Melting Behavior of Compression Molded Poly(ester amide) from 2,5-Furandicarboxylic Acid
Abstract
1. Introduction
2. Materials and Methods
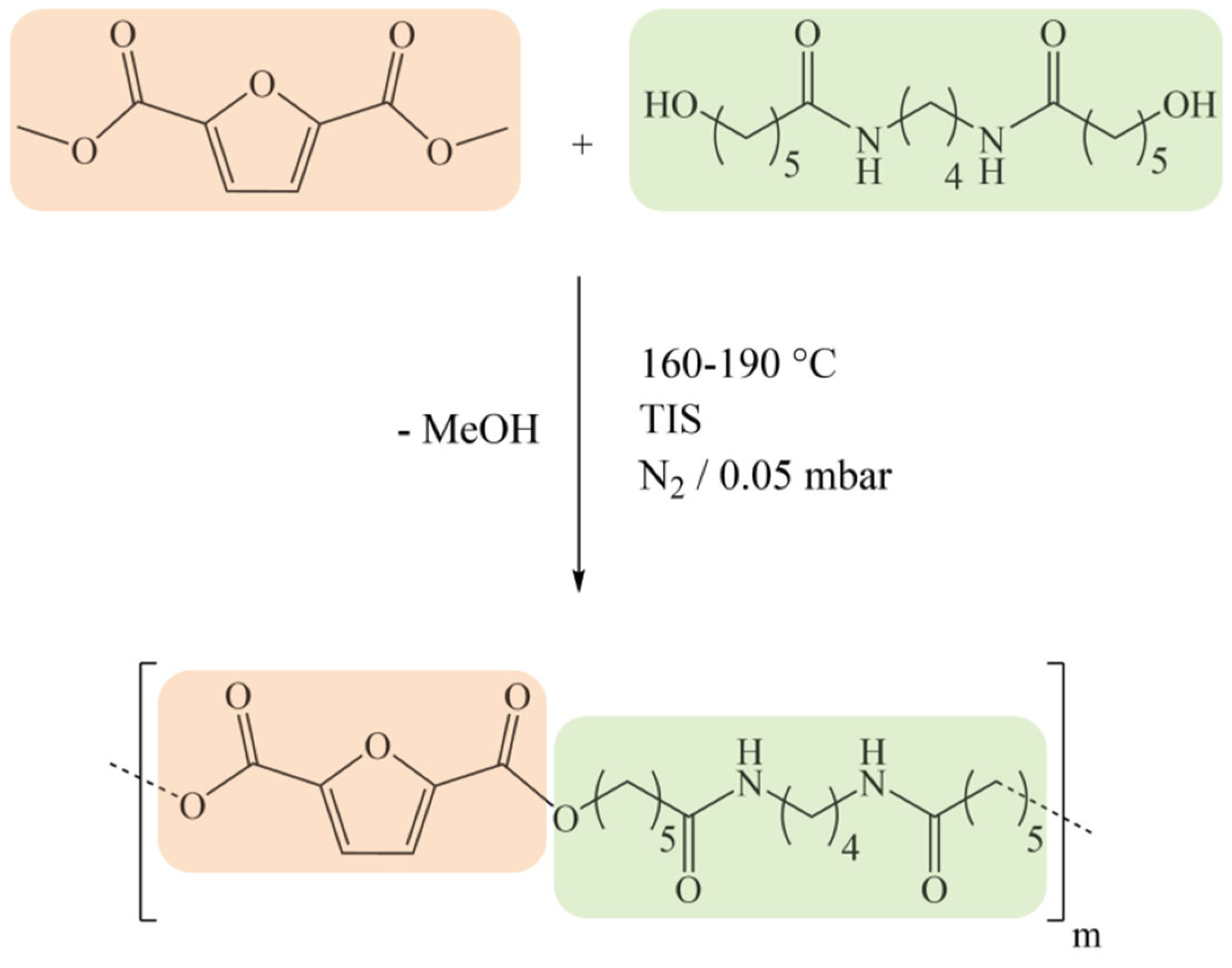
2.1. Materials and Sample Preparation
2.2. Thermal Characterization
2.3. Diffractometric Characterization
3. Results and Discussion
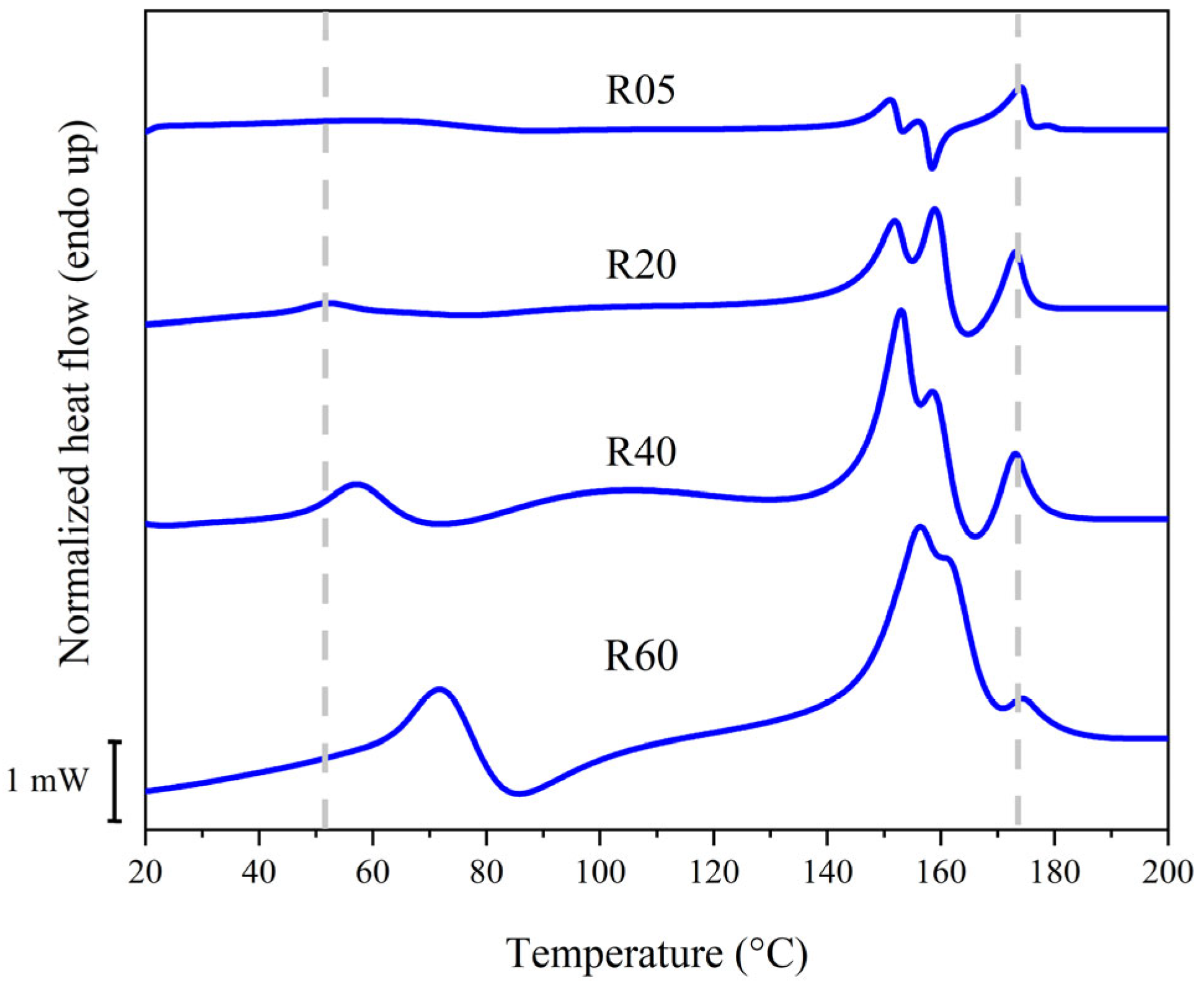
3.1. Preliminary Investigation
3.2. Results of Experiment 1
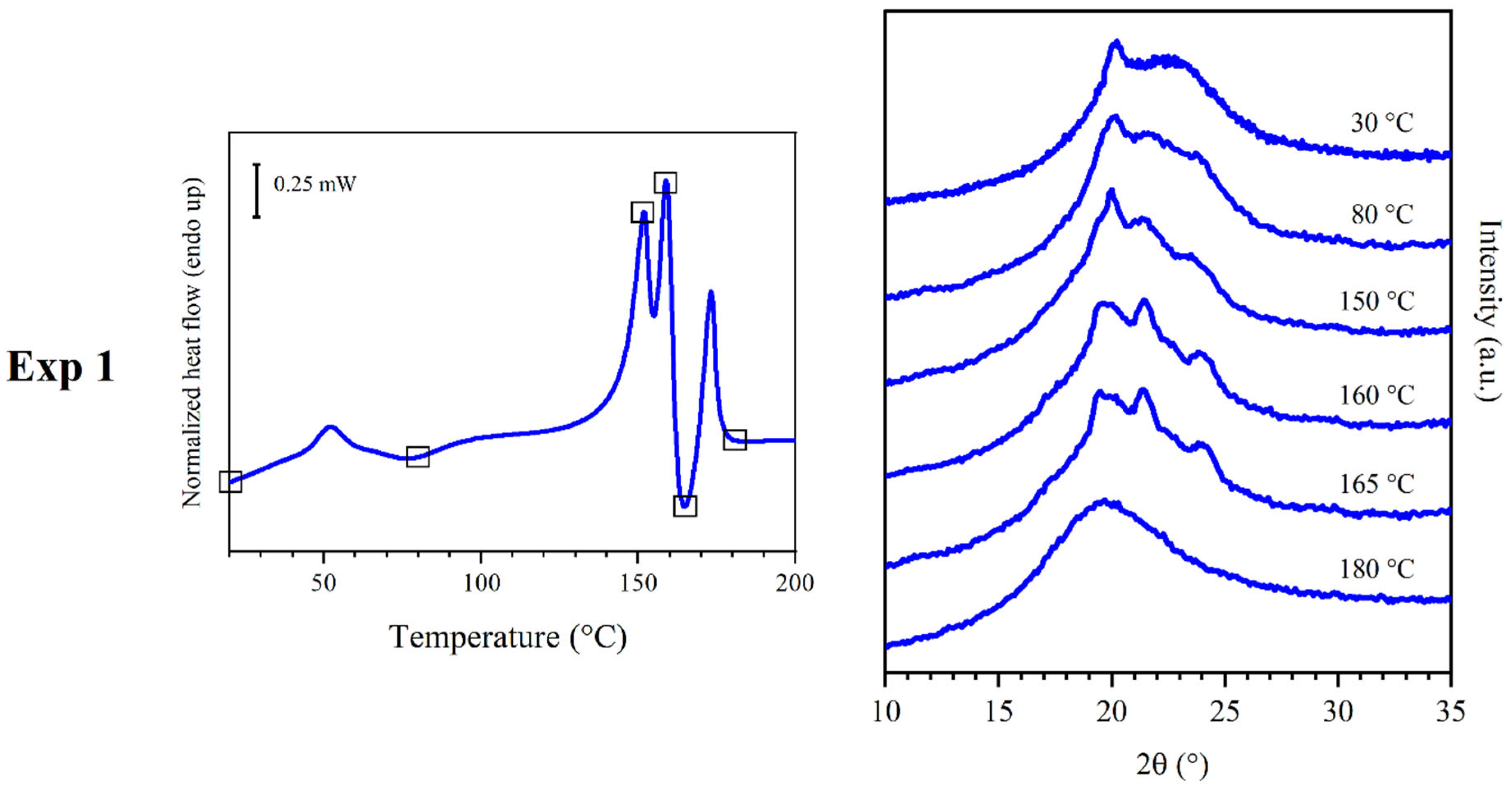
3.3. Results of Experiment 2

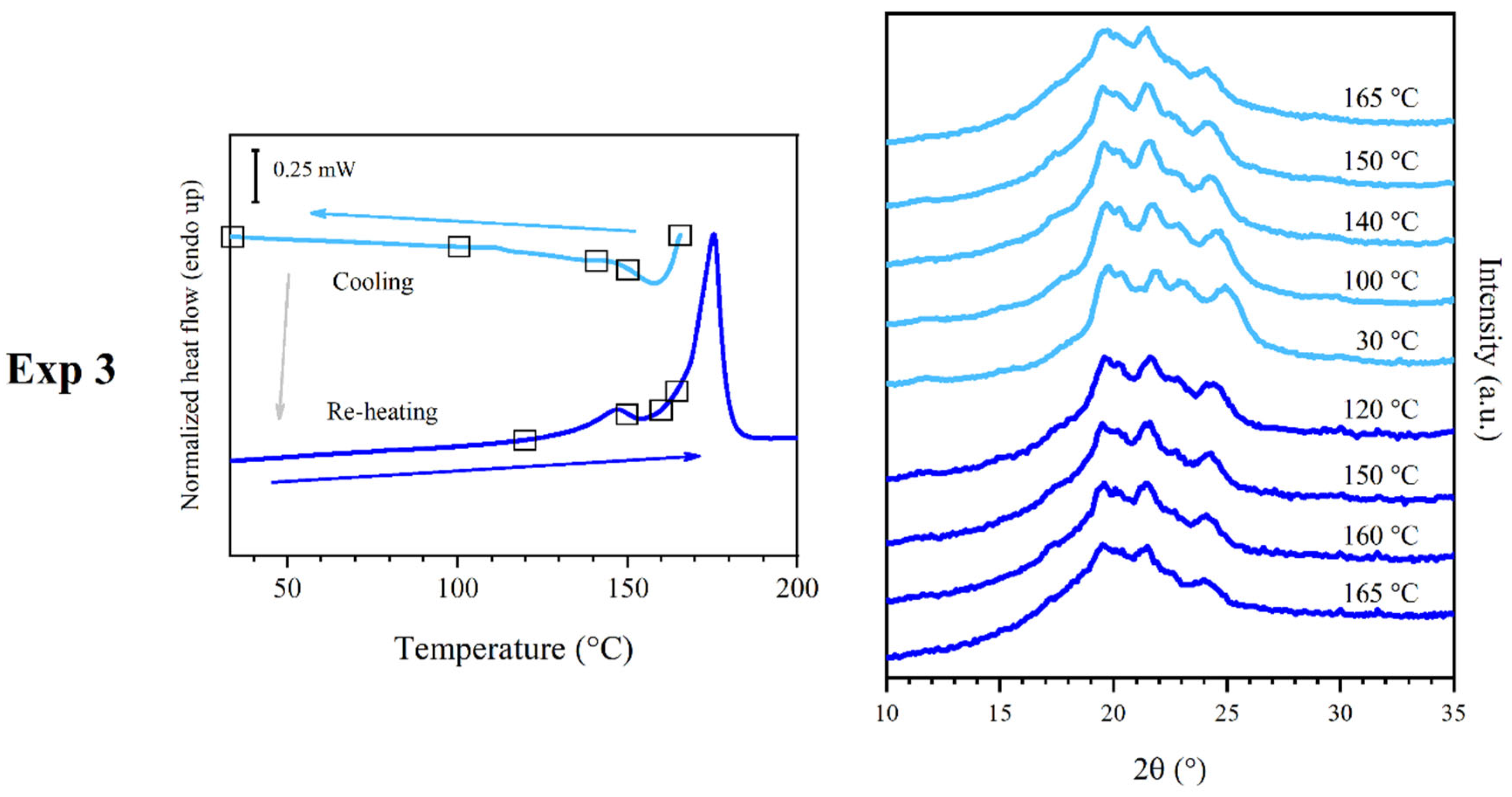
3.4. Results of Experiment 3
3.5. Discussion of Experiment 1
3.6. Discussion of Experiment 2
3.7. Discussion of Experiment 3
3.8. Overview
- ▪
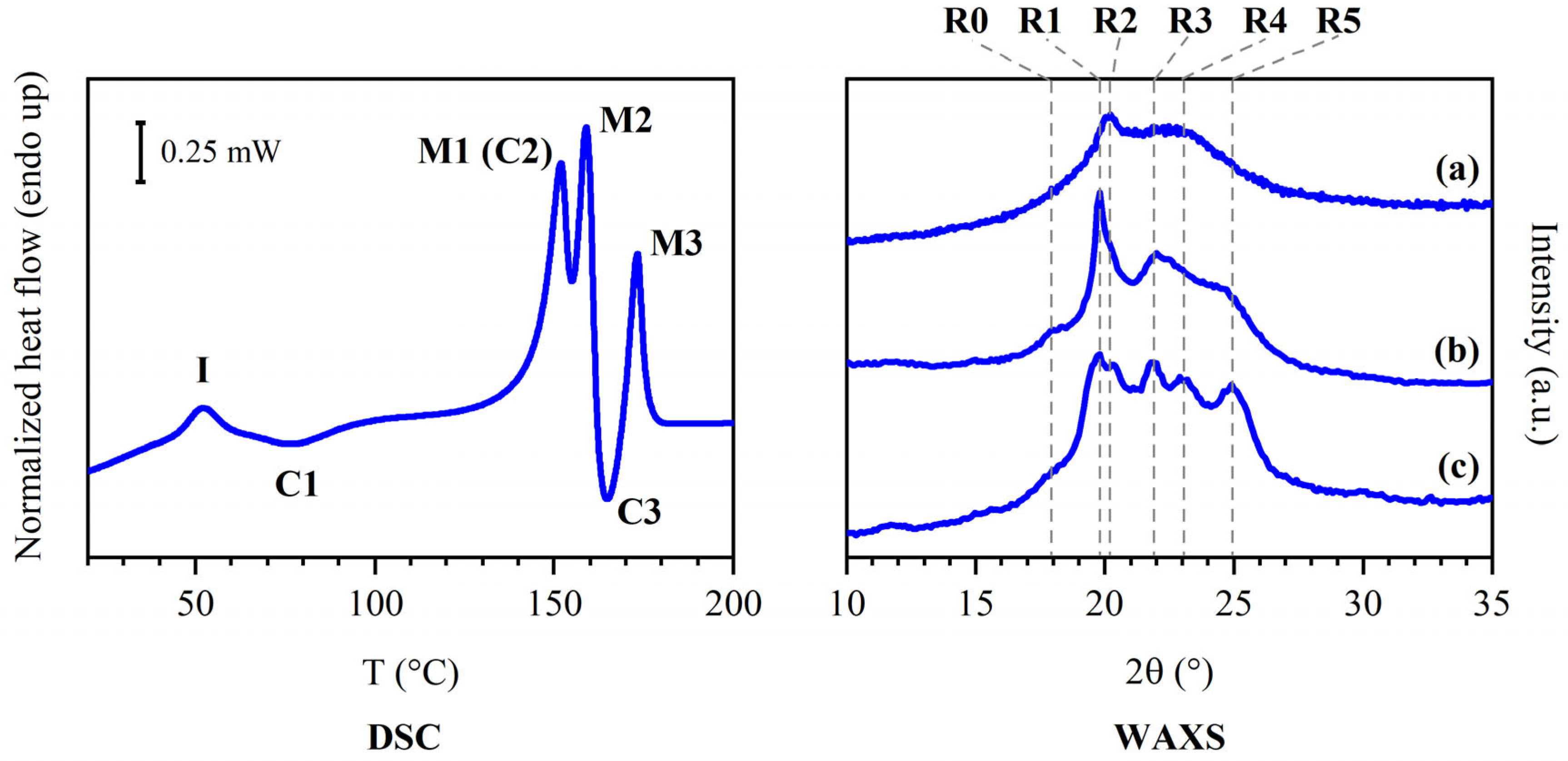
- Mesophase: this phase formed after compression molding. It underwent isotropization at I and showed the WAXS pattern reported in Figure 2a.
- ▪
- Phase α’ (phase α with low crystallinity): this phase crystallized at C1 and might have undergone multiple melting and recrystallization phenomena leading to the conversion into phase α. Its pattern was recorded in situ during the heating process.
- ▪
- Phase α: this phase crystallized at C2 and melted at M1, showing the WAXS pattern reported in Figure 2b; the peaks were sharper than the ones found in phase α’. Crystallization C2 was likely superimposed to melting M1.
- ▪
- Phase β’ (phase β with low crystallinity): this phase melted at M2; its diffraction pattern was recorded in situ. Crystallization C3 was likely partly superimposed to melting M2.
- ▪
- Phase β: this phase was obtained by favoring the crystallization of the sample via melt crystallization, over a temperature range corresponding to C4, and it melted at M3, showing the WAXS pattern reported in Figure 2c. It should effectively have the same structure as phase β’, but with enhanced degree of crystallinity.

4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Norton, M. Green Chemistry and the Plastic Pollution Challenge: Towards a Circular Economy. Green Chem. 2020, 22, 6310–6322. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Manheim, A.; Elliot, D.; Lasure, L.; Jones, S.; et al. Top Value Added Chemicals from Biomass—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Werpy, T., Petersen, G., Eds.; U.S. Department of Energy: Washington, DC, USA, 2004. [Google Scholar]
- Papamokos, G.; Dimitriadis, T.; Bikiaris, D.N.; Papageorgiou, G.Z.; Floudas, G. Chain Conformation, Molecular Dynamics, and Thermal Properties of Poly(n-Methylene 2,5-Furanoates) as a Function of Methylene Unit Sequence Length. Macromolecules 2019, 52, 6533–6546. [Google Scholar] [CrossRef]
- Robles-Hernández, B.; Soccio, M.; Castrillo, I.; Guidotti, G.; Lotti, N.; Alegria, A.; Martínez-Tong, D.E. Poly(Alkylene 2,5-Furanoate)s Thin Films: Morphology, Crystallinity and Nanomechanical Properties. Polymer 2020, 204, 122825. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Interpretation and Physical Meaning of Kinetic Parameters Obtained from Isoconversional Kinetic Analysis of Polymers. Polymers 2020, 12, 1280. [Google Scholar] [CrossRef]
- Maini, L.; Gigli, M.; Gazzano, M.; Lotti, N.; Bikiaris, D.; Papageorgiou, G. Structural Investigation of Poly(Ethylene Furanoate) Polymorphs. Polymers 2018, 10, 296. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Bikiaris, D.N.; Papageorgiou, G.Z. Crystallization and Polymorphism of Poly(Ethylene Furanoate). Cryst. Growth Des. 2015, 15, 5505–5512. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the Bio-Based Polyester Poly(Propylene 2,5-Furan Dicarboxylate). Comparison of Thermal Behavior and Solid State Structure with Its Terephthalate and Naphthalate Homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Papageorgiou, M.; Bikiaris, D.N. Evaluation of Polyesters from Renewable Resources as Alternatives to the Current Fossil-Based Polymers. Phase Transitions of Poly(Butylene 2,5-Furan-Dicarboxylate). Polymer 2014, 55, 3846–3858. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Chrissafis, K.; Exarhopoulos, S.; Bikiaris, D.N. Furan-Based Polyesters from Renewable Resources: Crystallization and Thermal Degradation Behavior of Poly(Hexamethylene 2,5-Furan-Dicarboxylate). Eur. Polym. J. 2015, 67, 383–396. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast Crystallization and Melting Behavior of a Long-Spaced Aliphatic Furandicarboxylate Biobased Polyester, Poly(Dodecylene 2,5-Furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Righetti, M.C.; Marchese, P.; Vannini, M.; Celli, A.; Tricoli, F.; Lorenzetti, C. Temperature-Induced Polymorphism in Bio-Based Poly(Propylene 2,5-Furandicarboxylate). Thermochim. Acta 2019, 677, 186–193. [Google Scholar] [CrossRef]
- Righetti, M.C.; Marchese, P.; Vannini, M.; Celli, A.; Lorenzetti, C.; Cavallo, D.; Ocando, C.; Müller, A.J.; Androsch, R. Polymorphism and Multiple Melting Behavior of Bio-Based Poly(Propylene 2,5-Furandicarboxylate). Biomacromolecules 2020, 21, 2622–2634. [Google Scholar] [CrossRef]
- Shi, O.; Li, P.; Yang, C.; Jiang, H.; Qin, L.; Liu, W.; Li, X.; Chen, Z. Melting Behaviors of Bio-Based Poly(Propylene 2,5-Furan Dicarboxylate)-b-Poly(Ethylene Glycol) Co Polymers Related to Their Crystal Morphology. Polymers 2023, 16, 97. [Google Scholar] [CrossRef]
- Ye, B.; Shao, Q.; Long, L.; Wu, B.; Liu, Z.; Li, Y.; Wang, Z. Intermolecular ═C–H···O═C Hydrogen Bonding and Conformational Preference in Crystalline Poly(Butylene 2,5-Furandicarboxylate). Macromolecules 2023, 56, 3595–3606. [Google Scholar] [CrossRef]
- Forestier, E.; Combeaud, C.; Guigo, N.; Monge, G.; Haudin, J.-M.; Sbirrazzuoli, N.; Billon, N. Strain-Induced Crystallization of Poly(Ethylene 2,5-Furandicarboxylate). Mechanical and Crystallographic Analysis. Polymer 2020, 187, 122126. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Gutiérrez-Fernández, E.; Ezquerra, T.A.; Siracusa, V.; Munari, A.; Lotti, N. Evidence of a 2D-Ordered Structure in Biobased Poly(Pentamethylene Furanoate) Responsible for Its Outstanding Barrier and Mechanical Properties. ACS Sustain. Chem. Eng. 2019, 7, 17863–17871. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, C.; He, B.; Huang, M.; Jiang, S. Synthesis of 2,5-Furandicarboxylic Acid-Based Heat-Resistant Polyamides under Existing Industrialization Process. Macromol. Res. 2017, 25, 722–729. [Google Scholar] [CrossRef]
- Walkowiak, K.; Irska, I.; Zubkiewicz, A.; Dryzek, J.; Paszkiewicz, S. The Properties of Poly(Ester Amide)s Based on Dimethyl 2,5-Furanedicarboxylate as a Function of Methylene Sequence Length in Polymer Backbone. Polymers 2022, 14, 2295. [Google Scholar] [CrossRef] [PubMed]
- Zubkiewicz, A.; Irska, I.; Miadlicki, P.; Walkowiak, K.; Rozwadowski, Z.; Paszkiewicz, S. Structure, Thermal and Mechanical Properties of Copoly(Ester Amide)s Based on 2,5-furandicarboxylic Acid. J. Mater. Sci. 2021, 56, 19296–19309. [Google Scholar] [CrossRef]
- Silvianti, F.; Maniar, D.; Boetje, L.; Loos, K. Green Pathways for the Enzymatic Synthesis of Furan-Based Polyesters and Polyamides. In Sustainability & Green Polymer Chemistry; American Chemical Society: Washington, DC, USA, 2020; Volume 2, pp. 3–29. [Google Scholar]
- Jiang, Y.; Maniar, D.; Woortman, A.J.J.; Loos, K. Enzymatic Synthesis of 2,5-Furandicarboxylic Acid-Based Semi-Aromatic Polyamides: Enzymatic Polymerization Kinetics, Effect of Diamine Chain Length and Thermal Properties. RSC Adv. 2016, 6, 67941–67953. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Klonos, P.A.; Kluge, M.; Zamboulis, A.; Terzopoulou, Z.; Kourtidou, D.; Magaziotis, A.; Chrissafis, K.; Kyritsis, A.; Bikiaris, D.N.; et al. Unlocking the Potential of Furan-Based Poly(Ester Amide)s: An Investigation of Crystallization, Molecular Dynamics and Degradation Kinetics of Novel Poly(Ester Amide)s Based on Renewable Poly(Propylene Furanoate). Polym. Chem. 2021, 12, 5518–5534. [Google Scholar] [CrossRef]
- Kluge, M.; Papadopoulos, L.; Magaziotis, A.; Tzetzis, D.; Zamboulis, A.; Bikiaris, D.N.; Robert, T. A Facile Method to Synthesize Semicrystalline Poly(Ester Amide)s from 2,5-Furandicarboxylic Acid, 1,10-Decanediol, and Crystallizable Amido Diols. ACS Sustain. Chem. Eng. 2020, 8, 10812–10821. [Google Scholar] [CrossRef]
- Bianchi, E.; Papadopoulos, L.; Soccio, M.; Siracusa, V.; Gazzano, M.; Robert, T.; Bikiaris, D.N.; Lotti, N. Mechanical Properties, Gas Permeability and Biodegradation Mechanism of Biobased Poly(Ester Amide)s from 2,5-Furandicarboxylic Acid and Amido Diols for Sustainable Food Packaging. Polym. Degrad. Stab. 2024, 230, 111049. [Google Scholar] [CrossRef]
- Tencé-Girault, S.; Lebreton, S.; Bunau, O.; Dang, P.; Bargain, F. Simultaneous SAXS-WAXS Experiments on Semi-Crystalline Polymers: Example of PA11 and Its Brill Transition. Crystals 2019, 9, 271. [Google Scholar] [CrossRef]
- Ishangulyyev, R.; Kim, S.; Lee, S. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging—Principles and Practice; CRC Press: Boca Raton, FL, USA, 2013; ISBN 1-4398-6241-9. [Google Scholar]
- Yamamoto, Y.; Inoue, Y.; Onai, T.; Doshu, C.; Takahashi, H.; Uehara, H. Deconvolution Analyses of Differential Scanning Calorimetry Profiles of β-Crystallized Polypropylenes with Synchronized X-Ray Measurements. Macromolecules 2007, 40, 2745–2750. [Google Scholar] [CrossRef]
- Hall, C.L.; Potticary, J.; Hamilton, V.; Gaisford, S.; Buanz, A.; Hall, S.R. Metastable Crystalline Phase Formation in Deep Eutectic Systems Revealed by Simultaneous Synchrotron XRD and DSC. Chem. Commun. 2020, 56, 10726–10729. [Google Scholar] [CrossRef]
- Wunderlich, B.; Czornyj, G. A Study of Equilibrium Melting of Polyethylene. Macromolecules 1977, 10, 906–913. [Google Scholar] [CrossRef]
- Yamada, K.; Hikosaka, M.; Toda, A.; Yamazaki, S.; Tagashira, K. Equilibrium Melting Temperature of Isotactic Polypropylene with High Tacticity: 1. Determination by Differential Scanning Calorimetry. Macromolecules 2003, 36, 4790–4801. [Google Scholar] [CrossRef]
- Toda, A.; Hikosaka, M.; Yamada, K. Superheating of the Melting Kinetics in Polymer Crystals: A Possible Nucleation Mechanism. Polymer 2002, 43, 1667–1679. [Google Scholar] [CrossRef]
- Toda, A.; Androsch, R.; Schick, C. Melting Kinetics of Superheated Polymer Crystals Examined by Isothermal and Nonisothermal Fast Scanning Calorimetry. Macromolecules 2021, 54, 8770–8779. [Google Scholar] [CrossRef]
- Soccio, M.; Lotti, N.; Munari, A.; Rebollar, E.; Martínez-Tong, D.E. Wrinkling Poly(Trimethylene 2,5-Furanoate) Free-Standing Films: Nanostructure Formation and Physical Properties. Polymer 2020, 202, 122666. [Google Scholar] [CrossRef]
- Muljajew, I.; Erlebach, A.; Weber, C.; Buchheim, J.R.; Sierka, M.; Schubert, U.S. A Polyesteramide Library from Dicarboxylic Acids and 2,2′-Bis(2-Oxazoline): Synthesis, Characterization, Nanoparticle Formulation and Molecular Dynamics Simulations. Polym. Chem. 2020, 11, 112–124. [Google Scholar] [CrossRef]
- Jones, N.A.; Atkins, E.D.T.; Hill, M.J. Comparison of Structures and Behavior on Heating of Solution-Grown, Chain-Folded Lamellar Crystals of 31 Even−Even Nylons. Macromolecules 2000, 33, 2642–2650. [Google Scholar] [CrossRef]
| Label | I | C1 | M1 | M2 | C3 | M3 |
|---|---|---|---|---|---|---|
| 5 °C/min | ||||||
| T (°C) | ND * | 88 | 151 | 156 | 158 | 173 |
| ΔH (J/g) | ND * | 24 | 23 | 3 | 14 | 30 |
| 20 °C/min | ||||||
| T (°C) | 52 | 76 | 152 | 159 | 165 | 173 |
| ΔH (J/g) | 9 | 4 | 23 | 15 | 6 | 7 |
| 40 °C/min | ||||||
| T (°C) | 57 | 75 | 153 | 159 | 166 | 173 |
| ΔH (J/g) | 5 | 6 | 31 | 8 | 4 | 4 |
| 60 °C/min | ||||||
| T (°C) | 72 | 86 | 156 | 162 | - | 173 |
| ΔH (J/g) | 6 | 16 | 22 | 10 | - | 1 |
| Label | I | C1 | M1 | M2 | C3 | M3 | |
|---|---|---|---|---|---|---|---|
| Experiment 1 | |||||||
| Heating | T (°C) | 52 | 76 | 152 | 159 | 165 | 173 |
| ΔH (J/g) | 9 | 4 | 23 | 15 | 6 | 7 | |
| Experiment 2 | |||||||
| Heating | T (°C) | - | - | 148 | 158 | 163 | 174 |
| ΔH (J/g) | - | - | 3 | 13 | 9 | 17 | |
| Experiment 3 | |||||||
| Cooling | T (°C) | - | - | - | - | 158 | - |
| ΔH (J/g) | 31 | ||||||
| Re-heating | T (°C) | - | - | 147 | - | - | 175 |
| ΔH (J/g) | - | - | 2 | - | - | 38 | |
| Label T (°C) | R0 2θ (°) | R1 2θ (°) | R2 2θ (°) | R3 2θ (°) | R4 2θ (°) | R5 2θ (°) | Xc (%) |
|---|---|---|---|---|---|---|---|
| Experiment 1 | |||||||
| 30 | - | - | 20.2 | - | - | - | 5 ± 2 |
| 80 * | - | - | 20.1 | 21.6 | - | 23.7 | 7 ± 2 |
| 150 * | - | - | 20.0 | 21.4 | - | 23.5 | 8 ± 2 |
| 160 * | - | 19.5 | 20.0 | 21.4 | - | 23.9 | 10 ± 1 |
| 165 * | - | 19.5 | 20.1 | 21.4 | - | 24.0 | 10 ± 1 |
| 180 * | - | - | - | - | - | - | 0 |
| Experiment 2 | |||||||
| 30 | 18.2 | 19.8 | - | 22.0 | - | 24.6 | 18 ± 1 |
| 80 * | 18.2 | 19.8 | - | 21.9 | - | 24.3 | 18 ± 1 |
| 150 * | - | 20.1 | - | 21.6 | - | 23.9 | 16 ± 1 |
| 160 * | - | 19.6 | 20.0 | 21.4 | - | 23.9 | 15 ± 1 |
| 165 * | - | 19.5 | 20.1 | 21.4 | - | 23.9 | 15 ± 1 |
| 170 * | - | 19.7 | 19.7 | 21.4 | - | 23.8 | 13 ± 1 |
| Experiment 3 | |||||||
| Cooling | |||||||
| 165 * | - | 19.5 | 20.0 | 21.5 | 22.8 | 24.0 | 9 ± 2 |
| 150 * | - | 19.5 | 20.1 | 21.5 | 22.7 | 24.2 | 12 ± 1 |
| 140 * | - | 19.6 | 20.2 | 21.6 | 22.8 | 24.3 | 13 ± 1 |
| 100 * | - | 19.7 | 20.2 | 21.7 | 22.9 | 24.5 | 14 ± 1 |
| 30 | - | 19.8 | 20.4 | 21.8 | 23.0 | 24.9 | 15 ± 1 |
| Re-heating | |||||||
| 120 * | - | 19.6 | 20.2 | 21.6 | 22.8 | 24.3 | 14 ± 1 |
| 150 * | - | 19.5 | 20.2 | 21.5 | 22.7 | 24.2 | 13 ± 1 |
| 160 * | - | 19.5 | 20.1 | 21.4 | 22.6 | 24.1 | 12 ± 1 |
| 165 * | - | 19.5 | 20.1 | 21.4 | 22.6 | 24.0 | 10 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, E.; Soccio, M.; Gazzano, M.; Papadopoulos, L.; Robert, T.; Bikiaris, D.N.; Lotti, N. Melting Behavior of Compression Molded Poly(ester amide) from 2,5-Furandicarboxylic Acid. Polymers 2024, 16, 3459. https://doi.org/10.3390/polym16243459
Bianchi E, Soccio M, Gazzano M, Papadopoulos L, Robert T, Bikiaris DN, Lotti N. Melting Behavior of Compression Molded Poly(ester amide) from 2,5-Furandicarboxylic Acid. Polymers. 2024; 16(24):3459. https://doi.org/10.3390/polym16243459
Chicago/Turabian StyleBianchi, Enrico, Michelina Soccio, Massimo Gazzano, Lazaros Papadopoulos, Tobias Robert, Dimitrios N. Bikiaris, and Nadia Lotti. 2024. "Melting Behavior of Compression Molded Poly(ester amide) from 2,5-Furandicarboxylic Acid" Polymers 16, no. 24: 3459. https://doi.org/10.3390/polym16243459
APA StyleBianchi, E., Soccio, M., Gazzano, M., Papadopoulos, L., Robert, T., Bikiaris, D. N., & Lotti, N. (2024). Melting Behavior of Compression Molded Poly(ester amide) from 2,5-Furandicarboxylic Acid. Polymers, 16(24), 3459. https://doi.org/10.3390/polym16243459











