Naphthalene-Containing Epoxy Resin: Phase Structure, Rheology, and Thermophysical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Phase State of Naphthalene/Epoxy Resin Blends
3.2. Rheological Properties of Uncured Blends
3.3. Thermal Curing of Blends
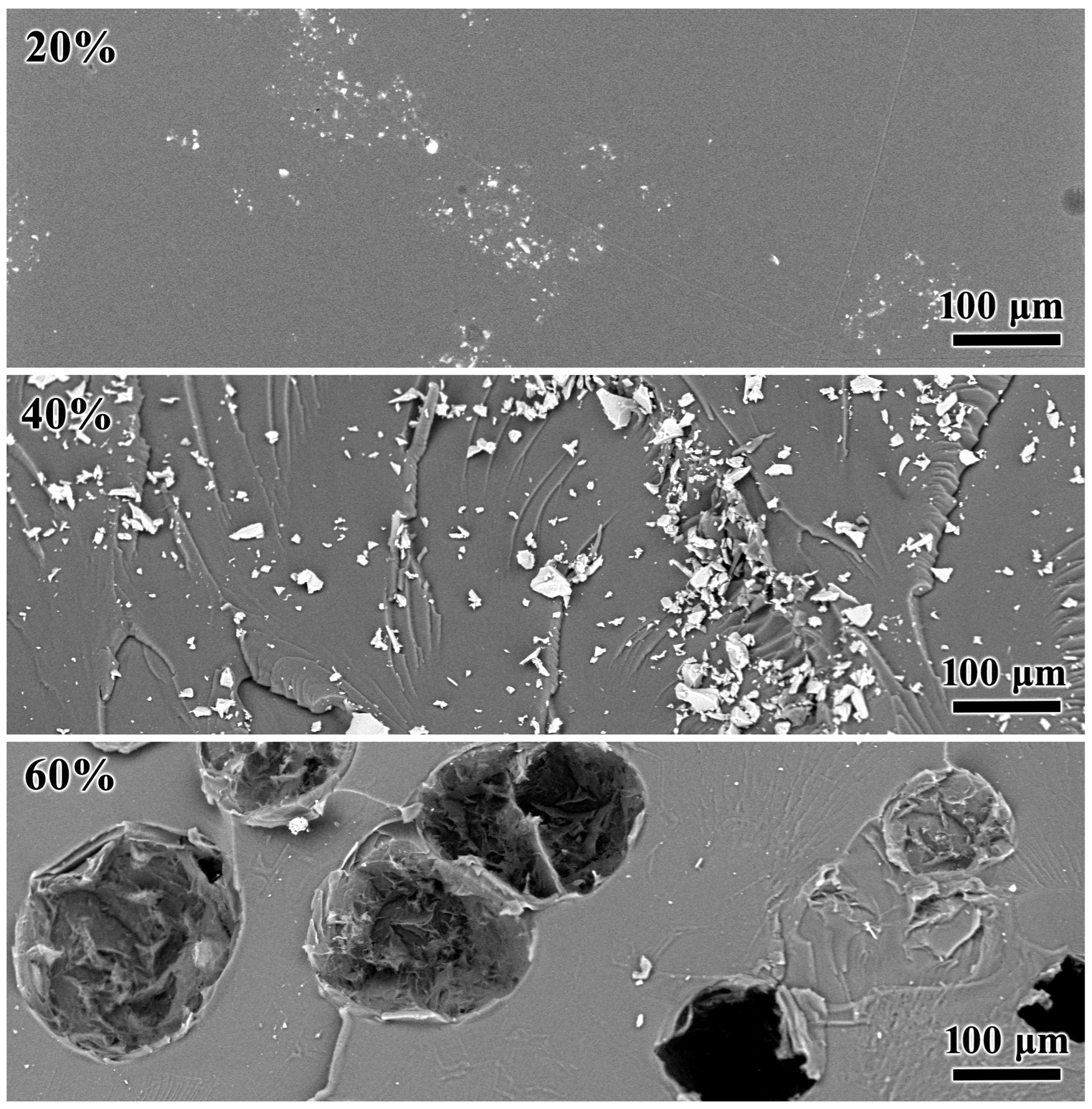
3.4. Phase Transitions and Morphology of Cured Blends
4. Conclusions
- Naphthalene is fully soluble in uncured epoxy resin at 80 °C, whereas cooling causes its crystallization and drop in solubility to 19% at 25 °C;
- Before curing, 20% of naphthalene reduces the viscosity of the epoxy blend at 25 °C because of dissolution. However, its higher content conversely increases the viscosity and induces yield stress behavior and gelation because of the percolation network from naphthalene and hardener particles;
- Naphthalene reduces the viscosity during high-temperature curing of epoxy resin because of solubility, which delays the moment of gelation, lowering the rate and completeness of cross-linking because of the accompanying decrease in the concentrations of reactants diluted with naphthalene;
- About 40% of naphthalene metastably dissolves within the cured epoxy polymer, lowering its glass transition temperature, while its higher concentrations form coarse particles capable of crystallization and thermal energy storage.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, C.; Batterman, S. A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air. Int. J. Environ. Res. Public Health 2010, 7, 2903–2939. [Google Scholar] [CrossRef] [PubMed]
- Sudakin, D.L.; Stone, D.L.; Power, L. Naphthalene Mothballs: Emerging and Recurring Issues and Their Relevance to Environmental Health. Curr. Top. Toxicol. 2011, 7, 13–19. [Google Scholar] [PubMed]
- Herrick, G.W.; Griswold, G.H. Naphthalene as a Fumigant for the Immature Stages of Clothes Moths and Carpet Beetles. J. Econ. Entomol. 1933, 26, 446–451. [Google Scholar] [CrossRef]
- Philleo, W.W.; Schonbrod, R.D.; Terriere, L.C. Mechanism of Insecticide Synergism, Methylenedioxyphenyl Compounds as Inhibitors of Hydroxylation of Naphthalene in Houseflies. J. Agric. Food Chem. 1965, 13, 113–115. [Google Scholar] [CrossRef]
- Travis, G.E. Naphthalene Paste. J. Econ. Entomol. 1943, 36, 477–478. [Google Scholar] [CrossRef]
- Linnie, M.J.; Keatinge, M.J. Pest Control in Museums: Toxicity of Para-Dichlorobenzene, ‘Vapona’TM, and Naphthalene against All Stages in the Life-Cycle of Museum Pests, Dermestes Maculatus Degeer, and Anthrenus verbasci (L.) (Coleoptera: Dermestidae). Int. Biodeterior. Biodegrad. 2000, 45, 1–13. [Google Scholar] [CrossRef]
- Ojianwuna, C.C.; Enwemiwe, V.N. Insecticidal Effectiveness of Naphthalene and Its Combination with Kerosene against the Emergence of Aedes Aegypti in Ika North East, LGA, Delta State, Nigeria. Parasite Epidemiol. Control 2022, 18, e00259. [Google Scholar] [CrossRef]
- Tattersfield, F. The decomposition of naphthalene in the soil and the effect upon its insecticidal action. Ann. Appl. Biol. 1928, 15, 57–80. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, D.A.; Ingham, E.R. Use of Sulfamethoxazole-Penicillin, Oxytetracycline, Carbofuran, Carbaryl, Naphthalene and Temik to Remove Key Organism Groups in Soil in a Corn Agroecosystem. J. Sustain. Agric. 1994, 4, 7–30. [Google Scholar] [CrossRef]
- Preuss, R.; Angerer, J.; Drexler, H. Naphthalene—An Environmental and Occupational Toxicant. Int. Arch. Occup. Environ. Health 2003, 76, 556–576. [Google Scholar] [CrossRef]
- Stohs, S. Naphthalene Toxicity and Antioxidant Nutrients. Toxicology 2002, 180, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.A.; Nascarella, M.A.; Kerper, L.E.; Rhomberg, L.R. Hypothesis-Based Weight-of-Evidence Evaluation and Risk Assessment for Naphthalene Carcinogenesis. Crit. Rev. Toxicol. 2016, 46, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, C. Genetic Toxicity of Naphthalene: A Review. J. Toxicol. Environ. Health Part B 2003, 6, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Yost, E.E.; Galizia, A.; Kapraun, D.F.; Persad, A.S.; Vulimiri, S.V.; Angrish, M.; Lee, J.S.; Druwe, I.L. Health Effects of Naphthalene Exposure: A Systematic Evidence Map and Analysis of Potential Considerations for Dose–Response Evaluation. Environ. Health Perspect. 2021, 129, 076002. [Google Scholar] [CrossRef]
- Buckpitt, A.; Boland, B.; Isbell, M.; Morin, D.; Shultz, M.; Baldwin, R.; Chan, K.; Karlsson, A.; Lin, C.; Taff, A.; et al. Naphthalene-induced respiratory tract toxicity: Metabolic mechanisms of toxicity. Drug Metab. Rev. 2002, 34, 791–820. [Google Scholar] [CrossRef]
- Bates, N. Mothball Poisoning: Nicola Bates Discusses Mothball Ingestion, a Condition Not Particularly Common in Emergency Units, but One Which Can Be Difficult to Manage Because the Active Ingredient Is Often Unknown. Emerg. Nurse 2002, 10, 24–28. [Google Scholar] [CrossRef]
- Hassan, A.; Shakeel Laghari, M.; Rashid, Y. Micro-Encapsulated Phase Change Materials: A Review of Encapsulation, Safety and Thermal Characteristics. Sustainability 2016, 8, 1046. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of Solid–Liquid Phase Change Materials and Their Encapsulation Technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Encapsulation Techniques for Organic Phase Change Materials as Thermal Energy Storage Medium: A Review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Ilyina, S.O.; Vlasova, A.V.; Gorbunova, I.Y.; Lukashov, N.I.; Kerber, M.L.; Ilyin, S.O. Epoxy Phase-Change Materials Based on Paraffin Wax Stabilized by Asphaltenes. Polymers 2023, 15, 3243. [Google Scholar] [CrossRef]
- Ilyina, S.O.; Gorbunova, I.Y.; Makarova, V.V.; Kerber, M.L.; Ilyin, S.O. Self-Lubricating and Shape-Stable Phase-Change Materials Based on Epoxy Resin and Vegetable Oils. Polymers 2023, 15, 4026. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, C.; Chen, K.; Liu, X.; Huang, Z. Preparation, Thermal, and Mechanical Properties of Polyethylene Glycol/Expoxy Resin Composites as Form-stable Phase Change Materials. Polym. Eng. Sci. 2022, 62, 520–529. [Google Scholar] [CrossRef]
- Wang, Z.; Situ, W.; Li, X.; Zhang, G.; Huang, Z.; Yuan, W.; Yang, C.; Yang, C. Novel Shape Stabilized Phase Change Material Based on Epoxy Matrix with Ultrahigh Cycle Life for Thermal Energy Storage. Appl. Therm. Eng. 2017, 123, 1006–1012. [Google Scholar] [CrossRef]
- Cheng, P.; Wei, K.; Shi, W.; Shi, J.; Wang, S.; Ma, B. Preparation and Performance Analysis of Phase Change Microcapsule/Epoxy Resin Composite Phase Change Material. J. Energy Storage 2022, 47, 103581. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, X.; Zhang, J.; Cheng, J.; Lian, Q. Relationship between Cross-Linking Network Structure and Phase Change Performances toward Multifunctional Epoxy/Bio-Based Wax Form-Stable Phase Change Materials. Chem. Eng. J. 2023, 454, 140221. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Wei, K.; Zhang, W. Thermal Stability and Mechanical Properties of Epoxy Resin/Microcapsule Composite Phase Change Materials. Constr. Build. Mater. 2021, 312, 125392. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Cao, X.; Du, Y.; Zhang, Z.; Gui, Y. Latent Heat Thermal Energy Storage Systems with Solid–Liquid Phase Change Materials: A Review. Adv. Eng. Mater. 2018, 20, 1700753. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent Thermal Energy Storage Technologies and Applications: A Review. Int. J. Thermofluids 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel Strategies and Supporting Materials Applied to Shape-Stabilize Organic Phase Change Materials for Thermal Energy Storage—A Review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Vasilyev, G.; Koifman, N.; Shuster, M.; Gishvoliner, M.; Cohen, Y.; Zussman, E. Synergistic Effect of Two Organogelators for the Creation of Bio-Based, Shape-Stable Phase-Change Materials. Langmuir 2020, 36, 15572–15582. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Dong, W.; Berardi, U.; Kim, S. Phase Change Materials Stabilized by Porous Metal Supramolecular Gels: Gelation Effect on Loading Capacity and Thermal Performance. Chem. Eng. J. 2020, 394, 124806. [Google Scholar] [CrossRef]
- Vasilyev, G.; Koifman, N.; Shuster, M.; Gishvoliner, M.; Cohen, Y.; Zussman, E. Phase Change Material with Gelation Imparting Shape Stability. ACS Omega 2022, 7, 11887–11902. [Google Scholar] [CrossRef] [PubMed]
- Abdeali, G.; Bahramian, A.R.; Abdollahi, M. Review on Nanostructure Supporting Material Strategies in Shape-Stabilized Phase Change Materials. J. Energy Storage 2020, 29, 101299. [Google Scholar] [CrossRef]
- Paul, J.; Pandey, A.K.; Mishra, Y.N.; Said, Z.; Mishra, Y.K.; Ma, Z.; Jacob, J.; Kadirgama, K.; Samykano, M.; Tyagi, V.V. Nano-Enhanced Organic Form Stable PCMs for Medium Temperature Solar Thermal Energy Harvesting: Recent Progresses, Challenges, and Opportunities. Renew. Sustain. Energy Rev. 2022, 161, 112321. [Google Scholar] [CrossRef]
- Sharwan, S.; Sikarwar, P.; Mazumdar, B. Recent Innovations in Support Materials for Shape-Stable Organic Composite Phase Change Materials: A Comprehensive Review. Sol. Energy Mater. Sol. Cells 2024, 272, 112910. [Google Scholar] [CrossRef]
- Rahmalina, D.; Rahman, R.A. Ismail Improving the Phase Transition Characteristic and Latent Heat Storage Efficiency by Forming Polymer-Based Shape-Stabilized PCM for Active Latent Storage System. Case Stud. Therm. Eng. 2022, 31, 101840. [Google Scholar] [CrossRef]
- Bidiyasar, R.; Kumar, R.; Jakhar, N. A Critical Review of Polymer Support-based Shape-stabilized Phase Change Materials for Thermal Energy Storage Applications. Energy Storage 2024, 6, e639. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Moqeet Hai, A.; Zhang, S.; Tang, B. Shape-Stabilization Micromechanisms of Form-Stable Phase Change Materials-A Review. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107047. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. Enhanced Thermophysical Properties of Organic PCM through Shape Stabilization for Thermal Energy Storage in Buildings: A State of the Art Review. Energy Build. 2021, 236, 110799. [Google Scholar] [CrossRef]
- Cai, R.; Sun, Z.; Yu, H.; Meng, E.; Wang, J.; Dai, M. Review on Optimization of Phase Change Parameters in Phase Change Material Building Envelopes. J. Build. Eng. 2021, 35, 101979. [Google Scholar] [CrossRef]
- Anisur, M.R.; Mahfuz, M.H.; Kibria, M.A.; Saidur, R.; Metselaar, I.H.S.C.; Mahlia, T.M.I. Curbing Global Warming with Phase Change Materials for Energy Storage. Renew. Sustain. Energy Rev. 2013, 18, 23–30. [Google Scholar] [CrossRef]
- Song, M.; Niu, F.; Mao, N.; Hu, Y.; Deng, S. Review on Building Energy Performance Improvement Using Phase Change Materials. Energy Build. 2018, 158, 776–793. [Google Scholar] [CrossRef]
- Da Cunha, S.R.L.; De Aguiar, J.L.B. Phase Change Materials and Energy Efficiency of Buildings: A Review of Knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Wang, C.-S.; Lee, M.-C. Synthesis, Characterization, and Properties of Multifunctional Naphthalene-Containing Epoxy Resins Cured with Cyanate Ester. J. Appl. Polym. Sci. 1999, 73, 1611–1622. [Google Scholar] [CrossRef]
- Ho, T.-H. Synthesis of Naphthalene Containing Aralkyl Novolac Epoxy Resins for Electronic Application. Macromol. Mater. Eng. 2000, 283, 57–61. [Google Scholar] [CrossRef]
- Wang, C.-S.; Lee, M.-C. Synthesis and Modification of a Naphthalene-Containing Trifunctional Epoxy Resin for Electronic Applications. J. Appl. Polym. Sci. 1998, 70, 1907–1921. [Google Scholar] [CrossRef]
- Wang, C.-S.; Lee, M.-C. Multifunctional Naphthalene Containing Epoxy Resins and Their Modification by Hydrosilation for Electronic Application. Polym. Bull. 1998, 40, 623–630. [Google Scholar] [CrossRef]
- Kaji, M.; Endo, T. Synthesis of a Novel Epoxy Resin Containing Naphthalene Moiety and Properties of Its Cured Polymer with Phenol Novolac. J. Polym. Sci. A Polym. Chem. 1999, 37, 3063–3069. [Google Scholar] [CrossRef]
- Kim, W.G.; Chun, H. Cure Properties of Naphthalene-Based Epoxy Resin Systems with Hardeners and Latent Catalysts for Semiconductor Packaging Materials. Mol. Cryst. Liq. Cryst. 2013, 579, 39–49. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, B.; Yuan, L. Structure and Properties of Novel Epoxy Resins Containing Naphthalene Units and Aliphatic Chains. Iran. Polym. J. 2013, 22, 325–334. [Google Scholar] [CrossRef]
- Duann, Y. Thermal Stability of Some Naphthalene- and Phenyl-Based Epoxy Resins. Polym. Degrad. Stab. 2004, 84, 305–310. [Google Scholar] [CrossRef]
- Pan, G.; Du, Z.; Zhang, C.; Li, C.; Yang, X.; Li, H. Synthesis, Characterization, and Properties of Novel Novolac Epoxy Resin Containing Naphthalene Moiety. Polymer 2007, 48, 3686–3693. [Google Scholar] [CrossRef]
- Xu, K.; Chen, M.; Zhang, K.; Hu, J. Synthesis and Characterization of Novel Epoxy Resin Bearing Naphthyl and Limonene Moieties, and Its Cured Polymer. Polymer 2004, 45, 1133–1140. [Google Scholar] [CrossRef]
- Mititelu-Mija, A.; Cascaval, C.N.; Navard, P. Liquid Crystalline Epoxy Thermosets with Naphthyl Mesogen. Des. Monomers Polym. 2005, 8, 487–499. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, J. Synthesis and Curing of Liquid Crystalline Epoxy Resin Based on Naphthalene Mesogen. J. Polym. Sci. A Polym. Chem. 1999, 37, 419–425. [Google Scholar] [CrossRef]
- Fila, K.; Gargol, M.; Goliszek, M.; Podkościelna, B. Synthesis of Epoxy Resins Derivatives of Naphthalene-2,7-Diol and Their Cross-Linked Products. J. Therm. Anal. Calorim. 2019, 138, 4349–4358. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Kostina, J.V.; Bakhtin, D.S.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Heavy Crude Oil Asphaltenes as a Nanofiller for Epoxy Resin. Polym. Eng. Sci. 2020, 60, 1530–1545. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Yadykova, A.Y.; Makarova, V.V.; Yashchenko, V.S.; Matveenko, Y.V. Sulfonated Polyoxadiazole Synthesis and Processing into Ion-conducting Films. Polym. Int. 2020, 69, 1243–1255. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase State and Rheology of Polyisobutylene Blends with Silicone Resin. Rheol. Acta 2020, 59, 375–386. [Google Scholar] [CrossRef]
- Schramm, G.A. Practical Approach to Rheology and Rheometry; Haake: Karlsruhe, Germany, 1994. [Google Scholar]
- Yadykova, A.Y.; Ilyin, S.O. Rheological and Adhesive Properties of Nanocomposite Bitumen Binders Based on Hydrophilic or Hydrophobic Silica and Modified with Bio-Oil. Constr. Build. Mater. 2022, 342, 127946. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kotomin, S.V. Mesophase State and Shear-Affected Phase Separation of Poly(p-Phenylene-Benzimidazole-Terephthalamide) Solutions in N,N-Dimethylacetamide. J. Polym. Res. 2022, 29, 326. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Smirnova, N.M.; Ilyin, S.O. Two-Functional Phase-Change Pressure-Sensitive Adhesives Based on Polyisobutylene Matrix Filled with Paraffin Wax. J. Energy Storage 2022, 52, 104797. [Google Scholar] [CrossRef]
- Arinina, M.P.; Kostenko, V.A.; Gorbunova, I.Y.; Il’in, S.O.; Malkin, A.Y. Kinetics of Curing of Epoxy Oligomer by Diaminodiphenyl Sulfone: Rheology and Calorimetry. Polym. Sci. Ser. A 2018, 60, 683–690. [Google Scholar] [CrossRef]
- John, N.A.S.; George, G.A. Diglycidyl Amine—Epoxy Resin Networks: Kinetics and Mechanisms of Cure. Prog. Polym. Sci. 1994, 19, 755–795. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Ilyin, S.O.; Kostyuk, A.V.; Bondarenko, G.N.; Antonov, S.V. Acceleration of Epoxy Resin Curing by Using a Combination of Aliphatic and Aromatic Amines. Polym. Bull. 2020, 77, 1519–1540. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Ilyin, S.O.; Arinina, M.P.; Kulichikhin, V.G. The Rheological State of Suspensions in Varying the Surface Area of Nano-Silica Particles and Molecular Weight of the Poly(Ethylene Oxide) Matrix. Colloid Polym. Sci. 2017, 295, 555–563. [Google Scholar] [CrossRef]
- Brantseva, T.V.; Ilyin, S.O.; Gorbunova, I.Y.; Antonov, S.V.; Korolev, Y.M.; Kerber, M.L. Epoxy Reinforcement with Silicate Particles: Rheological and Adhesive Properties—Part II: Characterization of Composites with Halloysite. Int. J. Adhes. Adhes. 2016, 68, 248–255. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Yadykova, A.Y.; Ilyin, S.O. A Novel Method for Producing Cellulose Nanoparticles and Their Possible Application as Thickeners for Biodegradable Low-Temperature Greases. Cellulose 2021, 28, 10203–10219. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Ignatenko, V.Y.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Polyethylene Wax as an Alternative to Mineral Fillers for Preparation of Reinforced Pressure-Sensitive Adhesives. Int. J. Adhes. Adhes. 2020, 102, 102689. [Google Scholar] [CrossRef]
- Rehbinder, P.A. Formation of Structures in Disperse Systems. Pure Appl. Chem. 1965, 10, 337–358. [Google Scholar] [CrossRef]
- Ilyin, S.O. Structural Rheology in the Development and Study of Complex Polymer Materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.I.; Dai, S. Edge Fracture in Non-Colloidal Suspensions. J. Non-Newton. Fluid. Mech. 2019, 272, 104171. [Google Scholar] [CrossRef]
- Chan, S.T.; Varchanis, S.; Haward, S.J.; Shen, A.Q. Perspective on Edge Fracture. J. Rheol. 2023, 67, 949–963. [Google Scholar] [CrossRef]
- Costanzo, S.; Parisi, D.; Schweizer, T.; Vlassopoulos, D. Review: Nonlinear Shear Rheometry: Brief History, Recent Progress, and Challenges. J. Rheol. 2024, 68, 1013–1036. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Arinina, M.P.; Malkin, A.Y.; Kulichikhin, V.G. Sol–Gel Transition and Rheological Properties of Silica Nanoparticle Dispersions. Colloid J. 2016, 78, 608–615. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Gorbacheva, S.N.; Yadykova, A.Y. Rheology and Tribology of Nanocellulose-Based Biodegradable Greases: Wear and Friction Protection Mechanisms of Cellulose Microfibrils. Tribol. Int. 2023, 178, 108080. [Google Scholar] [CrossRef]
- Michell, R.M.; Blaszczyk-Lezak, I.; Mijangos, C.; Müller, A.J. Confinement Effects on Polymer Crystallization: From Droplets to Alumina Nanopores. Polymer 2013, 54, 4059–4077. [Google Scholar] [CrossRef]
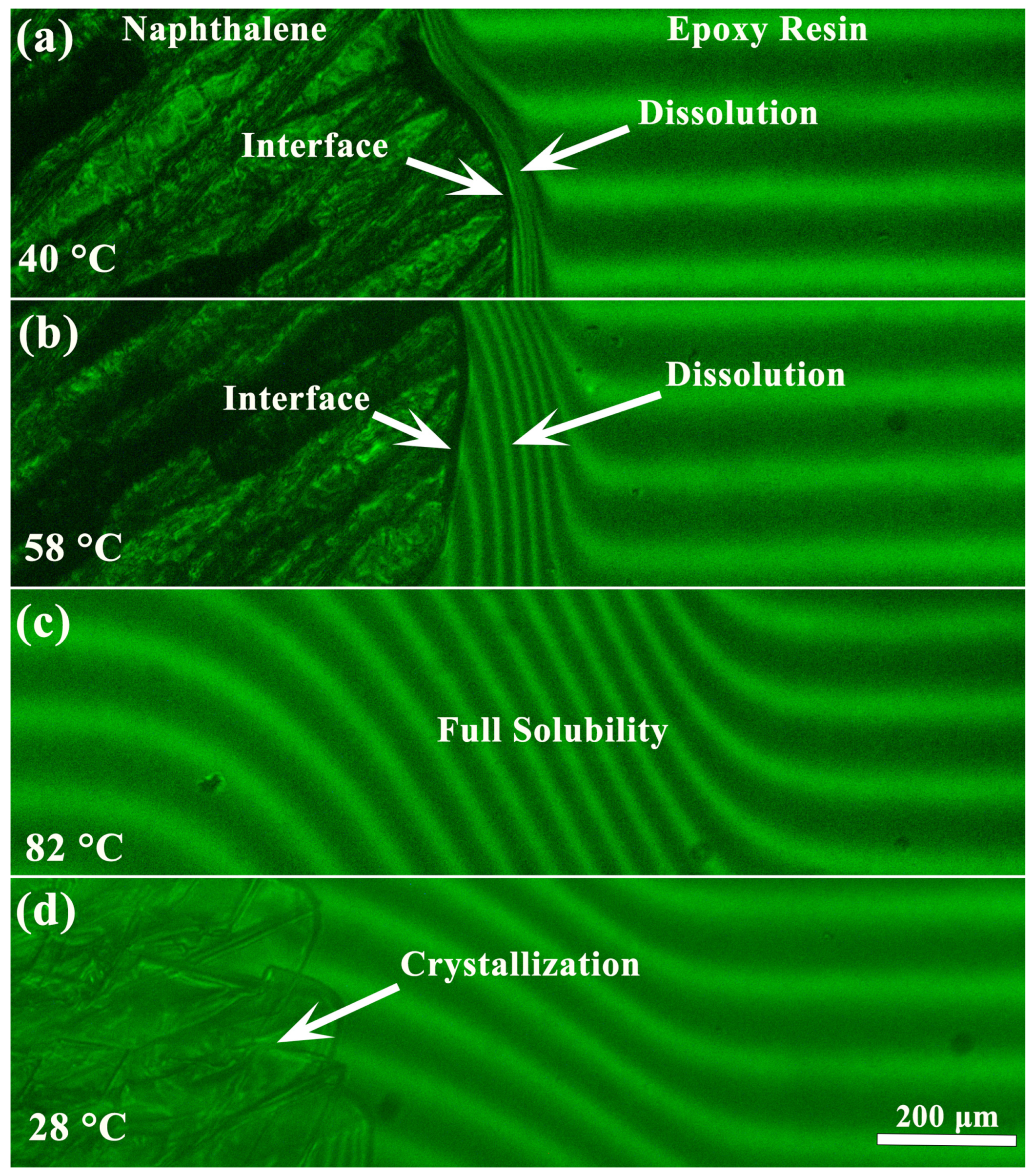



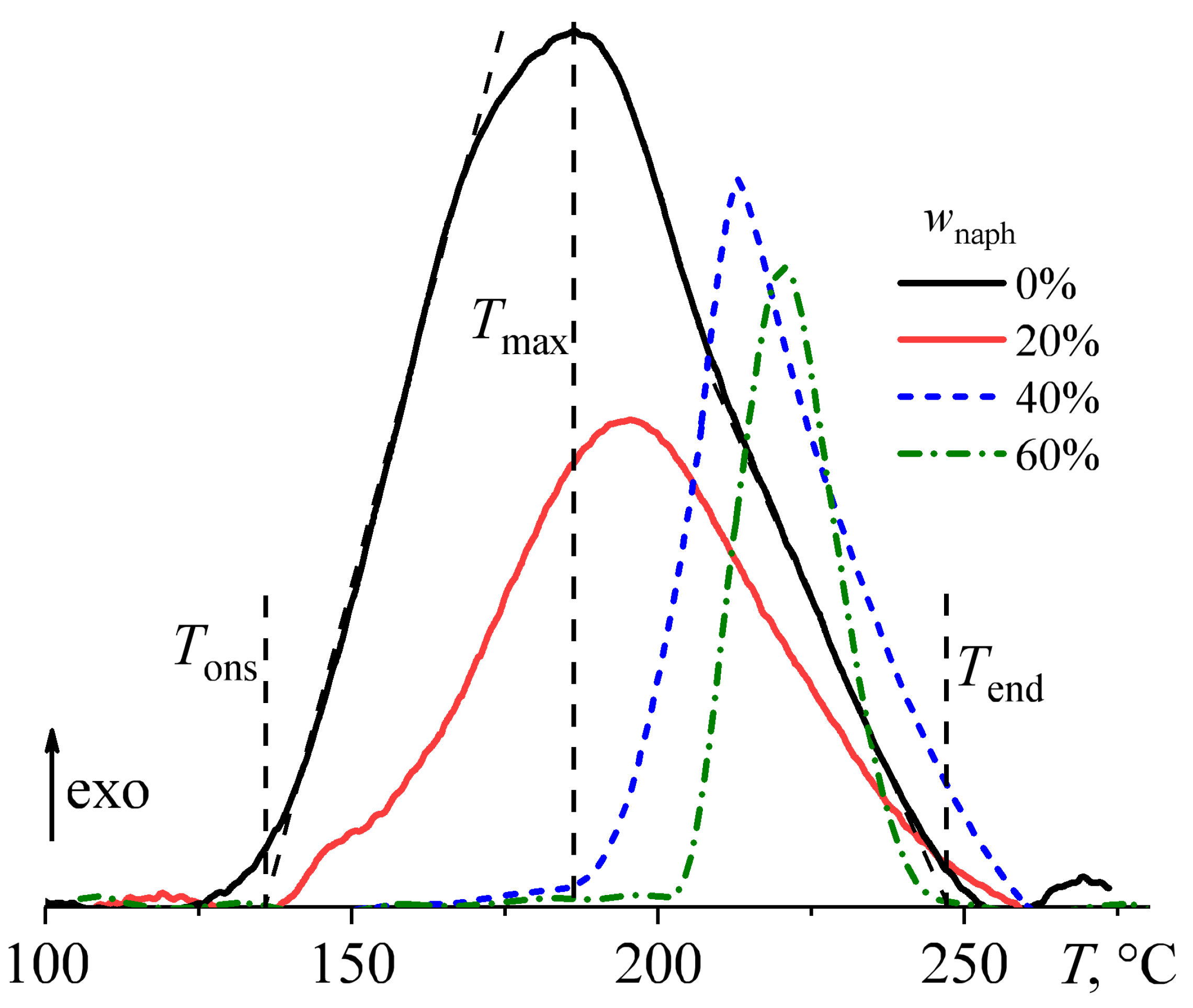
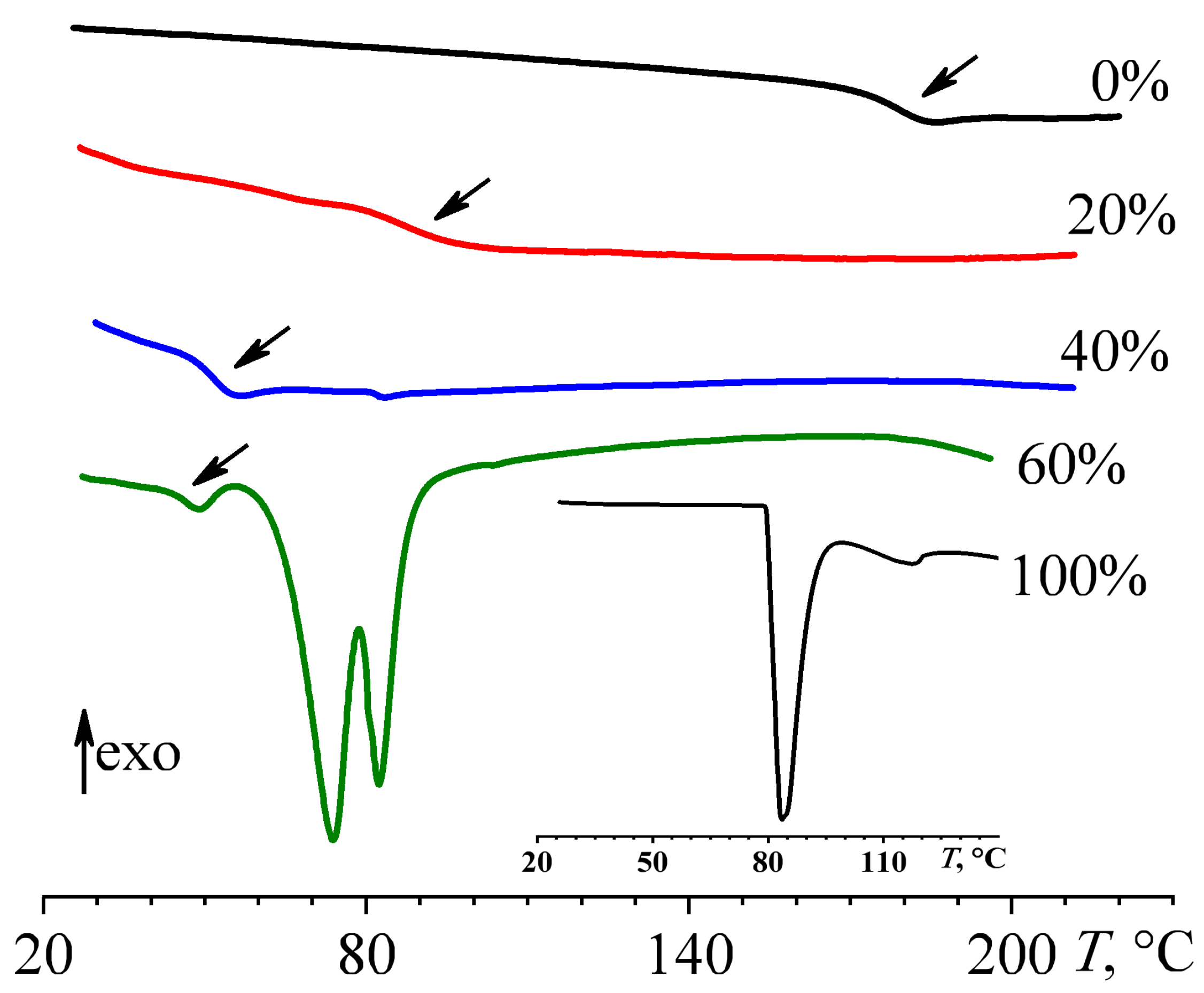
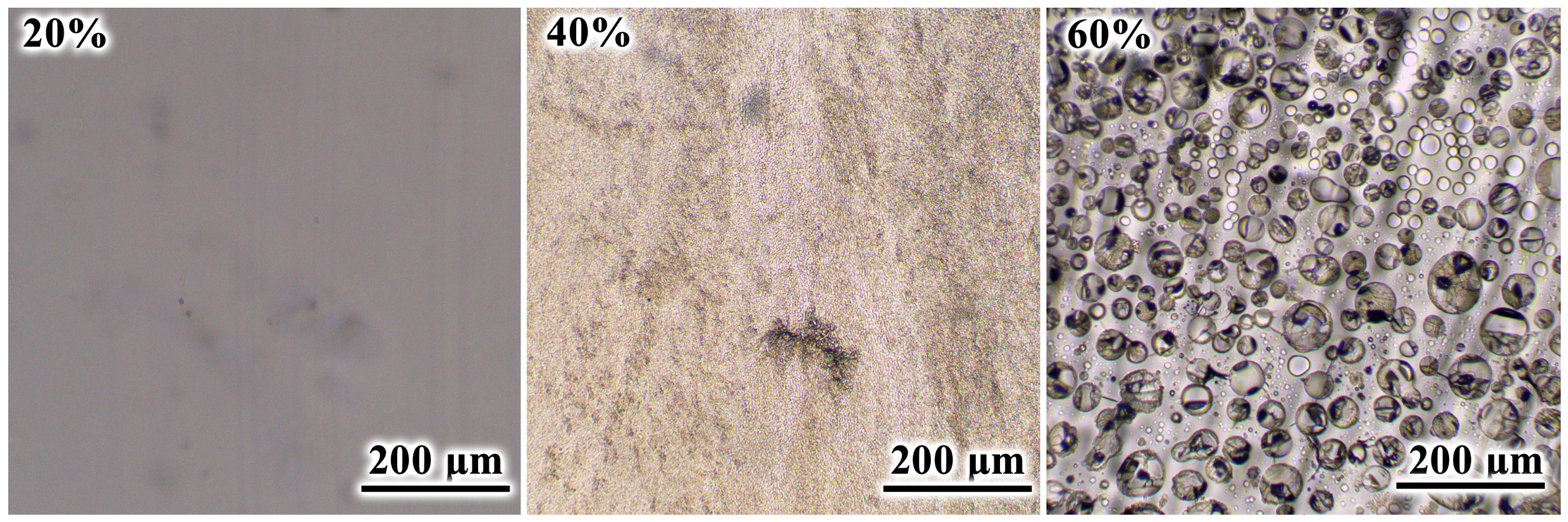
| wnaph, wt% | Tons, °C | Tmax, °C | Tgel, °C | Tend, °C | ΔH, J/g | ΔHred, J/g |
|---|---|---|---|---|---|---|
| 0 | 136.0 | 186.3 | 201 | 247 | 300 | 300 |
| 20 | 152.4 | 195.2 | 210 | 248 | 243 | 303 |
| 40 | 193.8 | 213.0 | 214 | 254 | 129 | 215 |
| 60 | 211.1 | 217.0 | 218 | 237 | 81 | 203 |
| Standard deviation | 0.3 | 0.2 | 1 | 1 | 15 | 15 |
| wnaph, wt% | Tg, °C | Tm, °C | ΔHm, J/g | NDC, % |
|---|---|---|---|---|
| 0 | 178.2 ± 0.2 | – | – | 0 |
| 20 | 86.7 ± 0.2 | – | – | 0 |
| 40 | 51.4 ± 0.2 | 83.2 ± 0.2 | 0.16 ± 0.01 | 0.30 ± 0.01 |
| 60 | 46.4 ± 0.2 | 73.8 ± 0.2, 82.3 ± 0.2 | 42.6 ± 0.3 | 46.8 ± 0.2 |
| 100 | – | 83.5 ± 0.2 * | 151.6 ± 0.7 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyina, S.O.; Gorbunova, I.Y.; Yadykova, A.Y.; Vlasova, A.V.; Kerber, M.L.; Ilyin, S.O. Naphthalene-Containing Epoxy Resin: Phase Structure, Rheology, and Thermophysical Properties. Polymers 2024, 16, 3264. https://doi.org/10.3390/polym16233264
Ilyina SO, Gorbunova IY, Yadykova AY, Vlasova AV, Kerber ML, Ilyin SO. Naphthalene-Containing Epoxy Resin: Phase Structure, Rheology, and Thermophysical Properties. Polymers. 2024; 16(23):3264. https://doi.org/10.3390/polym16233264
Chicago/Turabian StyleIlyina, Svetlana O., Irina Y. Gorbunova, Anastasiya Y. Yadykova, Anna V. Vlasova, Michael L. Kerber, and Sergey O. Ilyin. 2024. "Naphthalene-Containing Epoxy Resin: Phase Structure, Rheology, and Thermophysical Properties" Polymers 16, no. 23: 3264. https://doi.org/10.3390/polym16233264
APA StyleIlyina, S. O., Gorbunova, I. Y., Yadykova, A. Y., Vlasova, A. V., Kerber, M. L., & Ilyin, S. O. (2024). Naphthalene-Containing Epoxy Resin: Phase Structure, Rheology, and Thermophysical Properties. Polymers, 16(23), 3264. https://doi.org/10.3390/polym16233264




.png)



