Thermoplastic Composite Hot-Melt Adhesives with Metallic Nano-Particles for Reversible Bonding Techniques Utilizing Microwave Energy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods and Related Equipment
3. Results and Discussion
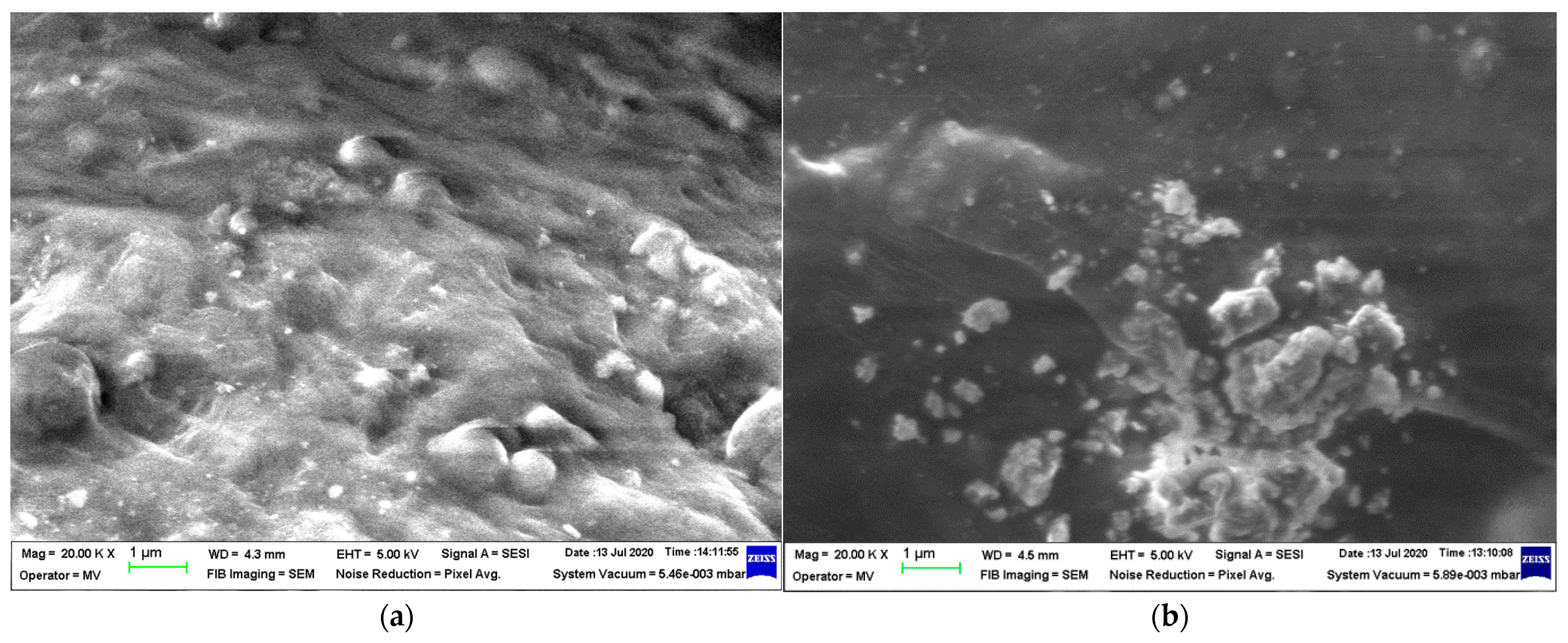
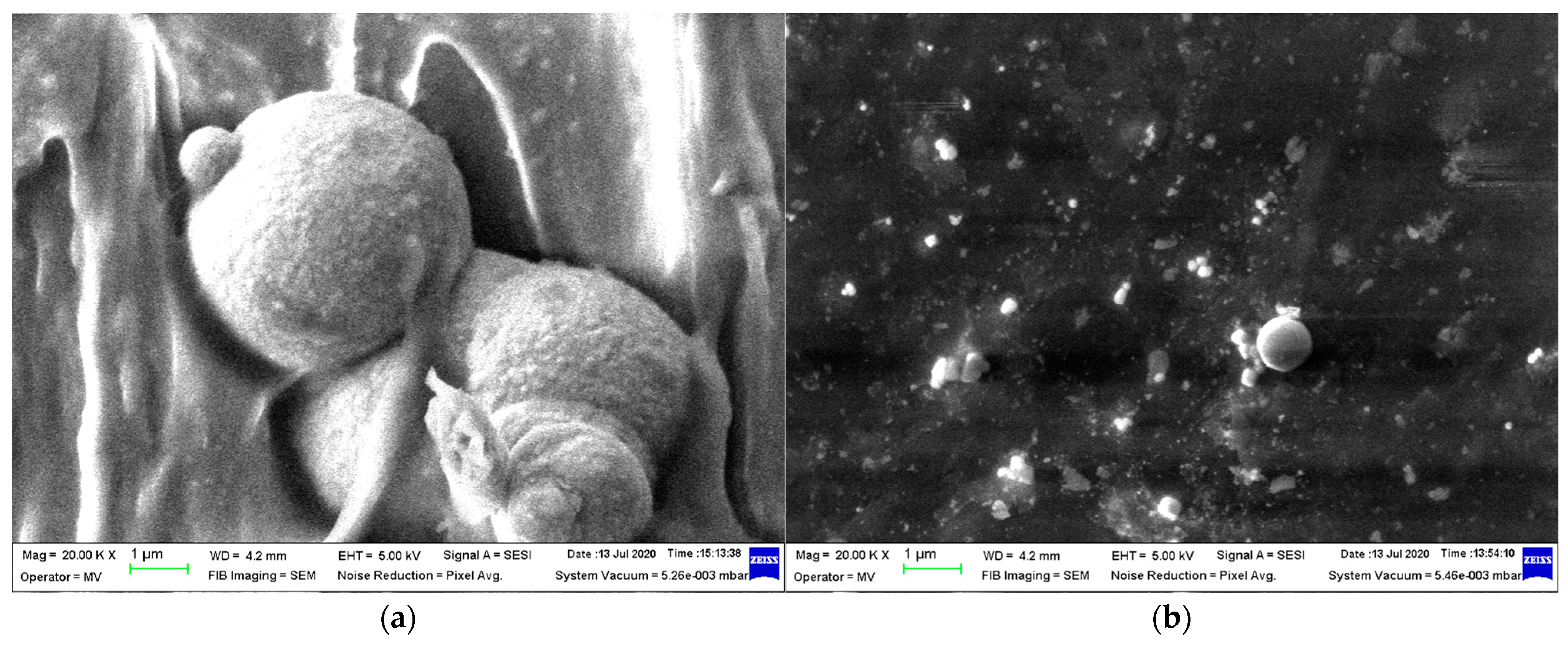
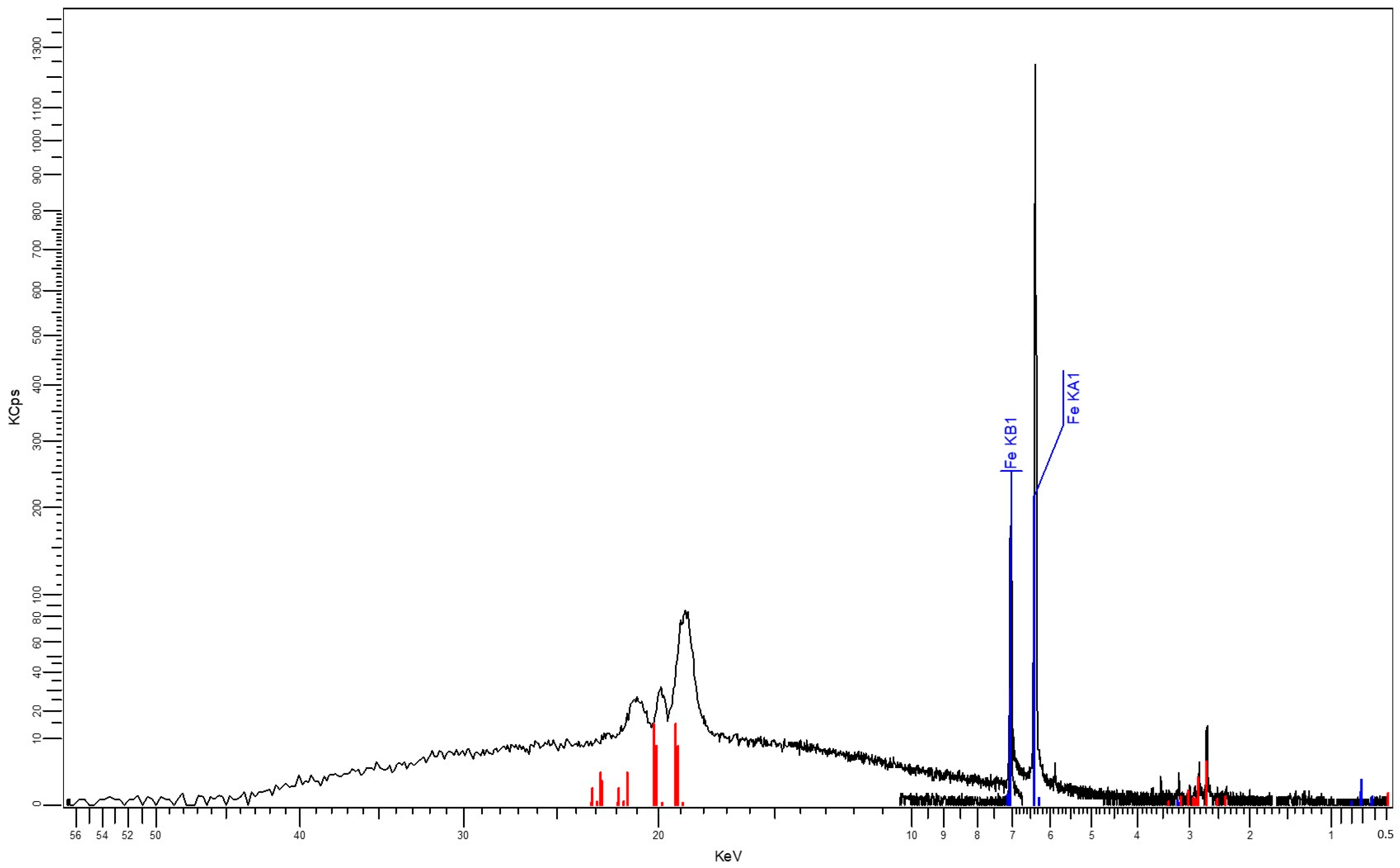
3.1. SEM and X-Ray Fluorescence (XRF)
3.2. Results Obtained from Micro-Indentation Tests
3.3. Results Obtained from Density Analysis
3.4. Results Obtained from Shore Hardness Tests
3.5. Results Obtained from the Mechanical Tests
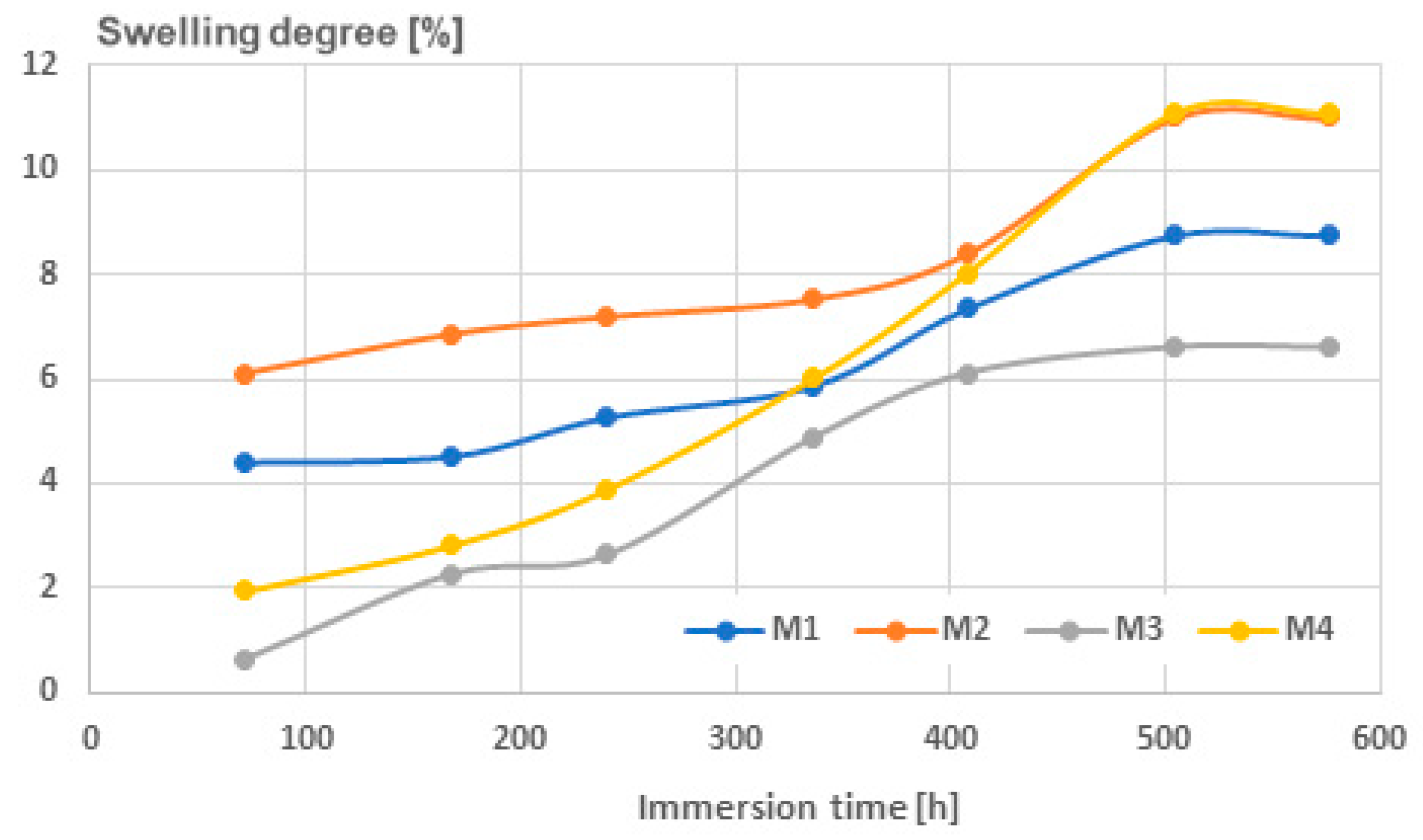
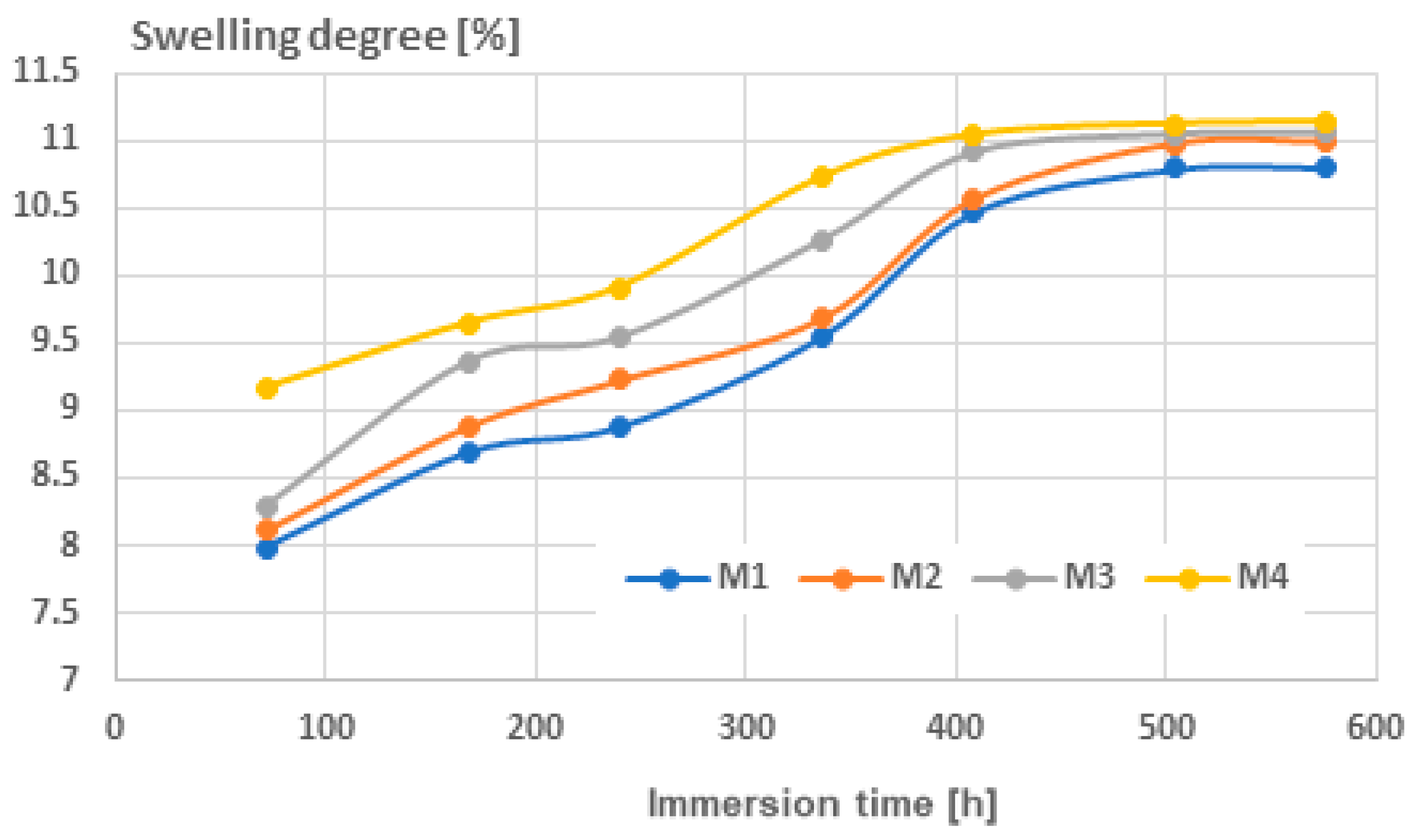
3.6. Results Obtained for the Degree of Swelling
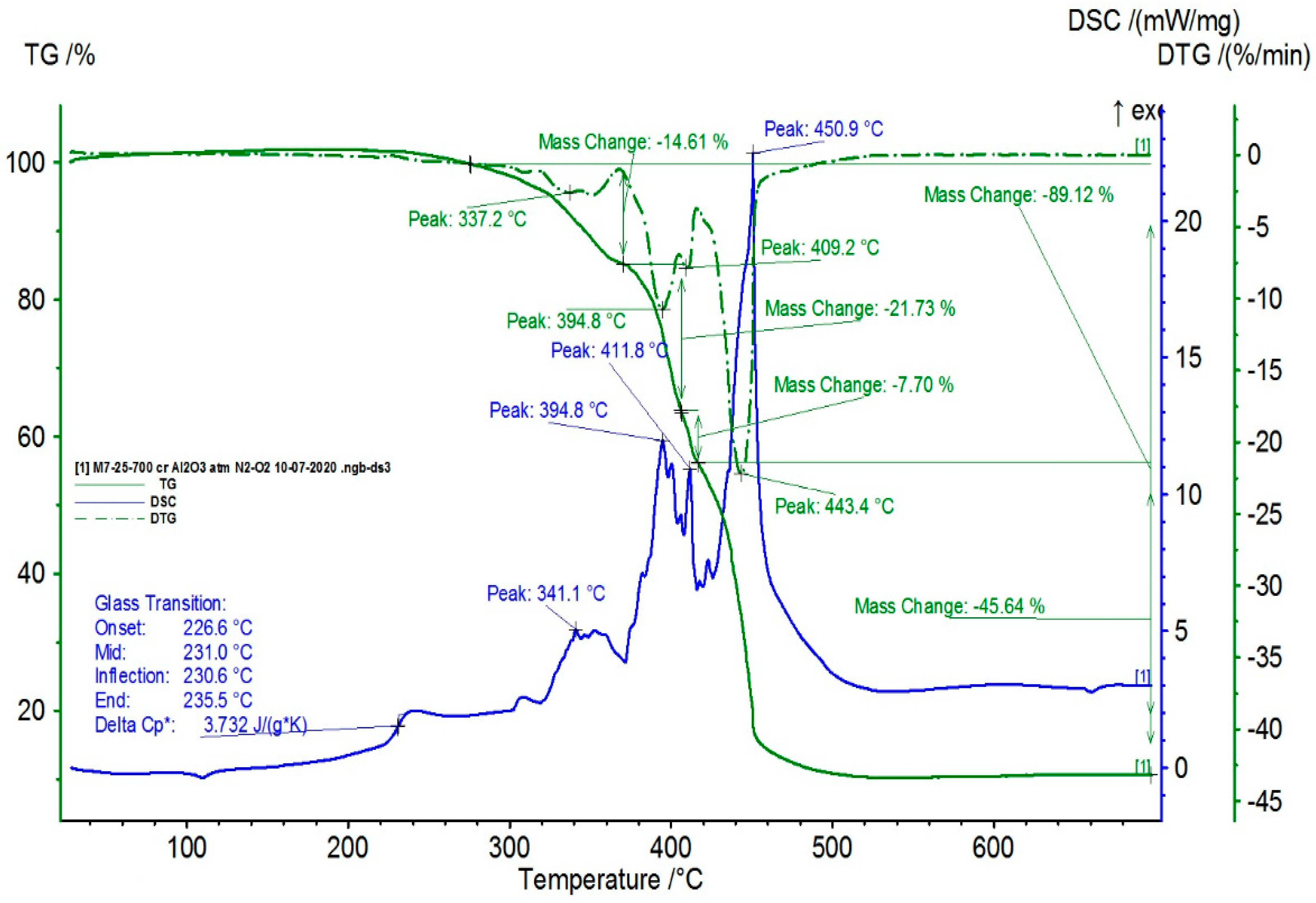
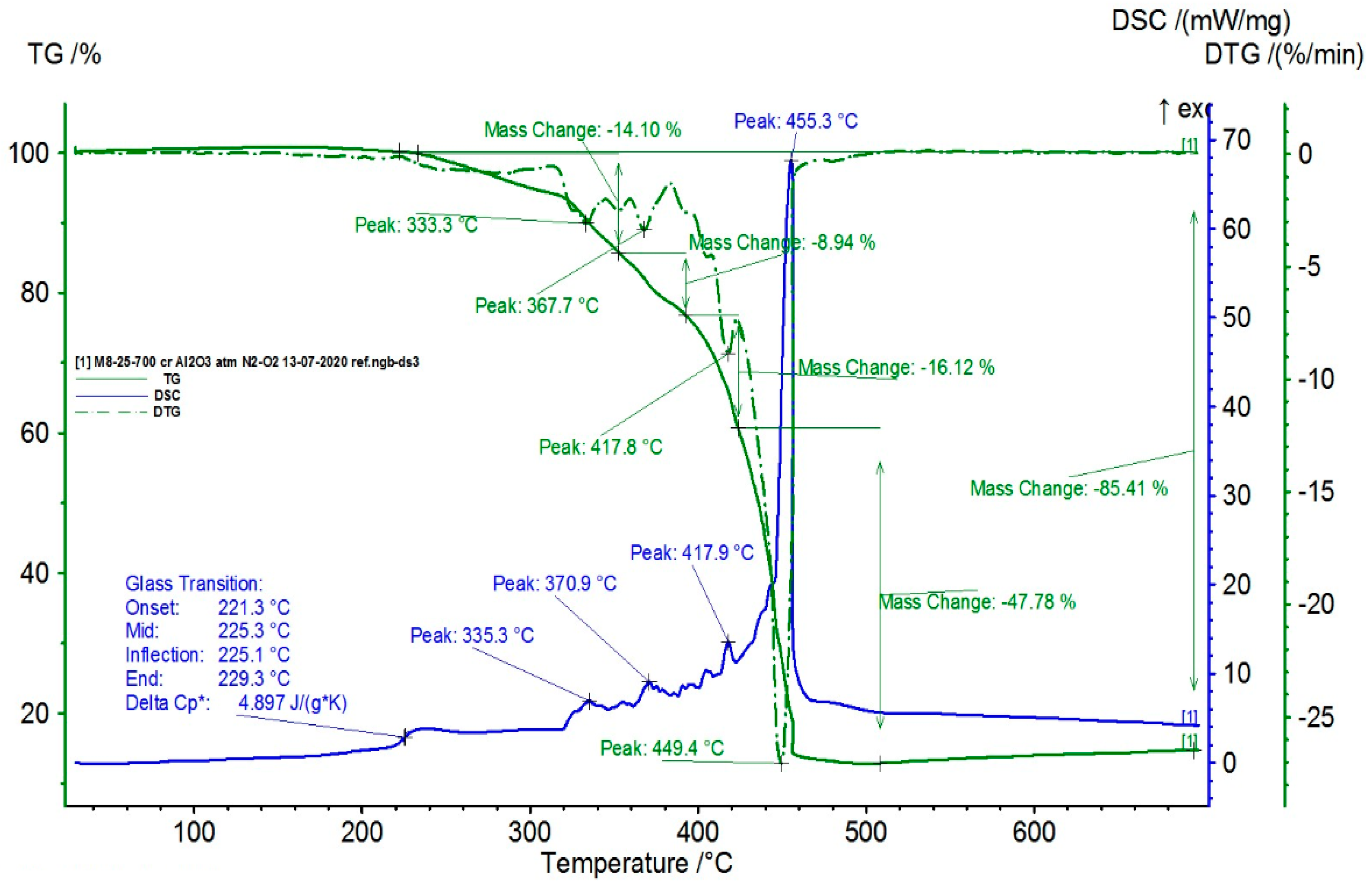
3.7. Results Obtained from Thermal Analysis
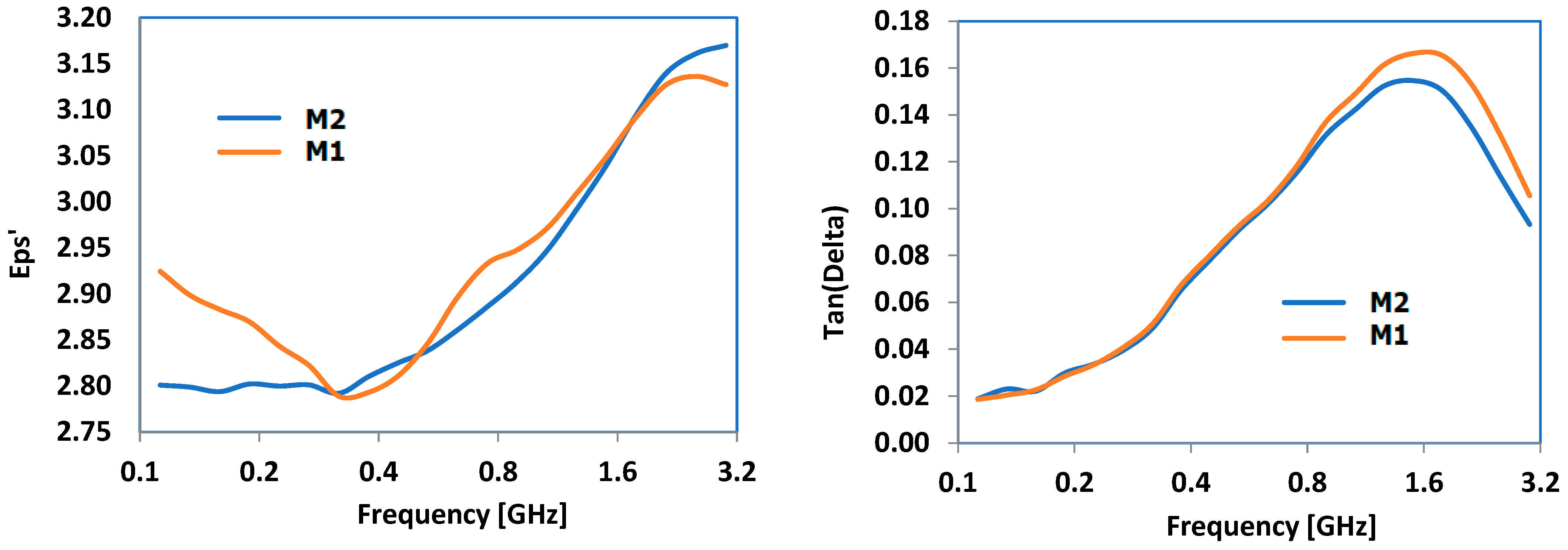
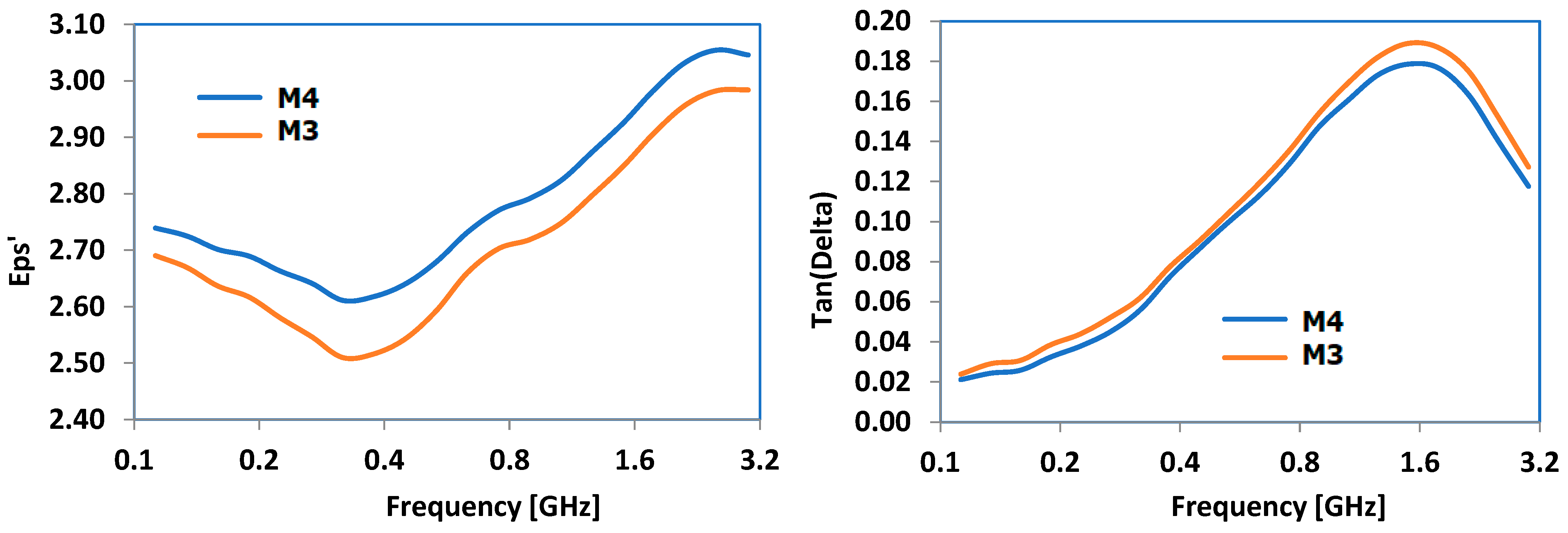
3.8. Results Obtained for Dielectric Properties
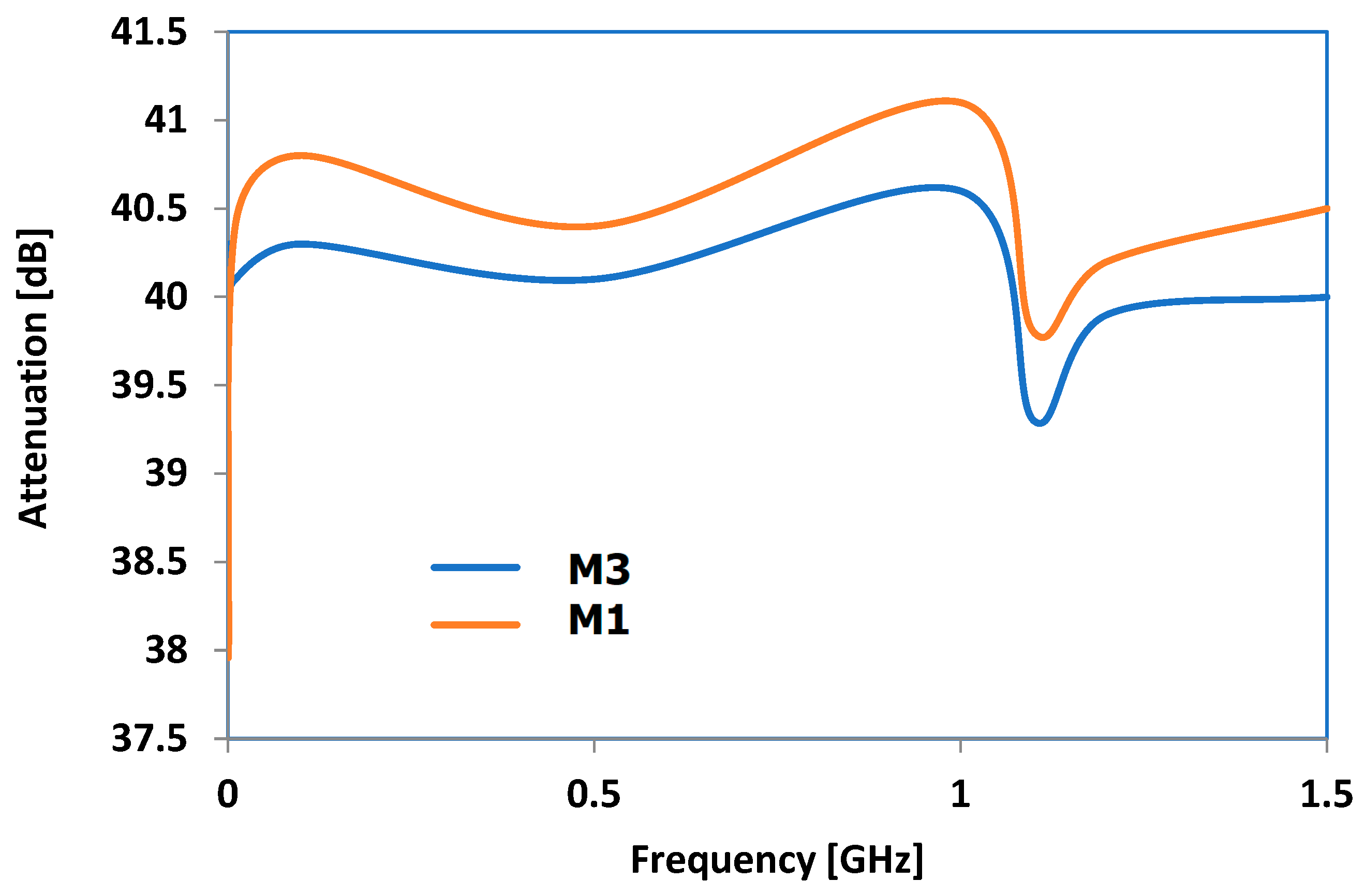
3.9. Results Obtained for Electromagnetic Radiation Attenuation
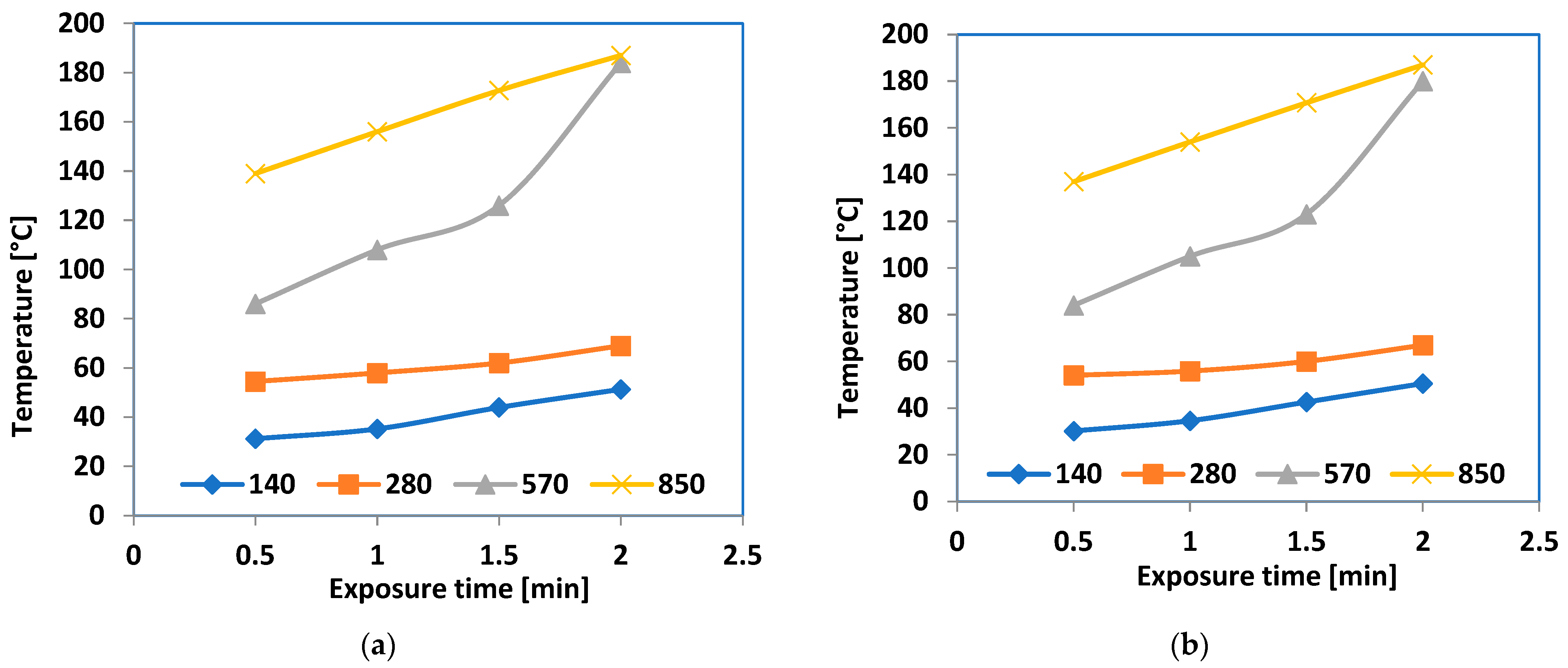
3.10. Tests upon the Feasibility of Reversible Bonding Techniques Utilizing Microwave Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- End of Life Vehicles Directive. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/end-life-vehicles_en (accessed on 28 October 2024).
- Global Hot Melt Adhesives Market Overview. Available online: https://www.marketresearchfuture.com/reports/hot-melt-adhesives-market-4640?utm_term=&utm_campaign=&utm_source=adwords&utm_medium=ppc&hsa_acc=2893753364&hsa_cam=20373674291&hsa_grp=150985931723&hsa_ad=665909881832&hsa_src=g&hsa_tgt=dsa-2089395910984&hsa_kw=&hsa_mt=&hsa_net=adwords&hsa_ver=3&gad_source=1 (accessed on 28 October 2024).
- What Is Hot Melt Made Of? Available online: https://www.hotmelt.com/blogs/blog/what-is-hot-melt?srsltid=AfmBOookNMHynvS8fmmLcf9jTMsXJid6Iv4_yc5m7DRh_2YPKojEEcfH (accessed on 28 October 2024).
- Gharde, S.; Sharma, G.; Kandasubramanian, B. Hot-Melt Adhesives: Fundamentals, Formulations, and Applications: A Critical Review. In Progress in Adhesion and Adhesives; Mittal, K.L., Ed.; Scrivener Publishing LLC: Austin, TX, USA, 2021; Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781119846703.ch1 (accessed on 28 October 2024).
- Budhe, S.; Banea, M.D.; de Barros, S.; da Silva, L.F.M. An updated review of adhesively bonded joints in composite materials. Int. J. Adhes. Adhes. 2017, 72, 30–42. [Google Scholar] [CrossRef]
- Amend, P.; Frick, T.; Schmidt, M. Experimental Studies on Laser-based Hot-melt Bonding of thermosetting Composites and Thermoplastics. Phys. Procedia 2011, 12, 166–173. [Google Scholar] [CrossRef]
- Gilbert, M.D. Electrically Disbonding Materials. U.S. patent 6620308, 16 September 2003. [Google Scholar]
- Gilbert, M.D. Electrically Disbonding Adhesive Compositions and Related Methods. U.S. patent 20080196828, 21 August 2008. [Google Scholar]
- Anduix-Canto, C.; Peral, D.; Pérez-Padilla, V.; Diaz-Rovira, A.M.; Lledó, A.B.; Orme, C.A.; Petrash, S.; Engels, T.; Chou, K.W. Unraveling the mechanism of electrically induced adhesive debonding: A spectro-microscopic study. Adv. Mater. Interfaces 2022, 9, 2101447. [Google Scholar] [CrossRef]
- Springer, M.; Bosco, N. Environmental Influence on Fracture and Delamination of Electrically Conductive Adhesives. In Proceedings of the 47th IEEE Photovoltaic Specialists Conference (PVSC), Calgary, Canada, 15–21 August 2020. [Google Scholar] [CrossRef]
- Bibi, I.; Ahmad, H.; Farid, A.; Iqbal, H.; Habib, N.; Atif, M. A comprehensive study of electrically switchable adhesives: Bonding and debonding on demand. Mater. Today Commun. 2023, 35, 106293. [Google Scholar] [CrossRef]
- Lupi, S.; Forzan, M.; Aliferov, A. Induction and Direct Resistance Heating: Theory and Numerical Modeling; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–22. [Google Scholar] [CrossRef]
- Leijonmarck, S.; Cornell, A.; Danielsson, C.-O.; Åkermark, T.; Brandner, B.D.; Lindbergh, G. Electrolytically assisted debonding of adhesives: An experimental investigation. Int. J. Adhes. Adhes. 2012, 32, 39–45. [Google Scholar] [CrossRef]
- Ciardiello, R. The Mechanical Performance of Re-Bonded and Healed Adhesive Joints Activable through Induction Heating Systems. Materials 2021, 14, 6351. [Google Scholar] [CrossRef]
- Ciardiello, R.; Belingardi, G.; Martorana, B.; Brunella, V. Physical and mechanical properties of a reversible adhesive for automotive applications. Int. J. Adhes. Adhes. 2019, 89, 117–128. [Google Scholar] [CrossRef]
- Ciardiello, R.; Tridello, A.; Brunella, V.; Martorana, B.; Paolino, D.S.; Belingardi, G. Impact response of adhesive reversible joints made of thermoplastic nanomodified adhesive. J. Adhes. 2017, 94, 1051–1066. [Google Scholar] [CrossRef]
- Verna, E.; Cannavaro, I.; Brunella, V.; Koricho, E.G.; Belingardi, G.; Roncato, D.; Martorana, B.; Lambertini, V.; Neamtu, V.A.; Ciobanu, R. Adhesive joining technologies activated by electro-magnetic external trims. Int. J. Adhes. Adhes. 2013, 46, 21–25. [Google Scholar] [CrossRef]
- Ciardiello, R.; Belingardi, G.; Litterio, F.; Brunella, V. Effect of iron oxide and graphene particles on joint strength and dismounting characteristics of a thermoplastic adhesive. Int. J. Adhes. Adhes. 2021, 107, 102850. [Google Scholar] [CrossRef]
- Salimi, S.; Babra, T.S.; Dines, G.S.; Baskerville, S.W.; Hayes, W.; Greenland, B.W. Composite polyurethane adhesives that debond-on-demand by hysteresis heating in an oscillating magnetic field. Eur. Polym. J. 2019, 121, 109264. [Google Scholar] [CrossRef]
- Liebezeit, S.; Mueller, A.; Leydolph, B.; Palzer, U. Microwave-induced interfacial failure to enable debonding of composite materials for recycling. Sustain. Mater. Technol. 2017, 14, 29–36. [Google Scholar]
- Hellmann, H.; Krieger, R. Microwave-Activatable Hot-Melt Adhesive. US patent 4906497A, 4 November 1988. [Google Scholar]
- Roth, M.; Padurschel, P.; Niehaus, E.; Hoffmann, G. Hot Melt Adhesive for Microwave Heating. Patent WO2008071469, 19 June 2008. [Google Scholar]
- Czakaj, J.; Sztorch, B.; Romanczuk-Ruszuk, E.; Brząkalski, D.; Przekop, R.E. Organosilicon Compounds in Hot-Melt Adhesive Technologies. Polymers 2023, 15, 3708. [Google Scholar] [CrossRef]
- Plasticisers: A Challenge for Bonding. 2024. Available online: https://www.buehnen.de/international/blog/2024/06/18/plasticisers-a-challenge-for-bonding/ (accessed on 25 October 2024).
- Chabert, F.; Tournilhac, F.; Sajot, N.; Tencé-Girault, S.; Leibler, L. Supramolecular polymer for enhancement of adhesion and processability of hot melt polyamides. Int. J. Adhes. Adhes. 2010, 30, 696–705. [Google Scholar] [CrossRef]
- Malysheva, G.V.; Bodrykh, N.V. Hot-melt adhesives. Polym. Sci. Ser. 2011, 4, 301–303. [Google Scholar] [CrossRef]
- Secrist, K.E.; Gray, S.D.; Hussein, N. Tackifier-Free Hot Melt Adhesive Compositions Suitable for Use in a Disposable Hygiene Article. Patent WO2021011390A1, 21 January 2021. [Google Scholar]
- Chou, R. Hot Melt Adhesive Composition. Patent WO2007079092A1, 12 July 2007. [Google Scholar]
- Nakatani, H.; Yamanoue, T. Hot-Melt Adhesive Composition. Patent EP 3 839 002 A1, 23 June 2021. [Google Scholar]
- Hu, Y.; Desai, D.R.; Chen, J.; Sharak, M.L. Hot Melt Adhesive Composition and Use Thereof. Patent EP 3 271 436 B1, 21 August 2019. [Google Scholar]
- Li, D.; Ye, X.; Shi, J. Hot Melt Adhesive Composition. Patent EP 4 186 956 A1, 31 May 2023. [Google Scholar]
- Srinivasan, D.V.; Idapalapati, S. Review of debonding techniques in adhesively bonded composite structures for sustainability. Sustain. Mater. Technol. 2021, 30, e00345. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Ursan, G.-A.; Aradoaer, M.; Ursan, M.; Schreiner, C. Life Cycle Assessment of Microwave Activated Hot-Melt Adhesives. In Proceedings of the 2018 International Conference and Exposition on Electrical and Power Engineering (EPE), Iasi, Romania, 19–19 October 2018. [Google Scholar] [CrossRef]
- European Commission. A European Strategy for Plastics in a Circular Economy. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2018; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2018%3A28%3AFIN (accessed on 28 October 2024).
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the Implementation of the Circular Economy Action Plan. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52019DC0190 (accessed on 28 October 2024).
- Ciobanu, R.C.; Damian, R.F.; Schreiner, C.M.; Aradoaei, M.; Sover, A.; Raichur, A.M. Simulation of Dielectric Properties of Nanocomposites with Non-Uniform Filler Distribution. Polymers 2023, 15, 1636. [Google Scholar] [CrossRef]
- ASTM E2546-15; Standard Practice for Instrumented Indentation Testing. ASTM: West Conshohocken, PA, USA, 2015. Available online: https://www.astm.org/e2546-15.html (accessed on 25 October 2024).
- ASTM D2240-00; Standard Test Method for Rubber Property—Durometer Hardness. ASTM: West Conshohocken, PA, USA, 2021. Available online: https://www.smithers.com/en-gb/services/testing/standard/astm/astm-d2240 (accessed on 25 October 2024).
- SR EN ISO 527-2:2000; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Injection and Extrusion Plastics. ISO: Geneva, Switzerland, 2012. Available online: https://cdn.standards.iteh.ai/samples/56046/c5c3ec20cc0741289bf6def861e0a40b/ISO-527-2-2012.pdf (accessed on 25 October 2024).
- SR EN ISO 175/2011; Plastics—Methods of Test for the Determination of the Effects of Immersion in Liquid Chemicals. ISO: Geneva, Switzerland, 2010. Available online: https://www.en-standard.eu/une-en-iso-175-2011-plastics-methods-of-test-for-the-determination-of-the-effects-of-immersion-in-liquid-chemicals-iso-175-2010/ (accessed on 25 October 2024).
- Meraje, W.C.; Huang, C.-C.; Ahmad, N.; Dewangga, G.R.S.; Kuo, C.-F.J. Hybrid sol-gel-derived method for the synthesis of silicon rubber composites with hBN for characteristic applications in elastomeric thermal pads. Text. Res. J. 2022, 92, 11–12. [Google Scholar] [CrossRef]
- Doner, S.; Paswan, R.; Das, S. The influence of metallic particulate inclusions on the mechanical and thermal performance of 3D printable acrylonitrile-butadiene-styrene/thermoplastic polyurethane fused polymer blends. Mater. Today Commun. 2023, 35, 106111. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H.; Saeed, A.M. A Review: Metal Filled Thermoplastic Composites. Polym. Plast. Technol. Mater. 2021, 60, 1033–1050. [Google Scholar] [CrossRef]
- Leon, A.; Perez, M.; Barasinski, A.; Abisset-Chavanne, E.; Defoort, B.; Chinesta, F. Multi-Scale Modeling and Simulation of Thermoplastic Automated Tape Placement: Effects of Metallic Particles Reinforcement on Part Consolidation. Nanomaterials 2019, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F.; Ahsan, S.; Budraa, N.; Li, W.J.; Mai, J.D. Microwave bonding of polymer-based substrates for potential encapsulated micro/nanofluidic device fabrication. Sens. Actuators A Phys. 2004, 114, 340–346. [Google Scholar] [CrossRef]
- Holmes, R.J.; McDonagh, C.; McLaughlin, J.A.D.; Mohr, S.; Goddard, N.J.; Fielden, P.R. Microwave bonding of poly(methylmethacrylate) microfluidic devices using a conductive polymer. J. Phys. Chem. Solids 2011, 72, 626–629. [Google Scholar] [CrossRef]
- Hasna, A. Microwave Processing Applications in Chemical Engineering: Cost Analysis. J. Appl. Sci. 2011, 11, 3613–3618. [Google Scholar] [CrossRef]
- Epure, E.-L.; Cojocaru, F.D.; Aradoaei, M.; Ciobanu, R.C.; Dodi, G. Exploring the Surface Potential of Recycled Polyethylene Terephthalate Composite Supports on the Collagen Contamination Level. Polymers 2023, 15, 776. [Google Scholar] [CrossRef]
- Ciobanu, R.C.; Schreiner, C.; Caramitu, A.R.; Aradoaei, S.; Aradoaei, M. Sustainability of the Technology for Obtaining Thermoplastic Building Materials from Non-Recyclable Mixed Plastic–Paper Packaging Waste. Sustainability 2024, 16, 3430. [Google Scholar] [CrossRef]
- Aradoaei, M.; Ciobanu, R.C.; Schreiner, C.; Ursan, A.G.; Hitruc, E.G.; Aflori, M. Thermoplastic Electromagnetic Shielding Materials from the Integral Recycling of Waste from Electronic Equipment. Polymers 2023, 15, 3859. [Google Scholar] [CrossRef] [PubMed]
- EU Construction and Demolition Waste Protocol and Guidelines. Available online: https://single-market-economy.ec.europa.eu/news/eu-construction-and-demolition-waste-protocol-2018-09-18_en (accessed on 25 October 2024).





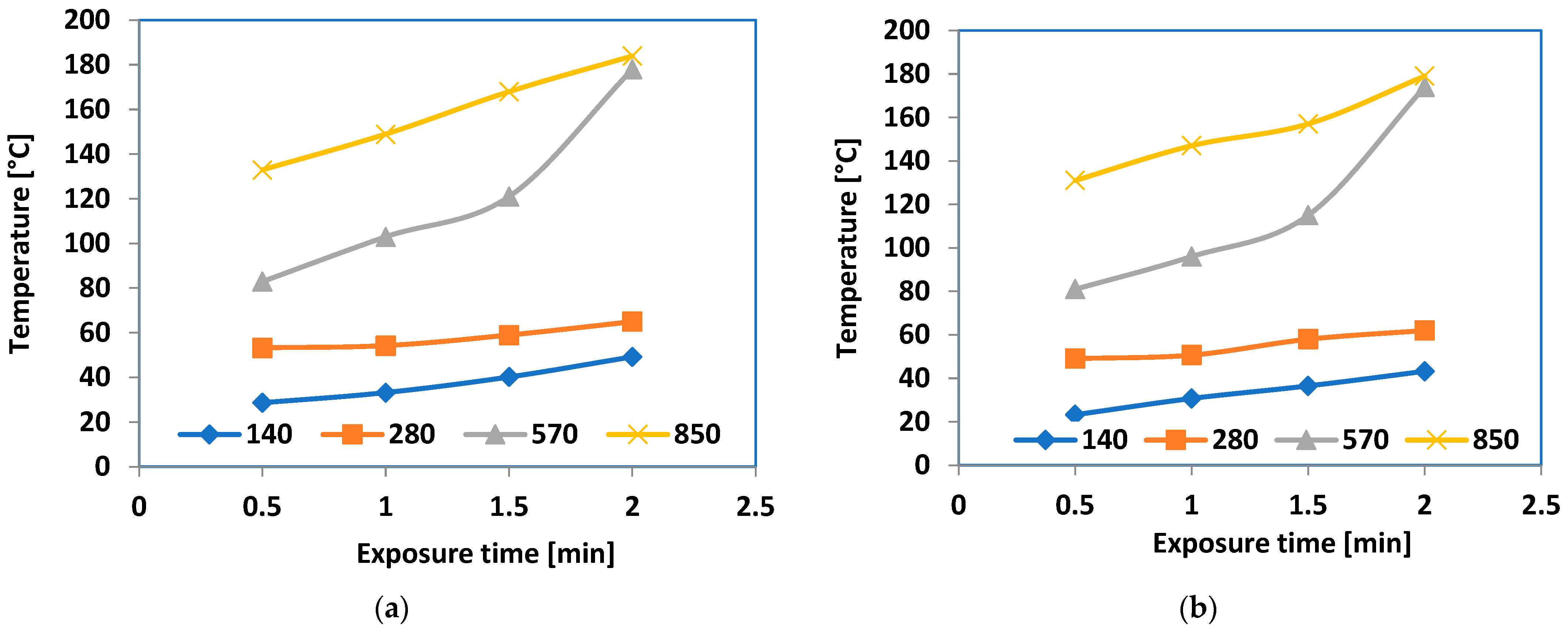
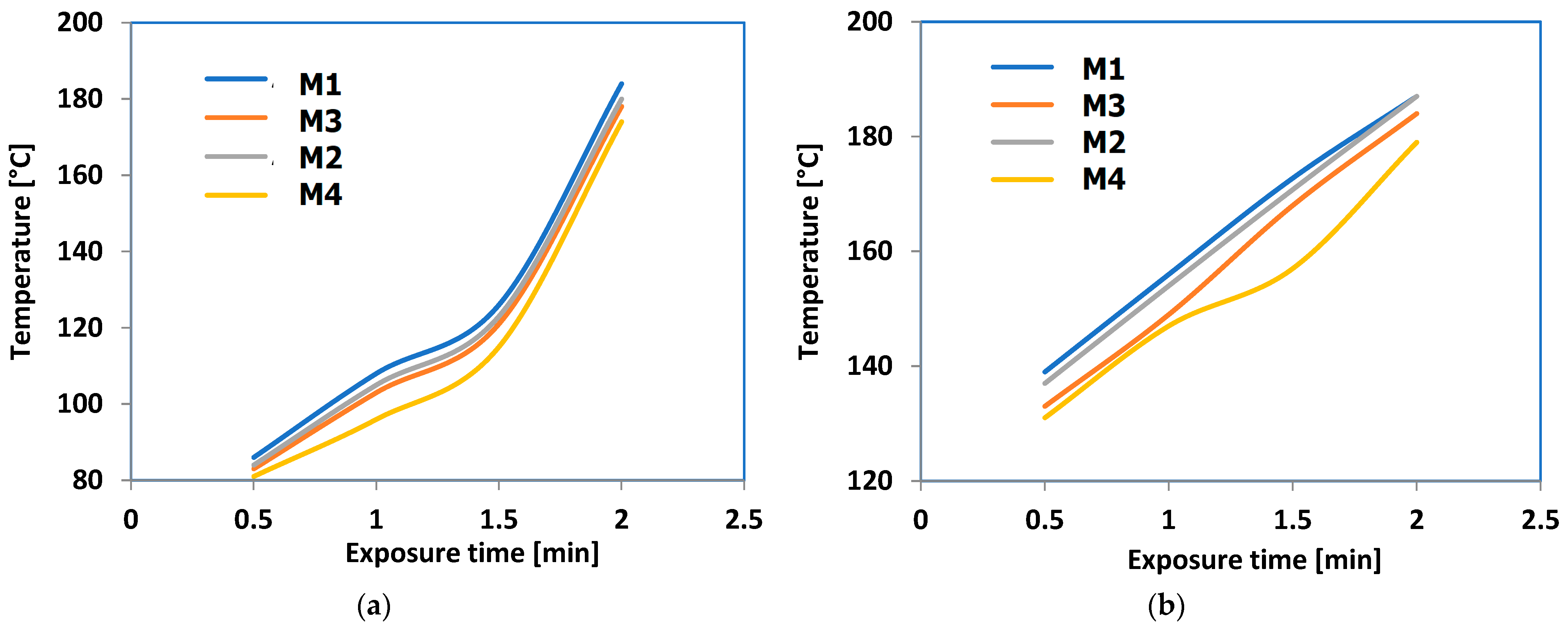

| Heating Zone | 5 | 4 | 3 | 2 | 1 |
|---|---|---|---|---|---|
| rLDPE—Al | 190 | 186 | 180 | 175 | 170 |
| rLDPE—Fe | 195 | 193 | 185 | 180 | 176 |
| Sample Code | Formulation |
|---|---|
| M1 | rLDPE + 7.5% Al/800 nm |
| M2 | rLDPE + 7.5% Al/50 nm |
| M3 | rLDPE + 7.5% Fe/800 nm |
| M4 | rLDPE + 7.5% Fe/50 nm |
| Formula | Z | Concentration | Most Intense Spectral Line | Statistical Measurement Error | Thickness of the Analyzed Layer |
|---|---|---|---|---|---|
| CH2 | - | 92.73% | Organic matter | - | - |
| Al | 13 | 7.19% | Al KA1-HR-Tr | 9.70% | 11.8 μm |
| Formula | Z | Concentration | Most Intense Spectral Line | Statistical Measurement Error | Thickness of the Analyzed Layer |
|---|---|---|---|---|---|
| CH2 | - | 92.64% | Organic matter | - | - |
| Fe | 26 | 7.35% | Fe KA1-HR-Tr | 2.06% | 0.95 μm |
| Sample | HIT (MPa) | HV | EIT (GPa) | S (N/µm) | hmax (µm) | Welastic (µJ) | Wplastic (µJ) | Wtotal (µJ) | ηIT (%) |
|---|---|---|---|---|---|---|---|---|---|
| M1 | 19.2 ± 0.2 | 1.8 ± 0.1 | 0.1 ± 0.01 | 0.04 ± 0.01 | 66.9 ± 0.5 | 16.9 ± 0.1 | 9.3 ± 0.3 | 26.1 ± 0.3 | 65.0 ± 0.6 |
| M2 | 20.0 ± 1.1 | 1.9 ± 0.1 | 0.1 ± 0.02 | 0.04 ± 0.01 | 65.4 ± 0.3 | 16.6 ± 0.3 | 9.1 ± 0.2 | 25.7 ± 0.5 | 64.6 ± 0.2 |
| M3 | 19.0 ± 0.5 | 1.8 ± 0.4 | 0.1 ± 0.02 | 0.04 ± 0.01 | 65.3 ± 0.4 | 15.8 ± 0.2 | 9.6 ± 0.1 | 25.4 ± 0.3 | 62.1 ± 0.2 |
| M4 | 21.2 ± 1.3 | 2.0 ± 0.1 | 0.1 ± 0.01 | 0.04 ± 0.01 | 61.0 ± 1.8 | 14.3 ± 0.4 | 9.0 ± 0.1 | 23.3 ± 0.4 | 61.2 ± 0.5 |
| Sample | Density [g/cm3] |
|---|---|
| M1 | 0.933 |
| M2 | 0.928 |
| M3 | 0.960 |
| M4 | 0.937 |
| Sample | Shore Hardness [MPa] |
|---|---|
| M1 | 58 |
| M2 | 64 |
| M3 | 59 |
| M4 | 69 |
| Sample | Mechanical Resistance [MPa] | Flow Resistance [MPa] | Elongation [%] | Young’s Modulus [GPa] |
|---|---|---|---|---|
| M1 | 5.12 | 0.17 | 133 | 0.01 |
| M2 | 7.42 | 0.14 | 100 | 0.03 |
| M3 | 6.74 | 0.18 | 104 | 0.01 |
| M4 | 8.93 | 0.15 | 96 | 0.03 |
| Sample | 72 h | 168 h | 240 h | 336 h | 408 h | 504 h | 576 h |
|---|---|---|---|---|---|---|---|
| M1 | 4.4147 | 4.5314 | 5.2729 | 5.8699 | 7.3488 | 8.7460 | 8.7460 |
| M2 | 6.1095 | 6.8577 | 7.1886 | 7.5236 | 8.3946 | 10.9999 | 10.9999 |
| M3 | 0.6470 | 2.2700 | 2.6299 | 4.8786 | 6.1141 | 6.6090 | 6.6095 |
| M4 | 1.9535 | 2.8318 | 3.8943 | 6.0172 | 8.0319 | 11.0827 | 11.0844 |
| Sample | 72 h | 168 h | 240 h | 336 h | 408 h | 504 h | 576 h |
|---|---|---|---|---|---|---|---|
| M1 | 7.9868 | 8.6907 | 8.8797 | 9.5472 | 10.4701 | 10.7932 | 10.8056 |
| M2 | 8.1148 | 8.8822 | 9.5250 | 9.6802 | 10.5641 | 10.9785 | 10.9908 |
| M3 | 8.2854 | 9.3585 | 9.5383 | 10.2608 | 10.9119 | 11.0490 | 11.0613 |
| M4 | 9.1654 | 9.6462 | 9.9119 | 10.7267 | 11.0407 | 11.1217 | 11.1341 |
| Sample | Tt (°C) | ΔHt (J/g) | χcr (%) | OOT1 (°C) | OOT2 (°C) |
|---|---|---|---|---|---|
| M1 | 103.7 | 40.1 | 14.9 | 212 | 315 |
| M2 | 104.5 | 36.4 | 13.5 | 209 | 307 |
| M3 | 102.9 | 46.3 | 17.2 | 204 | 251 |
| M4 | 104.5 | 40.1 | 14.3 | 188 | 239 |
| Bonded Items | Hot Melt Sample | Mechanical Resistance [MPa] | Elongation [%] |
|---|---|---|---|
| LDPE + LDPE | M1 | 11.38 | 33 |
| LDPE + HDPE | M1 | 10.84 | 28 |
| LDPE + PP | M1 | 11.11 | 31 |
| HDPE + PP | M1 | 10.75 | 25 |
| PP + PP | M1 | 10.31 | 21 |
| LDPE + LDPE | M3 | 11.12 | 32 |
| LDPE + HDPE | M3 | 10.78 | 27 |
| LDPE + PP | M3 | 10.93 | 28 |
| HDPE + PP | M3 | 10.83 | 24 |
| PP + PP | M3 | 10.68 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, R.C.; Aradoaei, M.; Ursan, G.A. Thermoplastic Composite Hot-Melt Adhesives with Metallic Nano-Particles for Reversible Bonding Techniques Utilizing Microwave Energy. Polymers 2024, 16, 3496. https://doi.org/10.3390/polym16243496
Ciobanu RC, Aradoaei M, Ursan GA. Thermoplastic Composite Hot-Melt Adhesives with Metallic Nano-Particles for Reversible Bonding Techniques Utilizing Microwave Energy. Polymers. 2024; 16(24):3496. https://doi.org/10.3390/polym16243496
Chicago/Turabian StyleCiobanu, Romeo Cristian, Mihaela Aradoaei, and George Andrei Ursan. 2024. "Thermoplastic Composite Hot-Melt Adhesives with Metallic Nano-Particles for Reversible Bonding Techniques Utilizing Microwave Energy" Polymers 16, no. 24: 3496. https://doi.org/10.3390/polym16243496
APA StyleCiobanu, R. C., Aradoaei, M., & Ursan, G. A. (2024). Thermoplastic Composite Hot-Melt Adhesives with Metallic Nano-Particles for Reversible Bonding Techniques Utilizing Microwave Energy. Polymers, 16(24), 3496. https://doi.org/10.3390/polym16243496





