NIPAm Microgels Synthesised in Water: Tailored Control of Particles’ Size and Thermoresponsive Properties
Abstract
1. Introduction
2. Materials and Methods
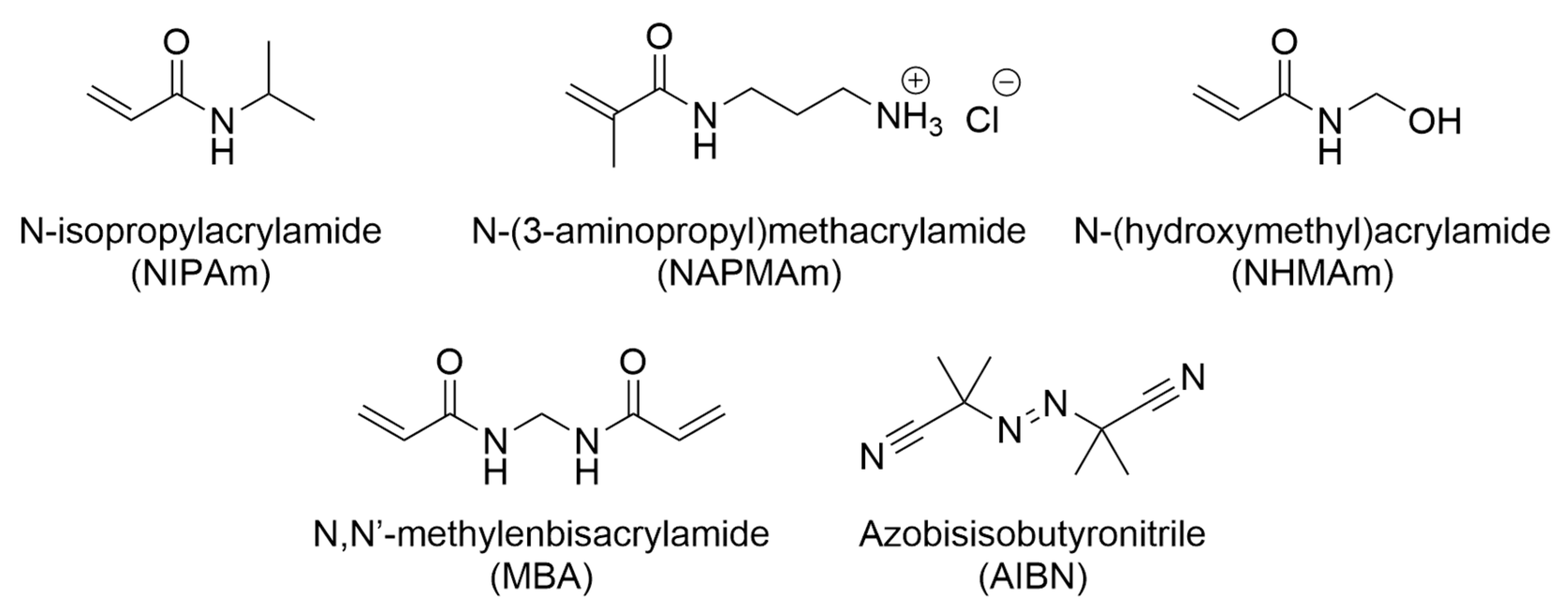
2.1. Materials
2.2. Synthesis of Microgels in Water
2.3. Characterisation of Microgels
2.3.1. Monomer Conversion via 1H-NMR
2.3.2. Size Measurements via DLS
2.3.3. VPTT Experiments
3. Results and Discussion
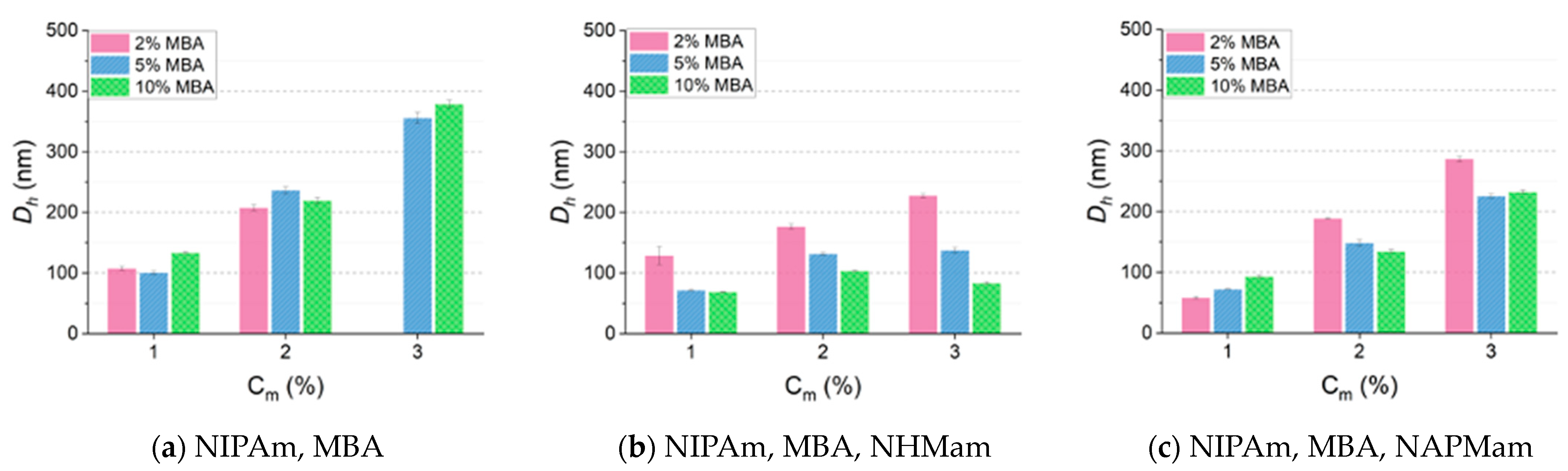
3.1. Effect of Total Monomer Concentration (Cm) on Particle Size
3.2. The Role of Hydrogen Bonding in Controlling Particle Size
3.3. The Role of Charge in Controlling Particle Size
3.4. Impact of Crosslinking Degree and Functional Groups on the Thermoresponsive Properties of NIPAm-Based Microgels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, S.; Avais, M.; Chattopadhyay, S. Microgels as Smart Polymer Colloids for Sensing and Environmental Remediation. ACS Appl. Polym. Mater. 2023, 5, 1626–1645. [Google Scholar] [CrossRef]
- Ahmad, Z.; Bashir, M.; Begum, R.; Irfan, A.; Chaudhry, A.R.; Farooqi, Z.H. Microgel Stabilized Palladium Nanostructures for Catalytic Applications. Mol. Catal. 2024, 559, 114061. [Google Scholar] [CrossRef]
- Papadimitriou, S.A.; Robin, M.P.; Ceric, D.; O’Reilly, R.K.; Marino, S.; Resmini, M. Fluorescent Polymeric Nanovehicles for Neural Stem Cell Modulation. Nanoscale 2016, 8, 17340–17349. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Tapase, S.R.; Kodam, K.M.; Shinde, V.S. Thermoresponsive Pluronic Based Microgels for Controlled Release of Curcumin against Breast Cancer Cell Line. Colloids Surf. B Biointerfaces 2021, 205, 111834. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Vdovchenko, A.; Pearce, A.K.; Freeley, M.; O’Reilly, R.K.; Resmini, M. Effect of Heterogeneous and Homogeneous Polymerisation on the Structure of pNIPAm Nanogels. Polym. Chem. 2021, 12, 6854–6864. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The Development of Microgels/Nanogels for Drug Delivery Applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- da Fonseca, C.O.; Simão, M.; Lins, I.R.; Caetano, R.O.; Futuro, D.; Quirico-Santos, T. Efficacy of Monoterpene Perillyl Alcohol upon Survival Rate of Patients with Recurrent Glioblastoma. J. Cancer Res. Clin. Oncol. 2011, 137, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic Concepts and Recent Advances in Nanogels as Carriers for Medical Applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jiang, C. Stimuli-Responsive Drug Delivery Systems Triggered by Intracellular or Subcellular Microenvironments. Adv. Drug Deliv. Rev. 2023, 196, 114773. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Koide, H.; Yoshimatsu, K.; Hoshino, Y.; Lee, S.-H.; Okajima, A.; Ariizumi, S.; Narita, Y.; Yonamine, Y.; Weisman, A.C.; Nishimura, Y.; et al. A Polymer Nanoparticle with Engineered Affinity for a Vascular Endothelial Growth Factor (VEGF165). Nat. Chem. 2017, 9, 715–722. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Switacz, V.K.; Wypysek, S.K.; Degen, R.; Crassous, J.J.; Spehr, M.; Richtering, W. Influence of Size and Cross-Linking Density of Microgels on Cellular Uptake and Uptake Kinetics. Biomacromolecules 2020, 21, 4532–4544. [Google Scholar] [CrossRef]
- Bilardo, R.; Traldi, F.; Vdovchenko, A.; Resmini, M. Influence of Surface Chemistry and Morphology of Nanoparticles on Protein Corona Formation. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol. 2022, 14, e1788. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Thickett, S.C.; Perrier, S.; Bourgeat-Lami, E.; Lansalot, M. Controlled/Living Radical Polymerisation in Dispersed Systems: An Update. Chem. Rev. 2015, 115, 9745–9800. [Google Scholar] [CrossRef]
- Parkatzidis, K.; Wang, H.S.; Truong, N.P.; Anastasaki, A. Recent Developments and Future Challenges in Controlled Radical Polymerisation: A 2020 Update. Chem 2020, 6, 1575–1588. [Google Scholar] [CrossRef]
- Fukuda, T.; Yoshikawa, C.; Kwak, Y.; Goto, A.; Tsujii, Y. Mechanisms and Kinetics of Living Radical Polymerisation: Absolute Comparison of Theory and Experiment. In Advances in Controlled/Living Radical Polymerisation; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2003; Volume 854, pp. 24–39. ISBN 978-0-8412-3854-1. [Google Scholar]
- Nishizawa, Y.; Minato, H.; Inui, T.; Uchihashi, T.; Suzuki, D. Nanostructures, Thermoresponsiveness, and Assembly Mechanism of Hydrogel Microspheres during Aqueous Free-Radical Precipitation Polymerisation. Langmuir 2021, 37, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Fandrich, P.; Wiehemeier, L.; Dirksen, M.; Wrede, O.; Kottke, T.; Hellweg, T. Acrylamide Precipitation Polymerisation in a Continuous Flow Reactor: An in Situ FTIR Study Reveals Kinetics. Colloid Polym. Sci. 2021, 299, 221–232. [Google Scholar] [CrossRef]
- El-Halah, A.; González, N.; Contreras, J.; López-Carrasquero, F. Effect of the Synthesis Solvent in Swelling Ability of Polyacrylamide Hydrogels. J. Polym. Res. 2019, 27, 21. [Google Scholar] [CrossRef]
- Alvarado Mendoza, A.G.; Zárate-Navarro, M.A.; Ceja, I.; Cortés-Ortega, J.A. The Effect of Water/Ethanol Ratio and Monomer Concentration on the Size and Morphology of Polyacrylamide Nanogels in a Surfactant-Free Synthesis. J. Macromol. Sci. Part A 2020, 57, 91–97. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Minato, H.; Inui, T.; Saito, I.; Kureha, T.; Shibayama, M.; Uchihashi, T.; Suzuki, D. Nanostructure and Thermoresponsiveness of Poly(N-Isopropyl Methacrylamide)-Based Hydrogel Microspheres Prepared via Aqueous Free Radical Precipitation Polymerisation. RSC Adv. 2021, 11, 13130–13137. [Google Scholar] [CrossRef]
- Sun, H.; Zielinska, K.; Resmini, M.; Zarbakhsh, A. Interactions of NIPAM Nanogels with Model Lipid Multi-Bilayers: A Neutron Reflectivity Study. J. Colloid Interface Sci. 2019, 536, 598–608. [Google Scholar] [CrossRef]
- Traldi, F.; Liu, P.; Albino, I.; Ferreira, L.; Zarbakhsh, A.; Resmini, M. Protein-Nanoparticle Interactions Govern the Interfacial Behavior of Polymeric Nanogels: Study of Protein Corona Formation at the Air/Water Interface. Int. J. Mol. Sci. 2023, 24, 2810. [Google Scholar] [CrossRef] [PubMed]
- Bilardo, R.; Traldi, F.; Brennan, C.H.; Resmini, M. The Role of Crosslinker Content of Positively Charged NIPAM Nanogels on the In Vivo Toxicity in Zebrafish. Pharmaceutics 2023, 15, 1900. [Google Scholar] [CrossRef]
- Anastasiadi, R.-M.; Traldi, F.; Resmini, M. Imidazole-Based Monomer as Functional Unit for the Specific Detection of Paraxanthine in Aqueous Environments. Chemosensors 2022, 10, 301. [Google Scholar] [CrossRef]
- Mazzali, D.; Rath, G.; Röntgen, A.; Chowdhury, V.R.; Vendruscolo, M.; Resmini, M. Sustainable and Surfactant-Free Synthesis of Negatively Charged Acrylamide Nanogels for Biomedical Applications. ChemRxiv 2024, preprint. [Google Scholar]
- Ramasamy, T.; Ruttala, H.; Kanu, B.; Poudel, B.; Choi, H.-G.; Yong, C.; Kim, J. Smart Chemistry-Based Nanosized Drug Delivery Systems for Systemic Applications: A Comprehensive Review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Yu, B.; Hu, H.; Xu, F.-J. Polysaccharide-Based Tumor Microenvironment-Responsive Drug Delivery Systems for Cancer Therapy. J. Control. Release 2023, 362, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Sharma, G. Nanogel: A Versatile Drug Delivery System for the Treatment of Various Diseases and Their Future Perspective. Drug Deliv. Transl. Res. 2024, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Özcan Bülbül, E.; Okur, M.E.; Karantas, I.D.; Üstündağ Okur, N. The Application of Nanogels as Efficient Drug Delivery Platforms for Dermal/Transdermal Delivery. Gels 2023, 9, 753. [Google Scholar] [CrossRef]
- Lu, I.-L.; Yu, T.-W.; Liu, T.-I.; Chen, H.-H.; Yang, Y.-C.; Lo, C.-L.; Wang, C.-Y.; Chiu, H.-C. Microfluidized Dextran Microgels Loaded with Cisplatin/SPION Lipid Nanotherapeutics for Local Colon Cancer Treatment via Oral Administration. Adv. Healthc. Mater. 2022, 11, 2201140. [Google Scholar] [CrossRef]
- Wypysek, S.K.; Centeno, S.P.; Gronemann, T.; Wöll, D.; Richtering, W. Hollow, pH-Sensitive Microgels as Nanocontainers for the Encapsulation of Proteins. Macromol. Biosci. 2023, 23, 2200456. [Google Scholar] [CrossRef]
- Palmese, L.L.; LeValley, P.J.; Pradhan, L.; Parsons, A.L.; Oakey, J.S.; Abraham, M.; D’Addio, S.M.; Kloxin, A.M.; Liang, Y.; Kiick, K.L. Injectable Liposome-Containing Click Hydrogel Microparticles for Release of Macromolecular Cargos. Soft Matter 2024, 20, 1736–1745. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Liu, W.; Liu, Y.; Li, A.; Qiu, D.; Zheng, X.; Gu, Q. 3D Bioprinting Microgels to Construct Implantable Vascular Tissue. Cell Prolif. 2023, 56, e13456. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Y.; Luan, T.; Shi, P.; Guo, L.; Zhang, Q.; Shi, G.; Hao, Z.; Chen, T.; Zhang, L.; et al. Hydrogel and Microgel Collaboration for Spatiotemporal Delivery of Biofactors to Awaken Nucleus Pulposus-Derived Stem Cells for Endogenous Repair of Disc. Small 2024, 20, 2404732. [Google Scholar] [CrossRef]
- Pruett, L.; Ellis, R.; McDermott, M.; Roosa, C.; Griffin, D. Spatially Heterogeneous Epidermal Growth Factor Release from Microporous Annealed Particle (MAP) Hydrogel for Improved Wound Closure. J. Mater. Chem. B 2021, 9, 7132–7139. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Wang, L.; Zhao, B.; Ma, Y.; Zhang, N.; Chen, W.; Huang, D. Size-Tailored and Acid-Degradable Polyvinyl Alcohol Microgels for Inhalation Therapy of Bacterial Pneumonia. J. Mater. Chem. B 2024, 12, 9325–9334. [Google Scholar] [CrossRef] [PubMed]
- Kanojiya, P.S.; Wadetwar, R.N.; Atole, P.G.; Thakrani, K.C.; Gawande, N.P. Sustained Delivery of Statistically Optimized Transfersomal Gel of Miconazole Nitrate for Vaginal Candidiasis. J. Dispers. Sci. Technol. 2023, 1–18. [Google Scholar] [CrossRef]
- Hamami, R.; Simaan-Yameen, H.; Gargioli, C.; Seliktar, D. Comparison of Four Different Preparation Methods for Making Injectable Microgels for Tissue Engineering and Cell Therapy. Regen. Eng. Transl. Med. 2022, 8, 615–629. [Google Scholar] [CrossRef]
- Hoque, J.; Zeng, Y.; Newman, H.; Gonzales, G.; Lee, C.; Varghese, S. Microgel-Assisted Delivery of Adenosine to Accelerate Fracture Healing. ACS Biomater. Sci. Eng. 2022, 8, 4863–4872. [Google Scholar] [CrossRef]
- Moghaddam, M.M.; Jooybar, E.; Imani, R.; Ehrbar, M. Development of Injectable Microgel-Based Scaffolds via Enzymatic Cross-Linking of Hyaluronic Acid-Tyramine/Gelatin-Tyramine for Potential Bone Tissue Engineering. Int. J. Biol. Macromol. 2024, 279, 135176. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermoresponsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Saunders, B.R.; Vincent, B. Microgel Particles as Model Colloids: Theory, Properties and Applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Liu, P.; Pearce, C.M.; Anastasiadi, R.-M.; Resmini, M.; Castilla, A.M. Covalently Crosslinked Nanogels: An NMR Study of the Effect of Monomer Reactivity on Composition and Structure. Polymers 2019, 11, 353. [Google Scholar] [CrossRef]
- Wan, T.; Xiong, J.; Zhao, Q.; Wu, D.; Tang, L.; Liao, L.; Chen, Q. Crosslinker Effects on Swelling and Gel Properties of pH- and Temperature-Responsive Poly (NIPAM/IA/AM) Hydrogels. Polym. Bull. 2016, 73, 1447–1458. [Google Scholar] [CrossRef]
- Lee, S.-H.; Hoshino, Y.; Randall, A.; Zeng, Z.; Baldi, P.; Doong, R.; Shea, K.J. Engineered Synthetic Polymer Nanoparticles as IgG Affinity Ligands. J. Am. Chem. Soc. 2012, 134, 15765–15772. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Mulmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryaskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Virtanen, O.L.J.; Richtering, W. Kinetics and Particle Size Control in Non-Stirred Precipitation Polymerisation of N-Isopropylacrylamide. Colloid Polym. Sci. 2014, 292, 1743–1756. [Google Scholar] [CrossRef]
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-3-642-85135-3. [Google Scholar]
- Dash, A.; Singh, S. Pharmaceutics: Basic Principles and Application to Pharmacy Practice; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-323-99797-3. [Google Scholar]
- Saadat, M.; Zahednezhad, F.; Zakeri-Milani, P.; Reza Heidari, H.; Shahbazi-Mojarrad, J.; Valizadeh, H. Drug Targeting Strategies Based on Charge Dependent Uptake of Nanoparticles into Cancer Cells. J. Pharm. Pharm. Sci. 2019, 22, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Zhang, C.; Bajpayee, A.G. Overcoming Negatively Charged Tissue Barriers: Drug Delivery Using Cationic Peptides and Proteins. Nano Today 2020, 34, 100898. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef]
- Vedadghavami, A.; He, T.; Zhang, C.; Amiji, S.M.; Hakim, B.; Bajpayee, A.G. Charge-Based Drug Delivery to Cartilage: Hydrophobic and Not Electrostatic Interactions Are the Dominant Cause of Competitive Binding of Cationic Carriers in Synovial Fluid. Acta Biomater. 2022, 151, 278–289. [Google Scholar] [CrossRef]
- Zielińska, K.; Sun, H.; Campbell, R.A.; Zarbakhsh, A.; Resmini, M. Smart Nanogels at the Air/Water Interface: Structural Studies by Neutron Reflectivity. Nanoscale 2016, 8, 4951–4960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Furyk, S.; Sagle, L.B.; Cho, Y.; Bergbreiter, D.E.; Cremer, P.S. Effects of Hofmeister Anions on the LCST of PNIPAM as a Function of Molecular Weight. J. Phys. Chem. C 2007, 111, 8916–8924. [Google Scholar] [CrossRef]
- Tymetska, S.; Shymborska, Y.; Stetsyshyn, Y.; Budkowski, A.; Bernasik, A.; Awsiuk, K.; Donchak, V.; Raczkowska, J. Thermoresponsive Smart Copolymer Coatings Based on P(NIPAM-Co-HEMA) and P(OEGMA-Co-HEMA) Brushes for Regenerative Medicine. ACS Biomater. Sci. Eng. 2023, 9, 6256–6272. [Google Scholar] [CrossRef]

| Code | NIPAm (molar%) | NAPMAm (molar%) | NHMAm (molar%) | MBA (molar%) | Size by Intensity (nm) | Size by Number (nm) | PDI | ζ-Potential (mV) | VPTT (°C) |
|---|---|---|---|---|---|---|---|---|---|
| MG-X2 | 98 | 0 | 0 | 2 | 168 | 108 | 0.165 | - | 33.5 |
| MG-X5 | 95 | 0 | 0 | 5 | 147 | 114 | 0.036 | - | 34.0 |
| MG-X10 | 90 | 0 | 0 | 10 | 162 | 134 | 0.020 | - | 34.5 |
| MG-X2O5 | 93 | 0 | 5 | 2 | 197 | 149 | 0.067 | - | 35.5 |
| MG-X5O5 | 90 | 0 | 5 | 5 | 193 | 132 | 0.026 | - | 36.0 |
| MG-X10O5 | 85 | 0 | 5 | 10 | 141 | 104 | 0.019 | - | 36.5 |
| MG-X2N5 | 93 | 5 | 0 | 2 | 246 | 189 | 0.143 | +3.9 | - |
| MG-X5N5 | 90 | 5 | 0 | 5 | 194 | 149 | 0.101 | +5.0 | - |
| MG-X10N5 | 85 | 5 | 0 | 10 | 185 | 135 | 0.096 | +7.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rath, G.; Mazzali, D.; Zarbakhsh, A.; Resmini, M. NIPAm Microgels Synthesised in Water: Tailored Control of Particles’ Size and Thermoresponsive Properties. Polymers 2024, 16, 3532. https://doi.org/10.3390/polym16243532
Rath G, Mazzali D, Zarbakhsh A, Resmini M. NIPAm Microgels Synthesised in Water: Tailored Control of Particles’ Size and Thermoresponsive Properties. Polymers. 2024; 16(24):3532. https://doi.org/10.3390/polym16243532
Chicago/Turabian StyleRath, Gabriela, Davide Mazzali, Ali Zarbakhsh, and Marina Resmini. 2024. "NIPAm Microgels Synthesised in Water: Tailored Control of Particles’ Size and Thermoresponsive Properties" Polymers 16, no. 24: 3532. https://doi.org/10.3390/polym16243532
APA StyleRath, G., Mazzali, D., Zarbakhsh, A., & Resmini, M. (2024). NIPAm Microgels Synthesised in Water: Tailored Control of Particles’ Size and Thermoresponsive Properties. Polymers, 16(24), 3532. https://doi.org/10.3390/polym16243532







