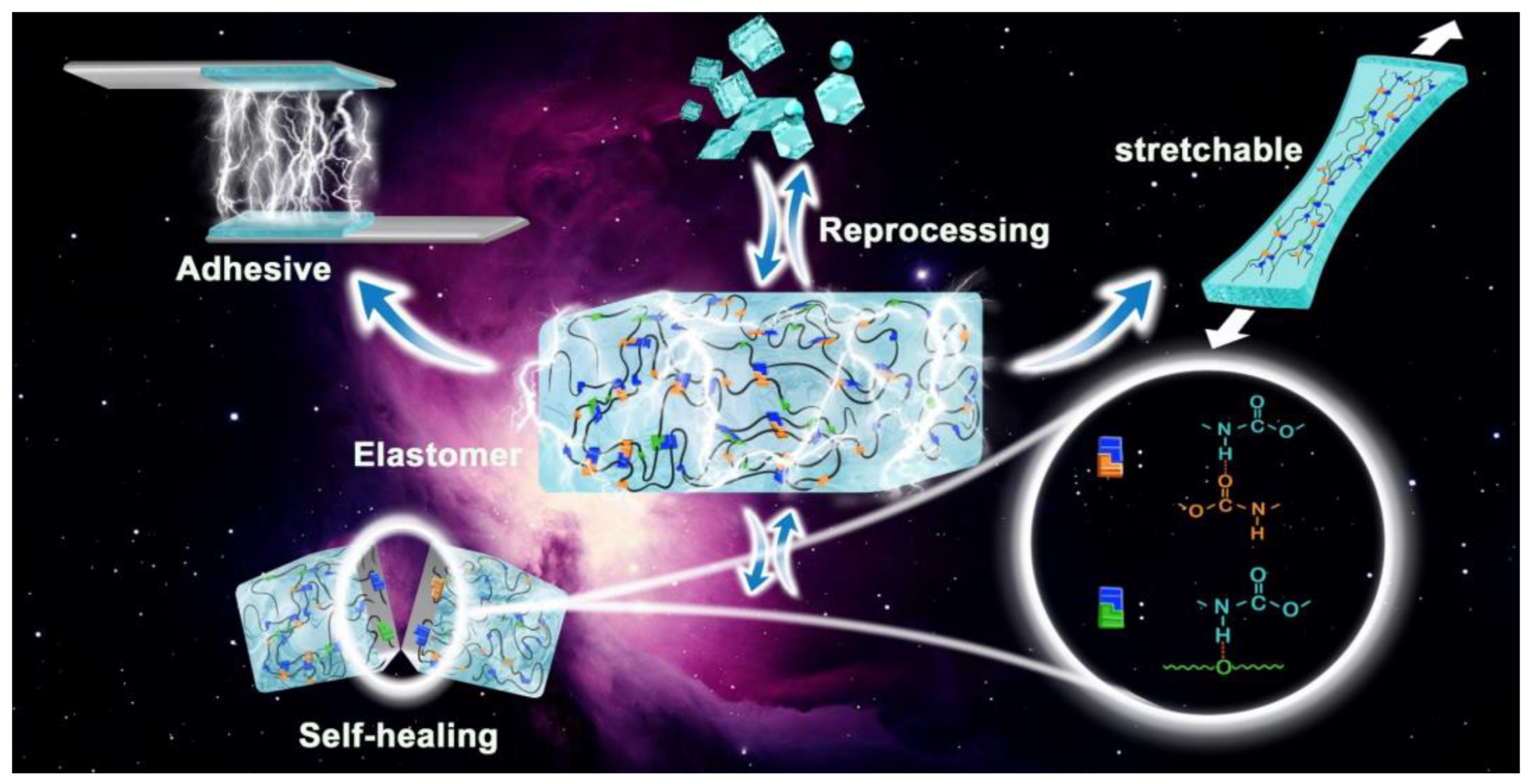
Effects of Isocyanate Structure on the Properties of Polyurethane: Synthesis, Performance, and Self-Healing Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
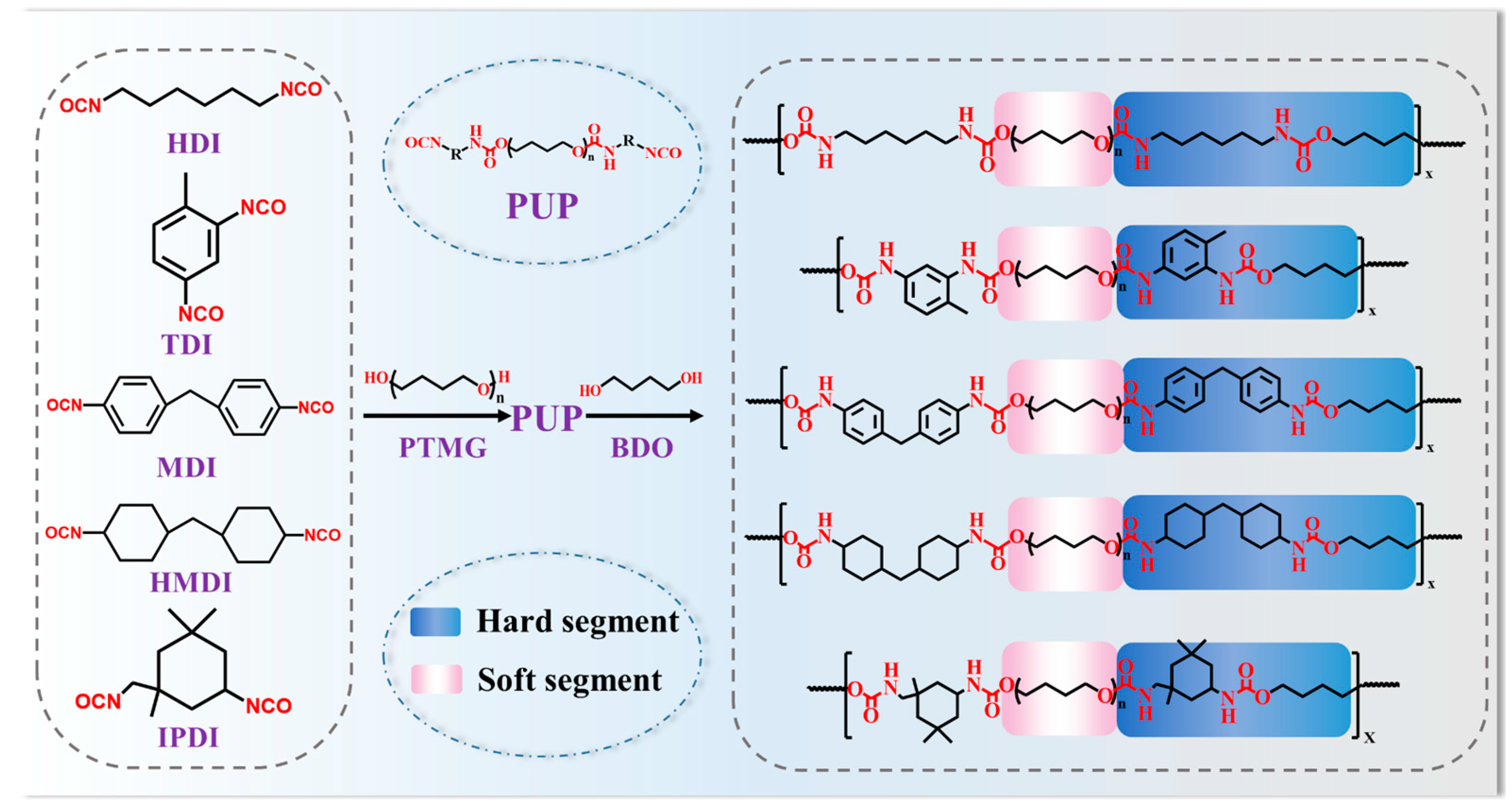
2.2. Preparation of PUs
2.3. Characterization
3. Results and Discussion
3.1. Prepolymerization Reaction Rate and Structural Analysis of PUs
3.2. Thermal Behavior and Crystallization
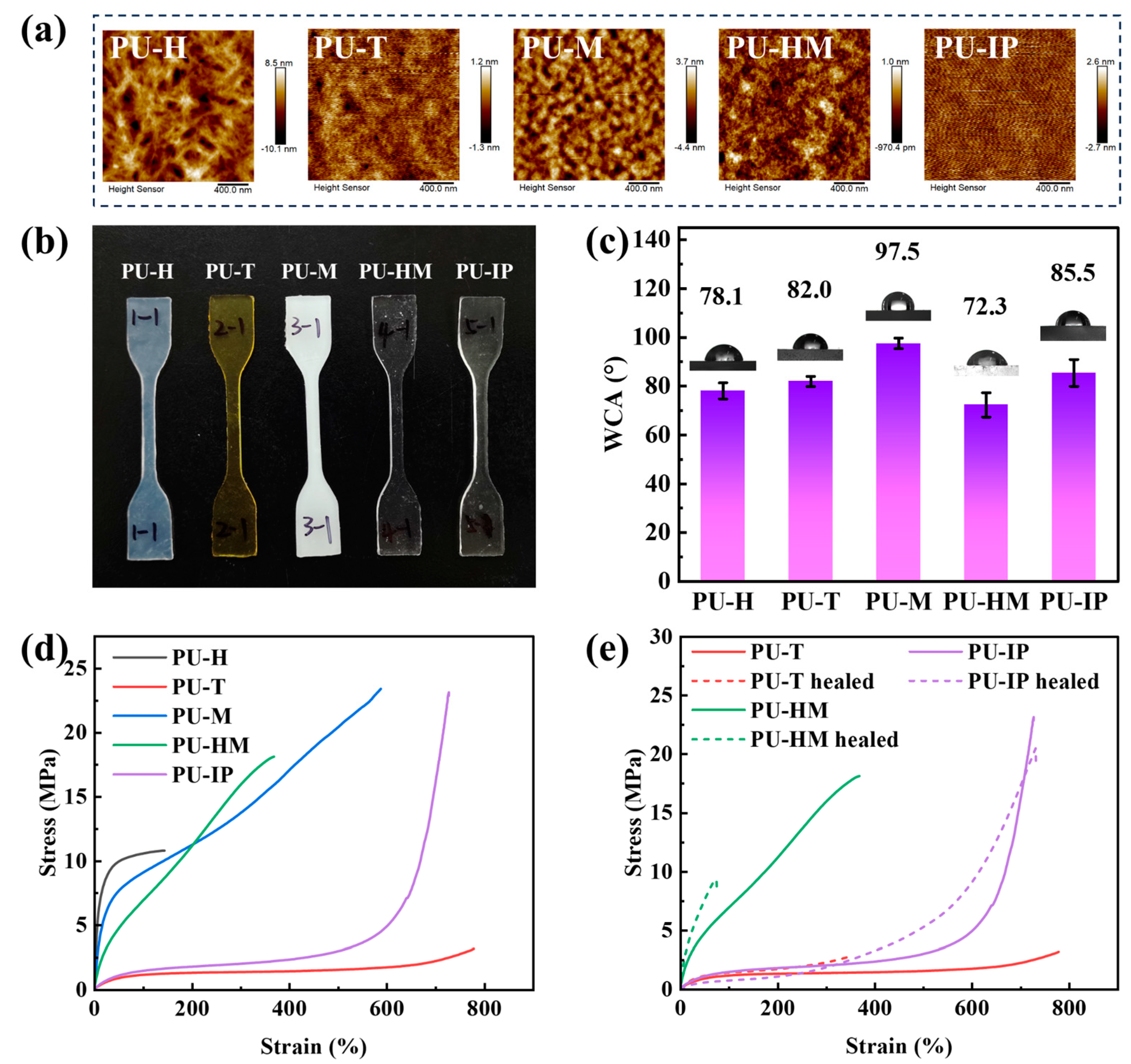
3.3. Morphology, Wettability, Mechanical Property, and Self-Healing Ability
3.4. Surface Scratch-Healing Properties
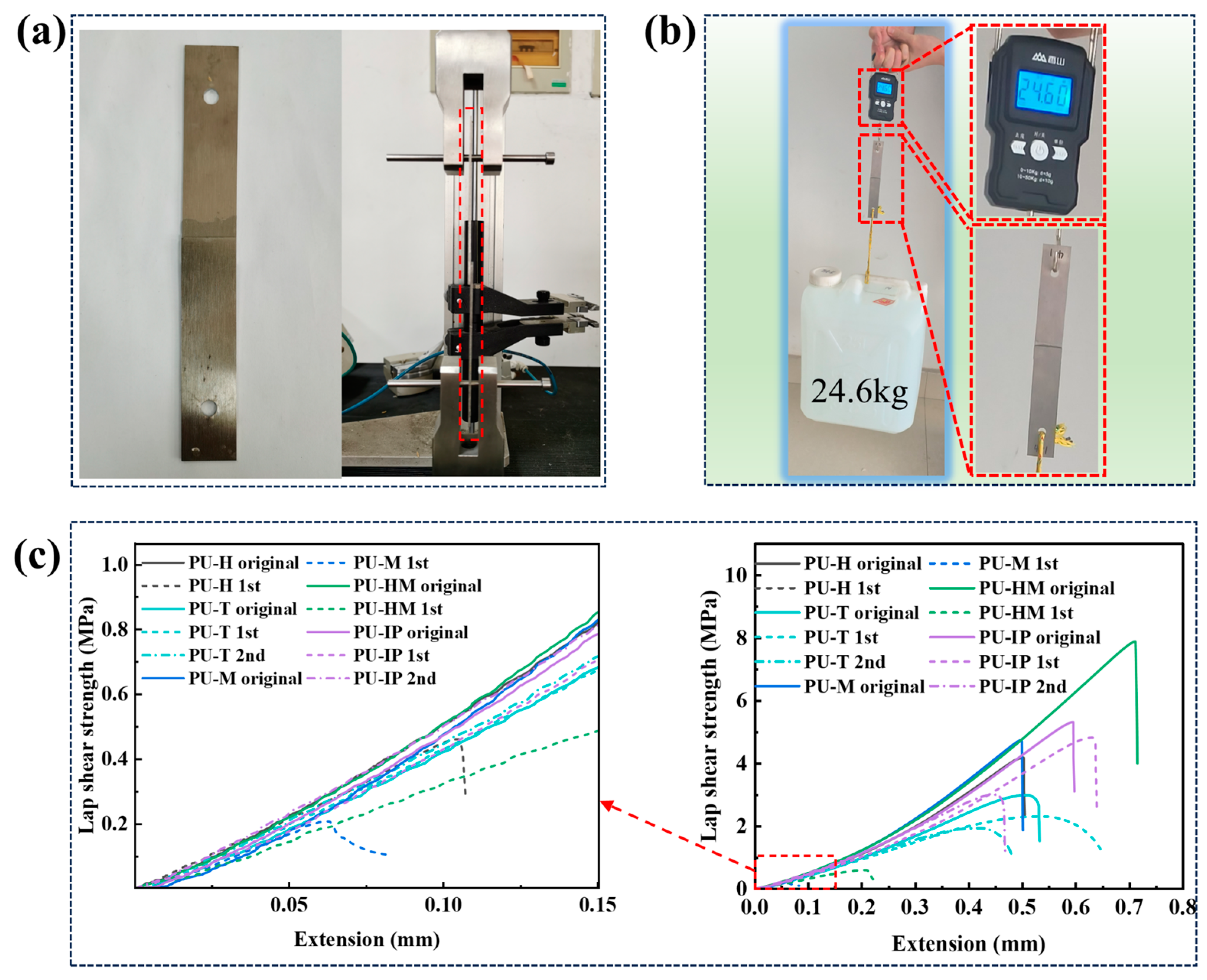
3.5. Adhesion Properties
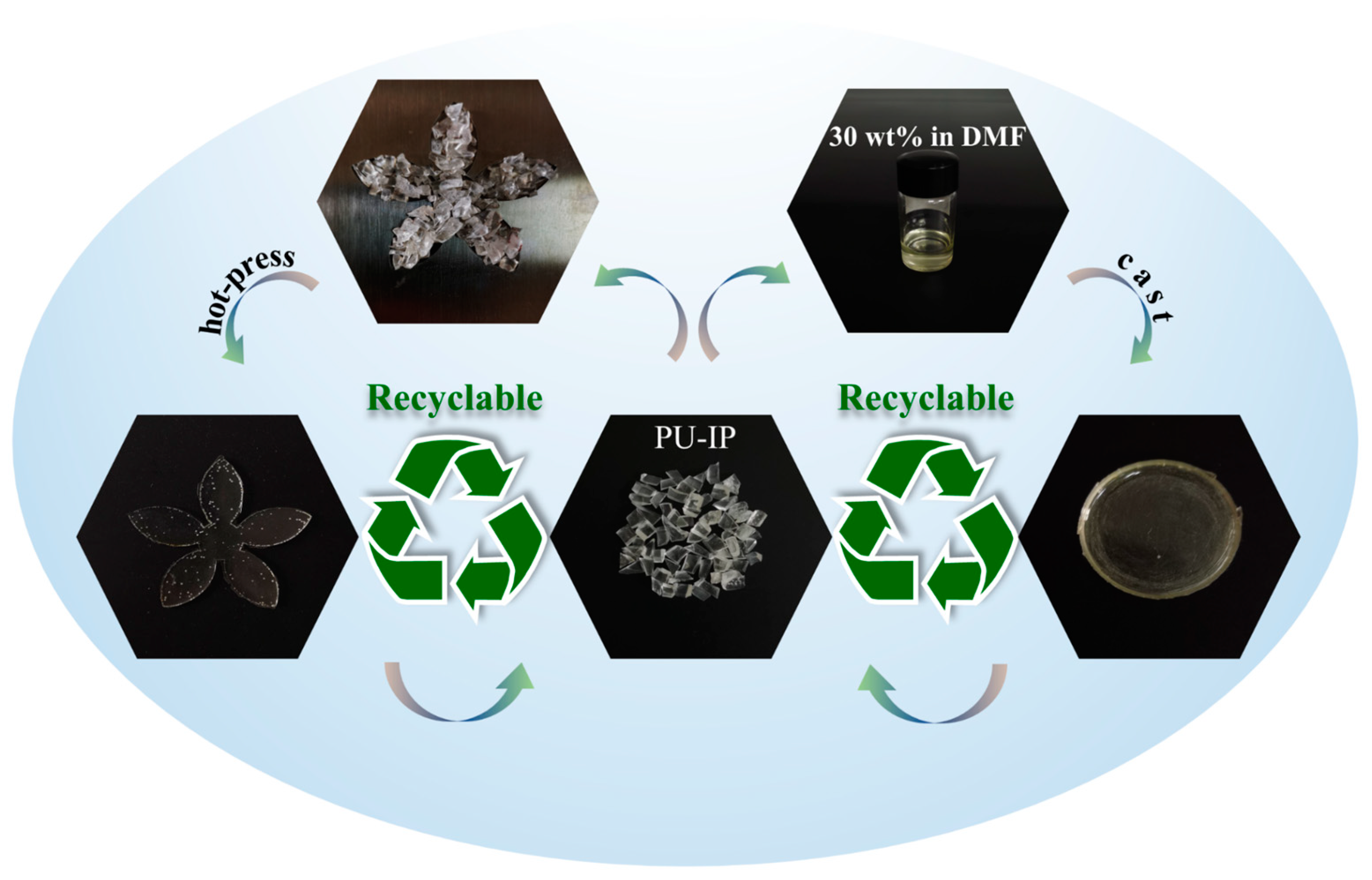
3.6. Recyclability and Reprocessability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polyurethane Market Size, Share & Industry Analysis, by Product Type (Rigid Foam, Flexible Foam, Molded Foam, Elastomers, Adhesives & Sealants, Coatings, and Others), by Application (Furniture, Construction, Electronics, Automotive, Packaging, Footwear, and Others), and Regional Forecast, 2024–2032. 2024. Available online: https://www.fortunebusinessinsights.com/industry-reports/polyurethane-pu-market-101801 (accessed on 8 October 2024).
- Delavarde, A.; Savin, G.; Derkenne, P.; Boursier, M.; Morales-Cerrada, R.; Nottelet, B.; Pinaud, J.; Caillol, S. Sustainable polyurethanes: Toward new cutting-edge opportunities. Prog. Polym. Sci. 2024, 151, 101805. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, W.; Zhang, S.; Dong, Z.; Lei, C. Synthesis and characterization of polyurethane with poly(ether-ester) diols soft segments consisted by ether and ester linkages in one repeating unit. Eur. Polym. J. 2022, 179, 111553. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, L.Y.; Zhai, J.X.; Yang, R.J.; Guo, X.Y. Analysis of the mechanical behavior of polyurethane thermoset elastomers based on hydrogen bonding between different crosslinking point structures. Polymer 2023, 285, 126356. [Google Scholar] [CrossRef]
- Li, X.J.; Xue, Z.Y.; Sun, W.T.; Chu, J.H.; Wang, Q.J.; Tong, L.B.; Wang, K.S. Bio-inspired self-healing MXene/polyurethane coating with superior active/passive anticorrosion performance for Mg alloy. Chem. Eng. J. 2023, 454, 140187. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, J.; Zhang, Y.M.; Wang, T.M.; Wang, Q.H.; Li, S.; Yang, Z.H.; Zhang, X.R. A stretchable, mechanically robust polymer exhibiting shape-memory-assisted self-healing and clustering-triggered emission. Nat. Commun. 2023, 14, 4712. [Google Scholar] [CrossRef]
- Liu, W.X.; Yang, Z.S.; Qiao, Z.; Zhang, L.; Zhao, N.; Luo, S.Z.; Xu, J. Dynamic multiphase semi-crystalline polymers based on thermally reversible pyrazole-urea bonds. Nat. Commun. 2019, 10, 4753. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.R.; Shao, Z.Y.; Zhao, N.F.; Zhang, R.Z.; Yuan, G.D.; Tian, L.L.; Zhang, Z.B.; Gao, W.W.; Bai, H. Biomimetic, knittable aerogel fiber for thermal insulation textile. Science 2023, 382, 1379–1383. [Google Scholar] [CrossRef]
- Qu, Q.; He, J.; Da, Y.S.; Zhu, M.H.; Liu, Y.Y.; Li, X.X.; Tian, X.Y.; Wang, H. High Toughness Polyurethane toward Artificial Muscles, Tuned by Mixing Dynamic Hard Domains. Macromolecules 2021, 54, 8243–8254. [Google Scholar] [CrossRef]
- Duan, X.Y.; Cao, W.H.; He, X.N.; Wang, M.Q.; Cong, R.Y.; Zhang, Z.C.; Ning, C.; Wang, C.S.; Zhao, S.L.; Li, Z.Q.; et al. Realization of dual crosslinked network robust, high toughness self-healing polyurethane elastomers for electronics applications. Chem. Eng. J. 2023, 476, 146536. [Google Scholar] [CrossRef]
- Boahen, E.K.; Pan, B.; Kweon, H.; Kim, J.S.; Choi, H.; Kong, Z.; Kim, D.J.; Zhu, J.; Ying, W.B.; Lee, K.J.; et al. Ultrafast, autonomous self-healable iontronic skin exhibiting piezo-ionic dynamics. Nat. Commun. 2022, 13, 7699. [Google Scholar] [CrossRef]
- Xu, H.; Tu, J.; Li, H.Z.; Ji, J.; Liang, L.; Tian, J.Q.; Guo, X.D. Room-temperature self-healing, high ductility, recyclable polyurethane elastomer fabricated via asymmetric dynamic hard segments strategy combined with self-cleaning function application. Chem. Eng. J. 2023, 454, 140101. [Google Scholar] [CrossRef]
- Ye, T.; Liu, J.Y.; Sun, J.J.; Tan, J.L.; Chen, X.; Yin, Y.J.; Wang, C.X. Healable, luminescent, notch-insensitive waterborne polyurethane via noncovalent crosslinking with hydrogen bonds and ionic interactions. Chem. Eng. J. 2023, 475, 146393. [Google Scholar] [CrossRef]
- Eom, Y.; Kim, S.M.; Lee, M.; Jeon, H.; Park, J.; Lee, E.S.; Hwang, S.Y.; Park, J.; Oh, D.X. Mechano-responsive hydrogen-bonding array of thermoplastic polyurethane elastomer captures both strength and self-healing. Nat. Commun. 2021, 12, 621. [Google Scholar] [CrossRef] [PubMed]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Sáenz-Pérez, M.; Lizundia, E.; Laza, J.M.; García-Barrasa, J.; Vilasa, J.L.; León, L.M. Methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) based polyurethanes: Thermal, shape-memory and mechanical behavior. RSC Adv. 2016, 6, 69094–69102. [Google Scholar] [CrossRef]
- Wang, Y.J.; Nie, H.F.; Wang, S.M.; Zhang, H.; Zhang, X.F.; Jiang, L.; Liu, W.T.; Huang, M.M. Dual-Functional Biopolyurethane Blends with Shape-Memory and Self-Healing Properties: Effects of Mixed Hard Domains on Structures and Properties. ACS Appl. Polym. Mater. 2023, 5, 9364–9374. [Google Scholar] [CrossRef]
- Wang, Y.J.; Nie, H.F.; Wang, S.M.; Zhang, H.; Zhang, X.F.; Huang, M.M.; Liu, W.T. Facile approach for imparting shape memory behavior to traditional thermoplastic polyurethane via interaction between different hard segments. J. Appl. Polym. Sci. 2023, 140, e54467. [Google Scholar] [CrossRef]
- Falco, G.; Simonin, L.; Pensec, S.; Dalmas, F.; Chenal, J.M.; Bouteiller, L.; Chazeau, L. Linear and nonlinear viscoelastic properties of segmented silicone-urea copolymers: Influence of the hard segment structure. Polymer 2020, 186, 122041. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, J.H. Shape memory characteristics of thermadapt polyurethane incorporated with two structurally distinctive aliphatic isocyanates. Polym. Test. 2021, 103, 107366. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Wang, H.R.; Pan, H.W.; Yan, T.T.; Zong, C.Z. The effect of mixed soft segment on the microstructure of thermoplastic polyurethane. J. Appl. Polym. Sci. 2021, 138, e51346. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, T.; Li, G.L. Towards Dynamic but Supertough Healable Polymers through Biomimetic Hierarchical Hydrogen-Bonding Interactions. Angew. Chem. Int. Ed. 2018, 57, 13838–13842. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, X.; Yang, X.; Zhou, P.; Guo, Q.; Zhang, X. Multi-Modal Melt-Processing of Birefringent Cellulosic Materials for Eco-Friendly Anti-Counterfeiting. Adv. Mater. 2024, 36, 2407170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.X.; Zhang, J.L.; Jiang, Y.; Wang, S.F.; Zhao, H. High Toughness, Multi-dynamic Self-Healing Polyurethane for Outstanding Energy Harvesting and Sensing. ACS Appl. Mater. Interfaces 2023, 15, 58806–58814. [Google Scholar] [CrossRef]
- Cai, Y.B.; Yan, L.W.; Wang, Y.; Ge, Y.; Liang, M.; Chen, Y.; Zou, H.W.; Zhou, S.T. A room temperature self-healing and thermally reprocessable cross-linked elastomer with unprecedented mechanical properties for ablation-resistant applications. Chem. Eng. J. 2022, 436, 135156. [Google Scholar] [CrossRef]
- Li, C.Y.; Wang, P.; Zhang, D.; Wang, S. Near-Infrared Responsive Smart Superhydrophobic Coating with Self-Healing and Robustness Enhanced by Disulfide-Bonded Polyurethane. ACS Appl. Mater. Interfaces 2022, 14, 45988–46000. [Google Scholar] [CrossRef]
- Yilgör, I.; Yilgör, E.; Wilkes, G.L. Critical parameters in designing segmented polyurethanes and their effect on morphology and properties: A comprehensive review. Polymer 2015, 58, A1–A36. [Google Scholar] [CrossRef]
- Jiang, Z.S.; Huang, L.L.; Fan, Y.X.; Zhou, S.F.; Zou, X.M. Contrasting effects of microplastic aging upon the adsorption of sulfonamides and its mechanism. Chem. Eng. J. 2022, 430, 132939. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, Y.; Kang, M.; Wang, J.; Wang, X.; Feng, Y.; Yin, N.; Li, Q. The effects of the molecular weight and structure of polycarbonatediols on the properties of waterborne polyurethanes. Prog. Org. Coat. 2015, 82, 46–56. [Google Scholar] [CrossRef]
- Lai, Y.; Kuang, X.; Zhu, P.; Huang, M.M.; Dong, X.; Wang, D.J. Colorless, Transparent, Robust, and Fast Scratch-Self-Healing Elastomers via a Phase-Locked Dynamic Bonds Design. Adv. Mater. 2018, 30, 1802556. [Google Scholar] [CrossRef]
- Xu, J.H.; Chen, J.Y.; Zhang, Y.N.; Liu, T.; Fu, J.J. A Fast Room-Temperature Self-Healing Glassy Polyurethane. Angew. Chem. Int. Edit. 2021, 60, 7947–7955. [Google Scholar] [CrossRef]
- Yang, S.W.; Wang, S.; Du, X.S.; Du, Z.L.; Cheng, X.; Wang, H.B. Mechanically robust self-healing and recyclable flame-retarded polyurethane elastomer based on thermoreversible crosslinking network and multiple hydrogen bonds. Chem. Eng. J. 2020, 391, 123544. [Google Scholar] [CrossRef]
- Castagna, A.M.; Pangon, A.; Choi, T.; Dillon, G.P.; Runt, J. The Role of Soft Segment Molecular Weight on Microphase Separation and Dynamics of Bulk Polymerized Polyureas. Macromolecules 2012, 45, 8438–8444. [Google Scholar] [CrossRef]
- Geng, Z.S.; Pang, A.M.; Ding, T.F.; Guo, X.Y.; Yang, R.J.; Luo, Y.J.; Zhai, J.X. Overlooked Impact of Interchain H-Bonding between Cross-Links on the Mechanical Properties of Thermoset Polyurethane Elastomers. Macromolecules 2022, 55, 8749–8756. [Google Scholar] [CrossRef]
- Cheng, B.X.; Gao, W.C.; Ren, X.M.; Ouyang, X.Y.; Zhao, Y.; Zhao, H.; Wu, W.; Huang, C.X.; Liu, Y.; Liu, X.Y.; et al. A review of microphase separation of polyurethane: Characterization and applications. Polym. Test. 2022, 107, 107489. [Google Scholar] [CrossRef]
- Cui, B.; Wu, Q.Y.; Gu, L.; Shen, L.; Yu, H.B. High performance bio-based polyurethane elastomers: Effect of different soft and hard segments. Chin. J. Polym. Sci. 2016, 34, 901–909. [Google Scholar] [CrossRef]
- Shen, R.L.; Long, M.J.; Lei, C.D.; Dong, L.M.; Yu, G.P.; Tang, J.T. Anticorrosive waterborne polyurethane coatings derived from castor oil and renewable diols. Chem. Eng. J. 2022, 433, 134470. [Google Scholar] [CrossRef]
- Zhang, E.D.; Liu, X.H.; Liu, Y.C.; Shi, J.; Li, X.B.; Xiong, X.Y.; Xu, C.A.; Wu, K.; Lu, M.G. Highly stretchable, bionic self-healing waterborne polyurethane elastic film enabled by multiple hydrogen bonds for flexible strain sensors. J. Mater. Chem. A 2021, 9, 23055–23071. [Google Scholar] [CrossRef]



| Sample | Isocyanate | Polyol | Chain Extender | Molar Ratio (Isocyanate/PTMG/BDO) | Molecular Weight of Isocyanate (g/mol) | Ch (%) |
|---|---|---|---|---|---|---|
| PU-H | HDI | PTMG | BDO | 3:1:2 | 168.19 | 41 |
| PU-T | TDI | PTMG | BDO | 3:1:2 | 174.16 | 41 |
| PU-M | MDI | PTMG | BDO | 3:1:2 | 250.26 | 48 |
| PU-HM | HMDI | PTMG | BDO | 3:1:2 | 262.35 | 49 |
| PU-IP | IPDI | PTMG | BDO | 3:1:2 | 222.29 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Cao, L.; Wang, X.; Lang, X.; Cong, W.; Han, L.; Zhang, H.; Zhou, H.; Sun, J.; Zong, C. Effects of Isocyanate Structure on the Properties of Polyurethane: Synthesis, Performance, and Self-Healing Characteristics. Polymers 2024, 16, 3045. https://doi.org/10.3390/polym16213045
Wang H, Cao L, Wang X, Lang X, Cong W, Han L, Zhang H, Zhou H, Sun J, Zong C. Effects of Isocyanate Structure on the Properties of Polyurethane: Synthesis, Performance, and Self-Healing Characteristics. Polymers. 2024; 16(21):3045. https://doi.org/10.3390/polym16213045
Chicago/Turabian StyleWang, Hairui, Lan Cao, Xiaolei Wang, Xiurui Lang, Wenwen Cong, Long Han, Hongyu Zhang, Huibin Zhou, Jujie Sun, and Chengzhong Zong. 2024. "Effects of Isocyanate Structure on the Properties of Polyurethane: Synthesis, Performance, and Self-Healing Characteristics" Polymers 16, no. 21: 3045. https://doi.org/10.3390/polym16213045
APA StyleWang, H., Cao, L., Wang, X., Lang, X., Cong, W., Han, L., Zhang, H., Zhou, H., Sun, J., & Zong, C. (2024). Effects of Isocyanate Structure on the Properties of Polyurethane: Synthesis, Performance, and Self-Healing Characteristics. Polymers, 16(21), 3045. https://doi.org/10.3390/polym16213045







