When a Side Reaction Is a Benefit: A Catalyst-Free Route to Obtain High-Molecular Cobaltocenium-Functionalized Polysiloxanes by Hydroamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Spectroscopy Equipment and Studies
2.2.2. Sedimentation Velocity Experiments
2.2.3. Cyclic Voltammetry
2.3. Synthesis of Cobaltocenium-Containing Polysiloxanes
3. Results and Discussion
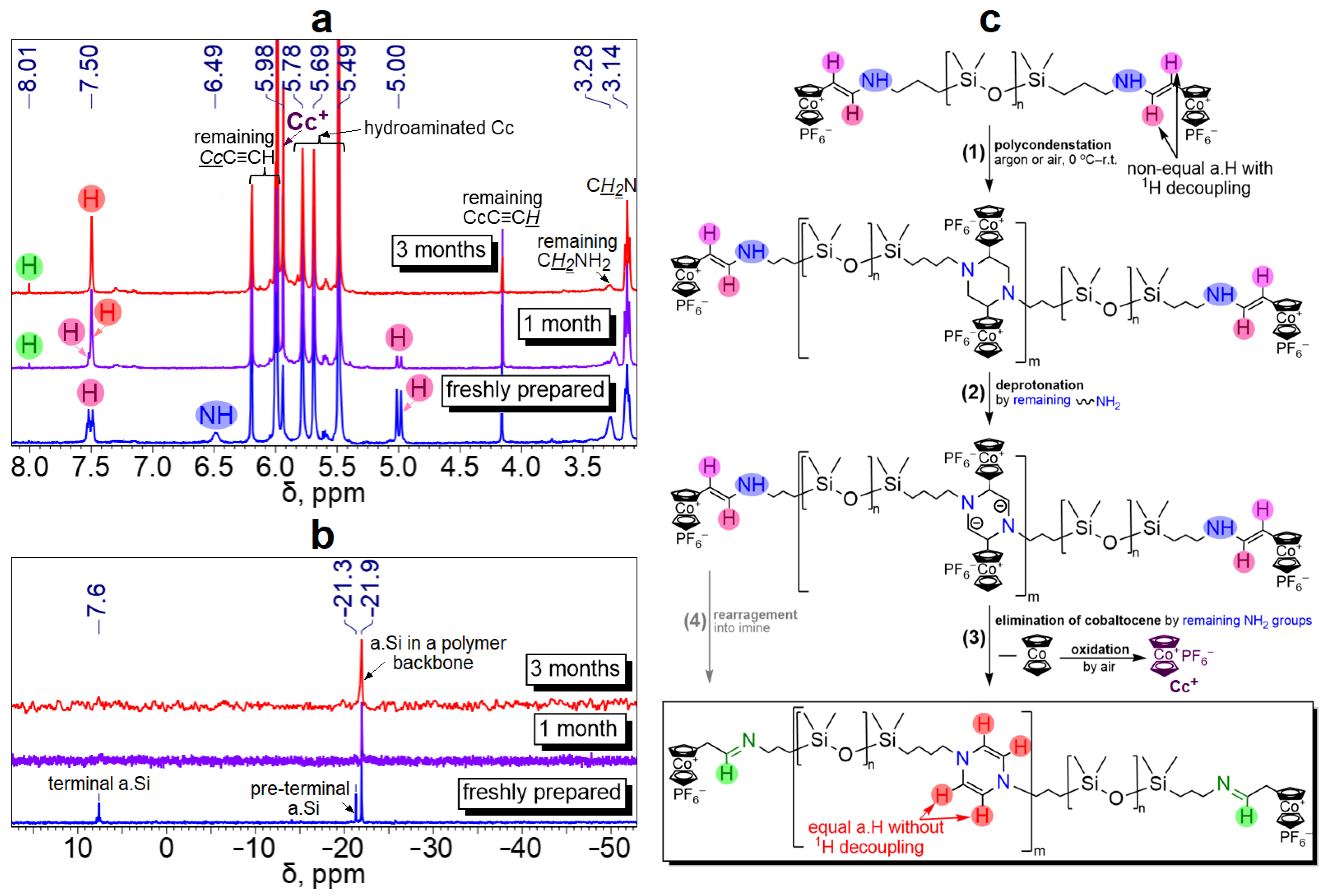
3.1. Synthesis and Characterization of Cobaltocenium-Containing Polysiloxanes
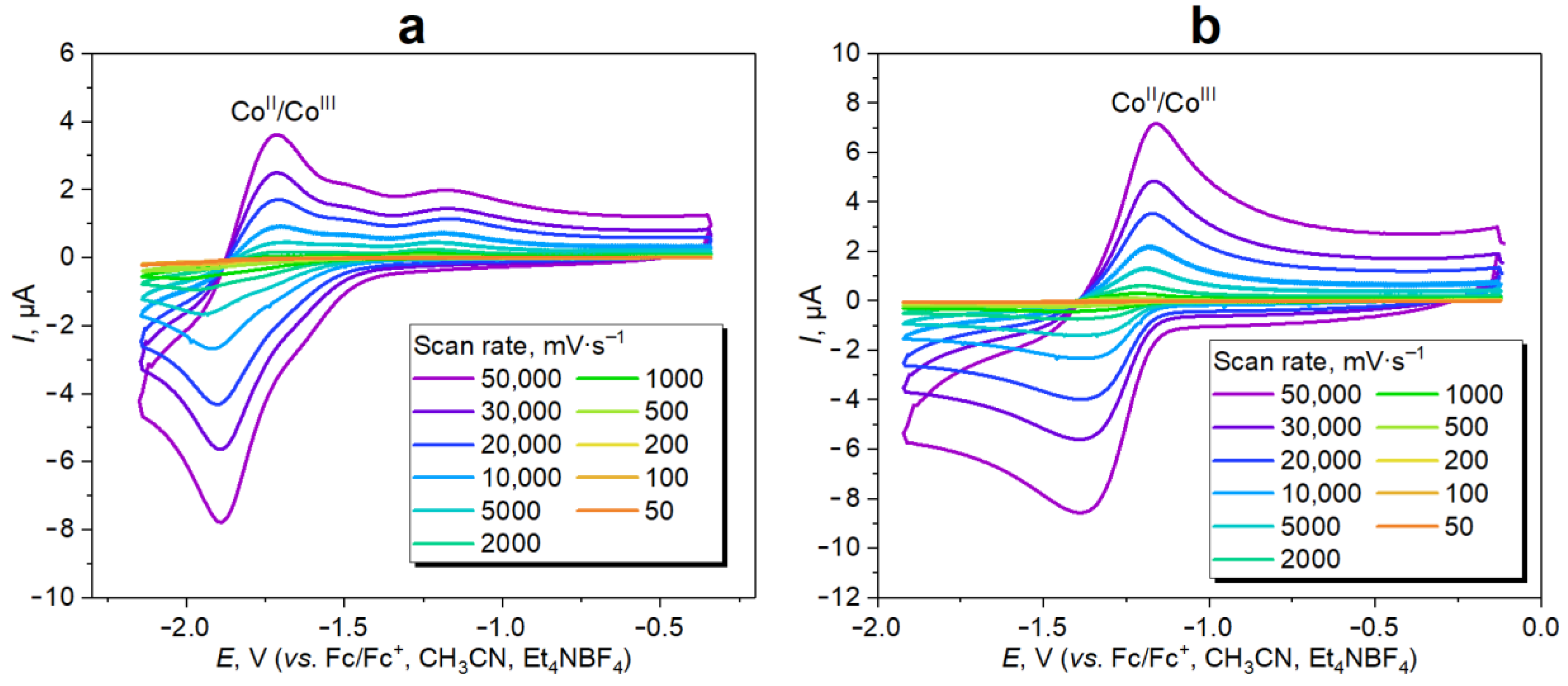
3.2. “Gelation” and Redox Properties of Cobaltocenium-Containing Polysiloxanes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gracia, R.; Mecerreyes, D. Polymers with Redox Properties: Materials for Batteries, Biosensors and More. Polym. Chem. 2013, 4, 2206. [Google Scholar] [CrossRef]
- Zhao, C.; Park, J.; Root, S.E.; Bao, Z. Skin-Inspired Soft Bioelectronic Materials, Devices and Systems. Nat. Rev. Bioeng. 2024, 2, 671–690. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Islamova, R.M. Ferrocenyl-Containing Oligosiloxanes and Polysiloxanes: Synthesis, Properties, and Application. Polym. Sci. Ser. C 2022, 64, 95–109. [Google Scholar] [CrossRef]
- Mark, J.E.; Schaefer, D.W.; Lin, G. The Polysiloxanes; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-518173-9. [Google Scholar]
- Yilgör, E.; Yilgör, I. Silicone Containing Copolymers: Synthesis, Properties and Applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Rashevskii, A.A.; Deriabin, K.V.; Parshina, E.K.; Islamova, R.M. Self-Healing Redox-Active Coatings Based on Ferrocenyl-Containing Polysiloxanes. Coatings 2023, 13, 1282. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Vereshchagin, A.A.; Kirichenko, S.O.; Rashevskii, A.A.; Levin, O.V.; Islamova, R.M. Self-Cross-Linkable Ferrocenyl-Containing Polysiloxanes as Flexible Electrochromic Materials. Mater. Today Chem. 2023, 29, 101399. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Lee, J.M.; Shin, W. Electrochemical Properties of Ferrocene Modified Polysiloxane/Chitosan Nanocomposite and Its Application to Glucose Sensor. Electrochim. Acta 2009, 54, 6508–6514. [Google Scholar] [CrossRef]
- Cazacu, M.; Vlad, A.; Marcu, M.; Racles, C.; Airinei, A.; Munteanu, G. New Organometallic Polymers by Polycondensation of Ferrocene and Siloxane Derivatives. Macromolecules 2006, 39, 3786–3793. [Google Scholar] [CrossRef]
- Islamova, R.M. Iron Compounds in Controlled Radical Polymerization: Ferrocenes, (Clathro)Chelates, and Porphyrins. Russ. J. Gen. Chem. 2016, 86, 125–143. [Google Scholar] [CrossRef]
- Yu, G.; Suzaki, Y.; Abe, T.; Osakada, K. Introduction of Ferrocene-Containing [2]Rotaxanes onto Siloxane, Silsesquioxane and Polysiloxanes via Click Chemistry. Dalton Trans. 2013, 42, 1476–1482. [Google Scholar] [CrossRef]
- Martínez-Montero, I.; Bruña, S.; González-Vadillo, A.M.; Cuadrado, I. Thiol–Ene Chemistry of Vinylferrocene: A Simple and Versatile Access Route to Novel Electroactive Sulfur- and Ferrocene-Containing Model Compounds and Polysiloxanes. Macromolecules 2014, 47, 1301–1315. [Google Scholar] [CrossRef]
- Inagaki, T.; Lee, H.S.; Skotheim, T.A.; Okamoto, Y. Syntheses and Electrochemical Properties of Siloxane Polymers Containing Ferrocene and Dimethylferrocene. J. Chem. Soc. Chem. Commun. 1989, 16, 1181–1183. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Zhang, L.; Qiu, G.; Astruc, D.; Gu, H. Metallomacromolecules Containing Cobalt Sandwich Complexes: Synthesis and Functional Materials Properties. Coord. Chem. Rev. 2017, 337, 34–79. [Google Scholar] [CrossRef]
- Kocheva, A.N.; Deriabin, K.V.; Volkov, A.I.; Levin, O.V.; Islamova, R.M. Cobaltocenium-Containing Polysiloxanes: Catalytic Synthesis, Structure, and Properties. ACS Appl. Polym. Mater. 2024, 6, 12112–12122. [Google Scholar] [CrossRef]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef]
- Baldaguez Medina, P.; Ardila Contreras, V.; Hartmann, F.; Schmitt, D.; Klimek, A.; Elbert, J.; Gallei, M.; Su, X. Investigating the Electrochemically Driven Capture and Release of Long-Chain PFAS by Redox Metallopolymer Sorbents. ACS Appl. Mater. Interfaces 2023, 15, 22112–22122. [Google Scholar] [CrossRef]
- Liu, X.; Rapakousiou, A.; Deraedt, C.; Ciganda, R.; Wang, Y.; Ruiz, J.; Gu, H.; Astruc, D. Multiple Applications of Polymers Containing Electron-Reservoir Metal-Sandwich Complexes. Chem. Commun. 2020, 56, 11374–11385. [Google Scholar] [CrossRef]
- Li, H.; Yang, P.; Hwang, J.; Pageni, P.; Decho, A.W.; Tang, C. Antifouling and Antimicrobial Cobaltocenium-Containing Metallopolymer Double-Network Hydrogels. Biomater. Transl. 2022, 3, 162–171. [Google Scholar] [CrossRef]
- Yang, P.; Luo, Y.; Kurnaz, L.B.; Bam, M.; Yang, X.; Decho, A.W.; Nagarkatti, M.; Tang, C. Biodegradable Polycaprolactone Metallopolymer–Antibiotic Bioconjugates Containing Phenylboronic Acid and Cobaltocenium for Antimicrobial Application. Biomater. Sci. 2021, 9, 7237–7246. [Google Scholar] [CrossRef]
- Cuadrado, I.; Casado, C.M.; Lobete, F.; Alonso, B.; González, B.; Losada, J.; Amador, U. Preparation and Redox Properties of Novel Polymerizable Pyrrole- and Allyl-Functionalized Cobaltocenium Monomers and Siloxane-Based Cobaltocenium Polymers. Organometallics 1999, 18, 4960–4969. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Zhu, M.; Liu, X.; Chao, D. Oligoaniline-Functionalized Polysiloxane/Prussian Blue Composite towards Bifunctional Electrochromic Supercapacitors. New J. Chem. 2020, 44, 8138–8147. [Google Scholar] [CrossRef]
- Pionteck, J.; Wypych, G. Handbook of Antistatics, 2nd ed.; ChemTec Publishing: Toronto, ON, Canada, 2016; ISBN 978-1-895198-95-9. [Google Scholar]
- Tan, Y.J.; Susanto, G.J.; Anwar Ali, H.P.; Tee, B.C.K. Progress and Roadmap for Intelligent Self-Healing Materials in Autonomous Robotics. Adv. Mater. 2021, 33, 2002800. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, X.; Zhang, X.; Liu, S.; Zhong, F.; Zhang, J.; Zhang, Q.; Yan, Y. Metallo-Polyelectrolyte-Based Waterborne Polyurethanes as Robust HCl Corrosion Inhibitor Mediated by Inter/Intramolecular Hydrogen Bond. ACS Appl. Polym. Mater. 2022, 4, 3844–3854. [Google Scholar] [CrossRef]
- Liu, S.; Yan, J.; Shi, J.; Li, X.; Zhang, J.; Wang, X.; Cai, N.; Fang, Q.; Zhang, Q.; Yan, Y. Rational Design of Cobaltocenium-Containing Polythioether Type Metallo-Polyelectrolytes as HCl Corrosion Inhibitors for Mild Steel. Polym. Chem. 2023, 14, 330–342. [Google Scholar] [CrossRef]
- Wei, J.; Ren, L.; Tang, C.; Su, Z. Electric-Stimulus-Responsive Multilayer Films Based on a Cobaltocenium-Containing Polymer. Polym. Chem. 2014, 5, 6480–6488. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.M.; Sadaf, S.; Hennecke, A.; Klein, J.; Mahmood, A.M.; Rüttiger, C.; Gallei, M.; Fu, F.; Fouquet, E.; Ruiz, J.; et al. The Metallocene Battery: Ultrafast Electron Transfer Self Exchange Rate Accompanied by a Harmonic Height Breathing. Angew. Chem. Int. Ed. 2021, 60, 13554–13558. [Google Scholar] [CrossRef]
- Escorihuela, J.; Lledós, A.; Ujaque, G. Anti-Markovnikov Intermolecular Hydroamination of Alkenes and Alkynes: A Mechanistic View. Chem. Rev. 2023, 123, 9139–9203. [Google Scholar] [CrossRef]
- Wang, Y.; Rapakousiou, A.; Ruiz, J.; Astruc, D. Metalation of Polyamine Dendrimers with Ethynylcobalticenium for the Construction of Mono- and Heterobimetallic Polycationic Metallodendrimers. Chem. Eur. J. 2014, 20, 11176–11186. [Google Scholar] [CrossRef]
- Wang, Y.; Rapakousiou, A.; Latouche, C.; Daran, J.-C.; Singh, A.; Ledoux-Rak, I.; Ruiz, J.; Saillard, J.-Y.; Astruc, D. Mild Uncatalyzed Hydroamination of an Electrophilic Alkyne, Ethynylcobalticinium. Chem. Commun. 2013, 49, 5862. [Google Scholar] [CrossRef]
- Vanicek, S.; Kopacka, H.; Wurst, K.; Müller, T.; Schottenberger, H.; Bildstein, B. Chemoselective, Practical Synthesis of Cobaltocenium Carboxylic Acid Hexafluorophosphate. Organometallics 2014, 33, 1152–1156. [Google Scholar] [CrossRef]
- Brown, P.H.; Schuck, P. Macromolecular Size-and-Shape Distributions by Sedimentation Velocity Analytical Ultracentrifugation. Biophys. J. 2006, 90, 4651–4661. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Ferreira, P.M.T.; Monteiro, L.S. Synthesis and Reactivity of a 1,4-Dihydropyrazine Derivative. Tetrahedron 2004, 60, 8489–8496. [Google Scholar] [CrossRef]
- Menia, D.; Pittracher, M.; Kopacka, H.; Wurst, K.; Neururer, F.R.; Leitner, D.; Hohloch, S.; Podewitz, M.; Bildstein, B. Curious Case of Cobaltocenium Carbaldehyde. Organometallics 2023, 42, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Perevyazko, I.; Gubarev, A.S.; Pavlov, G.M. Analytical Ultracentrifugation and Combined Molecular Hydrodynamic Approaches for Polymer Characterization. In Molecular Characterization of Polymers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 223–259. ISBN 978-0-12-819768-4. [Google Scholar]
- Feuerstein, A.; Boßmann, B.; Rittner, T.; Leiner, R.; Janka, O.; Gallei, M.; Schäfer, A. Polycobaltoceniumylmethylene—A Water-Soluble Polyelectrolyte Prepared by Ring-Opening Transmetalation Polymerization. ACS Macro Lett. 2023, 12, 1019–1024. [Google Scholar] [CrossRef]

| Abbreviations of (Poly)siloxanes | Mass of Used Amino-Containing (Poly)siloxane, g | Used solvents | Characteristics ** | |||
|---|---|---|---|---|---|---|
| Cobaltocenium- Containing (Poly)siloxane | Used Amino- Containing (Poly)siloxane | CH3CN/CH2Cl2 Ratio, V/V | Volume, * mL | Mn | Number of –[SiO]– Units | |
| Cc-APTMDS | APTMDS | 0.03 | 1:0 | 1.5 | 965 | 1 |
| Cc-APDMS850 | APDMS850 | 0.12 | 1:1 | 1.5 | 1630 | 9 |
| Cc-APDMS5000 | APDMS5000 | 0.70 | 1:4 | 3.5 | 5850 | 65 |
| Cc-APDMS25000 | APDMS25000 | 3.50 | 21.0 | 23,150 | 335 | |
| P(Cc-AMS-co-DMS) | P(AMS-co-DMS) | 1.05 | 5.5 | 7930 | 96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocheva, A.N.; Deriabin, K.V.; Perevyazko, I.; Bokach, N.A.; Boyarskiy, V.P.; Islamova, R.M. When a Side Reaction Is a Benefit: A Catalyst-Free Route to Obtain High-Molecular Cobaltocenium-Functionalized Polysiloxanes by Hydroamination. Polymers 2024, 16, 2887. https://doi.org/10.3390/polym16202887
Kocheva AN, Deriabin KV, Perevyazko I, Bokach NA, Boyarskiy VP, Islamova RM. When a Side Reaction Is a Benefit: A Catalyst-Free Route to Obtain High-Molecular Cobaltocenium-Functionalized Polysiloxanes by Hydroamination. Polymers. 2024; 16(20):2887. https://doi.org/10.3390/polym16202887
Chicago/Turabian StyleKocheva, Anastasia N., Konstantin V. Deriabin, Igor Perevyazko, Nadezhda A. Bokach, Vadim P. Boyarskiy, and Regina M. Islamova. 2024. "When a Side Reaction Is a Benefit: A Catalyst-Free Route to Obtain High-Molecular Cobaltocenium-Functionalized Polysiloxanes by Hydroamination" Polymers 16, no. 20: 2887. https://doi.org/10.3390/polym16202887
APA StyleKocheva, A. N., Deriabin, K. V., Perevyazko, I., Bokach, N. A., Boyarskiy, V. P., & Islamova, R. M. (2024). When a Side Reaction Is a Benefit: A Catalyst-Free Route to Obtain High-Molecular Cobaltocenium-Functionalized Polysiloxanes by Hydroamination. Polymers, 16(20), 2887. https://doi.org/10.3390/polym16202887








