while preserving normal differentiation process. | Non-fouling implant coatings that promote cell adhesion and proliferation | Not mentioned | 1991 | [119] |
| Linear RGD peptide (gRGDsc)-modified star PEG coatings. |
| Material | Topographical Property Features | Cell Type Affected | Effect of Added Topographical Feature | Key | Reference |
|---|---|---|---|---|---|
| Polydimethylsiloxane (PDMS) | 10 µm pitch (square and circular features), 5 µm pitch (parallel channels); heights: 21.1 nm and 117 nm | Staphylococcus epidermidis, Escherichia coli, Bacillus subtilis | Significant reduction in bacterial adhesion (30–45%) [103,104] | 2014 | [144] |
| Poly(dimethylsiloxan) (PDMS) | 10 µm tall square features, varied side lengths (2, 5, 10, 15, 20, 30, 40, 50, or 100 µm) and distances (5, 10, 15, or 20 µm) between features | Escherichia coli (E. coli) RP437/pRSH103 | E. coli formed biofilms mainly in valleys between features, with significant formation on protrusions only when plateaus were at least 20 µm × 20 µm (face-up) or 40 µm × 40 µm (face-down), indicating a size threshold for adhesion. Motility increased adhesion. | 2011 | [135] |
| Poly(dimethylsiloxan) (PDMS) | Hummock patterns (2.7 µm height, 3 µm diameter, 440 nm trenches) | E. coli (wild type and various mutants) | Wild-type E. coli showed increased adhesion over time, particularly with flagella exploring crevices. Mutants lacking flagella or motility showed reduced adhesion. The structured surfaces initially reduced adhesion, but wetting altered this. | 2013 | [145] |
| Polydimethylsiloxane (PDMS) | Line patterns (width: 5 µm, 10 µm, 20 µm; height: 5 µm; inter-pattern distance: 3 µm, 5 µm, 10 µm, 20 µm) and hexagon patterns (height: 10 µm, side length: 2 µm, 5 µm, 10 µm, 15 µm, 20 µm; inter-pattern distance: 2 µm, 5 µm, 10 µm, 15 µm, 20 µm). | Escherichia coli | Narrow patterns (5 µm) reduced cell cluster formation and biofilm formation; cells oriented perpendicularly on narrow patterns; flagella played a key role in cell orientation. Hexagon patterns with side length of 15 µm and inter-pattern distance of 2 µm reduced biofilm formation by 83.6% compared to flat PDMS. | 2016 | [134] |
| Nanocrystalline nickel | Solid core pillars (1000 nm diameter, height-to-diameter ratio 1.5), | Staphylococcus aureus | The X-shaped pillars exhibited the lowest success rate for bacterial adhesion, making them the most effective for preventing bacterial colonization. Mushroom-shaped nanostructures showed the highest bacterial attachment, making them the least effective. | 2014 | [133] |
| hollow pillars (1000 nm outer diameter, 840 nm inner diameter, height-to-diameter ratio 1.5), | |||||
| C-shaped pillars (1000 nm outer diameter, 760 nm inner diameter, height-to-diameter ratio 1.5), | |||||
| X-shaped pillars (1000 nm outer diameter, wall thickness 300 nm, height-to-diameter ratio 1.5), and | |||||
| mushroom-shaped nanostructures (stem diameter 220 nm, cap diameter 1400 nm). | |||||
| Aluminum oxide | Nanopores (15, 25, 50, 100 nm pore diameters) | Escherichia coli (E. coli), Listeria innocua (L. innocua) | Nanopores of 15 and 25 nm diameters significantly reduced bacterial attachment and biofilm formation. The 50 and 100 nm pores showed higher levels of bacterial adhesion and biofilm formation. | 2014 | [131] |
| Polyethylene terephthalate (PET) | Nanopillar arrays (diameter: ~250 nm, height: 1000 nm, interpillar spacing: 50 nm, 200 nm, 400 nm) | Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Helicobacter pylori (H. pylori) | The 50 nm spacing promoted adhesion, 200 nm spacing reduced adhesion compared to 50 nm, and 400 nm spacing resulted in the least adhesion; morphological changes in bacteria observed. | 2015 | [132] |
5.2. Techniques for Creating Micro- and Nanostructures
5.3. Combining Techniques for Enhanced Scaffold Fabrication
5.4. Soft Litography and Organ-on-a-Chip Designs
5.5. Electrospinning
| Techniques | Description | Advantages | Challenges | Applications | Reference |
|---|---|---|---|---|---|
| Soft lithography | Uses elastomeric stamps (most notably PDMS) to transfer patterns from a master template onto another material | Flexible (can be used for patterning a variety of materials) pillars, valves, and stretchable membranes. Capable of creating patterns on flexible and large-area substrates Reusable PDMS stamps Cost-effective High reproducibility High lateral resolution Can form temporary and adaptable contacts with complex geometries Suitable for modeling three-dimensional in vivo environments with few complications | Cannot reliably create features smaller than 100 nm. Transferring 2D resist patterns to a functional layer for 3D fabrication is as an arduous process. | Tissue engineering Microfluidic devices for cell cultivation and simulating tissue microenvironments Semiconductor manufacturing | [174,191,192,193,194] |
| Electron-beam lithography (EBL) | Uses a focused beam of electrons to create patterns on a surface covered with an electron-sensitive resist | Rapid prototyping High resolution down to 5 nm Versatile for substrates No need for templates Can pattern nonplanar and irregular surfaces Can be combined with other techniques like cryostases evaporation systems and metal deposition for enhanced capabilities Can expose a thick resist without ion contamination | Low fabrication speed Costly Requires complex proximity correction processes to compensate for light distortion and scattering of particles Challenging to apply for large-area patterning and on curved surfaces | Creation of detailed cellular scaffolds Fabrication of nanoscale biosensors Development of lab-on-a-chip devices Development of porous and fragile membranes for biological applications | [191,195,196] |
| Focused ion beam lithography (FIB) | Utilizes a focused beam of ions, typically gallium, to directly write or mill patterns onto a substrate | Rapid prototyping High resolution down to 5 nm Significant larger depth of focus compared to EBL Very small scattering of ions in a resist layer Can pattern on highly corrugated surfaces Can process a wide range of materials No need for masks Capable of fabricating suspended nanostructure | Achievable resolution lower than EBL Low fabrication speed Costly Potential damage to the substrate due to ion implantation | Fabrication of nanoscale biosensors Creation of microfluidic devices for cell studies Modification of biomaterials for tissue engineering Development of high-aspect-ratio nanostructures and suspended nanowires on complex geometries | [191,197,198,199] |
| Scanning probe lithography (SPL) | Employs a sharp probe (such as in atomic force microscopy) to create patterns on a surface by mechanically removing material or inducing a chemical reaction | Rapid prototyping Extremely high resolution down to the atomic scale Capable of patterning various materials No need for complex masks or stencils Versatile in creating 2D and 3D structures Cost effective | Very low throughput Limited to small areas Requires precise control of the probe and surface Mainly a laboratory-based technique with limited commercial application | Development of nanoscale drug delivery systems Patterning of biocompatible surfaces Research in cellular interaction with nanostructures | [200,201,202] |
| Nanoimprint lithography | Involves pressing a nanostructured stamp into a polymer resist to create nanoscale patterns, then hardening the resist with thermal or UV curing, and transferring the patterns to a substrate through etching or lift-off | Cost-effective for large-scale production High resolution down to the nanoscale Simplicity Rapid prototyping Capable of producing 3D nanostructures Versatile with a wide range of materials | Challenging to achieve uniformity over large areas Requires high precision in alignment Limited by the mechanical properties of the stamp and resist Difficulties in pattern transfer for complex 3D structures Requires a resist that can withstand mechanical deformation | Patterning of surfaces for cell culture Fabrication of biosensors Creation of microfluidic devices Development of drug delivery systems Production of optical devices and components Fabrication of superhydrophobic and oleophobic surfaces | [191,203] |
| Electrospinning | Produces continuous nanofibers using a high-voltage electric field applied between a needle and a collector | High productivity Simplicity Low cost Reproducibility Functionality Diversity Potential in scaffolds with drug delivery systems and shape memory polymer materials Great fiber alignment based on collector design, promoting natural cell phenotype, migration, and proliferation Suitability of electrospun fibers as thin layers (epithelial, corneal stroma, and endothelial) | Dense electrospun fibers can hinder cell migration Not suitable for full-thickness corneal scaffolds due to limited thickness of electrospun membranes Low light transmission when synthetic polymer fibers are used | ECM biomimetic structures Tissue engineering Disease modeling Drug delivery systems | [179,181,185,204,205,206,207] |
| Two-photon polymerization (2PP lithography) | Direct laser writing technique that uses femtosecond laser pulses to create 3D structures within a photosensitive material | High-resolution method (capable of creating features smaller than the diffraction limit of light) Rapid prototyping and flexible (can create any 3D structure from computer models) | High cost Complex setup Sensitivity to laser fluctuations Limited material choices (only certain photoresists are suitable for 2PP) Need for more biocompatible photoinitiators and photoresists | Biomedical scaffolds Tissue engineering (mimicking native 3D environments such as Descemet’s membrane, luminal walls of blood vessels, etc.) Microfluidic lab-on-chip devices Drug delivery systems Creating master molds for subsequent replication with other techniques | [107,208,209,210,211,212,213,214,215] |
| Spincoating | Used to create thin film coatings by depositing a liquid on a spinning substrate, allowing centrifugal force to spread it uniformly | Simple, fast, and cost-effective High reproducibility and scalability Produces smooth and homogeneous films with controllable thickness (10 nm to several µm) Versatility: can produce monolayer- and multilayer-thin coatings, as well as freestanding (FS) films | Limited to planar surfaces Inefficient material usage (only 2–5% of material is used) Difficulties with large substrates Challenges in creating freestanding films | Biomedical applications (surface modification, drug delivery, wound dressings, tissue engineering scaffolds) Microelectronics | [216] |
6. Emerging Technologies and Innovation
6.1. Peptide and Electroconductive Hydrogels
6.2. Carbon Nanotubes (CNTs)
6.3. Three-Dimensional Bioprinting
6.4. Four-Dimensional Bioprinting
6.5. Scaffolds with Drug Delivery Systems
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarnicola, C.; Farooq, A.V.; Colby, K. Fuchs Endothelial Corneal Dystrophy: Update on Pathogenesis and Future Directions. Eye Contact Lens 2019, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s Membrane Development, Structure, Function and Regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef] [PubMed]
- Volatier, T.; Cursiefen, C.; Notara, M. Current Advances in Corneal Stromal Stem Cell Biology and Therapeutic Applications. Cells 2024, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Bourne, W.M. Biology of the Corneal Endothelium in Health and Disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Jeang, L.J.; Margo, C.E.; Espana, E.M. Diseases of the Corneal Endothelium. Exp. Eye Res. 2021, 205, 108495. [Google Scholar] [CrossRef]
- Matthaei, M.; Hribek, A.; Clahsen, T.; Bachmann, B.; Cursiefen, C.; Jun, A.S. Fuchs Endothelial Corneal Dystrophy: Clinical, Genetic, Pathophysiologic, and Therapeutic Aspects. Annu. Rev. Vis. Sci. 2019, 5, 151–175. [Google Scholar] [CrossRef]
- Thaung, C.; Davidson, A.E. Fuchs Endothelial Corneal Dystrophy: Current Perspectives on Diagnostic Pathology and Genetics—Bowman Club Lecture. BMJ Open Ophthalmol. 2022, 7, e001103. [Google Scholar] [CrossRef]
- Patel, S.P.; Parker, M.D. SLC4A11 and the Pathophysiology of Congenital Hereditary Endothelial Dystrophy. BioMed Res. Int. 2015, 2015, 475392. [Google Scholar] [CrossRef]
- Liskova, P.; Palos, M.; Hardcastle, A.J.; Vincent, A.L. Further Genetic and Clinical Insights of Posterior Polymorphous Corneal Dystrophy 3. JAMA Ophthalmol. 2013, 131, 1296–1303. [Google Scholar] [CrossRef]
- Silva, L.; Najafi, A.; Suwan, Y.; Teekhasaenee, C.; Ritch, R. The Iridocorneal Endothelial Syndrome. Surv. Ophthalmol. 2018, 63, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Janson, B.J.; Alward, W.L.; Kwon, Y.H.; Bettis, D.I.; Fingert, J.H.; Provencher, L.M.; Goins, K.M.; Wagoner, M.D.; Greiner, M.A. Glaucoma-Associated Corneal Endothelial Cell Damage: A Review. Surv. Ophthalmol. 2018, 63, 500–506. [Google Scholar] [CrossRef]
- Goldstein, A.S.; Janson, B.J.; Skeie, J.M.; Ling, J.J.; Greiner, M.A. The Effects of Diabetes Mellitus on the Corneal Endothelium: A Review. Surv. Ophthalmol. 2020, 65, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Pseudophakic Bullous Keratopathy—EyeWiki. Available online: https://eyewiki.aao.org/Pseudophakic_Bullous_Keratopathy (accessed on 21 July 2024).
- Sikora, B.; Rafat, M.; Łos, M.J. Chapter 12—Examples of Successful Biomaterial-Based Artificial Tissues—Artificial Corneas. In Stem Cells and Biomaterials for Regenerative Medicine; Łos, M.J., Hudecki, A., Wiecheć, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 191–202. ISBN 978-0-12-812258-7. [Google Scholar]
- Català, P.; Thuret, G.; Skottman, H.; Mehta, J.S.; Parekh, M.; Ní Dhubhghaill, S.; Collin, R.W.J.; Nuijts, R.M.M.A.; Ferrari, S.; LaPointe, V.L.S.; et al. Approaches for Corneal Endothelium Regenerative Medicine. Prog. Retin. Eye Res. 2022, 87, 100987. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W. Corneal Endothelial Dysfunction: Evolving Understanding and Treatment Options. Prog. Retin. Eye Res. 2021, 82, 100904. [Google Scholar] [CrossRef] [PubMed]
- Statistical Report. Available online: https://restoresight.org/members/publications/statistical-report/ (accessed on 27 August 2024).
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yamashita, K.; Inagaki, E.; Hatou, S.; Higa, K.; Ogawa, A.; Miyashita, H.; Tsubota, K.; Shimmura, S. Corneal Endothelial Regeneration Using Mesenchymal Stem Cells Derived from Human Umbilical Cord. Stem Cells Dev. 2018, 27, 1097–1108. [Google Scholar] [CrossRef]
- Chi, M.; Yuan, B.; Xie, Z.; Hong, J. The Innovative Biomaterials and Technologies for Developing Corneal Endothelium Tissue Engineering Scaffolds: A Review and Prospect. Bioengineering 2023, 10, 1284. [Google Scholar] [CrossRef]
- Pavelka, M.; Roth, J. Descemet’s Membrane. In Functional Ultrastructure: Atlas of Tissue Biology and Pathology; Pavelka, M., Roth, J., Eds.; Springer: Vienna, Austria, 2010; pp. 184–185. ISBN 978-3-211-99390-3. [Google Scholar]
- Massoudi, D.; Malecaze, F.; Galiacy, S.D. Collagens and Proteoglycans of the Cornea: Importance in Transparency and Visual Disorders. Cell Tissue Res. 2016, 363, 337–349. [Google Scholar] [CrossRef]
- Saikia, P.; Medeiros, C.S.; Thangavadivel, S.; Wilson, S.E. Basement Membranes in the Cornea and Other Organs That Commonly Develop Fibrosis. Cell Tissue Res 2018, 374, 439–453. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Daniels, J.T. The Impact of Biomechanics on Corneal Endothelium Tissue Engineering. Exp. Eye Res. 2021, 209, 108690. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal Structure and Transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogerd, B.; Ní Dhubhghaill, S.; Zakaria, N. Characterizing Human Decellularized Crystalline Lens Capsules as a Scaffold for Corneal Endothelial Tissue Engineering. J. Tissue Eng. Regen. Med. 2018, 12, e2020–e2028. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Dennis, S.; Khan, S. Changes in the Refractive Index of the Stroma and Its Extrafibrillar Matrix When the Cornea Swells. Biophys. J. 2003, 85, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Arnalich-Montiel, F. Corneal Endothelium: Applied Anatomy. In Corneal Regeneration; Alió, J.L., Alió Del Barrio, J.L., Arnalich-Montiel, F., Eds.; Essentials in Ophthalmology; Springer International Publishing: Cham, Switzerland, 2019; pp. 419–424. ISBN 978-3-030-01303-5. [Google Scholar]
- Reizabal, A.; Gonçalves, S.; Pereira, N.; Costa, C.M.; Pérez, L.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Optically Transparent Silk Fibroin/Silver Nanowire Composites for Piezoresistive Sensing and Object Recognitions. J. Mater. Chem. C 2020, 8, 13053–13062. [Google Scholar] [CrossRef]
- Ji, D.; Lin, Y.; Guo, X.; Ramasubramanian, B.; Wang, R.; Radacsi, N.; Jose, R.; Qin, X.; Ramakrishna, S. Electrospinning of Nanofibres. Nat. Rev. Methods Primers 2024, 4, 1–21. [Google Scholar] [CrossRef]
- Zheng, J.; Su, Q.; Wang, C.; Cheng, G.; Zhu, R.; Shi, J.; Yao, K. Synthesis and Biological Evaluation of PMMA/MMT Nanocomposite as Denture Base Material. J. Mater. Sci. Mater. Med. 2011, 22, 1063–1071. [Google Scholar] [CrossRef]
- Balos, S.; Puskar, T.; Potran, M.; Milekic, B.; Djurovic Koprivica, D.; Laban Terzija, J.; Gusic, I. Modulus, Strength and Cytotoxicity of PMMA-Silica Nanocomposites. Coatings 2020, 10, 583. [Google Scholar] [CrossRef]
- Rafat, M.; Xeroudaki, M.; Koulikovska, M.; Sherrell, P.; Groth, F.; Fagerholm, P.; Lagali, N. Composite Core-and-Skirt Collagen Hydrogels with Differential Degradation for Corneal Therapeutic Applications. Biomaterials 2016, 83, 142–155. [Google Scholar] [CrossRef]
- Song, E.S.; Park, J.-H.; Ha, S.S.; Cha, P.-H.; Kang, J.-T.; Park, C.Y.; Park, K. Novel Corneal Endothelial Cell Carrier Couples a Biodegradable Polymer and a Mesenchymal Stem Cell-Derived Extracellular Matrix. ACS Appl. Mater. Interfaces 2022, 14, 12116–12129. [Google Scholar] [CrossRef]
- Jia, B.; Huang, H.; Dong, Z.; Ren, X.; Lu, Y.; Wang, W.; Zhou, S.; Zhao, X.; Guo, B. Degradable Biomedical Elastomers: Paving the Future of Tissue Repair and Regenerative Medicine. Chem. Soc. Rev. 2024, 53, 4086–4153. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Choi, S.-J.; Han, C.-M.; Kim, J.-J.; Shim, H.; Leong, K.W.; Kim, H.-W. Biomaterials Control of Pluripotent Stem Cell Fate for Regenerative Therapy. Prog. Mater. Sci. 2016, 82, 234–293. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Xie, H.; Liu, X.; Zhang, M. Substrate Stiffness Modulates Stemness and Differentiation of Rabbit Corneal Endothelium Through the Paxillin–YAP Pathway. Investig. Ophthalmol. Vis. Sci. 2024, 65, 15. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-H.; Frey, M.T.; Burnham, N.A.; Wang, Y.-L. Substrate Rigidity Regulates the Formation and Maintenance of Tissues. Biophys. J. 2006, 90, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Reinhart-King, C.A.; Dembo, M.; Hammer, D.A. Cell-Cell Mechanical Communication through Compliant Substrates. Biophys. J. 2008, 95, 6044–6051. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, C.C. Tensile Mechanical and Creep Properties of Descemet’s Membrane and Lens Capsule. Exp. Eye Res. 2004, 79, 343–350. [Google Scholar] [CrossRef]
- Du, R.; Li, D.; Huang, Y.; Xiao, H.; Xue, J.; Ji, J.; Feng, Y.; Fan, Y. Effect of Mechanical Stretching and Substrate Stiffness on the Morphology, Cytoskeleton and Nuclear Shape of Corneal Endothelial Cells. Med. Novel Technol. Dev. 2022, 16, 100180. [Google Scholar] [CrossRef]
- Myung, D.; Derr, K.; Huie, P.; Noolandi, J.; Ta, K.P.; Ta, C.N. Glucose Permeability of Human, Bovine, and Porcine Corneas in Vitro. Ophthalmic Res. 2006, 38, 158–163. [Google Scholar] [CrossRef]
- Charalel, R.A.; Engberg, K.; Noolandi, J.; Cochran, J.R.; Frank, C.; Ta, C.N. Diffusion of Protein through the Human Cornea. Ophthalmic Res. 2012, 48, 50–55. [Google Scholar] [CrossRef]
- Santana, C.P.; Matter, B.A.; Patil, M.A.; Silva-Cunha, A.; Kompella, U.B. Corneal Permeability and Uptake of Twenty-Five Drugs: Species Comparison and Quantitative Structure–Permeability Relationships. Pharmaceutics 2023, 15, 1646. [Google Scholar] [CrossRef]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the Cell–Material Interface for Controlling Stem Cell Adhesion, Migration, and Differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhang, F.; Li, G.; Wang, S. Smart Thin Hydrogel Coatings Harnessing Hydrophobicity and Topography to Capture and Release Cancer Cells. Small 2016, 12, 4697–4701. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Lourenço, B.N.; Marchioli, G.; Song, W.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.; Mano, J.F. Wettability Influences Cell Behavior on Superhydrophobic Surfaces with Different Topographies. Biointerphases 2012, 7, 46. [Google Scholar] [CrossRef]
- Wang, T.-J.; Wang, I.-J.; Chen, Y.-H.; Lu, J.-N.; Young, T.-H. Polyvinylidene Fluoride for Proliferation and Preservation of Bovine Corneal Endothelial Cells by Enhancing Type IV Collagen Production and Deposition. J. Biomed. Mater. Res. Part A 2012, 100A, 252–260. [Google Scholar] [CrossRef]
- Dorfmueller, S.; Tan, H.C.; Ngoh, Z.X.; Toh, K.Y.; Peh, G.; Ang, H.-P.; Seah, X.-Y.; Chin, A.; Choo, A.; Mehta, J.S.; et al. Isolation of a Recombinant Antibody Specific for a Surface Marker of the Corneal Endothelium by Phage Display. Sci. Rep. 2016, 6, 21661. [Google Scholar] [CrossRef]
- Ueno, M.; Asada, K.; Toda, M.; Hiraga, A.; Montoya, M.; Sotozono, C.; Kinoshita, S.; Hamuro, J. MicroRNA Profiles Qualify Phenotypic Features of Cultured Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5509–5517. [Google Scholar] [CrossRef]
- Cheong, Y.K.; Ngoh, Z.X.; Peh, G.S.L.; Ang, H.-P.; Seah, X.-Y.; Chng, Z.; Colman, A.; Mehta, J.S.; Sun, W. Identification of Cell Surface Markers Glypican-4 and CD200 That Differentiate Human Corneal Endothelium From Stromal Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4538–4547. [Google Scholar] [CrossRef]
- Okumura, N.; Hirano, H.; Numata, R.; Nakahara, M.; Ueno, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Cell Surface Markers of Functional Phenotypic Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7610–7618. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogerd, B.; Zakaria, N.; Adam, B.; Matthyssen, S.; Koppen, C.; Ní Dhubhghaill, S. Corneal Endothelial Cells Over the Past Decade: Are We Missing the Mark(Er)? Transl. Vis. Sci. Technol. 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Peh, G.; Mehta, J.S.; Ramos, T.; Ponzin, D.; Ahmad, S.; Ferrari, S. Passaging Capability of Human Corneal Endothelial Cells Derived from Old Donors with and without Accelerating Cell Attachment. Exp. Eye Res. 2019, 189, 107814. [Google Scholar] [CrossRef] [PubMed]
- Frausto, R.F.; Swamy, V.S.; Peh, G.S.L.; Boere, P.M.; Hanser, E.M.; Chung, D.D.; George, B.L.; Morselli, M.; Kao, L.; Azimov, R.; et al. Phenotypic and Functional Characterization of Corneal Endothelial Cells during in Vitro Expansion. Sci. Rep. 2020, 10, 7402. [Google Scholar] [CrossRef]
- He, Z.; Forest, F.; Bernard, A.; Gauthier, A.-S.; Montard, R.; Peoc’h, M.; Jumelle, C.; Courrier, E.; Perrache, C.; Gain, P.; et al. Cutting and Decellularization of Multiple Corneal Stromal Lamellae for the Bioengineering of Endothelial Grafts. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6639–6651. [Google Scholar] [CrossRef]
- Lu, Q.; Peng, R.-M.; Feng, N.; Wen, M.-D.; He, L.-H.; Hong, J. Evaluation of Reconstructed Human Corneal Endothelium Sheets Made with Porcine Descemet’s Membrane in Vitro and in Vivo. Exp. Eye Res. 2020, 197, 108125. [Google Scholar] [CrossRef]
- Parekh, M.; Van den Bogerd, B.; Zakaria, N.; Ponzin, D.; Ferrari, S. Fish Scale-Derived Scaffolds for Culturing Human Corneal Endothelial Cells. Stem Cells Int. 2018, 2018, 8146834. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, M.; Li, Y.; Su, W.; Fan, T. Construction of Tissue-Engineered Human Corneal Endothelium for Corneal Endothelial Regeneration Using a Crosslinked Amniotic Membrane Scaffold. Acta Biomater. 2022, 147, 185–197. [Google Scholar] [CrossRef]
- Parekh, M.; Romano, V.; Hassanin, K.; Testa, V.; Wongvisavavit, R.; Ferrari, S.; Haneef, A.; Willoughby, C.; Ponzin, D.; Jhanji, V.; et al. Biomaterials for Corneal Endothelial Cell Culture and Tissue Engineering. J. Tissue Eng. 2021, 12, 2041731421990536. [Google Scholar] [CrossRef]
- Bosch, B.M.; Bosch-Rue, E.; Perpiñan-Blasco, M.; Perez, R.A. Design of Functional Biomaterials as Substrates for Corneal Endothelium Tissue Engineering. Regen. Biomater. 2022, 9, rbac052. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Usui, T.; Tanaka, K.; Hattori, S.; Irie, S.; Miyata, K.; Araie, M.; Amano, S. Cultured Human Corneal Endothelial Cell Transplantation with a Collagen Sheet in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, N.; Chacón, M.; Rodríguez-Barrientos, C.A.; Merayo-Lloves, J.; Naveiras, M.; Baamonde, B.; Alfonso, J.F.; Zambrano-Andazol, I.; Riestra, A.C.; Meana, Á. Human Bone Derived Collagen for the Development of an Artificial Corneal Endothelial Graft. In Vivo Results in a Rabbit Model. PLoS ONE 2016, 11, e0167578. [Google Scholar] [CrossRef] [PubMed]
- Levis, H.J.; Peh, G.S.L.; Toh, K.-P.; Poh, R.; Shortt, A.J.; Drake, R.A.L.; Mehta, J.S.; Daniels, J.T. Plastic Compressed Collagen as a Novel Carrier for Expanded Human Corneal Endothelial Cells for Transplantation. PLoS ONE 2012, 7, e50993. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Pan, S.; Liu, X.; Zhu, X.; Connon, C.J.; Wu, J.; Mi, S. In Vivo Study of the Biocompatibility of a Novel Compressed Collagen Hydrogel Scaffold for Artificial Corneas. J. Biomed. Mater. Res. Part A 2014, 102, 1782–1787. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-Polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Watanabe, R.; Hayashi, R.; Kimura, Y.; Tanaka, Y.; Kageyama, T.; Hara, S.; Tabata, Y.; Nishida, K. A Novel Gelatin Hydrogel Carrier Sheet for Corneal Endothelial Transplantation. Tissue Eng. Part A 2011, 17, 2213–2219. [Google Scholar] [CrossRef]
- Niu, G.; Choi, J.-S.; Wang, Z.; Skardal, A.; Giegengack, M.; Soker, S. Heparin-Modified Gelatin Scaffolds for Human Corneal Endothelial Cell Transplantation. Biomaterials 2014, 35, 4005–4014. [Google Scholar] [CrossRef]
- Li, P.-C.; Chen, S.-C.; Hsueh, Y.-J.; Shen, Y.-C.; Tsai, M.-Y.; Hsu, L.-W.; Yeh, C.-K.; Chen, H.-C.; Huang, C.-C. Gelatin Scaffold with Multifunctional Curcumin-Loaded Lipid-PLGA Hybrid Microparticles for Regenerating Corneal Endothelium. Mater. Sci. Eng. C 2021, 120, 111753. [Google Scholar] [CrossRef]
- Yan, Y.; Cao, Y.; Cheng, R.; Shen, Z.; Zhao, Y.; Zhang, Y.; Zhou, G.; Sang, S. Preparation and In Vitro Characterization of Gelatin Methacrylate for Corneal Tissue Engineering. Tissue Eng. Regen. Med. 2022, 19, 59–72. [Google Scholar] [CrossRef]
- Farasatkia, A.; Kharaziha, M.; Ashrafizadeh, F.; Salehi, S. Transparent Silk/Gelatin Methacrylate (GelMA) Fibrillar Film for Corneal Regeneration. Mater. Sci. Eng. C 2021, 120, 111744. [Google Scholar] [CrossRef]
- Long, K.; Liu, Y.; Li, W.; Wang, L.; Liu, S.; Wang, Y.; Wang, Z.; Ren, L. Improving the Mechanical Properties of Collagen-Based Membranes Using Silk Fibroin for Corneal Tissue Engineering. J. Biomed. Mater. Res. Part A 2015, 103, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Tripathy, N.; Park, J.Y.; Lee, S.E.; Joo, C.-K.; Khang, G. Silk Fibroin Film as an Efficient Carrier for Corneal Endothelial Cells Regeneration. Macromol. Res. 2015, 23, 189–195. [Google Scholar] [CrossRef]
- Chen, J.; Yan, C.; Zhu, M.; Yao, Q.; Shao, C.; Lu, W.; Wang, J.; Mo, X.; Gu, P.; Fu, Y.; et al. Electrospun Nanofibrous SF/P(LLA-CL) Membrane: A Potential Substratum for Endothelial Keratoplasty. Int. J. Nanomed. 2015, 10, 3337–3350. [Google Scholar] [CrossRef]
- Vázquez, N.; Rodríguez-Barrientos, C.A.; Aznar-Cervantes, S.D.; Chacón, M.; Cenis, J.L.; Riestra, A.C.; Sánchez-Avila, R.M.; Persinal, M.; Brea-Pastor, A.; Fernández-Vega Cueto, L.; et al. Silk Fibroin Films for Corneal Endothelial Regeneration: Transplant in a Rabbit Descemet Membrane Endothelial Keratoplasty. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3357–3365. [Google Scholar] [CrossRef]
- Ramachandran, C.; Gupta, P.; Hazra, S.; Mandal, B.B. In Vitro Culture of Human Corneal Endothelium on Non-Mulberry Silk Fibroin Films for Tissue Regeneration. Transl. Vis. Sci. Technol. 2020, 9, 12. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, S.; Choi, J.H.; Jung, B.S.; Kim, K.S.; Song, J.E.; Reis, R.L.; Khang, G. Enhanced Silk Fibroin-Based Film Scaffold Using Curcumin for Corneal Endothelial Cell Regeneration. Macromol. Res. 2021, 29, 713–719. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Ma, D.H.-K.; Cheng, H.-Y.; Sun, C.-C.; Huang, S.-J.; Li, Y.-T.; Hsiue, G.-H. Ocular Biocompatibility of Carbodiimide Cross-Linked Hyaluronic Acid Hydrogels for Cell Sheet Delivery Carriers. J. Biomater. Sci. Polym. Ed. 2010, 21, 359–376. [Google Scholar] [CrossRef]
- Lai, J.-Y. Hyaluronic Acid Concentration-Mediated Changes in Structure and Function of Porous Carriers for Corneal Endothelial Cell Sheet Delivery. Mater. Sci. Eng. C 2016, 59, 411–419. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Yao, C.-H.; Luo, L.-J.; Chen, H.-C.; Hsueh, Y.-J.; Ma, D.H.-K.; Lai, J.-Y. Oxidation-Mediated Scaffold Engineering of Hyaluronic Acid-Based Microcarriers Enhances Corneal Stromal Regeneration. Carbohydr. Polym. 2022, 292, 119668. [Google Scholar] [CrossRef]
- Luo, Y.; Li, G.; Chen, L.; Hong, F.F. Preparation and Evaluation of Bacterial Nanocellulose/Hyaluronic Acid Composite Artificial Cornea for Application of Corneal Transplantation. Biomacromolecules 2023, 24, 201–212. [Google Scholar] [CrossRef]
- Zhao, S.; Hou, S.; Li, D.; Li, L.; Ding, X.; Huang, Y.; Li, Y.; Ji, J.; Wang, L.; Fan, Y. Injectable Magnetic Hyaluronic Acid Gel for Corneal Endothelial Cells Efficient Delivery and Retention. Appl. Mater. Today 2024, 37, 102090. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Liu, Y.; Long, K.; Liu, S.; Wang, Z.; Wang, Y.; Ren, L. Fabrication and Characterization of Chitosan–Collagen Crosslinked Membranes for Corneal Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2014, 25, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, B.; Brown, K.D.; Blencowe, A.; Daniell, M.; Stevens, G.W.; Qiao, G.G. Ultrathin Chitosan–Poly(Ethylene Glycol) Hydrogel Films for Corneal Tissue Engineering. Acta Biomater. 2013, 9, 6594–6605. [Google Scholar] [CrossRef] [PubMed]
- Young, T.-H.; Wang, I.-J.; Hu, F.-R.; Wang, T.-J. Fabrication of a Bioengineered Corneal Endothelial Cell Sheet Using Chitosan/Polycaprolactone Blend Membranes. Colloids Surf. B Biointerfaces 2014, 116, 403–410. [Google Scholar] [CrossRef]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of Chitosan/Chitosan Nanoparticles/Polycaprolactone Transparent Membrane for Corneal Endothelial Tissue Engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Seow, W.Y.; Kandasamy, K.; Peh, G.S.L.; Mehta, J.S.; Sun, W. Ultrathin, Strong, and Cell-Adhesive Agarose-Based Membranes Engineered as Substrates for Corneal Endothelial Cells. ACS Biomater. Sci. Eng. 2019, 5, 4067–4076. [Google Scholar] [CrossRef]
- Song, E.; Kwon, J.W.; Park, C.Y.; Kang, J.-T.; Park, K. Alginate Hydrogel Integrated with a Human Fibroblast-Derived Extracellular Matrix Supports Corneal Endothelial Cell Functionality and Suppresses Endothelial–Mesenchymal Transition. ACS Biomater. Sci. Eng. 2024, 10, 3855–3867. [Google Scholar] [CrossRef]
- Hsu, W.-M.; Chen, K.-H.; Lai, J.-Y.; Hsiue, G.-H. Transplantation of Human Corneal Endothelial Cells Using Functional Biomaterials: Poly(N-Isopropylacrylamide) and Gelatin. J. Exp. Clin. Med. 2013, 5, 56–64. [Google Scholar] [CrossRef]
- Salehi, S.; Grünert, A.K.; Bahners, T.; Gutmann, J.S.; Steuhl, K.P.; Czugala, M.; Singer, B.B.; Fuchsluger, T.A. [New nanofibrous scaffold for corneal tissue engineering]. Klin Monbl Augenheilkd 2014, 231, 626–630. [Google Scholar] [CrossRef]
- Kruse, M.; Walter, P.; Bauer, B.; Rütten, S.; Schaefer, K.; Plange, N.; Gries, T.; Jockenhoevel, S.; Fuest, M. Electro-Spun Membranes as Scaffolds for Human Corneal Endothelial Cells. Curr. Eye Res 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Park, S.; Nam, S.H.; Koh, W.-G. Preparation of Collagen-Immobilized Poly(Ethylene Glycol)/Poly(2-Hydroxyethyl Methacrylate) Interpenetrating Network Hydrogels for Potential Application of Artificial Cornea. J. Appl. Polym. Sci. 2012, 123, 637–645. [Google Scholar] [CrossRef]
- Rahman, C.V.; Kuhn, G.; White, L.J.; Kirby, G.T.S.; Varghese, O.P.; McLaren, J.S.; Cox, H.C.; Rose, F.R.A.J.; Müller, R.; Hilborn, J.; et al. PLGA/PEG-Hydrogel Composite Scaffolds with Controllable Mechanical Properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 648–655. [Google Scholar] [CrossRef]
- Kim, E.Y.; Tripathy, N.; Cho, S.A.; Lee, D.; Khang, G. Collagen Type I–PLGA Film as an Efficient Substratum for Corneal Endothelial Cells Regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 2471–2478. [Google Scholar] [CrossRef]
- Ozcelik, B.; Brown, K.D.; Blencowe, A.; Ladewig, K.; Stevens, G.W.; Scheerlinck, J.-P.Y.; Abberton, K.; Daniell, M.; Qiao, G.G. Biodegradable and Biocompatible Poly(Ethylene Glycol)-Based Hydrogel Films for the Regeneration of Corneal Endothelium. Adv. Healthc. Mater. 2014, 3, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Himmler, M.; Garreis, F.; Paulsen, F.; Schubert, D.W.; Fuchsluger, T.A. Optimization of Polycaprolactone—Based Nanofiber Matrices for the Cultivation of Corneal Endothelial Cells. Sci. Rep. 2021, 11, 18858. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Lace, R.; Carserides, C.; Gallagher, A.G.; Wellings, D.A.; Williams, R.L.; Levis, H.J. Poly-ε-Lysine Based Hydrogels as Synthetic Substrates for the Expansion of Corneal Endothelial Cells for Transplantation. J. Mater. Sci. Mater. Med. 2019, 30, 102. [Google Scholar] [CrossRef]
- Lace, R.; Duffy, G.L.; Gallagher, A.G.; Doherty, K.G.; Maklad, O.; Wellings, D.A.; Williams, R.L. Characterization of Tunable Poly-ε-Lysine-Based Hydrogels for Corneal Tissue Engineering. Macromol. Biosci. 2021, 21, 2100036. [Google Scholar] [CrossRef]
- Ratemi, E. 5—pH-Responsive Polymers for Drug Delivery Applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, Volume 1; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 121–141. ISBN 978-0-08-101997-9. [Google Scholar]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting Structure-Property Relationship of pH-Responsive Polymers for Drug Delivery Applications. J. Control Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The Role of Extracellular Matrix in Biomechanics and Its Impact on Bioengineering of Cells and 3D Tissues. Matrix Biol. 2020, 85–86, 1–14. [Google Scholar] [CrossRef]
- Khanna, A.; Oropeza, B.P.; Jain, I.; Huang, N.F. Extracellular Matrix Bioactive Molecules and Cell Behavior Modeling. In Handbook of the Extracellular Matrix; Maia, F.R.A., Oliveira, J.M., Reis, R.L., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Gutermuth, A.; Maassen, J.; Harnisch, E.; Kuhlen, D.; Sauer-Budge, A.; Skazik-Voogt, C.; Engelmann, K. Descemet’s Membrane Biomimetic Microtopography Differentiates Human Mesenchymal Stem Cells Into Corneal Endothelial-Like Cells. Cornea 2019, 38, 110. [Google Scholar] [CrossRef]
- Öztürk-Öncel, M.Ö.; Erkoc-Biradli, F.Z.; Rasier, R.; Marcali, M.; Elbuken, C.; Garipcan, B. Rose Petal Topography Mimicked Poly(Dimethylsiloxane) Substrates for Enhanced Corneal Endothelial Cell Behavior. Mater. Sci. Eng. C 2021, 126, 112147. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.; Peh, G.S.L.; Adnan, K.; Law, J.B.K.; Mehta, J.S.; Yim, E.K.F. Micro- and Nano-Topography to Enhance Proliferation and Sustain Functional Markers of Donor-Derived Primary Human Corneal Endothelial Cells. Acta Biomater. 2015, 19, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.K.K.; Goh, K.J.; Ng, Z.J.; Koo, S.; Yim, E.K.F. Functional Reconstruction of Corneal Endothelium Using Nanotopography for Tissue-Engineering Applications. Acta Biomater. 2012, 8, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.; Muhammad, R.; Peh, G.S.L.; Mehta, J.S.; Yim, E.K.F. Micro- and Nanotopography with Extracellular Matrix Coating Modulate Human Corneal Endothelial Cell Behavior. Acta Biomater. 2014, 10, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Peh, G.S.L.; Ang, H.-P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-Crosslinked Bioactive Hydrogels as Nano-Patterned Substrates with Customizable Stiffness and Degradation for Corneal Tissue Engineering Applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Cutiongco, M.F.A.; Goh, S.H.; Aid-Launais, R.; Le Visage, C.; Low, H.Y.; Yim, E.K.F. Planar and Tubular Patterning of Micro and Nano-Topographies on Poly(Vinyl Alcohol) Hydrogel for Improved Endothelial Cell Responses. Biomaterials 2016, 84, 184–195. [Google Scholar] [CrossRef]
- Xiong, S.; Gao, H.; Qin, L.; Jia, Y.; Gao, M.; Ren, L. Microgrooved Collagen-Based Corneal Scaffold for Promoting Collective Cell Migration and Antifibrosis. RSC Adv. 2019, 9, 29463–29473. [Google Scholar] [CrossRef]
- Scott, M.D.; Murad, K.L. Cellular Camouflage: Fooling the Immune System with Polymers. Curr. Pharm. Des. 1998, 4, 423–438. [Google Scholar] [CrossRef]
- Csucs, G.; Michel, R.; Lussi, J.W.; Textor, M.; Danuser, G. Microcontact Printing of Novel Co-Polymers in Combination with Proteins for Cell-Biological Applications. Biomaterials 2003, 24, 1713–1720. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Self-Assembled Organic Monolayers: Model Systems for Studying Adsorption of Proteins at Aurfaces. Science 1991, 252, 1164–1167. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Adsorption of Proteins onto Surfaces Containing End-Attached Oligo(Ethylene Oxide): A Model System Using Self-Assembled Monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein—Surface Interactions in the Presence of Polyethylene Oxide: I. Simplified Theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Groll, J.; Fiedler, J.; Engelhard, E.; Ameringer, T.; Tugulu, S.; Klok, H.-A.; Brenner, R.E.; Moeller, M. A Novel Star PEG–Derived Surface Coating for Specific Cell Adhesion. J. Biomed. Mater. Res. Part A 2005, 74A, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Luensmann, D.; Jones, L. Protein Deposition on Contact Lenses: The Past, the Present, and the Future. Contact Lens Anterior Eye 2012, 35, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Schulte, V.A.; Diez, M.; Möller, M.; Lensen, M.C. Topography-Induced Cell Adhesion to Acr-sP(EO-Stat-PO) Hydrogels: The Role of Protein Adsorption. Macromol. Biosci. 2011, 11, 1378–1386. [Google Scholar] [CrossRef]
- Schulte, V.A.; Díez, M.; Möller, M.; Lensen, M.C. Surface Topography Induces Fibroblast Adhesion on Intrinsically Nonadhesive Poly(Ethylene Glycol) Substrates. Biomacromolecules 2009, 10, 2795–2801. [Google Scholar] [CrossRef]
- Shi, D.; Xu, X.; Ye, Y.; Song, K.; Cheng, Y.; Di, J.; Hu, Q.; Li, J.; Ju, H.; Jiang, Q.; et al. Photo-Cross-Linked Scaffold with Kartogenin-Encapsulated Nanoparticles for Cartilage Regeneration. ACS Nano 2016, 10, 1292–1299. [Google Scholar] [CrossRef]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold Nanoparticle-Decorated Scaffolds Promote Neuronal Differentiation and Maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef]
- Childs, A.; Hemraz, U.D.; Castro, N.J.; Fenniri, H.; Zhang, L.G. Novel Biologically-Inspired Rosette Nanotube PLLA Scaffolds for Improving Human Mesenchymal Stem Cell Chondrogenic Differentiation. Biomed. Mater. 2013, 8, 065003. [Google Scholar] [CrossRef]
- Hopley, E.L.; Salmasi, S.; Kalaskar, D.M.; Seifalian, A.M. Carbon Nanotubes Leading the Way Forward in New Generation 3D Tissue Engineering. Biotechnol. Adv. 2014, 32, 1000–1014. [Google Scholar] [CrossRef]
- Shin, Y.C.; Lee, J.H.; Kim, M.J.; Hong, S.W.; Kim, B.; Hyun, J.K.; Choi, Y.S.; Park, J.-C.; Han, D.-W. Stimulating Effect of Graphene Oxide on Myogenesis of C2C12 Myoblasts on RGD Peptide-Decorated PLGA Nanofiber Matrices. J. Biol. Eng. 2015, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Raspa, A.; Marchini, A.; Pugliese, R.; Mauri, M.; Maleki, M.; Vasita, R.; Gelain, F. A Biocompatibility Study of New Nanofibrous Scaffolds for Nervous System Regeneration. Nanoscale 2015, 8, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, J.M.; Jallerat, Q.; Feinberg, A.W. ECM Protein Nanofibers and Nanostructures Engineered Using Surface-Initiated Assembly. J. Vis. Exp. 2014, 51176. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Chen, Y.; Wang, S.Y.; Hsu, L.C.; Feliz, Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Alumina Surfaces with Nanoscale Topography Reduce Attachment and Biofilm Formation by Escherichia Coli and Listeria Spp. Biofouling 2014, 30, 1253–1268. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Guo, W.; Xue, P.; Gao, H.; Zhao, M.; Zheng, C.; Zhang, Y.; Han, D. Quantitative Assay for the Colonization Ability of Heterogeneous Bacteria on Controlled Nanopillar Structures. Nanotechnology 2015, 26, 055702. [Google Scholar] [CrossRef]
- Jahed, Z.; Lin, P.; Seo, B.B.; Verma, M.S.; Gu, F.X.; Tsui, T.Y.; Mofrad, M.R.K. Responses of Staphylococcus Aureus Bacterial Cells to Nanocrystalline Nickel Nanostructures. Biomaterials 2014, 35, 4249–4254. [Google Scholar] [CrossRef]
- Gu, H.; Chen, A.; Song, X.; Brasch, M.E.; Henderson, J.H.; Ren, D. How Escherichia Coli Lands and Forms Cell Clusters on a Surface: A New Role of Surface Topography. Sci. Rep. 2016, 6, 29516. [Google Scholar] [CrossRef]
- Hou, S.; Gu, H.; Smith, C.; Ren, D. Microtopographic Patterns Affect Escherichia Coli Biofilm Formation on Poly(Dimethylsiloxane) Surfaces. Langmuir 2011, 27, 2686–2691. [Google Scholar] [CrossRef]
- Wallqvist, V.; Claesson, P.M.; Swerin, A.; Östlund, C.; Schoelkopf, J.; Gane, P.A.C. Influence of Surface Topography on Adhesive and Long-Range Capillary Forces between Hydrophobic Surfaces in Water. Langmuir 2009, 25, 9197–9207. [Google Scholar] [CrossRef]
- Hansson, P.M.; Swerin, A.; Schoelkopf, J.; Gane, P.A.C.; Thormann, E. Influence of Surface Topography on the Interactions between Nanostructured Hydrophobic Surfaces. Langmuir 2012, 28, 8026–8034. [Google Scholar] [CrossRef]
- Heydari, G.; Thormann, E.; Järn, M.; Tyrode, E.; Claesson, P.M. Hydrophobic Surfaces: Topography Effects on Wetting by Supercooled Water and Freezing Delay. J. Phys. Chem. C 2013, 117, 21752–21762. [Google Scholar] [CrossRef]
- Xin, B.; Hao, J. Reversibly Switchable Wettability. Chem. Soc. Rev. 2010, 39, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.; Winandy, J.E.; Shupe, T.F. From Hydrophilicity to Hydrophobicity: A Critical Review: Part I. Wettability and Surface Behavior. Wood Fiber Sci. 2010, 490–510. [Google Scholar]
- Dai, Z.; Lu, L.; Sun, Y.; Tang, Z.; Lu, X. Wetting Control through Topography and Surface Hydrophilic/Hydrophobic Property Changes by Coarse Grained Simulation. Mol. Simul. 2017, 43, 1202–1208. [Google Scholar] [CrossRef]
- Santander-Borrego, M.; Taran, E.; Shadforth, A.M.A.; Whittaker, A.K.; Chirila, T.V.; Blakey, I. Hydrogels with Lotus Leaf Topography: Investigating Surface Properties and Cell Adhesion. Langmuir 2017, 33, 485–493. [Google Scholar] [CrossRef]
- Mock, U.; Förster, R.; Menz, W.; Rühe, J. Towards Ultrahydrophobic Surfaces: A Biomimetic Approach. J. Phys. Condens. Matter 2005, 17, S639. [Google Scholar] [CrossRef]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the Influence of Surface Topography on Bacterial Adhesion Using Spatially Organized Microtopographic Surface Patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial Flagella Explore Microscale Hummocks and Hollows to Increase Adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef]
- Limongi, T.; Tirinato, L.; Pagliari, F.; Giugni, A.; Allione, M.; Perozziello, G.; Candeloro, P.; Di Fabrizio, E. Fabrication and Applications of Micro/Nanostructured Devices for Tissue Engineering. Nano-Micro Lett. 2016, 9, 1. [Google Scholar] [CrossRef]
- McMahon, R.E.; Qu, X.; Jimenez-Vergara, A.C.; Bashur, C.A.; Guelcher, S.A.; Goldstein, A.S.; Hahn, M.S. Hydrogel–Electrospun Mesh Composites for Coronary Artery Bypass Grafts. Tissue Eng. Part C Methods 2011, 17, 451–461. [Google Scholar] [CrossRef]
- Ainslie, K.M.; Desai, T.A. Microfabricated Implants for Applications in Therapeutic Delivery, Tissue Engineering, and Biosensing. Lab Chip 2008, 8, 1864–1878. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.J.; Correlo, V.M.; Reis, R.L. Micro/Nano Replication and 3D Assembling Techniques for Scaffold Fabrication. Mater. Sci. Eng. C 2014, 42, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Formisano, N.; Sahin, G.; Català, P.; Truckenmüller, R.; Nuijts, R.M.M.A.; Dickman, M.M.; LaPointe, V.L.S.; Giselbrecht, S. Nanoscale Topographies for Corneal Endothelial Regeneration. Appl. Sci. 2021, 11, 827. [Google Scholar] [CrossRef]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New Approaches to Nanofabrication: Molding, Printing, and Other Techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Limongi, T.; Schipani, R.; Di Vito, A.; Giugni, A.; Francardi, M.; Torre, B.; Allione, M.; Miele, E.; Malara, N.; Alrasheed, S.; et al. Photolithography and Micromolding Techniques for the Realization of 3D Polycaprolactone Scaffolds for Tissue Engineering Applications. Microelectron. Eng. 2015, 141, 135–139. [Google Scholar] [CrossRef]
- Dalton, P.D.; Woodfield, T.B.F.; Mironov, V.; Groll, J. Advances in Hybrid Fabrication toward Hierarchical Tissue Constructs. Adv. Sci. 2020, 7, 1902953. [Google Scholar] [CrossRef]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication Methods for Reconstructing Extracellular Matrix Mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef]
- Moroni, L.; Schotel, R.; Hamann, D.; de Wijn, J.R.; van Blitterswijk, C.A. 3D Fiber-Deposited Electrospun Integrated Scaffolds Enhance Cartilage Tissue Formation. Adv. Funct. Mater. 2008, 18, 53–60. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, T.G.; Kim, H.C.; Yang, D.-Y.; Park, T.G. Development of Dual Scale Scaffolds via Direct Polymer Melt Deposition and Electrospinning for Applications in Tissue Regeneration. Acta Biomater. 2008, 4, 1198–1207. [Google Scholar] [CrossRef]
- Mota, C.; Puppi, D.; Dinucci, D.; Errico, C.; Bártolo, P.; Chiellini, F. Dual-Scale Polymeric Constructs as Scaffolds for Tissue Engineering. Materials 2011, 4, 527–542. [Google Scholar] [CrossRef]
- Centola, M.; Rainer, A.; Spadaccio, C.; Porcellinis, S.D.; Genovese, J.A.; Trombetta, M. Combining Electrospinning and Fused Deposition Modeling for the Fabrication of a Hybrid Vascular Graft. Biofabrication 2010, 2, 014102. [Google Scholar] [CrossRef] [PubMed]
- Giselbrecht, S.; Gietzelt, T.; Gottwald, E.; Trautmann, C.; Truckenmüller, R.; Weibezahn, K.F.; Welle, A. 3D Tissue Culture Substrates Produced by Microthermoforming of Pre-Processed Polymer Films. Biomed. Microdevices 2006, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Truckenmüller, R.; Giselbrecht, S.; Escalante-Marun, M.; Groenendijk, M.; Papenburg, B.; Rivron, N.; Unadkat, H.; Saile, V.; Subramaniam, V.; van den Berg, A.; et al. Fabrication of Cell Container Arrays with Overlaid Surface Topographies. Biomed Microdevices 2012, 14, 95–107. [Google Scholar] [CrossRef]
- Li, Z. Adaptive Fabrication of Biofunctional Decellularized Extracellular Matrix Niche towards Complex Engineered Tissues. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Holmberg, S.; Garza-Flores, N.A.; Almajhadi, M.A.; Chávez-Madero, C.; Lujambio-Angeles, A.; Jind, B.; Bautista-Flores, C.; Mendoza-Buenrostro, C.; Pérez-Carrillo, E.; Wickramasinghe, H.K.; et al. Fabrication of Multilayered Composite Nanofibers Using Continuous Chaotic Printing and Electrospinning: Chaotic Electrospinning. ACS Appl. Mater. Interfaces 2021, 13, 37455–37465. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gao, C.; Gritsenko, D.; Zhou, R.; Xu, J. Soft Lithography Based on Photolithography and Two-Photon Polymerization. Microfluid Nanofluid 2018, 22, 97. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Goss, J.A.; Cho, A.; McCain, M.L.; Parker, K.K. Microfluidic Heart on a Chip for Higher Throughput Pharmacological Studies. Lab Chip 2013, 13, 3599–3608. [Google Scholar] [CrossRef]
- Wu, G.; Wu, J.; Li, Z.; Shi, S.; Wu, D.; Wang, X.; Xu, H.; Liu, H.; Huang, Y.; Wang, R.; et al. Development of Digital Organ-on-a-Chip to Assess Hepatotoxicity and Extracellular Vesicle-Based Anti-Liver Cancer Immunotherapy. Bio-Des. Manuf. 2022, 5, 437–450. [Google Scholar] [CrossRef]
- Jang, K.-J.; Mehr, A.P.; Hamilton, G.A.; McPartlin, L.A.; Chung, S.; Suh, K.-Y.; Ingber, D.E. Human Kidney Proximal Tubule-on-a-Chip for Drug Transport and Nephrotoxicity Assessment. Integr. Biol. 2013, 5, 1119–1129. [Google Scholar] [CrossRef]
- Agarwal, T.; Onesto, V.; Lamboni, L.; Ansari, A.; Maiti, T.K.; Makvandi, P.; Vosough, M.; Yang, G. Engineering Biomimetic Intestinal Topological Features in 3D Tissue Models: Retrospects and Prospects. Bio-Des. Manuf. 2021, 4, 568–595. [Google Scholar] [CrossRef]
- Berdichevsky, Y.; Staley, K.J.; Yarmush, M.L. Building and Manipulating Neural Pathways with Microfluidics. Lab Chip 2010, 10, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Aazmi, A.; Zhou, H.; Li, Y.; Yu, M.; Xu, X.; Wu, Y.; Ma, L.; Zhang, B.; Yang, H. Engineered Vasculature for Organ-on-a-Chip Systems. Engineering 2022, 9, 131–147. [Google Scholar] [CrossRef]
- Haderspeck, J.C.; Chuchuy, J.; Kustermann, S.; Liebau, S.; Loskill, P. Organ-on-a-Chip Technologies That Can Transform Ophthalmic Drug Discovery and Disease Modeling. Expert Opin. Drug Discov. 2019, 14, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhou, L.; Wong, J.K.W.; Chan, Y.K. Eye-on-a-Chip (EOC) Models and Their Role in the Future of Ophthalmic Drug Discovery. Expert Rev. Ophthalmol. 2020, 15, 259–261. [Google Scholar] [CrossRef]
- Manafi, N.; Shokri, F.; Achberger, K.; Hirayama, M.; Mohammadi, M.H.; Noorizadeh, F.; Hong, J.; Liebau, S.; Tsuji, T.; Quinn, P.M.J.; et al. Organoids and Organ Chips in Ophthalmology. Ocul. Surf. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Li, Z.; Hui, J.; Yang, P.; Mao, H. Microfluidic Organ-on-a-Chip System for Disease Modeling and Drug Development. Biosensors 2022, 12, 370. [Google Scholar] [CrossRef]
- Li, Q.; Wong, H.L.; Ip, Y.L.; Chu, W.Y.; Li, M.S.; Saha, C.; Shih, K.C.; Chan, Y.K. Current Microfluidic Platforms for Reverse Engineering of Cornea. Mater. Today Bio 2023, 20, 100634. [Google Scholar] [CrossRef]
- Abdalkader, R.; Chaleckis, R.; Wheelock, C.E.; Kamei, K. Spatiotemporal Determination of Metabolite Activities in the Corneal Epithelium on a Chip. Exp. Eye Res. 2021, 209, 108646. [Google Scholar] [CrossRef]
- Yu, Z.; Hao, R.; Du, J.; Wu, X.; Chen, X.; Zhang, Y.; Li, W.; Gu, Z.; Yang, H. A Human Cornea-on-a-Chip for the Study of Epithelial Wound Healing by Extracellular Vesicles. iScience 2022, 25, 104200. [Google Scholar] [CrossRef]
- Bennet, D.; Estlack, Z.; Reid, T.; Kim, J. A Microengineered Human Corneal Epithelium-on-a-Chip for Eye Drops Mass Transport Evaluation. Lab Chip 2018, 18, 1539–1551. [Google Scholar] [CrossRef]
- Al-Hazeem, N.Z.A. Nanofibers and Electrospinning Method. In Novel Nanomaterials—Synthesis and Applications; IntechOpen: London, UK, 2018; ISBN 978-1-78923-089-5. [Google Scholar]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Arica, T.A.; Guzelgulgen, M.; Yildiz, A.A.; Demir, M.M. Electrospun GelMA Fibers and p(HEMA) Matrix Composite for Corneal Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111720. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Chen, K.M.; Margolis, M.S.; Wungcharoen, T.; Koh, W.-G.; Myung, D. Electrospun Nanofiber Membrane for Cultured Corneal Endothelial Cell Transplantation. Bioengineering 2024, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Kurpanik, R.; Gajek, M.; Gryń, K.; Jeleń, P.; Ścisłowska-Czarnecka, A.; Stodolak-Zych, E. Multiscale Characterization of Electrospun Non-Wovens for Corneal Regeneration: Impact of Microstructure on Mechanical, Optical and Biological Properties. J. Mech. Behav. Biomed. Mater. 2024, 152, 106437. [Google Scholar] [CrossRef] [PubMed]
- Momenzadeh, D.; Baradaran-Rafii, A.; Keshel, S.H.; Ebrahimi, M.; Biazar, E. Electrospun Mat with Eyelid Fat-Derived Stem Cells as a Scaffold for Ocular Epithelial Regeneration. Artif. Cells Nanomed. Biotechnol. 2017, 45, 120–127. [Google Scholar] [CrossRef]
- Patel, G.; Na, K.-S.; Lee, H.J.; Koh, W.-G. Electrospun Fibers for Corneal Regeneration. Curr. Ophthalmol. Rep. 2021, 9, 146–157. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, D.; Mohanty, S.; Jassal, M.; Agrawal, A.K.; Tandon, R. Surface-Modified Electrospun Poly(ε-Caprolactone) Scaffold With Improved Optical Transparency and Bioactivity for Damaged Ocular Surface Reconstruction. Investig. Ophthalmol. Vis. Sci. 2014, 55, 899–907. [Google Scholar] [CrossRef]
- Kong, B.; Sun, W.; Chen, G.; Tang, S.; Li, M.; Shao, Z.; Mi, S. Tissue-Engineered Cornea Constructed with Compressed Collagen and Laser-Perforated Electrospun Mat. Sci. Rep. 2017, 7, 970. [Google Scholar] [CrossRef]
- Kong, B.; Mi, S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. [Google Scholar] [CrossRef]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 1908996. [Google Scholar] [CrossRef]
- Carter, K.; Lee, H.J.; Na, K.-S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the Impact of 2D and 3D Culture Conditions on the Therapeutic Effects of Human Mesenchymal Stem Cell Secretome on Corneal Wound Healing In Vitro and Ex Vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, D.; Yang, Y.; Oh, D.K.; Seong, J.; Kim, J.; Jeon, N.; Kang, D.; Rho, J. Emerging Low-Cost, Large-Scale Photonic Platforms with Soft Lithography and Self-Assembly. Photonics Insights 2023, 2, R04. [Google Scholar] [CrossRef]
- Tony, A.; Badea, I.; Yang, C.; Liu, Y.; Wells, G.; Wang, K.; Yin, R.; Zhang, H.; Zhang, W. The Additive Manufacturing Approach to Polydimethylsiloxane (PDMS) Microfluidic Devices: Review and Future Directions. Polymers 2023, 15, 1926. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Tu, Q.; Liu, W.; Wang, J. Recent Advances in Microfluidic Technologies for Organ-on-a-Chip. TrAC Trends Anal. Chem. 2019, 117, 146–156. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Gangnaik, A.S.; Georgiev, Y.M.; Collins, G.; Holmes, J.D. Novel Germanium Surface Modification for Sub-10 Nm Patterning with Electron Beam Lithography and Hydrogen Silsesquioxane Resist. J. Vac. Sci. Technol. B 2016, 34, 041603. [Google Scholar] [CrossRef]
- Zhu, C.; Ekinci, H.; Pan, A.; Cui, B.; Zhu, X. Electron Beam Lithography on Nonplanar and Irregular Surfaces. Microsyst. Nanoeng. 2024, 10, 52. [Google Scholar] [CrossRef]
- Rajput, N.S.; Luo, X. FIB Micro-/Nano-Fabrication; Qin, Y., Ed.; Elsevier Limited: Amsterdam, The Netherlands, 2015; pp. 61–80. ISBN 978-0-323-31149-6. [Google Scholar]
- Erdmanis, M.; Sievilä, P.; Shah, A.; Chekurov, N.; Ovchinnikov, V.; Tittonen, I. Focused Ion Beam Lithography for Fabrication of Suspended Nanostructures on Highly Corrugated Surfaces. Nanotechnology 2014, 25, 335302. [Google Scholar] [CrossRef]
- Li, P.; Chen, S.; Dai, H.; Yang, Z.; Chen, Z.; Wang, Y.; Chen, Y.; Peng, W.; Shan, W.; Duan, H. Recent Advances in Focused Ion Beam Nanofabrication for Nanostructures and Devices: Fundamentals and Applications. Nanoscale 2021, 13, 1529–1565. [Google Scholar] [CrossRef]
- Fan, P.; Gao, J.; Mao, H.; Geng, Y.; Yan, Y.; Wang, Y.; Goel, S.; Luo, X. Scanning Probe Lithography: State-of-the-Art and Future Perspectives. Micromachines 2022, 13, 228. [Google Scholar] [CrossRef]
- Albisetti, E.; Petti, D.; Pancaldi, M.; Madami, M.; Tacchi, S.; Curtis, J.; King, W.P.; Papp, A.; Csaba, G.; Porod, W.; et al. Nanopatterning Reconfigurable Magnetic Landscapes via Thermally Assisted Scanning Probe Lithography. Nat. Nanotechnol. 2016, 11, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Albisetti, E.; Carroll, K.M.; Lu, X.; Curtis, J.E.; Petti, D.; Bertacco, R.; Riedo, E. Thermochemical Scanning Probe Lithography of Protein Gradients at the Nanoscale. Nanotechnology 2016, 27, 315302. [Google Scholar] [CrossRef] [PubMed]
- Vigneswaran, N.; Samsuri, F.; Ranganathan, B. Padmapriya Recent Advances in Nano Patterning and Nano Imprint Lithography for Biological Applications. Procedia Eng. 2014, 97, 1387–1398. [Google Scholar] [CrossRef]
- Elsadek, N.E.; Nagah, A.; Ibrahim, T.M.; Chopra, H.; Ghonaim, G.A.; Emam, S.E.; Cavalu, S.; Attia, M.S. Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine. Materials 2022, 15, 1934. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, L. An Overview on Electrospinning and Its Advancement toward Hard and Soft Tissue Engineering Applications. Colloid Polym. Sci. 2022, 300, 875–901. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Sabra, S.; Ragab, D.M.; Agwa, M.M.; Rohani, S. Recent Advances in Electrospun Nanofibers for Some Biomedical Applications. Eur. J. Pharm. Sci. 2020, 144, 105224. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Narayan, R.J. Two-Photon Polymerization for Biological Applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Raimondi, M.T.; Eaton, S.M.; Nava, M.M.; Laganà, M.; Cerullo, G.; Osellame, R. Two-Photon Laser Polymerization: From Fundamentals to Biomedical Application in Tissue Engineering and Regenerative Medicine. J. Appl. Biomater. Funct. Mater. 2012, 10, 56–66. [Google Scholar] [CrossRef]
- Cheng, D.; Jayne, R.K.; Tamborini, A.; Eyckmans, J.; White, A.E.; Chen, C.S. Studies of 3D Directed Cell Migration Enabled by Direct Laser Writing of Curved Wave Topography. Biofabrication 2019, 11, 021001. [Google Scholar] [CrossRef]
- Prina, E.; Amer, M.H.; Sidney, L.; Tromayer, M.; Moore, J.; Liska, R.; Bertolin, M.; Ferrari, S.; Hopkinson, A.; Dua, H.; et al. Bioinspired Precision Engineering of Three-Dimensional Epithelial Stem Cell Microniches. Adv. Biosyst. 2020, 4, 2000016. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, A.; Gittard, S.; Schlie, S.; Deiwick, A.; Jockenhoevel, S.; Chichkov, B. Fabrication of Fibrin Scaffolds with Controlled Microscale Architecture by a Two-Photon Polymerization–Micromolding Technique. Biofabrication 2012, 4, 015001. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Malinauskas, M.; Schlie, S.; Chichkov, B.; Gittard, S.; Narayan, R.; Löbler, M.; Sternberg, K.; Schmitz, K.-P.; Haverich, A. Three-Dimensional Laser Micro- and Nano-Structuring of Acrylated Poly(Ethylene Glycol) Materials and Evaluation of Their Cytoxicity for Tissue Engineering Applications. Acta Biomater. 2011, 7, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Gruene, M.; Pflaum, M.; Koch, L.; Maiorana, F.; Wilhelmi, M.; Haverich, A.; Chichkov, B. Laser Printing of Cells into 3D Scaffolds. Biofabrication 2010, 2, 014104. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, S.; Pandit, A.; Heise, A.; Kellett, A. Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies. Adv. Sci. (Weinh.) 2023, 10, e2204072. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Vale, A.C.; Alves, N.M. Spin-Coated Freestanding Films for Biomedical Applications. J. Mater. Chem. B 2021, 9, 3778–3799. [Google Scholar] [CrossRef]
- Kasai, R.D.; Radhika, D.; Archana, S.; Shanavaz, H.; Koutavarapu, R.; Lee, D.-Y.; Shim, J. A Review on Hydrogels Classification and Recent Developments in Biomedical Applications. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1059–1069. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Ghalandari, B.; Ghorbani, F.; Varma, S.N.; Liu, C. Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers 2023, 15, 1068. [Google Scholar] [CrossRef]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, H.; Li, Y.; Lee, A.L.; Li, Z.; Fu, M.; Li, C.; Yang, Y.Y.; Yuan, P. Synthetic Peptide Hydrogels as 3D Scaffolds for Tissue Engineering. Adv. Drug Deliv. Rev. 2020, 160, 78–104. [Google Scholar] [CrossRef]
- Gao, C.; Song, S.; Lv, Y.; Huang, J.; Zhang, Z. Recent Development of Conductive Hydrogels for Tissue Engineering: Review and Perspective. Macromol. Biosci. 2022, 22, 2200051. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent Advances of Injectable Hydrogels for Drug Delivery and Tissue Engineering Applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Shar, A.; Shar, A.; Joung, D. Carbon Nanotube Nanocomposite Scaffolds: Advances in Fabrication and Applications for Tissue Regeneration and Cancer Therapy. Front. Bioeng. Biotechnol. 2023, 11, 1299166. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, M.; Lee, S.K.; Kang, J.; Nam, J.-D.; Ci, L.; Suhr, J. Tensile Properties of Millimeter-Long Multi-Walled Carbon Nanotubes. Sci. Rep. 2017, 7, 9512. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An Overview of Recent Development in Therapeutic Drug Carrier System Using Carbon Nanotubes. J. Drug Deliv. Sci. Technol. 2020, 59, 101855. [Google Scholar] [CrossRef]
- Akasaka, T.; Yokoyama, A.; Matsuoka, M.; Hashimoto, T.; Watari, F. Maintenance of Hemiround Colonies and Undifferentiated State of Mouse Induced Pluripotent Stem Cells on Carbon Nanotube-Coated Dishes. Carbon 2011, 49, 2287–2299. [Google Scholar] [CrossRef]
- Brunner, E.W.; Jurewicz, I.; Heister, E.; Fahimi, A.; Bo, C.; Sear, R.P.; Donovan, P.J.; Dalton, A.B. Growth and Proliferation of Human Embryonic Stem Cells on Fully Synthetic Scaffolds Based on Carbon Nanotubes. ACS Appl. Mater. Interfaces 2014, 6, 2598–2603. [Google Scholar] [CrossRef]
- Hirata, E.; Uo, M.; Nodasaka, Y.; Takita, H.; Ushijima, N.; Akasaka, T.; Watari, F.; Yokoyama, A. 3D Collagen Scaffolds Coated with Multiwalled Carbon Nanotubes: Initial Cell Attachment to Internal Surface. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93B, 544–550. [Google Scholar] [CrossRef]
- da Silva, E.E.; Della Colleta, H.H.M.; Ferlauto, A.S.; Moreira, R.L.; Resende, R.R.; Oliveira, S.; Kitten, G.T.; Lacerda, R.G.; Ladeira, L.O. Nanostructured 3-D Collagen/Nanotube Biocomposites for Future Bone Regeneration Scaffolds. Nano Res. 2009, 2, 462–473. [Google Scholar] [CrossRef]
- Santoni, S.; Gugliandolo, S.G.; Sponchioni, M.; Moscatelli, D.; Colosimo, B.M. 3D Bioprinting: Current Status and Trends—a Guide to the Literature and Industrial Practice. Bio-Des. Manuf. 2022, 5, 14–42. [Google Scholar] [CrossRef]
- Wu, C.A.; Zhu, Y.; Woo, Y.J. Advances in 3D Bioprinting: Techniques, Applications, and Future Directions for Cardiac Tissue Engineering. Bioengineering 2023, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D Bioprinting of a Corneal Stroma Equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human Stem Cell Based Corneal Tissue Mimicking Structures Using Laser-Assisted 3D Bioprinting and Functional Bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, M.-N.; Kim, J.; Jang, J.; Kim, H.-K.; Cho, D.-W. Characterization of Cornea-Specific Bioink: High Transparency, Improved in Vivo Safety. J. Tissue Eng. 2019, 10, 2041731418823382. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.-F.; Mehta, J.S.; Fischer, H.; et al. Corneal Bioprinting Utilizing Collagen-Based Bioinks and Primary Human Keratocytes. J. Biomed. Mater. Res. Part A 2019, 107, 1945–1953. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber Reinforced GelMA Hydrogel to Induce the Regeneration of Corneal Stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef]
- Bektas, C.K.; Hasirci, V. Cell Loaded 3D Bioprinted GelMA Hydrogels for Corneal Stroma Engineering. Biomater. Sci. 2019, 8, 438–449. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Park, J.; Lee, K.-P.; Lee, S.; Lee, D.-M.; Kim, K.H.; Kim, H.K.; Cho, D.-W. Shear-Induced Alignment of Collagen Fibrils Using 3D Cell Printing for Corneal Stroma Tissue Engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting Three-Dimensional Cell-Laden Tissue Constructs with Controllable Degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Q.; Hu, H.; Yu, M.; Gao, L.; Luo, Y.; Li, Y.; Li, J.; Ma, L.; Yao, Y.; et al. Integrated 3D Bioprinting-Based Geometry-Control Strategy for Fabricating Corneal Substitutes. J. Zhejiang Univ. Sci. B 2019, 20, 945–959. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S.J.; Park, S.H.; Kim, J.C. Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells. Adv. Healthc. Mater. 2018, 7, 1800398. [Google Scholar] [CrossRef] [PubMed]
- Grönroos, P.; Mörö, A.; Puistola, P.; Hopia, K.; Huuskonen, M.; Viheriälä, T.; Ilmarinen, T.; Skottman, H. Bioprinting of Human Pluripotent Stem Cell Derived Corneal Endothelial Cells with Hydrazone Crosslinked Hyaluronic Acid Bioink. Stem Cell Res. Ther. 2024, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lu, B.; Li, M.; Yang, X.; Qin, M.; Wu, Y. Development of Digital Light Processing-Based Multi-Material Bioprinting for Fabrication of Heterogeneous Tissue Constructs. Biomater. Sci. 2023, 11, 6663–6673. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Kuang, X.; Roach, D.J.; Wang, Y.; Hamel, C.M.; Lu, C.; Qi, H.J. Integrating Digital Light Processing with Direct Ink Writing for Hybrid 3D Printing of Functional Structures and Devices. Addit. Manuf. 2021, 40, 101911. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Mille, L.S.; Ching, T.; Luo, Z.; Tang, G.; Garciamendez, C.E.; Lesha, A.; Hashimoto, M.; Zhang, Y.S. Digital Light Processing Based Bioprinting with Composable Gradients. Adv. Mater. 2022, 34, e2107038. [Google Scholar] [CrossRef]
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Tran, Q.; Ajeti, V.; Freeman, B.T.; Fast, V.G.; et al. Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef]
- Ramiah, P.; du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Hydrogel-Based Bioinks for 3D Bioprinting in Tissue Regeneration. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; Araujo, W.A.; Zorlutuna, P.; Vrana, N.E.; Ghaemmaghami, A.M.; Dokmeci, M.R.; et al. Direct-Write Bioprinting of Cell-Laden Methacrylated Gelatin Hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Rad, S.H.; Naderi, G.; Saeb, M.R.; et al. Chitosan-Based Inks for 3D Printing and Bioprinting. Green Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Raza, A.; Xue, Y.-Q.; Yang, G.; Hayat, U.; Yu, J.; Liu, C.; Wang, H.-J.; Wang, J.-Y. Water-Responsive 4D Printing Based on Self-Assembly of Hydrophobic Protein “Zein” for the Control of Degradation Rate and Drug Release. Bioact. Mater. 2023, 23, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, D.; Liao, P.; Su, J.-W.; Deng, H.; Vardhanabhuti, B.; Ulery, B.D.; Chen, S.-Y.; Lin, J. 4D Printing of Shape-Memory Polymeric Scaffolds for Adaptive Biomedical Implantation. Acta Biomater. 2021, 122, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kabirian, F.; Mela, P.; Heying, R. 4D Printing Applications in the Development of Smart Cardiovascular Implants. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef]
- Roberts, C.T.; Beck, S.K.; Prejean, C.M.; Graul, L.M.; Maitland, D.J.; Grunlan, M.A. Star-PCL Shape Memory Polymer (SMP) Scaffolds with Tunable Transition Temperatures for Enhanced Utility. J. Mater. Chem. B 2024, 12, 3694–3702. [Google Scholar] [CrossRef]
- Namathoti, S.; Vakkalagadda, M.R.K. Development of Multiwalled Carbon Nanotubes/Halloysite Nanotubes Reinforced Thermal Responsive Shape Memory Polymer Nanocomposites for Enhanced Mechanical and Shape Recovery Characteristics in 4D Printing Applications. Polymers 2023, 15, 1371. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H.; Sheng, J.; Agyenim-Boateng, E.; Wang, C.; Yang, H.; Wei, J.; Jiang, G.; Zhou, J.; Lu, J.; et al. Digital Light Processing 4D Printing Multilayer Polymers with Tunable Mechanical Properties and Shape Memory Behavior. Chem. Eng. J. 2023, 465, 142830. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. 4D Printing-Encapsulated Polycaprolactone–Thermoplastic Polyurethane with High Shape Memory Performances. Adv. Eng. Mater. 2023, 25, 2201309. [Google Scholar] [CrossRef]
- Thakur, V.; Singh, R.; Kumar, R.; Gehlot, A. 4D Printing of Thermoresponsive Materials: A State-of-the-Art Review and Prospective Applications. Int. J. Interact. Des. Manuf. 2023, 17, 2075–2094. [Google Scholar] [CrossRef]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-Responsive Polymers: Applications of Smart Materials in Drug Delivery and Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Mu, X.; Feng, W.; Lu, Y.; Yu, W.; Zhou, X. Reducing Thermal Damage to Adjacent Normal Tissue with Dual Thermo-Responsive Polymer via Thermo-Induced Phase Transition for Precise Photothermal Theranosis. Acta Biomater. 2022, 148, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Chiesa, I.; Costantini, M.; Lopamarda, A.; Tirelli, M.C.; Borra, O.P.; Varshapally, S.V.S.; Kumar, Y.A.V.; Koteswara Reddy, G.; De Maria, C.; et al. Chitosan and Its Derivatives in 3D/4D (Bio) Printing for Tissue Engineering and Drug Delivery Applications. Int. J. Biol. Macromol. 2023, 246, 125669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, F.; Leng, J.; Liu, Y. Personalized 4D Printing of Bioinspired Tracheal Scaffold Concept Based on Magnetic Stimulated Shape Memory Composites. Compos. Sci. Technol. 2019, 184, 107866. [Google Scholar] [CrossRef]
- Simińska-Stanny, J.; Nizioł, M.; Szymczyk-Ziółkowska, P.; Brożyna, M.; Junka, A.; Shavandi, A.; Podstawczyk, D. 4D Printing of Patterned Multimaterial Magnetic Hydrogel Actuators. Addit. Manuf. 2022, 49, 102506. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Huang, W.; Lei, Y. Injectable Smart Stimuli-Responsive Hydrogels: Pioneering Advancements in Biomedical Applications. Biomater. Sci. 2023, 12, 8–56. [Google Scholar] [CrossRef]
- Breakthrough Injectable Hydrogel Drug Delivery System for Advanced Medical Treatment Applications. Available online: https://phys.org/news/2023-08-breakthrough-hydrogel-drug-delivery-advanced.html (accessed on 13 August 2024).
- Abdullah, T.; Okay, O. 4D Printing of Body Temperature-Responsive Hydrogels Based on Poly(Acrylic Acid) with Shape-Memory and Self-Healing Abilities. ACS Appl. Bio Mater. 2023, 6, 703–711. [Google Scholar] [CrossRef]
- Zu, S.; Zhang, Z.; Liu, Q.; Wang, Z.; Song, Z.; Guo, Y.; Xin, Y.; Zhang, S. 4D Printing of Core–Shell Hydrogel Capsules for Smart Controlled Drug Release. Bio-Des. Manuf. 2022, 5, 294–304. [Google Scholar] [CrossRef]
- Ramezani, M.; Mohd Ripin, Z. 4D Printing in Biomedical Engineering: Advancements, Challenges, and Future Directions. J. Funct. Biomater. 2023, 14, 347. [Google Scholar] [CrossRef]
- Parın, F.N. 3D and 4D Bioprinting Technology for Tissue Engineering Applications. In Interaction of Nanomaterials With Living Cells; Sheikh, F.A., Majeed, S., Beigh, M.A., Eds.; Springer Nature: Singapore, 2023; pp. 213–250. ISBN 978-981-9921-19-5. [Google Scholar]
- Wang, Z.; Jiang, C.; Fan, Y.; Hao, X.; Dong, Y.; He, X.; Gao, J.; Zhang, Y.; Li, M.; Wang, M.; et al. The Application of a 4D-Printed Chitosan-Based Stem Cell Carrier for the Repair of Corneal Alkali Burns. Stem Cell Res. Ther. 2024, 15, 41. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Wang, X.; Shang, Z.; Liu, Y.; Pan, N.; Sun, X.; Xu, W. Limbal Stem Cells Carried by a Four-Dimensional -Printed Chitosan-Based Scaffold for Corneal Epithelium Injury in Diabetic Rabbits. Front. Physiol. 2024, 15, 1285850. [Google Scholar] [CrossRef]
- McLoughlin, S.T.; McKenna, A.R.; Fisher, J.P. 4D Bioprinting via Molecular Network Contraction for Membranous Tissue Fabrication. Adv. Healthc. Mater. 2023, 12, e2300642. [Google Scholar] [CrossRef] [PubMed]
- Miotto, M.; Gouveia, R.M.; Ionescu, A.M.; Figueiredo, F.; Hamley, I.W.; Connon, C.J. 4D Corneal Tissue Engineering: Achieving Time-Dependent Tissue Self-Curvature through Localized Control of Cell Actuators. Adv. Funct. Mater. 2019, 29, 1807334. [Google Scholar] [CrossRef]
- Natu, M.V.; Gaspar, M.N.; Ribeiro, C.A.F.; Correia, I.J.; Silva, D.; de Sousa, H.C.; Gil, M.H. A Poly(ε-Caprolactone) Device for Sustained Release of an Anti-Glaucoma Drug. Biomed. Mater. 2011, 6, 025003. [Google Scholar] [CrossRef] [PubMed]
- Ilka, R.; Mohseni, M.; Kianirad, M.; Naseripour, M.; Ashtari, K.; Mehravi, B. Nanogel-Based Natural Polymers as Smart Carriers for the Controlled Delivery of Timolol Maleate through the Cornea for Glaucoma. Int. J. Biol. Macromol. 2018, 109, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S. Interpenetrating Polymeric Network (IPNs) in Ophthalmic Drug Delivery: Breaking the Barriers. Int. Ophthalmol. 2023, 43, 1063–1074. [Google Scholar] [CrossRef]
- Cegielska, O.; Sierakowski, M.; Sajkiewicz, P.; Lorenz, K.; Kogermann, K. Mucoadhesive Brinzolamide-Loaded Nanofibers for Alternative Glaucoma Treatment. Eur. J. Pharm. Biopharm. 2022, 180, 48–62. [Google Scholar] [CrossRef]
- Taka, E.; Karavasili, C.; Bouropoulos, N.; Moschakis, T.; Andreadis, D.D.; Zacharis, C.K.; Fatouros, D.G. Ocular Co-Delivery of Timolol and Brimonidine from a Self-Assembling Peptide Hydrogel for the Treatment of Glaucoma: In Vitro and Ex Vivo Evaluation. Pharmaceuticals 2020, 13, 126. [Google Scholar] [CrossRef]
- Da Silva, G.R.; Lima, T.H.; Fernandes-Cunha, G.M.; Oréfice, R.L.; Da Silva-Cunha, A.; Zhao, M.; Behar-Cohen, F. Ocular Biocompatibility of Dexamethasone Acetate Loaded Poly(ɛ-Caprolactone) Nanofibers. Eur. J. Pharm. Biopharm. 2019, 142, 20–30. [Google Scholar] [CrossRef]
- Göttel, B.; Lucas, H.; Syrowatka, F.; Knolle, W.; Kuntsche, J.; Heinzelmann, J.; Viestenz, A.; Mäder, K. In Situ Gelling Amphotericin B Nanofibers: A New Option for the Treatment of Keratomycosis. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Tawfik, E.A.; Alshamsan, A.; Abul Kalam, M.; Raish, M.; Alkholief, M.; Stapleton, P.; Harvey, K.; Craig, D.Q.M.; Barker, S.A. In Vitro and In Vivo Biological Assessment of Dual Drug-Loaded Coaxial Nanofibers for the Treatment of Corneal Abrasion. Int. J. Pharm. 2021, 604, 120732. [Google Scholar] [CrossRef]
- De Hoon, I.; Barras, A.; Swebocki, T.; Vanmeerhaeghe, B.; Bogaert, B.; Muntean, C.; Abderrahmani, A.; Boukherroub, R.; De Smedt, S.; Sauvage, F.; et al. Influence of the Size and Charge of Carbon Quantum Dots on Their Corneal Penetration and Permeation Enhancing Properties. ACS Appl. Mater. Interfaces 2023, 15, 3760–3771. [Google Scholar] [CrossRef] [PubMed]
- Nekoueian, K.; Amiri, M.; Sillanpää, M.; Marken, F.; Boukherroub, R.; Szunerits, S. Carbon-Based Quantum Particles: An Electroanalytical and Biomedical Perspective. Chem. Soc. Rev. 2019, 48, 4281–4316. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Okumura, N.; Kitano, J.; Koizumi, N.; Tabata, Y. Development of Poly Lactic/Glycolic Acid (PLGA) Microspheres for Controlled Release of Rho-Associated Kinase Inhibitor. J. Ophthalmol. 2017. [Google Scholar] [CrossRef]
- Wang, L.; Li, A.; Zhang, D.; Zhang, M.; Ma, L.; Li, Y.; Wang, W.; Nan, K.; Chen, H.; Li, L. Injectable Double-Network Hydrogel for Corneal Repair. Chem. Eng. J. 2023, 455, 140698. [Google Scholar] [CrossRef]
- Muhindo, D.; Elkanayati, R.; Srinivasan, P.; Repka, M.A.; Ashour, E.A. Recent Advances in the Applications of Additive Manufacturing (3D Printing) in Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Zu, S.; Wang, Z.; Zhang, S.; Guo, Y.; Chen, C.; Zhang, Q.; Wang, Z.; Liu, T.; Liu, Q.; Zhang, Z. A Bioinspired 4D Printed Hydrogel Capsule for Smart Controlled Drug Release. Mater. Today Chem. 2022, 24, 100789. [Google Scholar] [CrossRef]
- Willemen, N.G.A.; Morsink, M.A.J.; Veerman, D.; da Silva, C.F.; Cardoso, J.C.; Souto, E.B.; Severino, P. From Oral Formulations to Drug-Eluting Implants: Using 3D and 4D Printing to Develop Drug Delivery Systems and Personalized Medicine. Bio-des. Manuf. 2022, 5, 85–106. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mettu, S.; Roy Choudhury, N.; Dutta, N.K. 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Pharmaceuticals 2022, 15, 1282. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Schulz-Siegmund, M. Utilizing 4D Printing to Design Smart Gastroretentive, Esophageal, and Intravesical Drug Delivery Systems. Adv. Healthc. Mater. 2023, 12, 2202631. [Google Scholar] [CrossRef]
- Uboldi, M.; Perrotta, C.; Moscheni, C.; Zecchini, S.; Napoli, A.; Castiglioni, C.; Gazzaniga, A.; Melocchi, A.; Zema, L. Insights into the Safety and Versatility of 4D Printed Intravesical Drug Delivery Systems. Pharmaceutics 2023, 15, 757. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, S.; Ke, Y.; Ding, L.; Zeng, X.; Magdassi, S.; Long, Y. 4D Printed Hydrogels: Fabrication, Materials, and Applications. Adv. Mater. Technol. 2020, 5, 2000034. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, Q.; Wu, W. Application and Prospects of Hydrogel Additive Manufacturing. Gels 2022, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-H.; Huang, S.-M.; Liu, S.-M.; Chen, J.-C.; Chen, W.-C. Hybrid-Aligned Fibers of Electrospun Gelatin with Antibiotic and Polycaprolactone Composite Membranes as an In Vitro Drug Delivery System to Assess the Potential Repair Capacity of Damaged Cornea. Polymers 2024, 16, 448. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Multifluid Electrospinning for Multi-Drug Delivery Systems: Pros and Cons, Challenges, and Future Directions. Biomater. Sci. 2022, 11, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Hatton, F.L.; Willcock, H.; Mele, E. Electrospinning of Stimuli-Responsive Polymers for Controlled Drug Delivery: pH- and Temperature-Driven Release. Biotechnol. Bioeng. 2022, 119, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.O.L.; Guerra, M.C.A.; Heneine, L.G.D.; de Oliveira, C.R.; da, S. Cunha Junior, A.; Fialho, S.L.; Oréfice, R.L. Biodegradable Core-Shell Electrospun Nanofibers Containing Bevacizumab to Treat Age-Related Macular Degeneration. J. Mater. Sci. Mater. Med. 2018, 29, 173. [Google Scholar] [CrossRef]
- Romeo, A.; Kazsoki, A.; Omer, S.; Pinke, B.; Mészáros, L.; Musumeci, T.; Zelkó, R. Formulation and Characterization of Electrospun Nanofibers for Melatonin Ocular Delivery. Pharmaceutics 2023, 15, 1296. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-Free Cell-Based Tissue Engineering Therapies: Advances, Shortfalls and Forecast. NPJ Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Melde, K.; Athanassiadis, A.G.; Missirlis, D.; Shi, M.; Seneca, S.; Fischer, P. Ultrasound-Assisted Tissue Engineering. Nat. Rev. Bioeng. 2024, 2, 486–500. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered Biomaterials for in Situ Tissue Regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Rosellini, E.; Cascone, M.G. Microfluidic Fabrication of Natural Polymer-Based Scaffolds for Tissue Engineering Applications: A Review. Biomimetics 2023, 8, 74. [Google Scholar] [CrossRef] [PubMed]

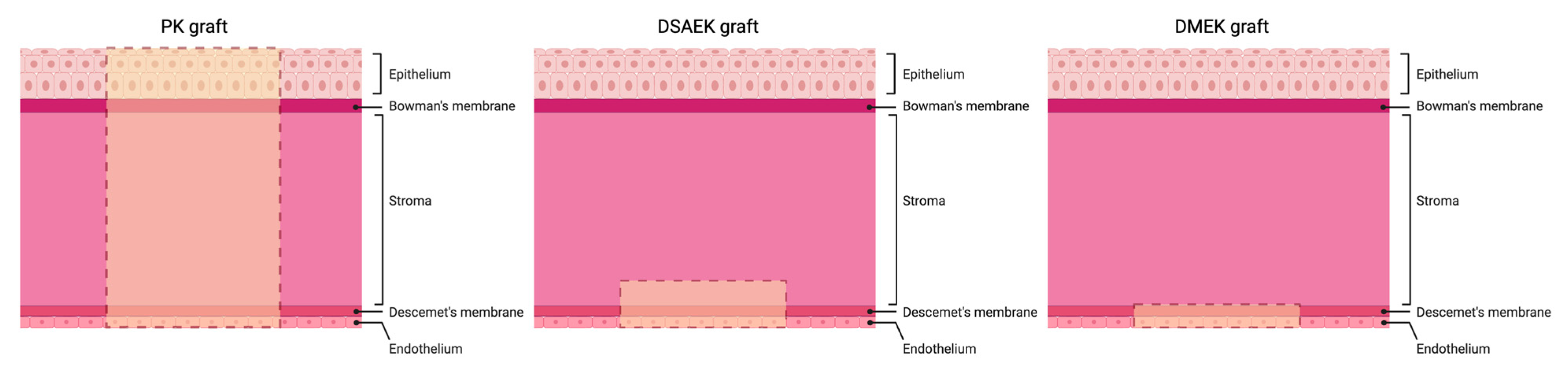

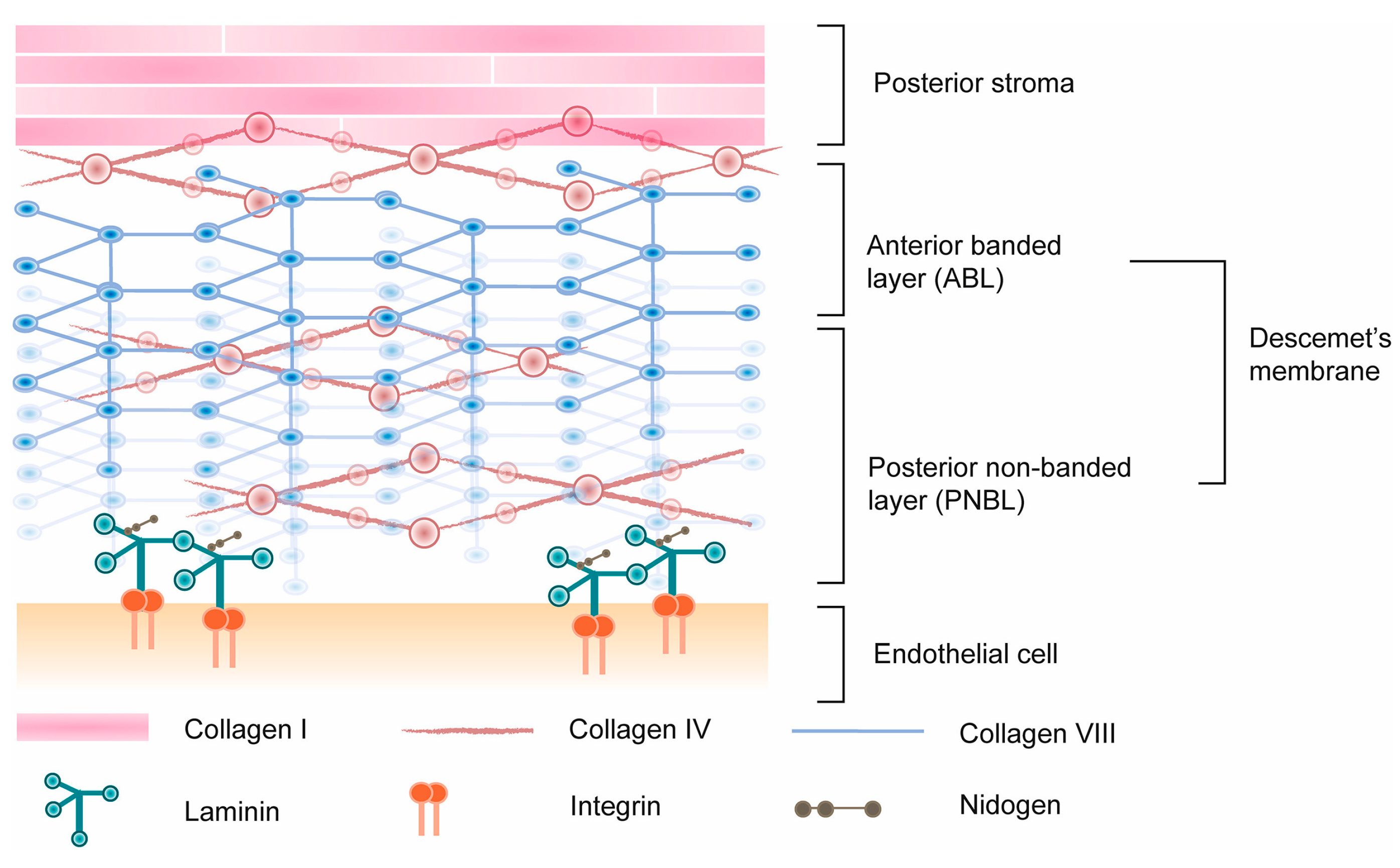
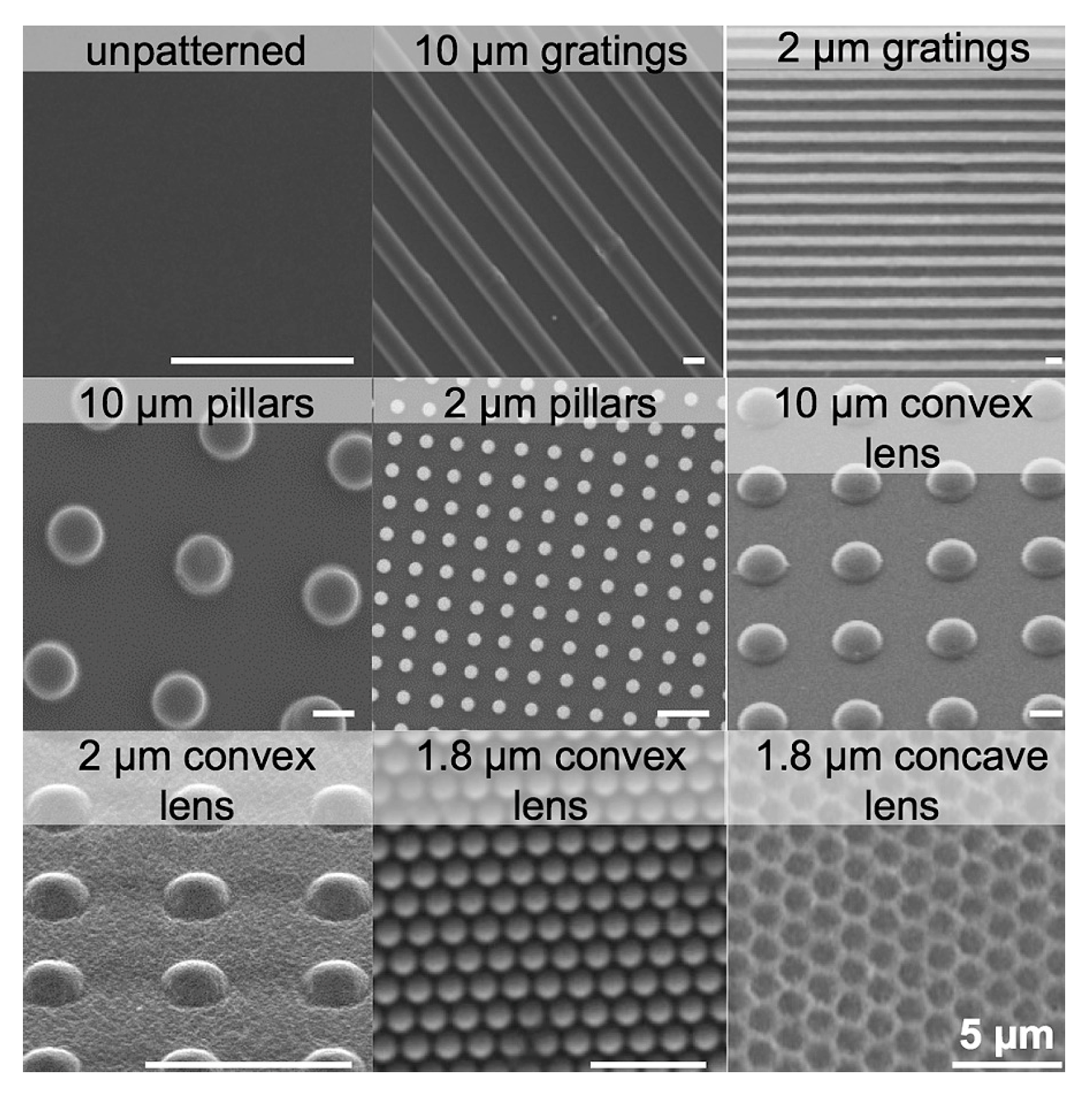


| Corneal Layer | Corneal Endothelium | Corneal Endothelium |
|---|---|---|
| Year | 2018 | 2024 |
| Bioink Method | Extrusion-based 3D printing | Extrusion-based 3D printing |
| Bioink Substrate | Lyophilized amniotic membrane | Hydrazone crosslinked hyaluronic acid |
| Cell Source | Ribonuclease 5-overexpressing human corneal endothelial cells (HCECs) and control HCECs | Human pluripotent stem cell-derived corneal endothelial cells |
| Advantage | Improved cell confluency and functionality; enhanced corneal clarity and reduced edema | High viability and printability of cells; biocompatibility with various corneal tissues; good morphology and phenotype maintenance |
| Limitation | Potential for graft shrinkage; challenges with consistent cell attachment and survival | Some areas showed mesenchymal-like cell growth; more research needed to confirm adhesive properties in vivo |
| In vivo Study | No (ex vivo transplantation on rabbit corneas) | No (ex vivo transplantation on rat and porcine corneas) |
| Use with HCECs (Yes/No) | Yes | Yes |
| Reference | [241] | [242] |
| Year | Corneal Layer | Material/Technology | Applications | Study Type | Advantages | Challenges/Limitations | Reference |
|---|---|---|---|---|---|---|---|
| 2024 | Epithelium | 4D printed chitosan-based thermosensitive hydrogel scaffold for rat limbal epithelium stem cells (LESCs) | Corneal alkali burns repair | In vitro | Excellent cytocompatibility, enhanced proliferation and differentiation of cells, temperature-sensitive properties for better adaptation to corneal environment, improved repair efficacy (lower corneal opacity, reduced neovascularization, and higher corneal epithelial wound healing rate) | Further research needed to confirm long-term effectiveness and integration in clinical settings | [271] |
| 2024 | Epithelium | 4D printed chitosan-based scaffold with LSCs (limbal stem cells) | Treatment of corneal epithelium injury in diabetic rabbits | In vivo | Rapid wound healing, improved corneal nerve repair, significant decrease in inflammation, enhanced epithelialization | Potential issues with long-term integration and stability | [272] |
| 2023 | Thin membranous tissues which include the cornea, epidermis, and periosteum (corneal layer not specified) | 4D printed anionic gelatin methacrylate (GelMA) hydrogels treated with cationic poly-l-lysine (PLL) | Generating cell-laden thin membranous tissues like the cornea, epidermis, and periosteum | In vitro | Charge-driven shrinking (which depends on the molecular weight of PLL) improves the resolution of printed structures to 65 µm, generation of macroscale and microscale tissues within the same construct | Cytotoxicity of higher molecular weight PLL, optimization needed for cell viability and shrinking capabilities | [273] |
| 2019 | Stroma | 4D printed collagen-based hydrogel containing localized bio-actuators (contractile cells) that use a contraction-inhibiting peptide amphiphile | Creating a self-curving biomaterial through localized control of cell actuators | In vitro | Structural and mechanical properties are more similar to natural corneal tissue compared to flat 3D scaffolds; production of cornea-shaped, curved stromal tissue equivalents | Requires precise control of bio-actuator activity | [274] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Belaiche, M.; Wen, Y.; Choulakian, M.Y.; Tran, S.D. Advancements in Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering. Polymers 2024, 16, 2882. https://doi.org/10.3390/polym16202882
Wu KY, Belaiche M, Wen Y, Choulakian MY, Tran SD. Advancements in Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering. Polymers. 2024; 16(20):2882. https://doi.org/10.3390/polym16202882
Chicago/Turabian StyleWu, Kevin Y., Myriam Belaiche, Ying Wen, Mazen Y. Choulakian, and Simon D. Tran. 2024. "Advancements in Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering" Polymers 16, no. 20: 2882. https://doi.org/10.3390/polym16202882
APA StyleWu, K. Y., Belaiche, M., Wen, Y., Choulakian, M. Y., & Tran, S. D. (2024). Advancements in Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering. Polymers, 16(20), 2882. https://doi.org/10.3390/polym16202882







