Preparation of Montmorillonite–Melamine Cyanurate and Inhibition of the Emission of Phosphine from PA6/Aluminum Hypophosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MMT-MCA
- (1)
- In situ intercalation
- (2)
- Water intercalation
- (3)
- Mechanical intercalation
2.3. Preparation of PA6/FR
2.4. Characterization
3. Results and Discussion
3.1. Structure of MMT-MCA
3.2. Morphology of MMT-MCA
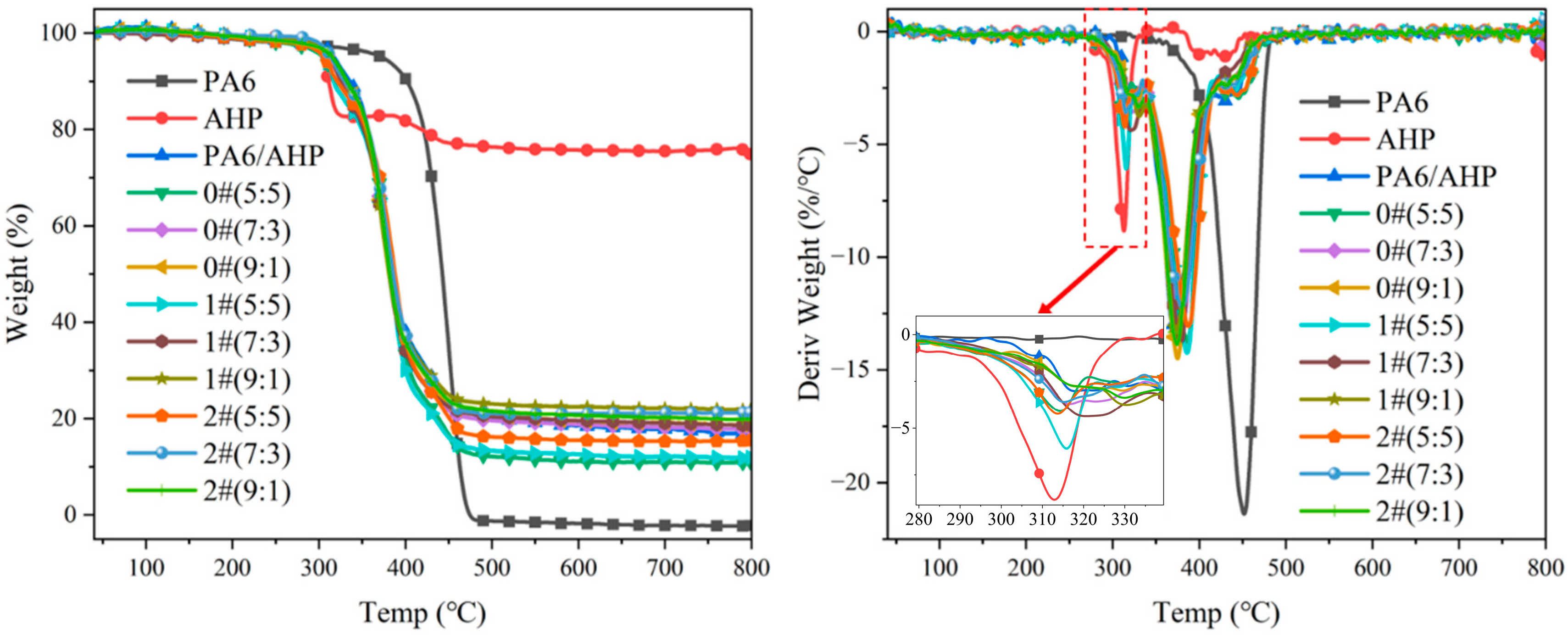
3.3. Thermal Stability of PA6/FR
3.4. LOI and UL 94 of PA6/FR
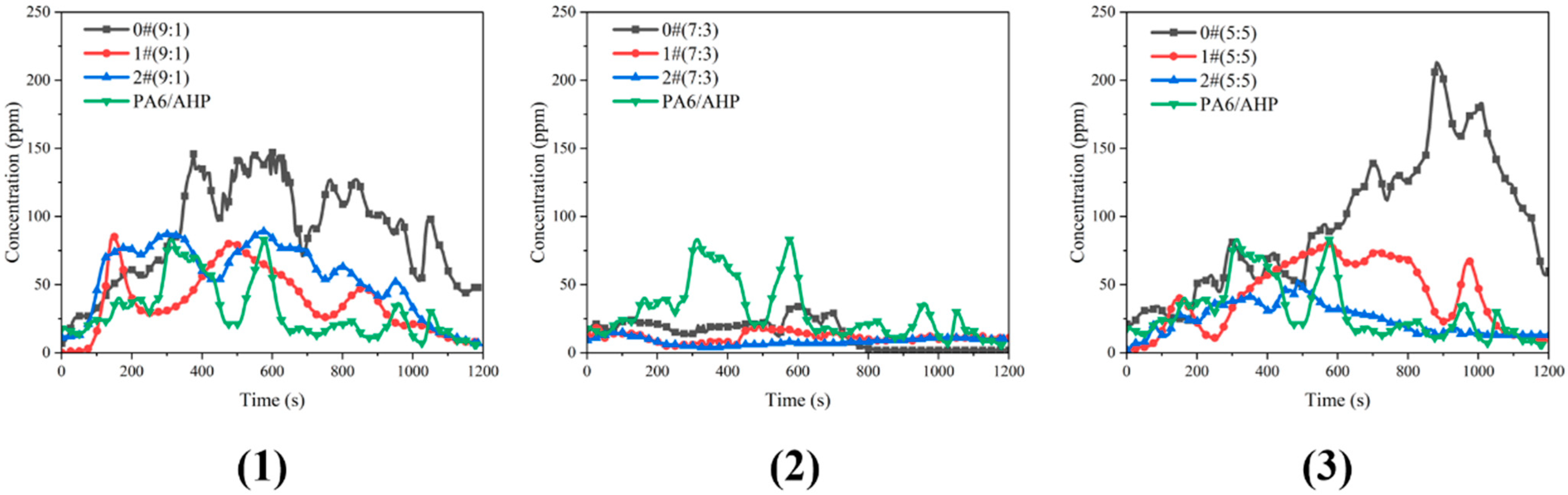
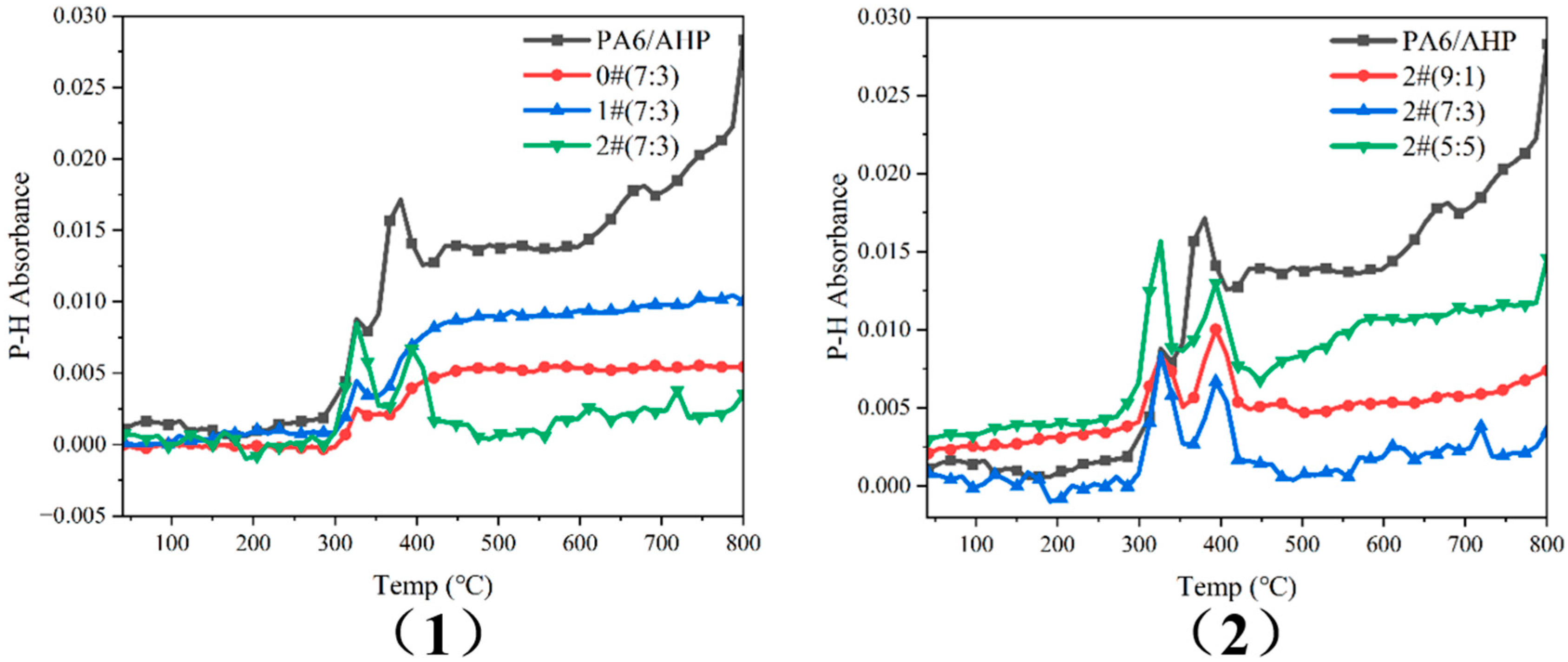
3.5. PH3 Release of PA6/FR
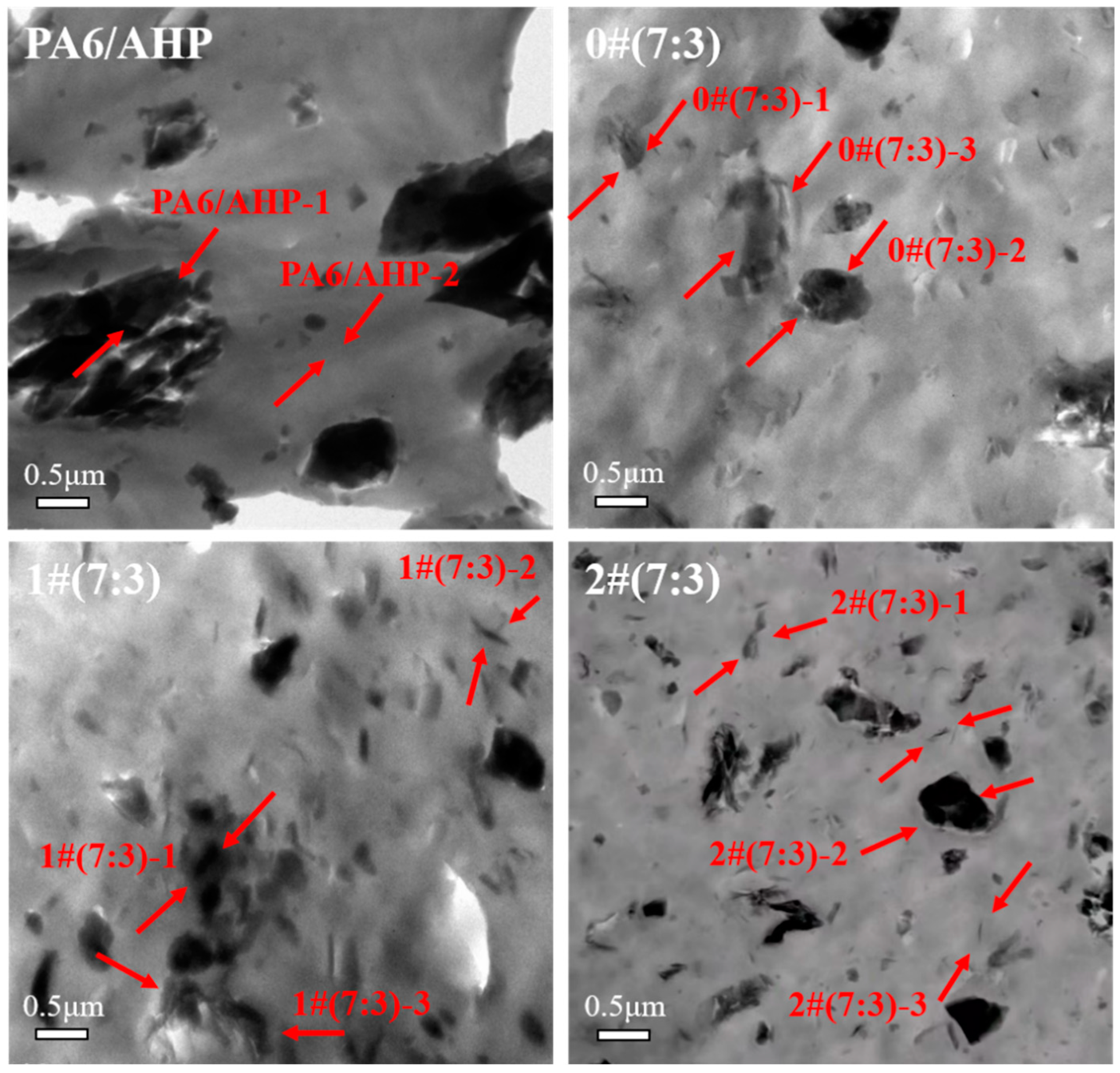
3.6. MMT-MCA Distribution in PA6
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, W.; Fu, Y.; Zeng, C.; Liu, N.; Yin, C. Enhancement of flame retardancy and mechanical properties of polyamide 6 by incorporating melamine cyanurate combined with attapulgite. J. Appl. Polym. Sci. 2020, 137, 47298. [Google Scholar] [CrossRef]
- Boonkongkaew, M.; Sirisinha, K. Halloysite nanotubes loaded with liquid organophosphate for enhanced flame retardancy and mechanical properties of polyamide 6. J. Mater. Sci. 2018, 53, 10181–10193. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q. Applications of micro-scale combustion calorimetry to the studies of cotton and nylon fabrics treated with organophosphorus flame retardants. J. Anal. Appl. Pyrolysis 2011, 91, 125–133. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular fireFigurehting—How modern phosphorus chemistry can help solve the challenge of flame retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Shen, K.K. Review of Recent Advances on the Use of Boron-based Flame Retardants. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 367–388. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, R. Pyrolysis and fire behaviour of epoxy resin composites based on a phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS). Polym. Degrad. Stab. 2011, 96, 1821–1832. [Google Scholar] [CrossRef]
- Liu, K.; Gao, X.; Liu, Z.; Hu, W.; Li, A.; Xu, Y.; Zhang, W. Flame retardant polyurethane microbubble elastomer based on ionic liquid/ammonium polyphosphate/aluminum hypophosphite ternary flame retardant system. J. Appl. Polym. Sci. 2024, 141, e55535. [Google Scholar] [CrossRef]
- Savas, L.A.; Dogan, M. Flame retardant effect of zinc borate in polyamide 6 containing aluminum hypophosphite. Polym. Degrad. Stab. 2019, 165, 101–109. [Google Scholar] [CrossRef]
- Savas, L.A.; Hacioglu, F.; Hancer, M.; Dogan, M. Flame retardant effect of aluminum hypophosphite in heteroatom-containing polymers. Polym. Bull. 2020, 77, 291–306. [Google Scholar] [CrossRef]
- Li, Y.; Qi, L.; Liu, Y.; Qiao, J.; Wang, M.; Liu, X.; Li, S. Recent Advances in Halogen-Free Flame Retardants for Polyolefin Cable Sheath Materials. Polymers 2022, 14, 2876. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, W.; Xie, D.; Wang, Y.; Sun, X.; Zhou, R.; Jiang, J. A flame retardant containing dicyandiamide and aluminum hypophosphite for polyethylene. Case Stud. Constr. Mater. 2023, 18, e01797. [Google Scholar] [CrossRef]
- Tian, S.; He, H.; Wang, D.; Yu, P.; Jia, Y.; Luo, Y. Study of using aluminum hypophosphite as a flame retardant for low-density polyethylene. Fire Mater. 2017, 41, 983–992. [Google Scholar] [CrossRef]
- Ge, H.; Tang, G.; Hu, W.-Z.; Wang, B.-B.; Pan, Y.; Song, L.; Hu, Y. Aluminum hypophosphite microencapsulated to improve its safety and application to flame retardant polyamide 6. J. Hazard. Mater. 2015, 294, 186–194. [Google Scholar] [CrossRef]
- Nath, N.S.; Bhattacharya, I.; Tuck, A.G.; Schlipalius, D.I.; Ebert, P.R. Mechanisms of phosphine toxicity. J. Toxicol. 2011, 2011, 494168. [Google Scholar] [CrossRef]
- Laoutid, F.; Jouyandeh, M.; Murariu, O.; Vahabi, H.; Saeb, M.R.; Brison, L.; Murariu, M.; Dubois, P. New Transparent Flame-Retardant (FR) Coatings Based on Epoxy-Aluminum Hypophosphite Nanocomposites. Coatings 2023, 13, 140. [Google Scholar] [CrossRef]
- Zheng, Z.; Sun, H.; Li, W.; Zhong, S.; Yan, J.; Cui, X.; Wang, H. Co-microencapsulation of ammonium polyphosphate and aluminum hydroxide in halogen-free and intumescent flame retarding polypropylene. Polym. Compos. 2014, 35, 715–729. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, Y.; Hu, W.; Lian, J.; Hu, Y. Influence of ammonium polyphosphate microencapsulation on flame retardancy, thermal degradation and crystal structure of polypropylene composite. Compos. Sci. Technol. 2013, 81, 17–23. [Google Scholar] [CrossRef]
- Yang, W.; Song, L.; Hu, Y.; Lu, H.; Yuen, R.K. Enhancement of fire retardancy performance of glass-fibre reinforced poly (ethylene terephthalate) composites with the incorporation of aluminum hypophosphite and melamine cyanurate. Compos. Part B Eng. 2011, 42, 1057–1065. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Pan, Y.-T.; Xu, X.; Song, Y.; Yang, R. Mitigation the release of toxic PH3 and the fire hazard of PA6/AHP composite by MOFs. J. Hazard. Mater. 2020, 395, 122604. [Google Scholar] [CrossRef]
- Zhao, M.; Yi, D.; Camino, G.; Frache, A.; Yang, R. Interdigitated crystalline MMT–MCA in polyamide 6. RSC Adv. 2017, 7, 861–869. [Google Scholar] [CrossRef]
- Dong, W.; Wen, P.; Wang, C.; Tian, H.; Mou, D.; Huang, J.; Hu, R.; Xue, Z.; Jiang, D.; Li, D. 2D-3D cyclodextrin-modified montmorillonite assembly for efficient directional capture of amines. J. Water Process Eng. 2024, 66, 106029. [Google Scholar] [CrossRef]
- Ajbary, M.; Santos, A.; Morales-Flórez, V.; Esquivias, L. Removal of basic yellow cationic dye by an aqueous dispersion of Moroccan stevensite. Appl. Clay Sci. 2013, 80–81, 46–51. [Google Scholar] [CrossRef]
- Borah, D.; Nath, H.; Saikia, H. Modification of bentonite clay & its applications: A review. Rev. Inorg. Chem. 2022, 42, 265–282. [Google Scholar] [CrossRef]
- Li, D.; Zuo, X.; Zhang, X.; Tang, Y.; Zhao, X.; Zhang, Y.; Yang, H. Emerging urchin-like core-shell mineral microspheres with efficient photothermal conversion and solar energy storage. J. Energy Storage 2023, 68, 107661. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Cheng, J.; Hou, W.; Zheng, X.; Fediuk, R.; Qin, Y.; Chen, Z.; Luo, Y.; Mojahidul Islam, M. Modified space suspension bentonite-based powder for suppression dust explosion in moist conditions: Experimental testing and validations. Fuel 2024, 373, 132190. [Google Scholar] [CrossRef]
- Fouda, S.R.; Hassan, S.A. Impact of LaZnFe2O4 supported NiWO4@D400-MMT@CMS/MMA nanocomposites as a catalytic system in remediation of dyes from wastewater. Sci. Rep. 2024, 14, 11644. [Google Scholar] [CrossRef]
- Sreekanth Reddy, O.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Chowdoji Rao, K. Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. 2021, 11, 191–199. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; El-Naggar, M.E.; Radwan, E.K.; Mohamed, I.M.; Azaam, M.; Kenawy, E.R. High-performance mixed-matrix membranes enabled by organically/inorganic modified montmorillonite for the treatment of hazardous textile wastewater. Chem. Eng. J. 2021, 405, 126964. [Google Scholar] [CrossRef]
- Schiessl, S.; Kucukpinar, E.; Schwiddessen, R.; Langowski, H.C.; Eisner, P. Mechanisms of permeation of helium, hydrogen, oxygen, and water vapor through silicate-based composite barrier coating layers. Surf. Coat. Technol. 2024, 483, 130800. [Google Scholar] [CrossRef]
- Cui, Y.; Kumar, S.; Kona, B.R.; van Houcke, D. Gas barrier properties of polymer/clay nanocomposites. Rsc Adv. 2015, 5, 63669–63690. [Google Scholar] [CrossRef]
- Hua, S.; Yang, H.; Wang, A. A pH-sensitive nanocomposite microsphere based on chitosan and montmorillonite with in vitro reduction of the burst release effect. Drug Dev. Ind. Pharm. 2010, 36, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhu, Y.; Wu, Q.; Zhu, M.; Guo, F.; Yu, H.; Yu, J. Enhanced storage stability of different polymer modified asphalt binders through nano-montmorillonite modification. Nanomaterials 2020, 10, 641. [Google Scholar] [CrossRef]
- Gijsman, P.; Steenbakkers, R.; Fürst, C.; Kersjes, J. Differences in the flame retardant mechanism of melamine cyanurate in polyamide 6 and polyamide 66. Polym. Degrad. Stab. 2002, 78, 219–224. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Yan, W.; Tang, H. Preparation of flame retardant polyamide 6 composite with melamine cyanurate nanoparticles in situ formed in extrusion process. Polym. Degrad. Stab. 2006, 91, 2632–2643. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Y.; Sha, K.; Xiao, R. Preparation and characterizations of flame retardant melamine cyanurate/polyamide 6 composite fibers via in situ polymerization. Text. Res. J. 2017, 87, 561–569. [Google Scholar] [CrossRef]
- Zhao, M.; Yi, D.; Camino, G.; Frache, A.; Yang, R. Interdigitated crystalline MMT-MCA: Preparation and characterization. Polym. Adv. Technol. 2018, 29, 22–29. [Google Scholar] [CrossRef]
- Xu, W.; Dong, M.; Gersen, H.; Rauls, E.; Vázquez-Campos, S.; Crego-Calama, M.; Reinhoudt, D.N.; Stensgaard, I.; Laegsgaard, E.; Linderoth, T.R.; et al. Cyanuric acid and melamine on Au(111): Structure and energetics of hydrogen-bonded networks. Small 2007, 3, 854–858. [Google Scholar] [CrossRef]
- Zhang, J.; Lewin, M.; Pearce, E.; Zammarano, M.; Gilman, J.W. Flame retarding polyamide 6 with melamine cyanurate and layered silicates. Polym. Adv. Technol. 2008, 19, 928–936. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q. Preparation, properties and characterizations of halogen-free nitrogen-phosphorous flame-retarded glass fiber reinforced polyamide 6 composite. Polym. Degrad. Stab. 2006, 91, 2003–2013. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Xu, W.; Liu, Y.C.; Xia, J.K.; Wu, Q.X.; Xu, W.J. Preparation and characterization of flame-retardant melamine cyanurate/polyamide 6 nanocomposites by in situ polymerization. J. Appl. Polym. Sci. 2009, 113, 2109–2116. [Google Scholar] [CrossRef]
- Im, J.S.; Lee, S.K.; In, S.J.; Lee, Y.S. Improved flame retardant properties of epoxy resin by fluorinated MMT/MWCNT additives. J. Anal. Appl. Pyrolysis 2010, 89, 225–232. [Google Scholar] [CrossRef]
- Liang, C.-Y.; Uchytil, P.; Petrychkovych, R.; Lai, Y.-C.; Friess, K.; Sipek, M.; Reddy, M.M.; Suen, S.-Y. A comparison on gas separation between PES (polyethersulfone)/MMT (Na-montmorillonite) and PES/TiO2 mixed matrix membranes. Sep. Purif. Technol. 2012, 92, 57–63. [Google Scholar] [CrossRef]
- Rezaei, M.; Ismail, A.F.; Bakeri, G.; Hashemifard, S.A.; Matsuura, T. Effect of general montmorillonite and Cloisite 15A on structural parameters and performance of mixed matrix membranes contactor for CO2 absorption. Chem. Eng. J. 2015, 260, 875–885. [Google Scholar] [CrossRef]

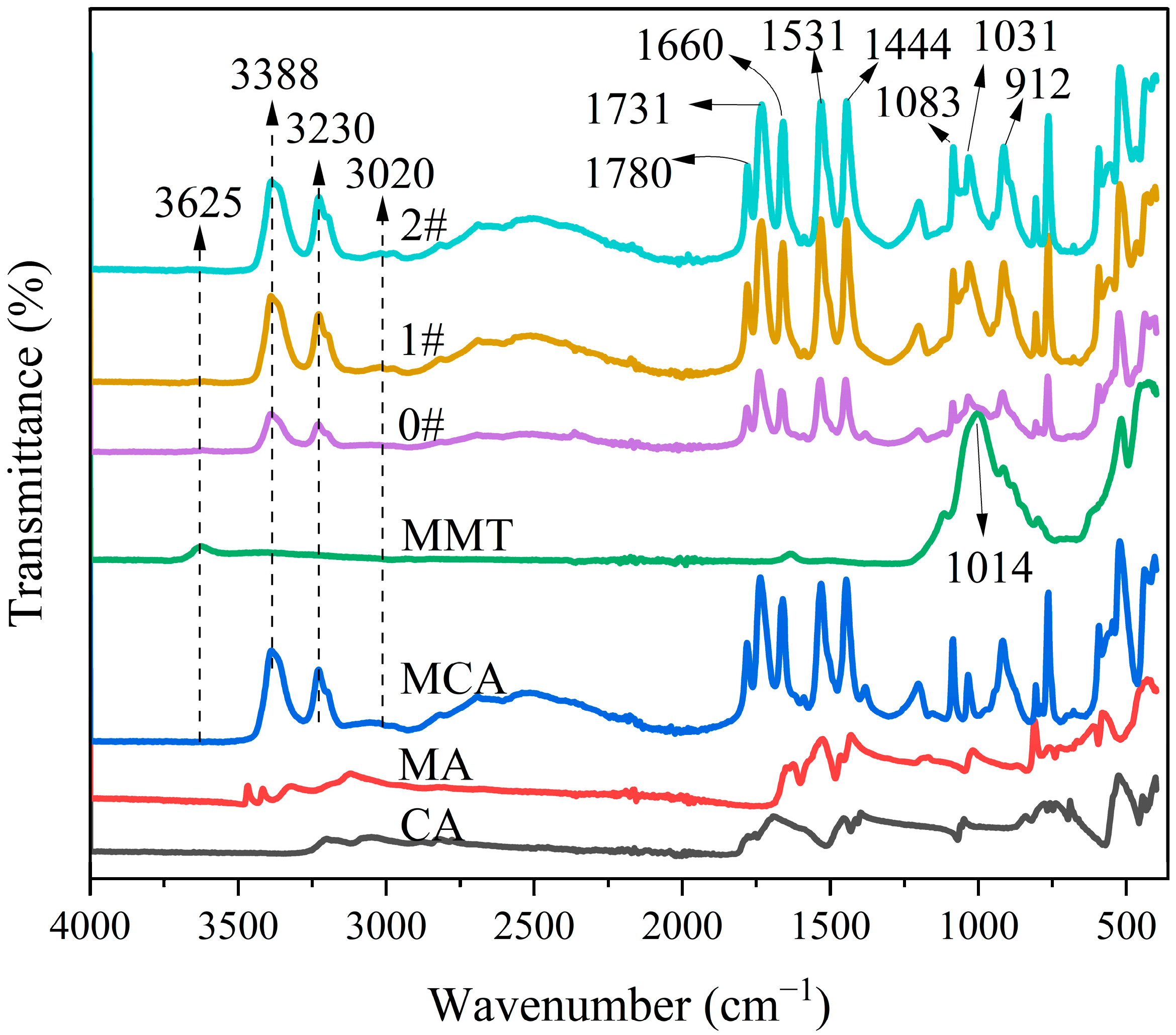
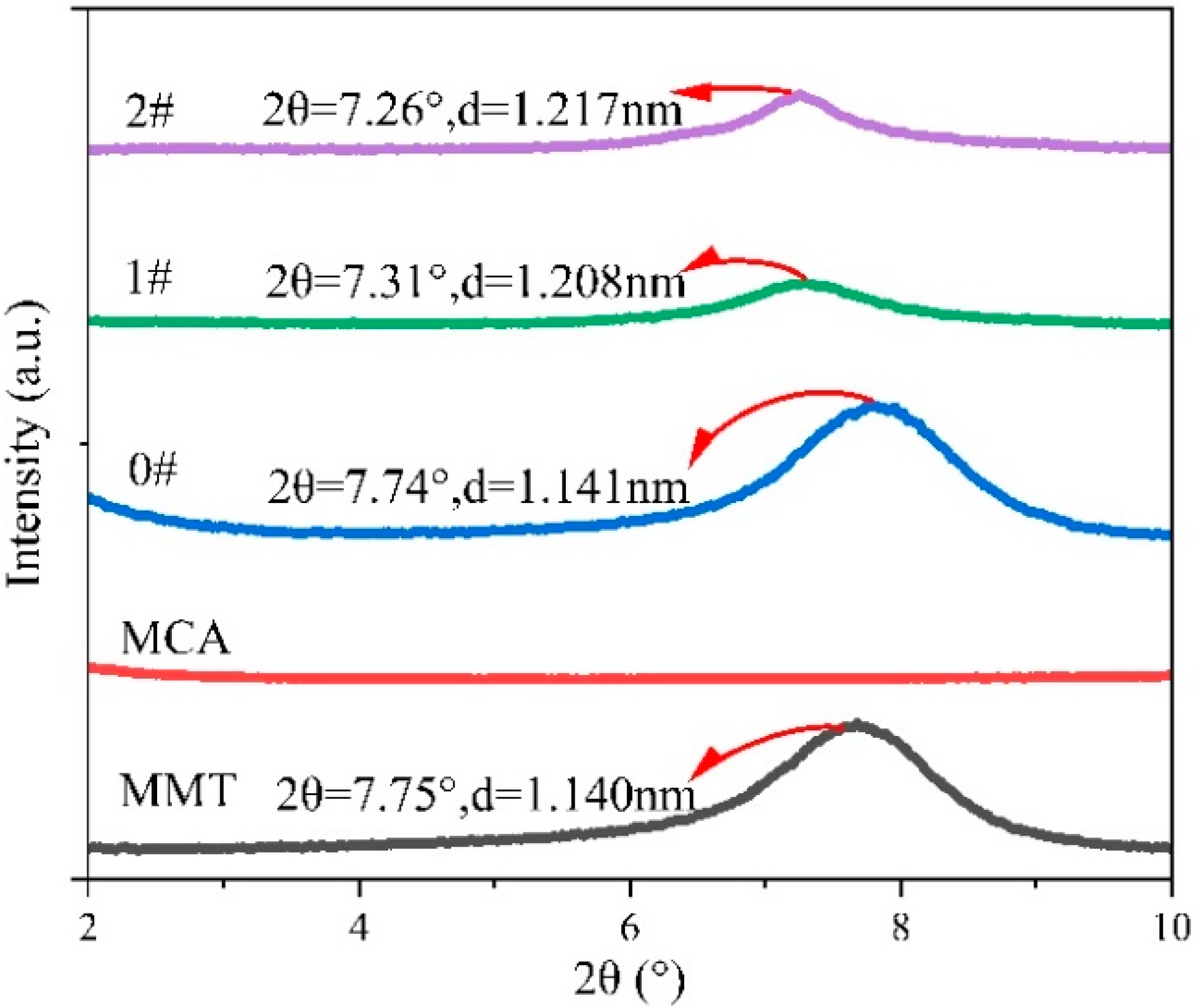
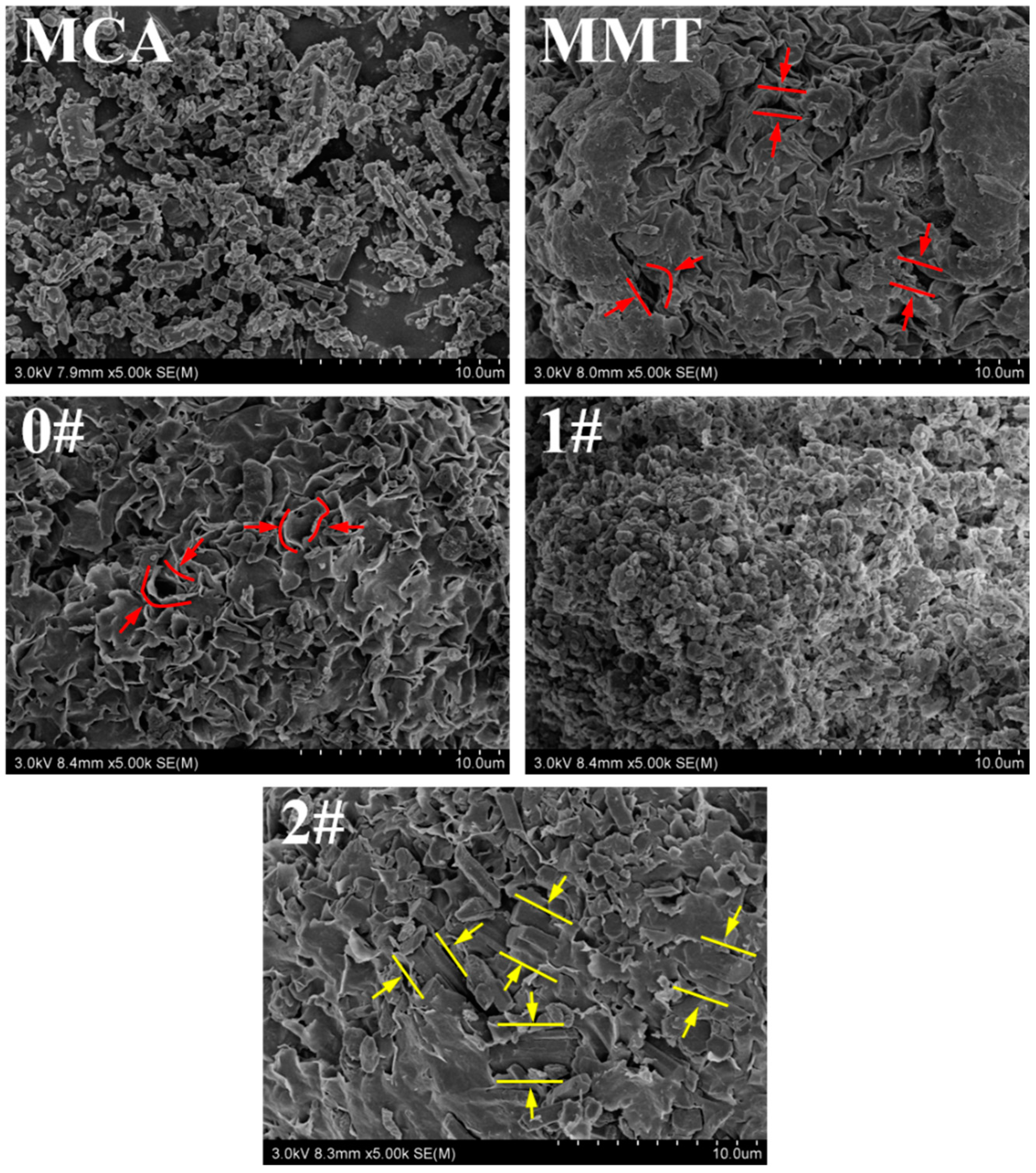
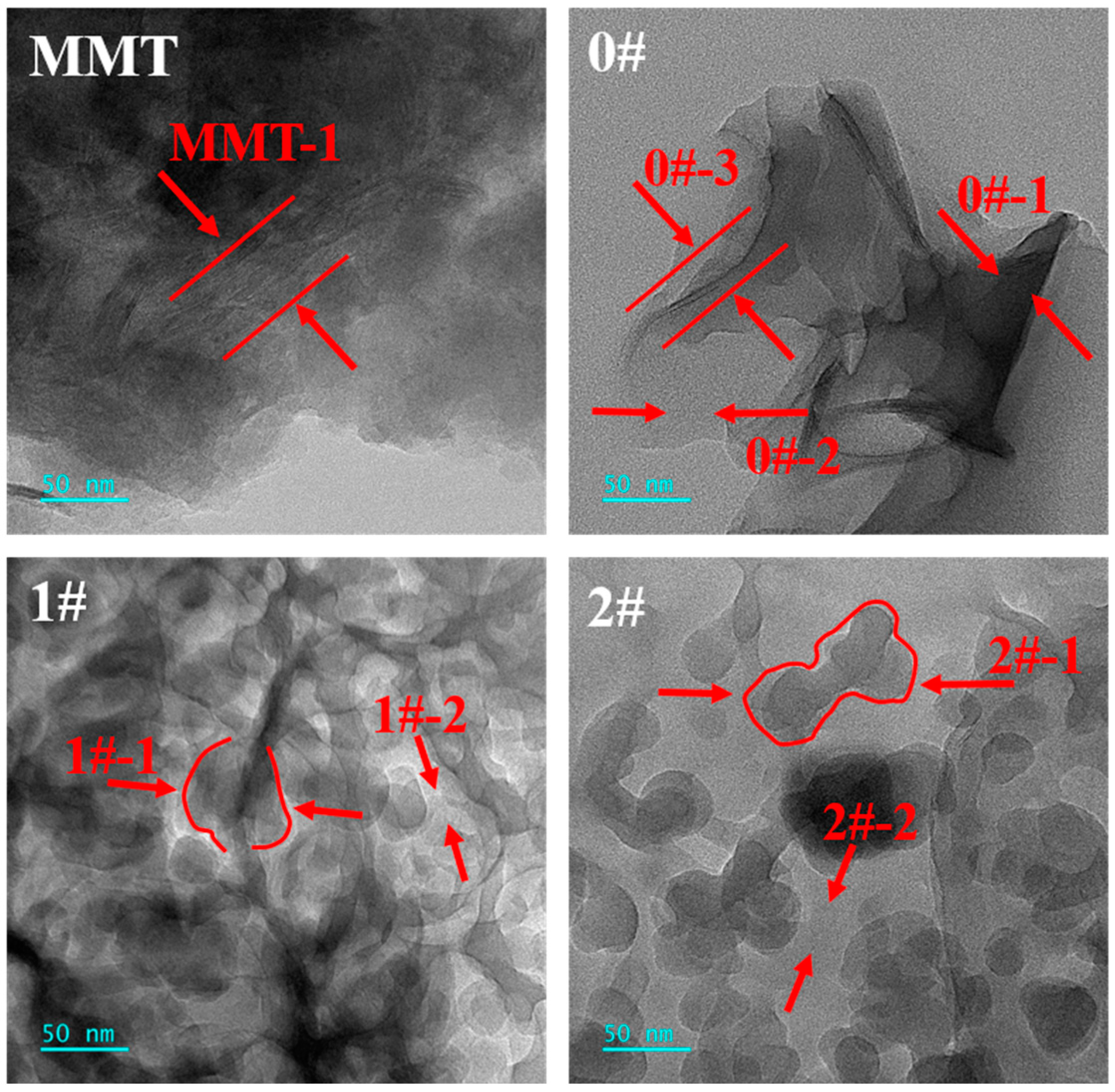
| Samples | PA6 (g) | AHP (g) | MMT-MCA (g) | Antioxid1010 (g) | Antioxid168 (g) | Calcium Stearate (g) | ||
|---|---|---|---|---|---|---|---|---|
| 0# | 1# | 2# | ||||||
| PA6 | 792 | 0 | 0 | 0 | 0 | 1 | 2 | 5 |
| PA6/AHP | 742 | 250 | 0 | 0 | 0 | 1 | 2 | 5 |
| 0#(9:1) | 742 | 225 | 25 | 0 | 0 | 1 | 2 | 5 |
| 0#(7:3) | 742 | 175 | 75 | 0 | 0 | 1 | 2 | 5 |
| 0#(5:5) | 742 | 125 | 125 | 0 | 0 | 1 | 2 | 5 |
| 1#(9:1) | 742 | 225 | 0 | 25 | 0 | 1 | 2 | 5 |
| 1#(7:3) | 742 | 175 | 0 | 75 | 0 | 1 | 2 | 5 |
| 1#(5:5) | 742 | 125 | 0 | 125 | 0 | 1 | 2 | 5 |
| 2#(9:1) | 742 | 225 | 0 | 0 | 25 | 1 | 2 | 5 |
| 2#(7:3) | 742 | 175 | 0 | 0 | 75 | 1 | 2 | 5 |
| 2#(5:5) | 742 | 125 | 0 | 0 | 125 | 1 | 2 | 5 |
| Positions | C (wt%) | N (wt%) | Si (wt%) |
|---|---|---|---|
| MMT-1 | 39.69 | 1.19 | 59.12 |
| 0#-1 | 59.13 | 36.90 | 3.96 |
| 0#-2 | 61.92 | 3.31 | 34.77 |
| 1#-1 | 63.40 | 27.47 | 9.13 |
| 1#-2 | 62.18 | 19.66 | 18.16 |
| 2#-1 | 56.86 | 26.10 | 17.04 |
| 2#-2 | 57.77 | 16.78 | 25.45 |
| Samples | T-5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | TR at 800 °C (%) | ER at 800 °C (%) | GR (%) |
|---|---|---|---|---|---|---|---|
| PA6 | 372.6 | \ | \ | 451.9 | \ | 0 | \ |
| AHP | 303.5 | 312.9 | \ | 434.0 | \ | 74.86 | \ |
| PA6/AHP | 317.4 | 319.8 | 380.2 | 437.0 | 25.47 | 25.47 | 0 |
| 0#(5:5) | 304.4 | 314.4 | 386.5 | 442.4 | 18.98 | 10.98 | 42.2 |
| 0#(7:3) | 311.6 | 316.5 | 379.5 | 436.1 | 21.58 | 17.70 | 18.0 |
| 0#(9:1) | 312.5 | 321.5 | 374.9 | 432.7 | 24.17 | 18.74 | 22.5 |
| 1#(5:5) | 306.3 | 315.8 | 385.7 | 441.3 | 18.98 | 11.91 | 37.2 |
| 1#(7:3) | 312.8 | 320.5 | 380.6 | 433.1 | 21.58 | 18.58 | 13.9 |
| 1#(9:1) | 313.8 | 329.8 | 372.4 | 432.0 | 24.17 | 21.84 | 9.6 |
| 2#(5:5) | 309.2 | 314.9 | 381.7 | 445.3 | 18.98 | 11.15 | 41.2 |
| 2#(7:3) | 314.0 | 315.3 | 380.0 | 441.0 | 21.58 | 21.24 | 1.6 |
| 2#(9:1) | 314.8 | 331.2 | 378.3 | 433.4 | 24.17 | 23.32 | 3.5 |
| Samples | UL-94 | LOI (%) | |
|---|---|---|---|
| Dripping | Grade | ||
| PA6 | Yes | None | 20% |
| PA6/AHP | No | V-0 | 26.0 |
| 0#(5:5) | Yes | None | 24.5 |
| 0#(7:3) | No | V-0 | 24.8 |
| 0#(9:1) | No | V-0 | 25.2 |
| 1#(5:5) | Yes | None | 24.7 |
| 1#(7:3) | No | V-0 | 25.5 |
| 1#(9:1) | No | V-0 | 25.3 |
| 2#(5:5) | No | V-0 | 25.0 |
| 2#(7:3) | No | V-0 | 25.8 |
| 2#(9:1) | No | V-0 | 25.7 |
| Samples | Peaks of PH3 (ppm) | Means of PH3 (ppm) | Integral of PH3 (ppm·s) |
|---|---|---|---|
| PA6/AHP | 83 | 29.8 | 35,830 |
| 0#(9:1) | 147 | 86.8 | 104,147 |
| 0#(7:3) | 34 | 13.9 | 16,673 |
| 0#(5:5) | 213 | 94.5 | 113,468 |
| 1#(9:1) | 86 | 37.0 | 44,410 |
| 1#(7:3) | 19 | 11.4 | 13,694 |
| 1#(5:5) | 80 | 42.0 | 50,385 |
| 2#(9:1) | 89 | 54.4 | 65,345 |
| 2#(7:3) | 15 | 8.3 | 9977 |
| 2#(5:5) | 53 | 24.4 | 29,244 |
| Positions | C (wt%) | N (wt%) | Al (wt%) | Si (wt%) | P (wt%) |
|---|---|---|---|---|---|
| PA6/AHP-1 | 10.95 | 65.85 | 6.58 | 0.04 | 16.58 |
| PA6/AHP-2 | 53.93 | 45.95 | 0.04 | 0.05 | 0.03 |
| 0#(7:3)-1 | 15.65 | 66.56 | 3.43 | 14.21 | 0.14 |
| 0#(7:3)-2 | 18.01 | 37.63 | 7.18 | 0.42 | 36.77 |
| 0#(7:3)-3 | 79.00 | 3.02 | 5.64 | 10.32 | 2.02 |
| 1#(7:3)-1 | 11.99 | 65.95 | 6.14 | 0.9 | 15.02 |
| 1#(7:3)-2 | 33.90 | 56.28 | 3.41 | 6.17 | 0.19 |
| 1#(7:3)-3 | 23.53 | 65.07 | 1.50 | 9.85 | 0.06 |
| 2#(7:3)-1 | 29.57 | 60.11 | 3.89 | 6.15 | 0.28 |
| 2#(7:3)-2 | 52.80 | 38.40 | 2.77 | 0.09 | 5.94 |
| 2#(7:3)-3 | 43.33 | 56.35 | 0.02 | 0.38 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, Y.; Yan, C.; Li, X.; He, J.; Yang, R. Preparation of Montmorillonite–Melamine Cyanurate and Inhibition of the Emission of Phosphine from PA6/Aluminum Hypophosphate. Polymers 2024, 16, 2946. https://doi.org/10.3390/polym16202946
Wang L, Li Y, Yan C, Li X, He J, Yang R. Preparation of Montmorillonite–Melamine Cyanurate and Inhibition of the Emission of Phosphine from PA6/Aluminum Hypophosphate. Polymers. 2024; 16(20):2946. https://doi.org/10.3390/polym16202946
Chicago/Turabian StyleWang, Lin, Yuyang Li, Chenyang Yan, Xiangmei Li, Jiyu He, and Rongjie Yang. 2024. "Preparation of Montmorillonite–Melamine Cyanurate and Inhibition of the Emission of Phosphine from PA6/Aluminum Hypophosphate" Polymers 16, no. 20: 2946. https://doi.org/10.3390/polym16202946
APA StyleWang, L., Li, Y., Yan, C., Li, X., He, J., & Yang, R. (2024). Preparation of Montmorillonite–Melamine Cyanurate and Inhibition of the Emission of Phosphine from PA6/Aluminum Hypophosphate. Polymers, 16(20), 2946. https://doi.org/10.3390/polym16202946






