Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of pSB and pSBCC
2.3. Preparation and Characterization of pSB and pSBCC Coatings
2.4. Quantitative Analysis of FITC-BSA Adsorption on pSB-Coated Substrates
2.5. Evaluation of L929 Cell Adhesion and Cytotoxicity of pSB-Coated Substrates
2.6. Assessment of Bacteria Adhesion to pSB or pSBCC Coated PDMS
2.7. Evaluation of Bactericidal Effects Under Light Illumination
2.8. Statistical Analysis
3. Results and Discussion
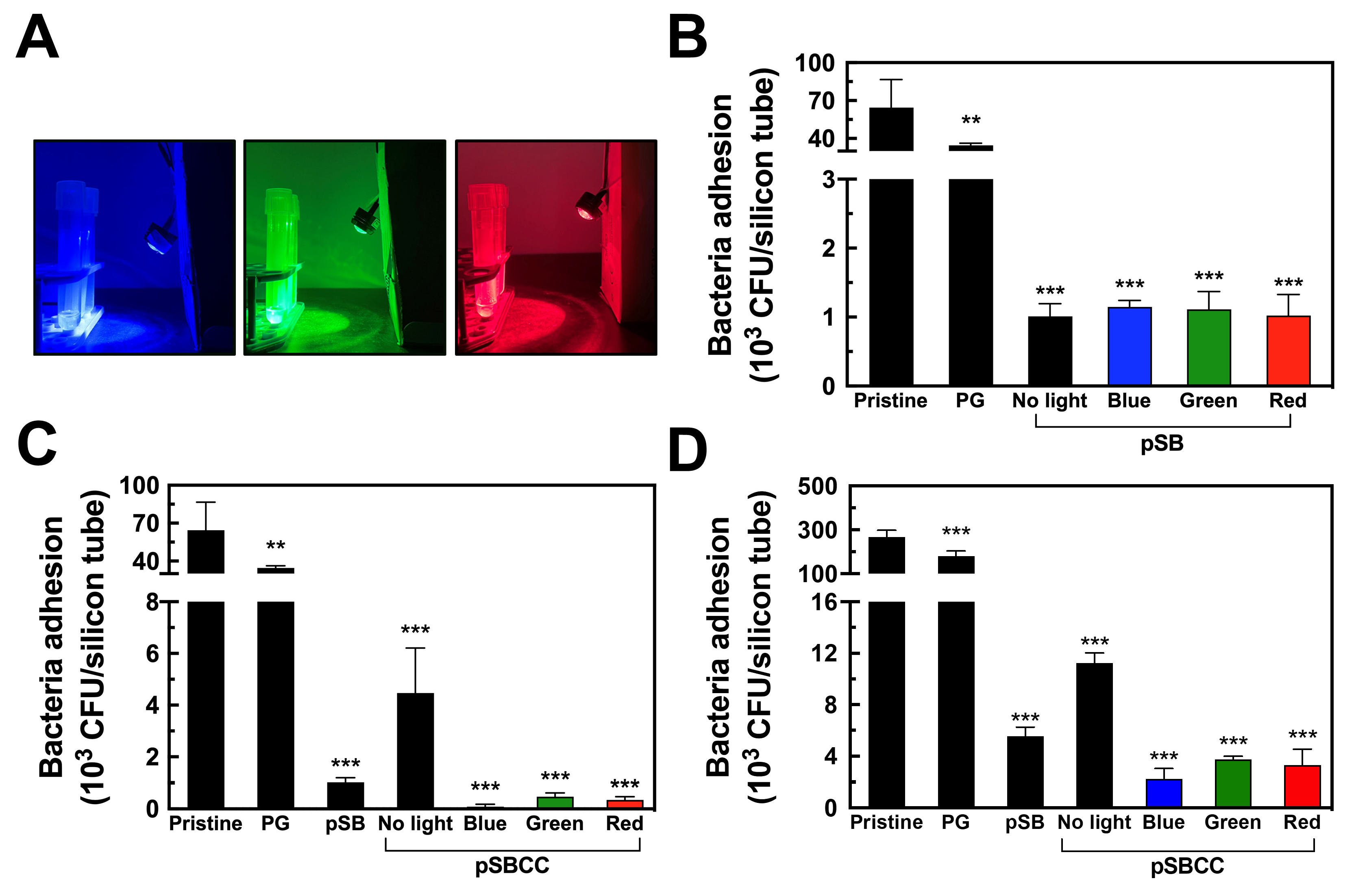
3.1. Light-Induced Bactericidal Properties of CC
3.2. Characterization of pSB and pSBCC
3.3. Surface Characterization of pSB-Coated Substrates
3.4. Antifouling Efficacy of pSB and pSBCC Coated Surfaces
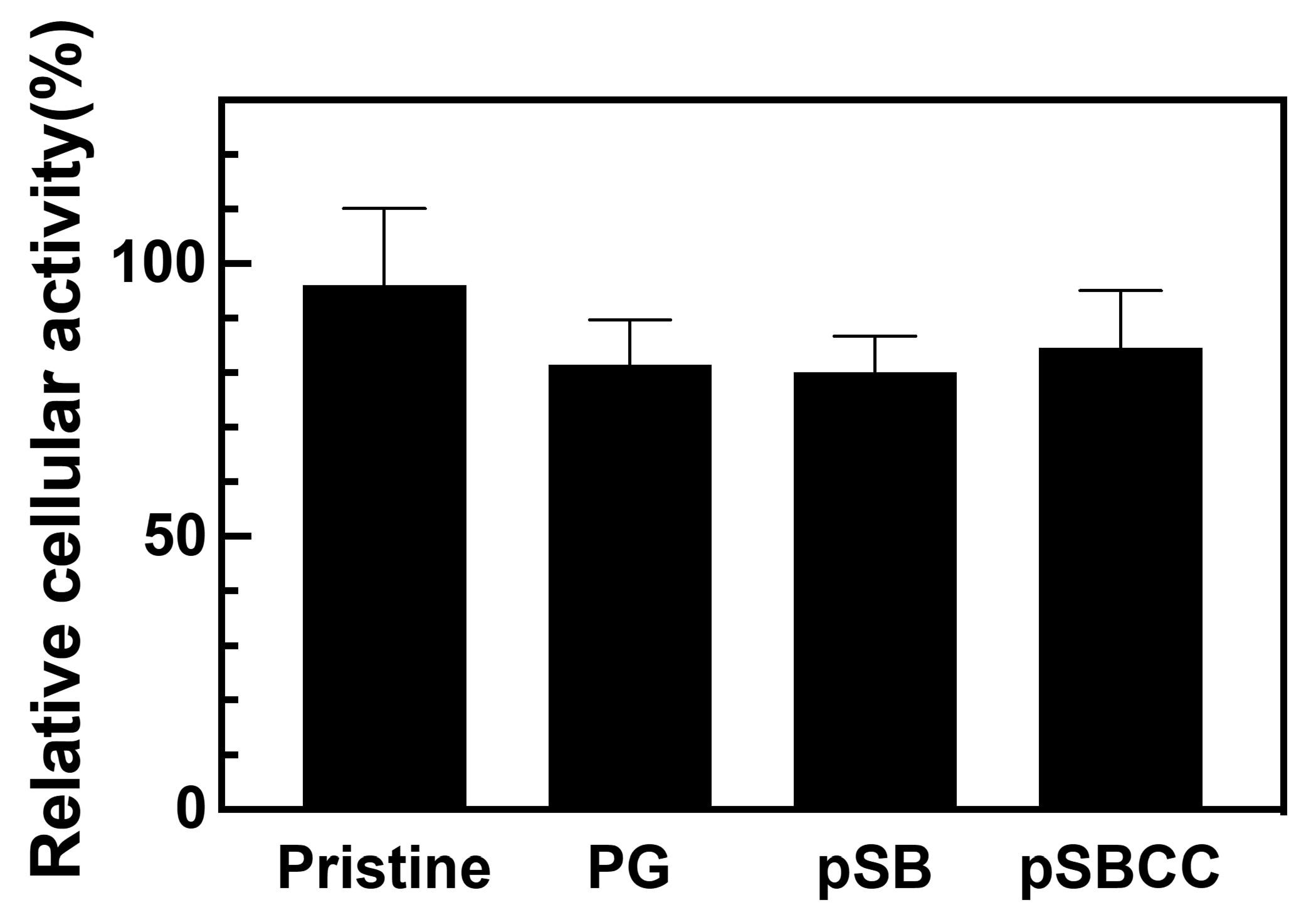
3.5. Cytotoxicity Assessment of pSB-Coated Silicone Tubes
3.6. Bactericidal Efficacy of pSBCC Coating with Light Illumination
3.7. Irradiation of Bacteria Through Optical Fiber-Guided Green Laser Illumination Inside Silicone Tubes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, J.W. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 2001, 17, 299–303. [Google Scholar] [CrossRef]
- Tambyah, P.A.; Oon, J. Catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 2012, 25, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Saint, S.; Greene, M.T.; Krein, S.L.; Rogers, M.A.; Ratz, D.; Fowler, K.E.; Edson, B.S.; Watson, S.R.; Meyer-Lucas, B.; Masuga, M.; et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. N. Engl. J. Med. 2016, 374, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting Against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef] [PubMed]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Yeh, S.L.; Deval, P.; Tsai, W.B. Fabrication of Transparent PEGylated Antifouling Coatings via One-Step Pyrogallol Deposition. Polymers 2023, 15, 2731. [Google Scholar] [CrossRef]
- Venault, A.; Wu, Y.L.; Maggay, I.V.; Chang, Y. Rapid attainment of full wettability through surface PEGylation of hydrophobic polyvinylidene fluoride membranes via spray-coating for enhanced anti-biofouling performances. Sep. Purif. Technol. 2024, 349, 127917. [Google Scholar] [CrossRef]
- Yeh, S.L.; Wang, T.C.; Yusa, S.; Thissen, H.; Tsai, W.B. Conjugation of Polysulfobetaine via Poly(pyrogallol) Coatings for Improving the Antifouling Efficacy of Biomaterials. Acs Omega 2021, 6, 3517–3524. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Lin, H.T.; Ang, M.B.M.Y.; Teow, Y.H.; Wickramasinghe, S.R.; Chang, Y. Surface Zwitterionization via Grafting of Epoxylated Sulfobetaine Copolymers onto PVDF Membranes for Improved Permeability and Biofouling Mitigation. Ind. Eng. Chem. Res. 2023, 62, 2913–2923. [Google Scholar] [CrossRef]
- Song, B.Y.; Zhang, E.S.; Shi, Y.J.; Wang, W.; Zhu, H.; Gallagher, S.J.; Fischer, S.; Rigney, J.; Kim, E.; Cao, Z.Q. Zwitterionic Hydrogel Coating with Antisediment Properties for Marine Antifouling Applications. Acs Appl. Mater. Inter. 2024, 16, 27908–27916. [Google Scholar] [CrossRef]
- Yoshikawa, C.; Takagi, R.; Nakaji-Hirabayashi, T.; Ochi, T.; Kawamura, Y.; Thissen, H. Marine Antifouling Coatings Based on Durable Bottlebrush Polymers. Acs Appl. Mater. Inter. 2022, 14, 32497–32509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chao, T.; Chen, S.F.; Jiang, S.Y. Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 2006, 22, 10072–10077. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Ziats, N.P.; Tierney, B.P.; Nakabayashi, N.; Anderson, J.M. Protein adsorption from human plasma is reduced on phospholipid polymers. J. Biomed. Mater. Res. 1991, 25, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.Y.; Easton, C.D.; Thissen, H.; Tsai, W.B. Aminomalononitrile-Assisted Multifunctional Antibacterial Coatings. Acs Biomater. Sci. Eng. 2020, 6, 3349–3360. [Google Scholar] [CrossRef]
- Sakala, G.P.; Reches, M. Peptide-Based Approaches to Fight Biofouling. Adv. Mater. Interfaces 2018, 5, 1800073. [Google Scholar] [CrossRef]
- Li, F.Y.; Huang, T.; Pasic, P.; Easton, C.D.; Voelcker, N.H.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J.; Thissen, H. One step antimicrobial coatings for medical device applications based on low fouling polymers containing selenium nanoparticles. Chem. Eng. J. 2023, 467, 143546. [Google Scholar] [CrossRef]
- Riool, M.; de Breij, A.; de Boer, L.; Kwakman, P.H.S.; Cordfunke, R.A.; Cohen, O.; Malanovic, N.; Emanuel, N.; Lohner, K.; Drijfhout, J.W.; et al. Controlled Release of LL-37-Derived Synthetic Antimicrobial and Anti-Biofilm Peptides SAAP-145 and SAAP-276 Prevents Experimental Biomaterial-Associated Infection. Adv. Funct. Mater. 2017, 27, 1606623. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.T.; Neoh, K.G. Polymer-Based Coatings with Integrated Antifouling and Bactericidal Properties for Targeted Biomedical Applications. Acs Appl. Polym. Mater. 2021, 3, 2233–2263. [Google Scholar] [CrossRef]
- Su, C.C.; Ye, Y.M.; Qiu, H.F.; Zhu, Y.B. Solvent-Free Fabrication of Self-Regenerating Antibacterial Surfaces Resisting Biofilm Formation. Acs Appl. Mater. Inter. 2021, 13, 10553–10563. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.; Zhong, P.; Chen, Y.Y.; Zhao, Z.Z.; Liu, W.Q. Multimechanism Long-Term Antibacterial Coating with Simultaneous Antifouling, Contact-Active, and Release-Kill Properties. Acs Appl. Polym. Mater. 2024, 6, 5726–5737. [Google Scholar] [CrossRef]
- Li, W.L.; Hua, G.P.; Cai, J.F.; Zhou, Y.M.; Zhou, X.; Wang, M.; Wang, X.M.; Fu, B.Q.; Ren, L. Multi-Stimulus Responsive Multilayer Coating for Treatment of Device-Associated Infections. J. Funct. Biomater. 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, K.J.; Tang, H.B.; Peng, J.Y.; Gao, S.; Du, Z.L.; Chen, G.; Wu, D.M.; Liu, G.Y. Smart Lubricant Coating with Urease-Responsive Antibacterial Functions for Ureteral Stents to Inhibit Infectious Encrustation. Adv. Funct. Mater. 2023, 34, 2307760. [Google Scholar] [CrossRef]
- Ricardo, S.I.C.; Anjos, I.I.L.; Monge, N.; Faustino, C.M.C.; Ribeiro, I.A.C. A glance at antimicrobial strategies to prevent catheter-associated medical infections. ACS Infect. Dis. 2020, 6, 3109–3130. [Google Scholar] [CrossRef] [PubMed]
- Misba, L.; Khan, A.U. Domestic LED bulb induced photodynamic effect of Toluidine Blue O-embedded silicone catheters against urinary tract infection. Photodiagn. Photodyn. Ther. 2023, 42, 103590. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef]
- Jiang, C.; Scholle, F.; Jin, F.; Wei, Q.; Wang, Q.; Ghiladi, R.A. Chlorophyllin as a photosensitizer in photodynamic antimicrobial materials. Cellulose 2024, 31, 2475–2491. [Google Scholar] [CrossRef]
- Clerici, D.J.; da Silveira, C.H.; Iglesias, B.A.; Santos, R.C.V. The first evidence of antibiofilm action of Proteus mirabilis with tetra-cationic porphyrins containing cisplatin by antimicrobial photodynamic therapy. Microb. Pathog. 2023, 174, 105859. [Google Scholar] [CrossRef] [PubMed]
- Caires, C.S.; Silva, C.M.; Lima, A.R.; Alves, L.M.; Lima, T.H.; Rodrigues, A.C.; Chang, M.R.; Oliveira, S.L.; Whitby, C.; Nascimento, V.A. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by a natural food colorant (E-141ii). Molecules 2020, 25, 4464. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, J.; Wang, Z.; Chen, F.; Liao, X.; Hu, X.; Dong, L. Sodium copper chlorophyll mediated photodynamic treatment inactivates Escherichia coli via oxidative damage. Food Res. Int. 2022, 157, 111472. [Google Scholar] [CrossRef]
- Phasupan, P.; Le, T.D.; Nguyen, L.T. Assessing the photodynamic efficacy of different photosensitizer-light treatments against foodborne bacteria based on the number of absorbed photons. J. Photochem. Photobiol. B Biol. 2021, 221, 112249. [Google Scholar] [CrossRef]
- Liu, X.X.; Yu, L.F.; Wei, J.F.; Huang, Y.Y.; Yang, L.; Ning, J.H.; Su, Q.P.; Li, H.L.; Xin, J.L.; Jia, K.L. Mussel-Inspired Antimicrobial and Antifouling Coating Constructed by the Combination of Zwitterionic Copolymers and Silver Nanoparticles. Langmuir 2024, 40, 8654–8664. [Google Scholar] [CrossRef]
- Song, B.Y.; Zhang, E.S.; Han, X.F.; Zhu, H.; Shi, Y.J.; Cao, Z.Q. Engineering and Application Perspectives on Designing an Antimicrobial Surface. Acs Appl. Mater. Inter. 2020, 12, 21330–21341. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.W.; Tsai, W.B. Fabrication of tunable micropatterned substrates for cell patterning via microcontact printing of polydopamine with poly(ethylene imine)-grafted copolymers. Acta Biomater. 2012, 8, 3678–3686. [Google Scholar] [CrossRef]
- Meng, L.W.; Huang, C.X.; Liu, X.; Qu, H.Y.; Wang, Q.L. Zwitterionic coating assisted by dopamine with metal-phenolic networks loaded on titanium with improved biocompatibility and antibacterial property for artificial heart. Front. Bioeng. Biotech. 2023, 11, 1167340. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Kim, H.-D.; Maniak, P.; Messersmith, P.B. Antibacterial Performance of Polydopamine-Modified Polymer Surfaces Containing Passive and Active Components. Acs Appl. Mater. Inter. 2011, 3, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.G.; Sileika, T.S.; Messersmith, P.B. Molecular diversity in phenolic and polyphenolic precursors of tannin-inspired nanocoatings. Chem. Commun. 2014, 50, 7265–7268. [Google Scholar] [CrossRef]
- Dhand, C.; Harini, S.; Venkatesh, M.; Dwivedi, N.; Ng, A.; Liu, S.P.; Verma, N.K.; Ramakrishna, S.; Beuerman, R.W.; Loh, X.J.; et al. Multifunctional Polyphenols- and Catecholamines-Based Self-Defensive Films for Health Care Applications. Acs Appl. Mater. Inter. 2016, 8, 1220–1232. [Google Scholar] [CrossRef]
- Drynan, J.W.; Clifford, M.N.; Obuchowicz, J.; Kuhnert, N. The chemistry of low molecular weight black tea polyphenols. Nat. Prod. Rep. 2010, 27, 417–462. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. Int. Edit. 2013, 52, 10766–10770. [Google Scholar] [CrossRef]
- Deval, P.; Lin, C.H.; Tsai, W.B. Fabrication of Polysulfobetaine Gradient Coating via Oxidation Polymerization of Pyrogallol to Modulate Biointerfaces. Acs Omega 2022, 7, 7125–7133. [Google Scholar] [CrossRef]
- Yeh, S.L.; Deval, P.; Wu, J.G.; Luo, S.C.; Tsai, W.B. One-step electrochemical deposition of antifouling polymers with pyrogallol for biosensing applications. J. Mater. Chem. B 2022, 10, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Liao, T.-Y.; Thissen, H.; Tsai, W.-B. One-step aminomalononitrile-based coatings containing zwitterionic copolymers for the reduction of biofouling and the foreign body response. Acs Biomater. Sci. Eng. 2019, 5, 6454–6462. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.; Neal, S.L. Efficiency comparison of the imidazole plus RNO method for singlet oxygen detection in biorelevant solvents. Anal. Bioanal. Chem. 2019, 411, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Garcez, A.; Barros, L.; Fernandes, M.; Fujii, D.; Suzuki, S.; Nepomuceno, R. Fluorescence image and microbiological analysis of biofilm retained around healthy and inflamed orthodontic miniscrews. Photodiagn. Photodyn. Ther. 2020, 30, 101707. [Google Scholar] [CrossRef]
- Chang, R.; Hsu, C.-F.; Tsai, W.-B. Fabrication of chlorophyll-incorporated nanogels for potential applications in photothermal cancer therapy. Acs Omega 2018, 3, 16057–16062. [Google Scholar] [CrossRef]
- Chang, R.; Tsai, W.B. Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy. Polymers 2018, 10, 1098. [Google Scholar] [CrossRef]
- An, T.; Lee, N.; Cho, H.-J.; Kim, S.; Shin, D.-S.; Lee, S.-M. Ultra-selective detection of Fe2+ ion by redox mechanism based on fluorescent polymerized dopamine derivatives. RSC Adv. 2017, 7, 30582–30587. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Lee, Y.; Kim, T.-H.; Heo, S.-J.; Shin, H.; Lee, J.; Yi, M.; Kang, H.W.; Jung, W.-K. Anti-Cancer Effect of Chlorophyllin-Assisted Photodynamic Therapy to Induce Apoptosis through Oxidative Stress on Human Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 11565. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Gao, J.; Jin, X.; Wang, Z.; Wang, B.; Li, K.; Li, Y. Detection and comparison of reactive oxygen species (ROS) generated by chlorophyllin metal (Fe, Mg and Cu) complexes under ultrasonic and visible-light irradiation. Ultrason. Sonochem. 2011, 18, 1028–1034. [Google Scholar] [CrossRef]
- Buchovec, I.; Lukseviciūtė, V.; Kokstaite, R.; Labeikyte, D.; Kaziukonyte, L.; Luksiene, Z. Inactivation of Gram (−) bacteria Salmonella enterica by chlorophyllin-based photosensitization: Mechanism of action and new strategies to enhance the inactivation efficiency. J. Photochem. Photobiol. B Biol. 2017, 172, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pablos, C.; Marugán, J.; van Grieken, R.; Hamilton, J.W.; Ternan, N.G.; Dunlop, P.S. Assessment of Photoactivated Chlorophyllin Production of Singlet Oxygen and Inactivation of Foodborne Pathogens. Catalysts 2024, 14, 507. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-H.; Chen, G.-H.; Tsai, W.-B. Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications. Polymers 2024, 16, 2974. https://doi.org/10.3390/polym16212974
Chen P-H, Chen G-H, Tsai W-B. Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications. Polymers. 2024; 16(21):2974. https://doi.org/10.3390/polym16212974
Chicago/Turabian StyleChen, Po-Hsun, Guan-Hua Chen, and Wei-Bor Tsai. 2024. "Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications" Polymers 16, no. 21: 2974. https://doi.org/10.3390/polym16212974
APA StyleChen, P.-H., Chen, G.-H., & Tsai, W.-B. (2024). Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications. Polymers, 16(21), 2974. https://doi.org/10.3390/polym16212974






