Modification of Processability and Shear-Induced Crystallization of Poly(lactic acid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Measurements
3. Results and Discussion
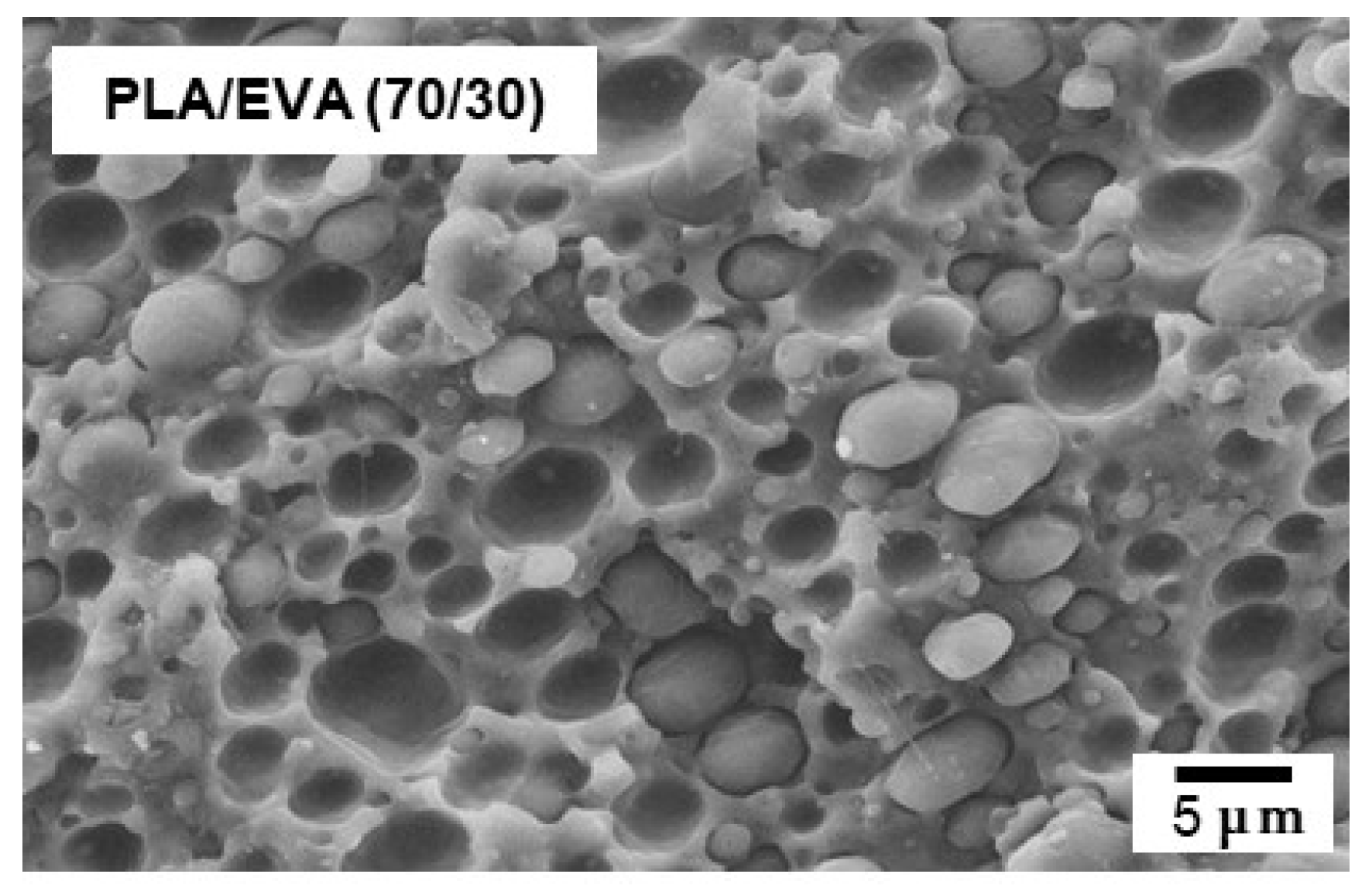
3.1. Structure of Blend Sample
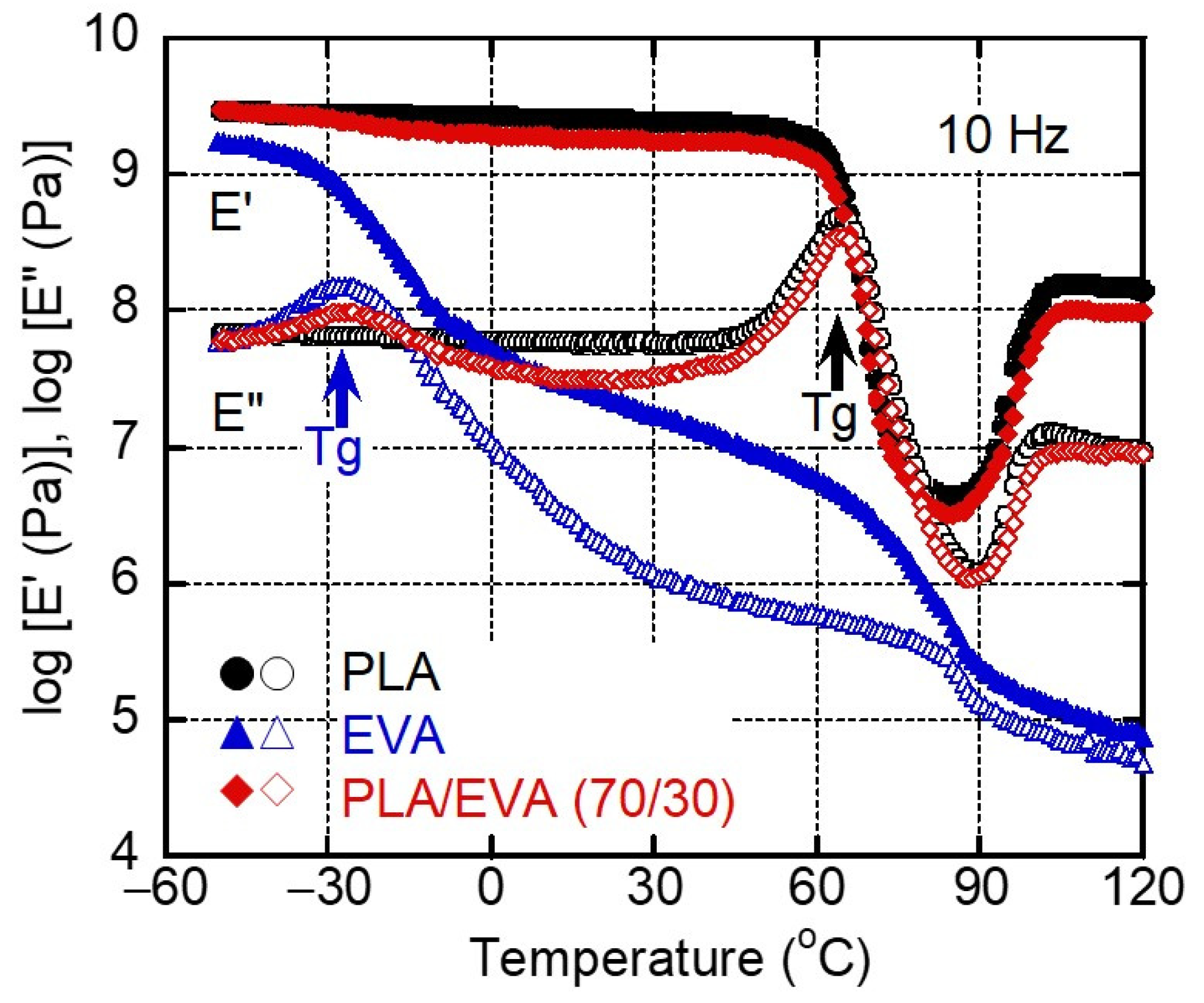
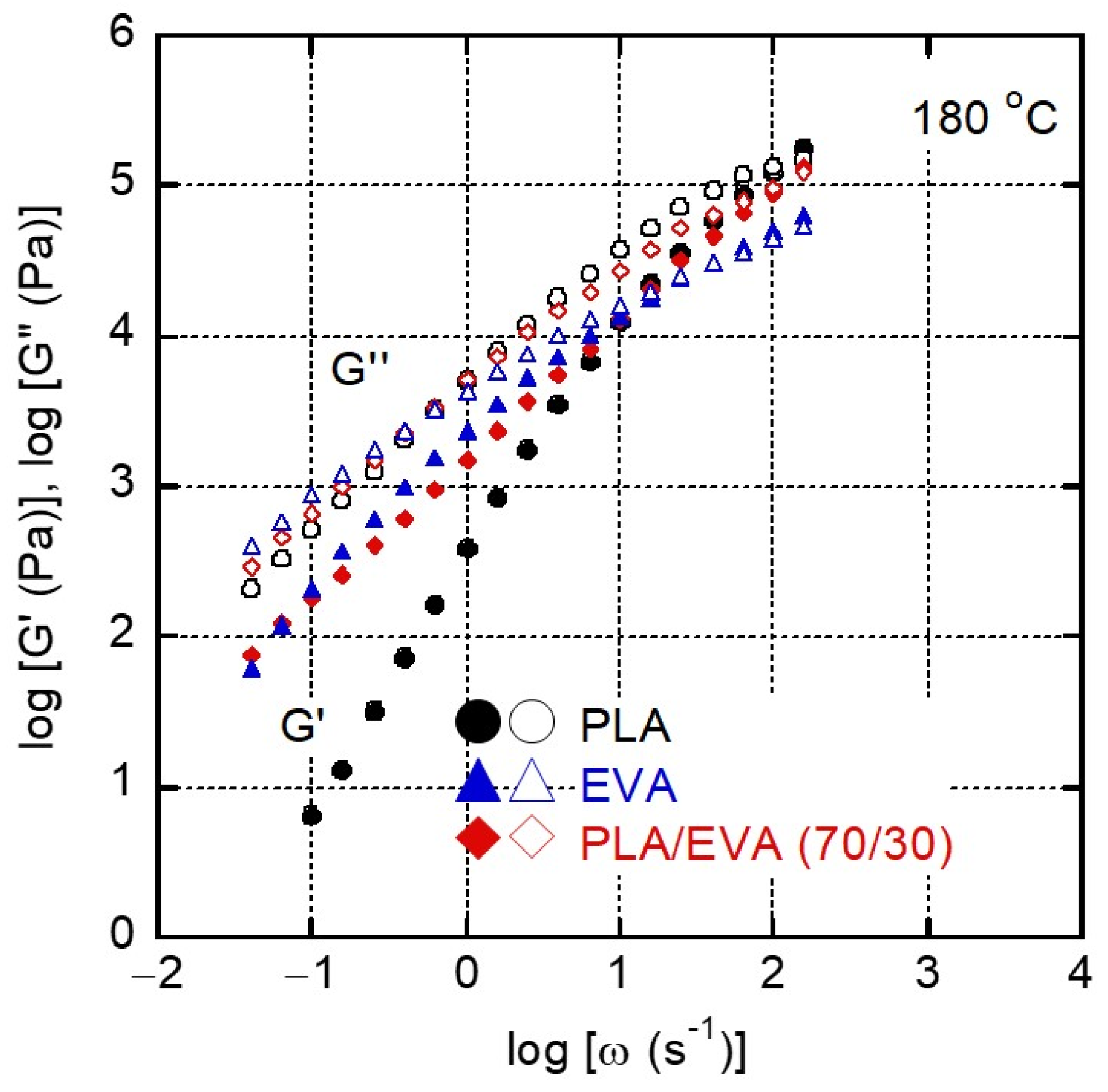
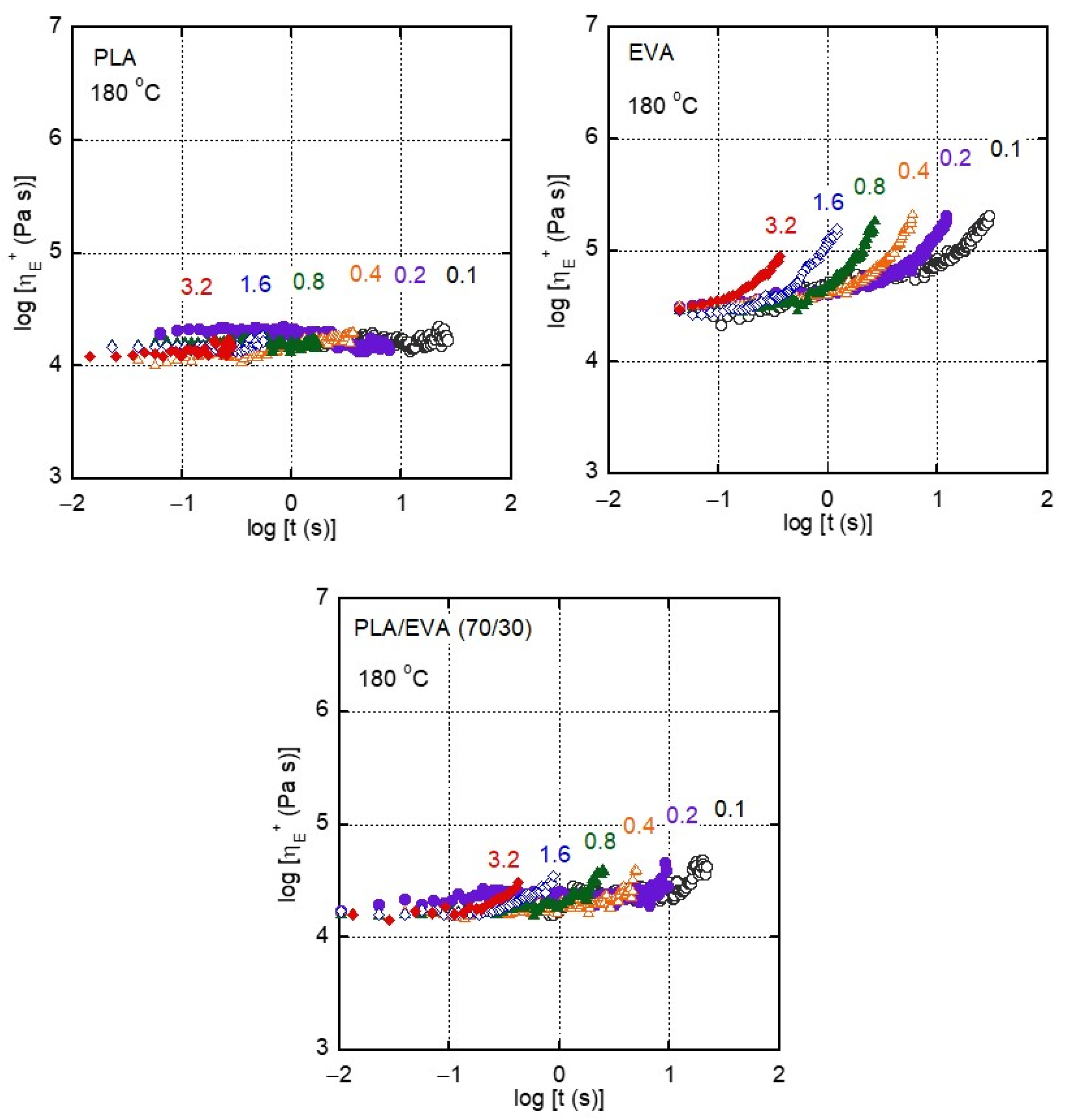
3.2. Rheological Properties
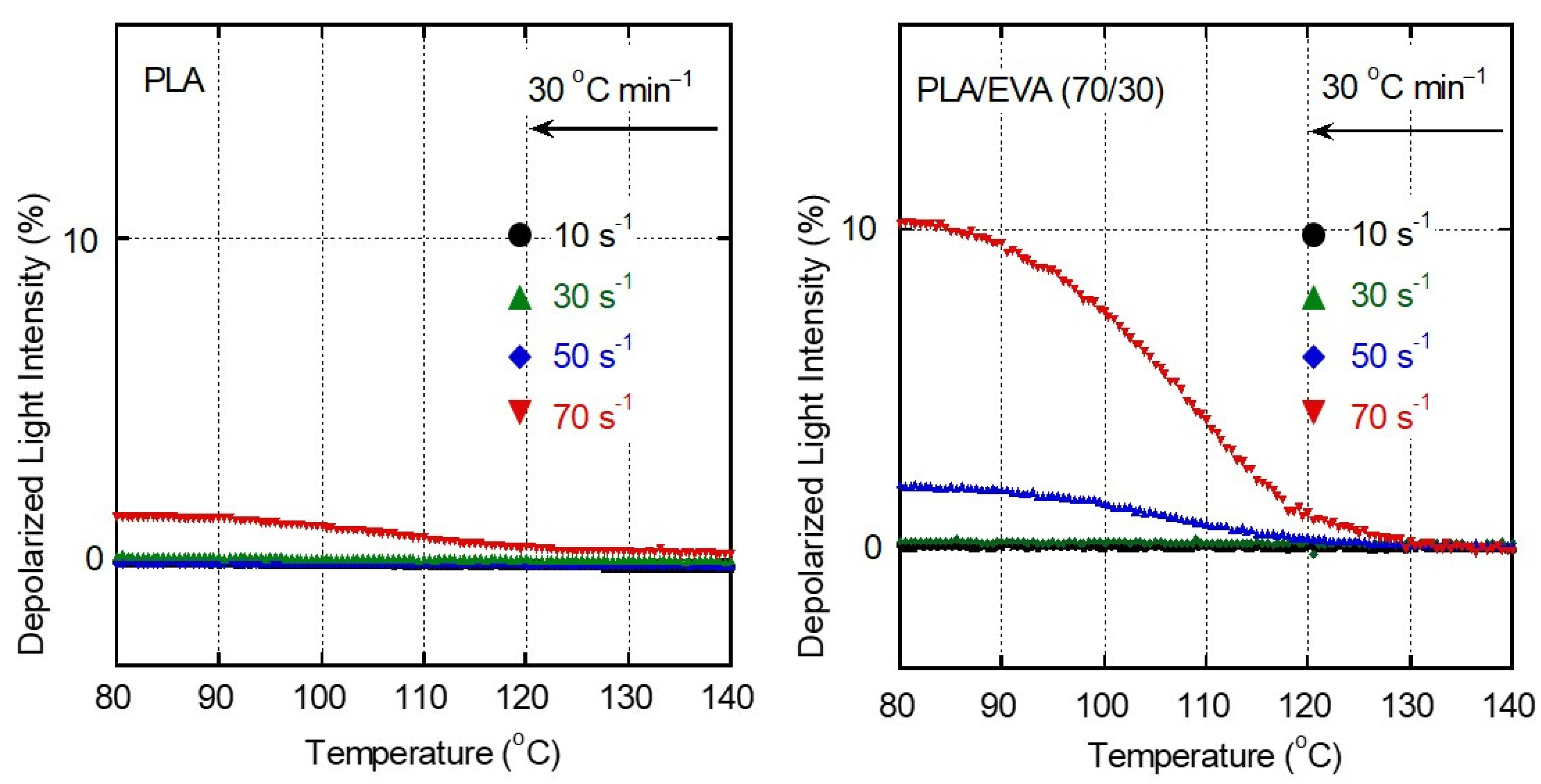
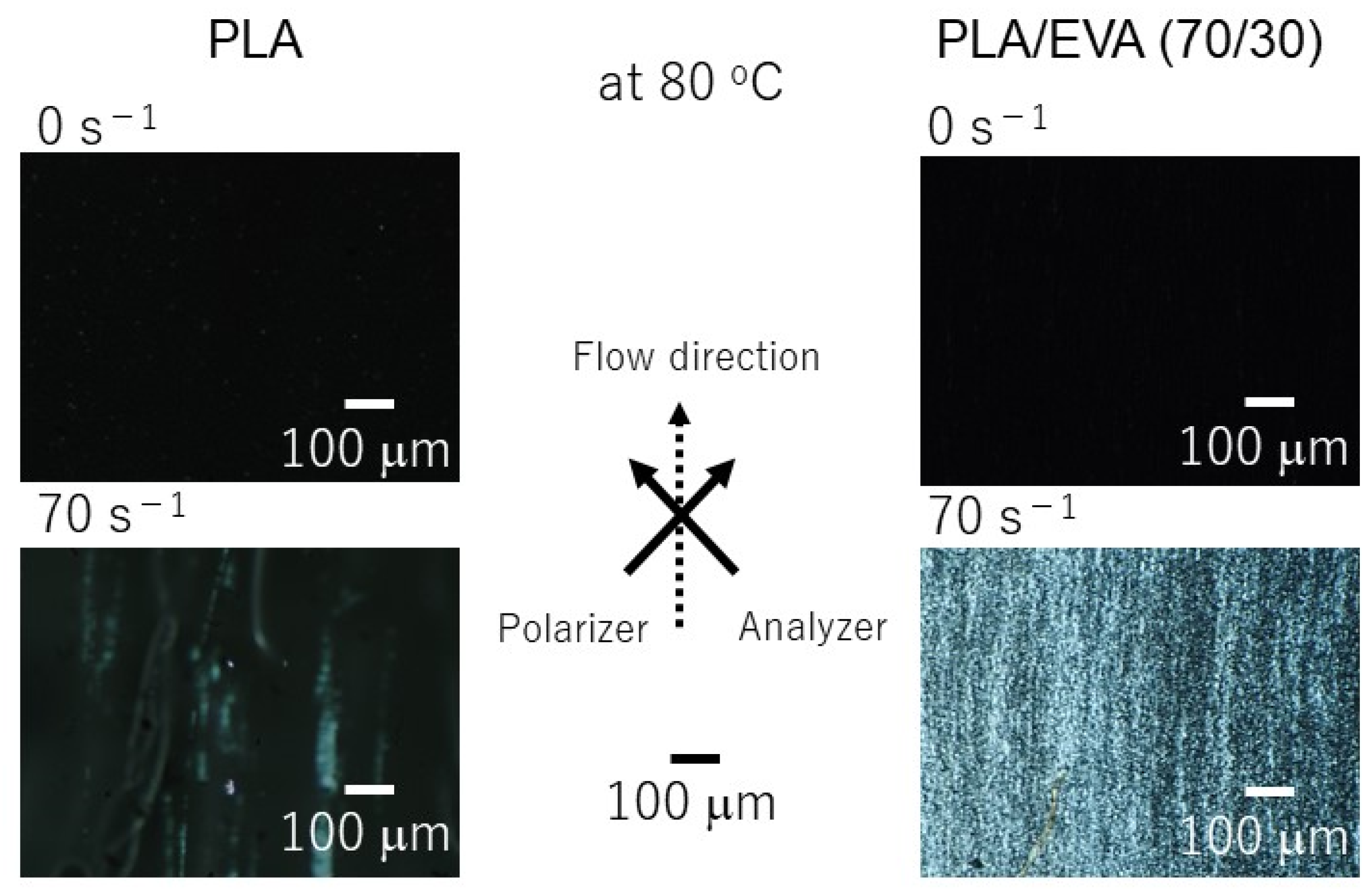
3.3. Shear-Induced Crystallization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Jimenez, A.; Peltzer, M.; Ruseckaite, R. Poly(lactic acid) Science and Technology: Processing, Properties, Additives, and Applications; RSC Publishing: Oxfordshire, UK, 2014. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Sin, L.T.; Trueen, B.S. Polylactic Acid. A Practical Guide for the Processing, Manufacturing, and Applications of PLA; Applied Science Publishers: Oxford, UK, 2019. [Google Scholar]
- Sharma, M. Biodegradable Polymers: Materials and Their Structures; CRC Press: Oxon, UK, 2021. [Google Scholar]
- Auras, R.A.; Lim, L.T.; Seike, S.E.M.; Tsuji, H. Poly(lactic acid): Synthesis, Structures, Properties, Processing, Applications, and End of Life; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Sangeetha, V.H.; Valapa, R.B.; Nayak, S.K.; Varghese, T.O. Investigation on the influence of EVA content on the mechanical and thermal characteristics of poly(lactic acid) blends. J. Polym. Environ. 2018, 26, 1–14. [Google Scholar] [CrossRef]
- Kugimoto, D.; Kouda, S.; Yamaguchi, M. Improvement of mechanical toughness of poly(lactic acid) by addition of ethylene-vinyl acetate copolymer. Polym. Test. 2019, 80, 106021. [Google Scholar] [CrossRef]
- Tabi, T. The application of the synergistic effect between the crystal structure of poly(lactic acid) (PLA) and the presence of ethylene vinyl acetate copolymer (EVA) to produce highly ductile PLA/EVA blends. J. Therm. Anal. Calorim. 2019, 138, 1287–1297. [Google Scholar] [CrossRef]
- Moradi, S.; Yeganeh, J.K. Highly toughened poly(lactic acid) (PLA) prepared through melt blending with ethylene-co-vinyl acetate (EVA) copolymer and simultaneous addition of hydrophilic silica nanoparticles and block copolymer compatibilizer. Polym. Test. 2020, 91, 106735. [Google Scholar] [CrossRef]
- Kugimoto, D.; Kouda, S.; Yamaguchi, M. Modification of poly(lactic acid) rheological properties using ethylene-vinyl acetate copolymer. J. Polym. Environ. 2020, 29, 121. [Google Scholar] [CrossRef]
- Ferreira, E.S.B.; Luna, C.B.B.; Siqueira, D.D.; Araujo, E.M.; Franca, D.C.; Wellen, R.M.R. Annealing effect on PLA/EVA blends performance. J. Polym. Environ. 2022, 30, 541. [Google Scholar] [CrossRef]
- Ferreira, E.S.B.; Luna, C.B.B.; Filho, E.A.S.; Wellen, R.M.R.; Araujo, E.M. Use of crosslinking agent to produce high-performance PLA/EVA blends via reactive processing. J. Vinyl Addit. Technol. 2023, 29, 161–175. [Google Scholar] [CrossRef]
- Salyer, I.O.; Kenyon, A.S. Structure and property relationships in ethylene-vinyl acetate copolymers. J. Polym. Sci. Part A-1 1971, 9, 3083–3103. [Google Scholar] [CrossRef]
- Arsac, A.; Carrot, C.; Guillet, J. Rheological characterization of ethylene vinyl acetate copolymers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2389–2400. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Tzankova, D.N. EVA copolymer-based nanocomposites: Rheological behavior under shear and isothermal and non-isothermal elongational flow. Polym. Test. 2006, 25, 701–708. [Google Scholar] [CrossRef]
- Mallet, B.; Lamnawar, K.; Maazouz, A. Improvement of blown film extrusion of poly(lactic acid): Structure–processing–properties relationships. Polym. Eng. Sci. 2014, 54, 840–857. [Google Scholar] [CrossRef]
- Schneider, J.R.; Shi, X.; Manjure, S.; Gravier, D.; Narayan, R. Epoxy functionalized poly(lactide) reactive modifier for blown film applications. J. Appl. Polym. Sci. 2015, 132, 42243. [Google Scholar] [CrossRef]
- Tadmor, Z.; Gogos, G.G. Principles of Polymer Processing, 2nd ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mahmood, S.H.; Keshtkar, M.; Park, C.B. Determination of carbon dioxide solubility in polylactide acid with accurate PVT properties. J. Chem. Thermodyn. 2014, 70, 13–23. [Google Scholar] [CrossRef]
- Janchai, K.; Kida, T.; Inoue, T.; Iwasaki, S.; Yamaguchi, M. Crystallization behavior of isotactic polypropylene containing a fibrous nucleating agent in a flow field. Polym. J. 2022, 54, 367–375. [Google Scholar] [CrossRef]
- Janchai, K.; Kida, T.; Yamaguchi, M.; Sunagawa, T.; Okura, T. Optimum processing conditions for the maximum crystallization rate of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Sci. Rep. 2023, 13, 497. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.G.D.; Yamaguchi, M. Growth of molecular orientation during post-process annealing of poly(lactic acid) containing a fibrous nucleating agent. J. Appl. Polym. Sci. 2024, 141, e55714. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Todd, D.B.; Gogos, C.G. Rheological properties of LDPE processed by conventional processing machines. Adv. Polym. Technol. 2003, 22, 179–187. [Google Scholar] [CrossRef]
- Sako, T.; Date, J.; Hagi, M.; Hiraoka, T.; Matsuoka, S.; Yamaguchi, M. Anomalous viscosity decrease of polycarbonate by addition of polystyrene. Polymer 2019, 170, 135–141. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sako, T.; Hiraoka, T.; Yamaguchi, M.; Yamaguchi, M. Effect of morphology on shear viscosity for binary blends of polycarbonate and polystyrene. J. Appl. Polym. Sci. 2020, 137, 49516. [Google Scholar] [CrossRef]
- Kuroda, Y.; Suzuki, K.; Kikuchi, G.; Moonprasith, N.; Kida, T.; Yamaguchi, M. Improvement of processability at injection-molding of bisphenol-A polycarbonate by addition of low-density polyethylene. Materials 2023, 16, 866. [Google Scholar] [CrossRef]
- Fujii, Y.; Nishikawa, R.; Phulkerd, P.; Yamaguchi, M. Modifying the rheological properties of polypropylene under elongational flow by adding polyethylene. J. Rheol. 2019, 63, 11–18. [Google Scholar] [CrossRef]
- Kang, G.B.; Kim, M.H.; Son, Y.; Park, O.O. Extrusion coating performances of iPP/LDPE blends. J. Appl. Polym. Sci. 2009, 111, 3121–3127. [Google Scholar] [CrossRef]
- Otsuki, Y.; Fujii, Y.; Sasaki, H.; Phulkerd, P.; Yamaguchi, M. Experimental and numerical study on transient elongational viscosity for polypropylene/low-density polyethylene blends. Polym. J. 2022, 52, 529–538. [Google Scholar] [CrossRef]
- Batchelor, G.K. The stress generated in a non-dilute suspension of elongated particles by pure straining motion. J. Fluid Mech. 1971, 46, 813–829. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Puopolo, M. Recycling of PLA-based bioplastics: The role of chain-extenders in twin-screw extrusion compounding and cast extrusion of sheets. J. Appl. Polym. Sci. 2020, 137, 49292. [Google Scholar] [CrossRef]
- Debroth, T.; Erwin, L. Causes of edge beads in cast films. Polym. Eng. Sci. 1986, 26, 462–467. [Google Scholar] [CrossRef]
- Satoh, N.; Tomiyama, H.; Kajiwara, T. Viscoelastic simulation of film casting process for a polymer melt. Polym. Eng. Sci. 2001, 41, 1564–1579. [Google Scholar] [CrossRef]
- Kouda, S. Prediction of processability at extrusion coating for low-density polyethylene. Polym. Eng. Sci. 2008, 48, 1094–1102. [Google Scholar] [CrossRef]
- Seo, Y.H.; Han, S.S.; Oh, T.H.; Khil, M.S. Annealing effects on structural changes and thermal shrinkage of poly (lactic acid) draw-textured filaments. J. Text. Inst. 2014, 105, 553–558. [Google Scholar] [CrossRef]
- Ogino, Y.; Fukushima, H.; Matsuba, G.; Takahashi, N.; Nishida, K.; Kanaya, T. Effects of high molecular weight component on crystallization of polyethylene under shear flow. Polymer 2006, 47, 5669–5677. [Google Scholar] [CrossRef]
- Balzano, L.; Kukalyekar, N.; Rastogi, S.; Peters, G.W.M.; Chadwick, J.C. Crystallization and dissolution of flow-induced precursors. Phys. Rev. Lett. 2008, 100, 048302. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, O.O.; Chambon, P.; Impradice, C.; Fairclough, J.P.A.; Terrill, N.J.; Ryan, A.J. Control of structural morphology in shear-induced crystallization of polymers. Macromolecules 2010, 43, 2389–2405. [Google Scholar] [CrossRef]
- Fernandez-Ballester, L.; Thurman, D.W.; Zhou, W.; Kornfield, J.A. Effect of long chains on the threshold stresses for flow-induced crystallization in iPP: Shish kebabs vs. Sausages. Macromolecules 2012, 45, 6557–6570. [Google Scholar] [CrossRef]
- Hamad, F.G.; Colby, R.H.; Milner, S.T. Onset of flow-induced crystallization kinetics of highly isotactic polypropylene. Macromolecules 2015, 48, 3725–3735. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, S.; Lei, J.; Hsiao, B.S.; Li, Z. Role of stably entangled chain network density in shish-kebab formation in polyethylene under an intense flow field. Macromolecules 2015, 48, 6652–6663. [Google Scholar] [CrossRef]
- Jalali, A.; Huneault, M.A.; Nofar, M.; Lee, P.C.; Park, C.B. Effect of branching on flow-induced crystallization of poly (lactic acid). Eur. Polym. J. 2019, 119, 410–420. [Google Scholar] [CrossRef]
- Nie, C.; Peng, F.; Cao, R.; Cui, K.; Sheng, J.; Chen, W.; Li, L. Recent progress in flow-induced polymer crystallization. J. Polym. Sci. 2022, 60, 3149–3163. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Ma, Z. Flow-induced crystallization starting from self-nucleated precursors. Macromolecules 2024, 57, 606–618. [Google Scholar] [CrossRef]
- Janchai, K.; Yamaguchi, M. Shear-induced crystallization of polypropylene/low-density polyethylene blend. J. Rheol. 2024, 68, 59–70. [Google Scholar] [CrossRef]
- Zülle, B.; Linster, J.; Meissner, J.; Hürlimann, H.P. Deformation hardening and thinning in both elongation and shear of a low density polyethylene melt. J. Rheol. 1987, 31, 583–597. [Google Scholar] [CrossRef]
- Doshi, S.; Dealy, J.M. Exponential shear: A strong flow. J. Rheol. 1987, 31, 563–574. [Google Scholar] [CrossRef]
- Venerus, D.C. Exponential shear flow of branched polymer melts. Rheol. Acta 2000, 39, 71–79. [Google Scholar] [CrossRef]
- Graham, R.S.; McLeish, T.C.B.; Harlen, O.G. Using the pom-pom equations to analyze polymer melts in exponential shear. J. Rheol. 2001, 45, 275–290. [Google Scholar] [CrossRef]
- Wagner, H.M.; Rolon-Garrido, V.H.; Chai, C.K. Exponential shear flow of branched polyethylenes in rotational parallel-plate geometry. Rheol. Acta 2005, 45, 164–175. [Google Scholar] [CrossRef]
- Liu, G.; Sun, H.; Rangou, S.; Ntetsikas, K.; Avgeropoulos, A.; Wang, S. Studying the origin of “strain hardening”: Basic difference between extension and shear. J. Rheol. 2013, 57, 89–104. [Google Scholar] [CrossRef]
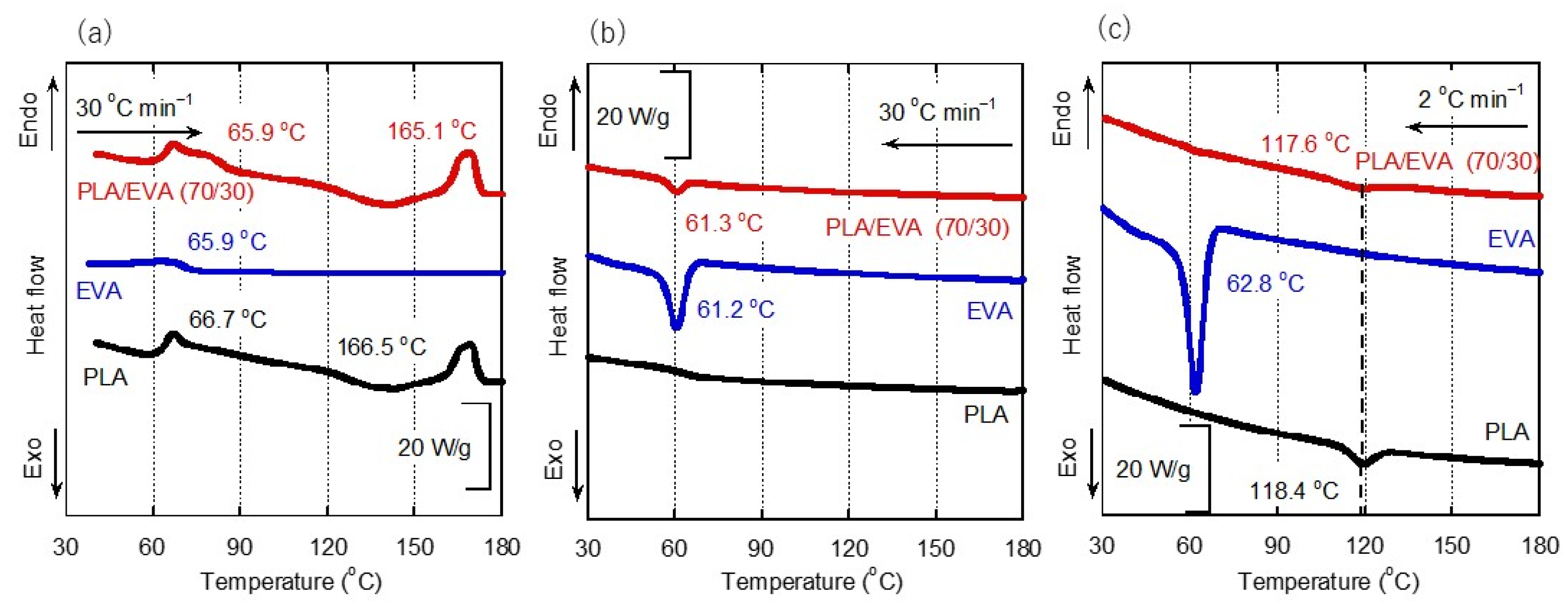


| Samples | Cold Crystallization of PLA at the First Heating (J g−1) | Melting of PLA at the First Heating (J g−1) | Crystallization of PLA at the Cooling 2 °C min−1 (J g−1) |
|---|---|---|---|
| PLA | 9.3 | 10.6 | 1.1 |
| PLA/EVA (70/30) | 6.5 | 7.8 | 0.8 |
| Width (mm) | Δn × 104 | |
|---|---|---|
| PLA | 28.2 | 1.1 |
| PLA/EVA (70/30) | 30.0 | 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, R.; Kugimoto, D.; Yamaguchi, M. Modification of Processability and Shear-Induced Crystallization of Poly(lactic acid). Polymers 2024, 16, 3487. https://doi.org/10.3390/polym16243487
Feng R, Kugimoto D, Yamaguchi M. Modification of Processability and Shear-Induced Crystallization of Poly(lactic acid). Polymers. 2024; 16(24):3487. https://doi.org/10.3390/polym16243487
Chicago/Turabian StyleFeng, Ruiqi, Daisuke Kugimoto, and Masayuki Yamaguchi. 2024. "Modification of Processability and Shear-Induced Crystallization of Poly(lactic acid)" Polymers 16, no. 24: 3487. https://doi.org/10.3390/polym16243487
APA StyleFeng, R., Kugimoto, D., & Yamaguchi, M. (2024). Modification of Processability and Shear-Induced Crystallization of Poly(lactic acid). Polymers, 16(24), 3487. https://doi.org/10.3390/polym16243487






