Diamine Crosslinked Addition-Type Diblock Poly(Norbornene)s-Based Anion Exchange Membranes with High Conductivity and Stability for Fuel Cell Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of NB-O-Br Monomer
2.3. Synthesis of NB-O-Hex Monomer
2.4. Synthesis of Allyl Palladium Chloride Complex Catalyst ((η3-allyl)Pd(Cl)PPh3)
2.5. Synthesis of Addition-Type Norbornene-Based Diblock Copolymer (aP(NB-O-Br-b-NB-O-Hex))
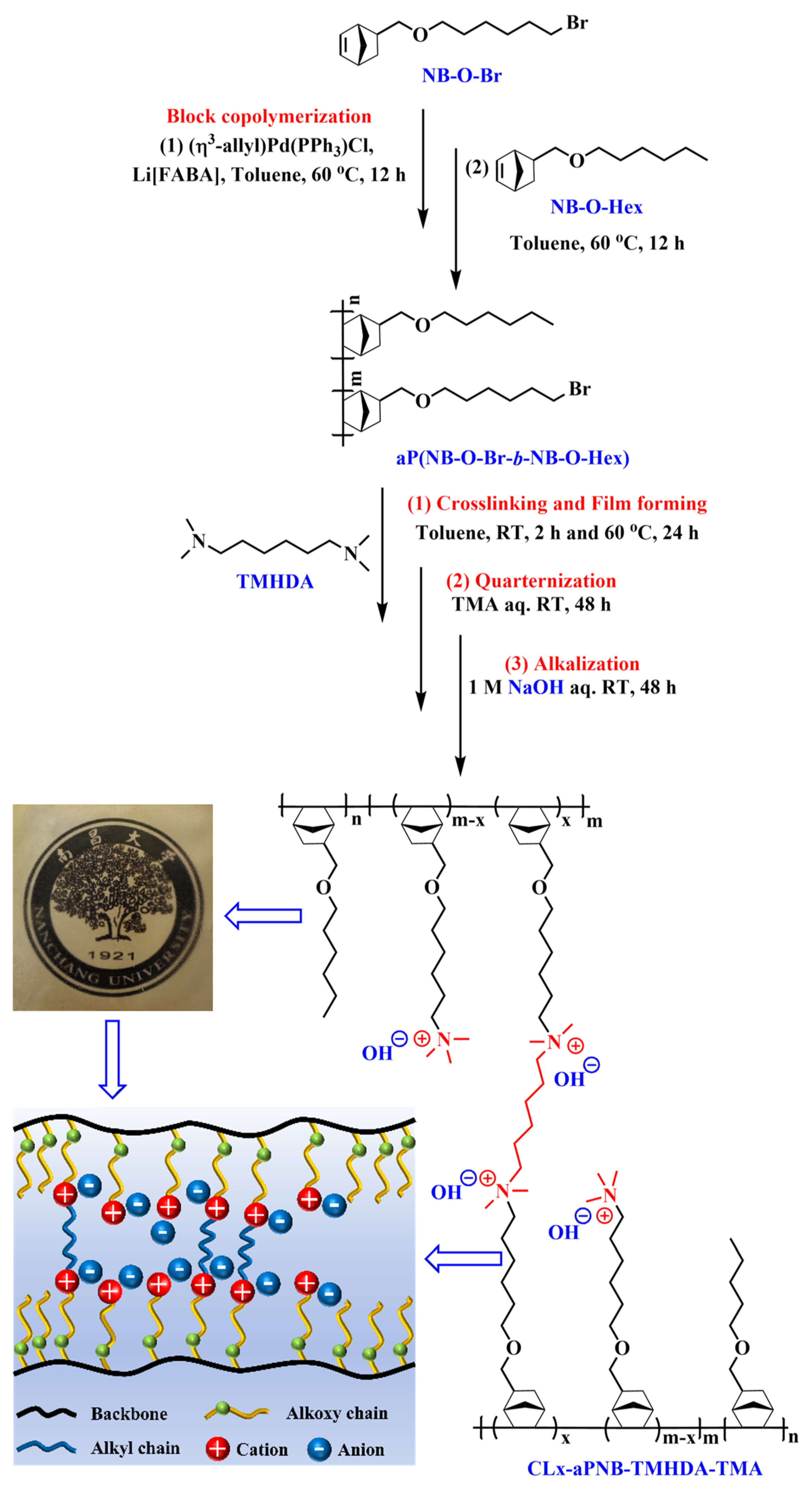
2.6. Preparation of Diamine Crosslinked Addition-Type Diblock Poly(Norbornene)s-Based AEMs (CLx-aPNB-TMHDA-TMA)
2.7. Preparation of Non-Crosslinked Addition-Type Diblock Poly(Norbornene)s-Based AEMs (aPNB-TMA)
2.8. Characterization and Measurements
3. Results and Discussion
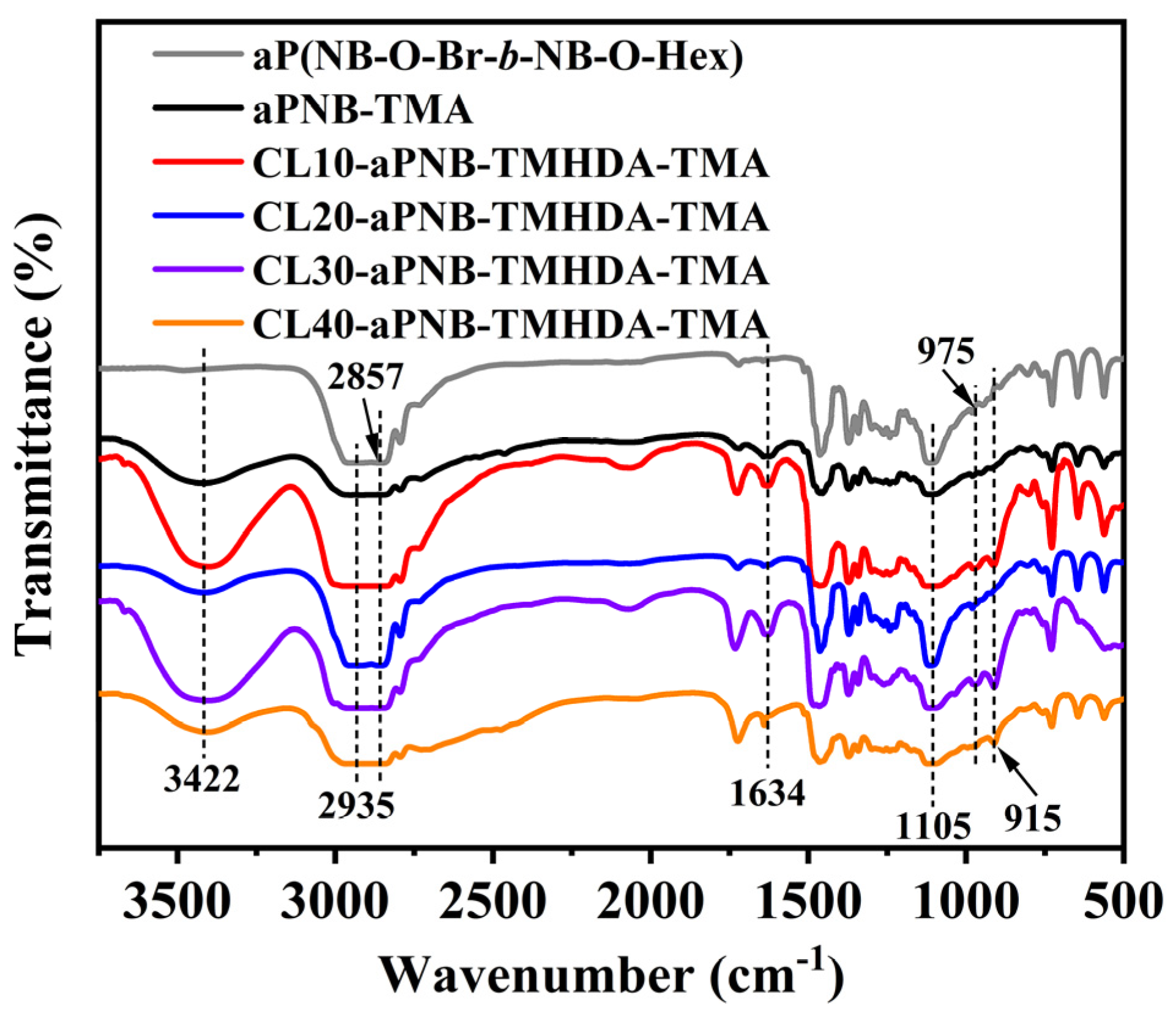
3.1. Structure Analysis
3.2. Solubility of Copolymers and Diamine Crosslinked AEMs
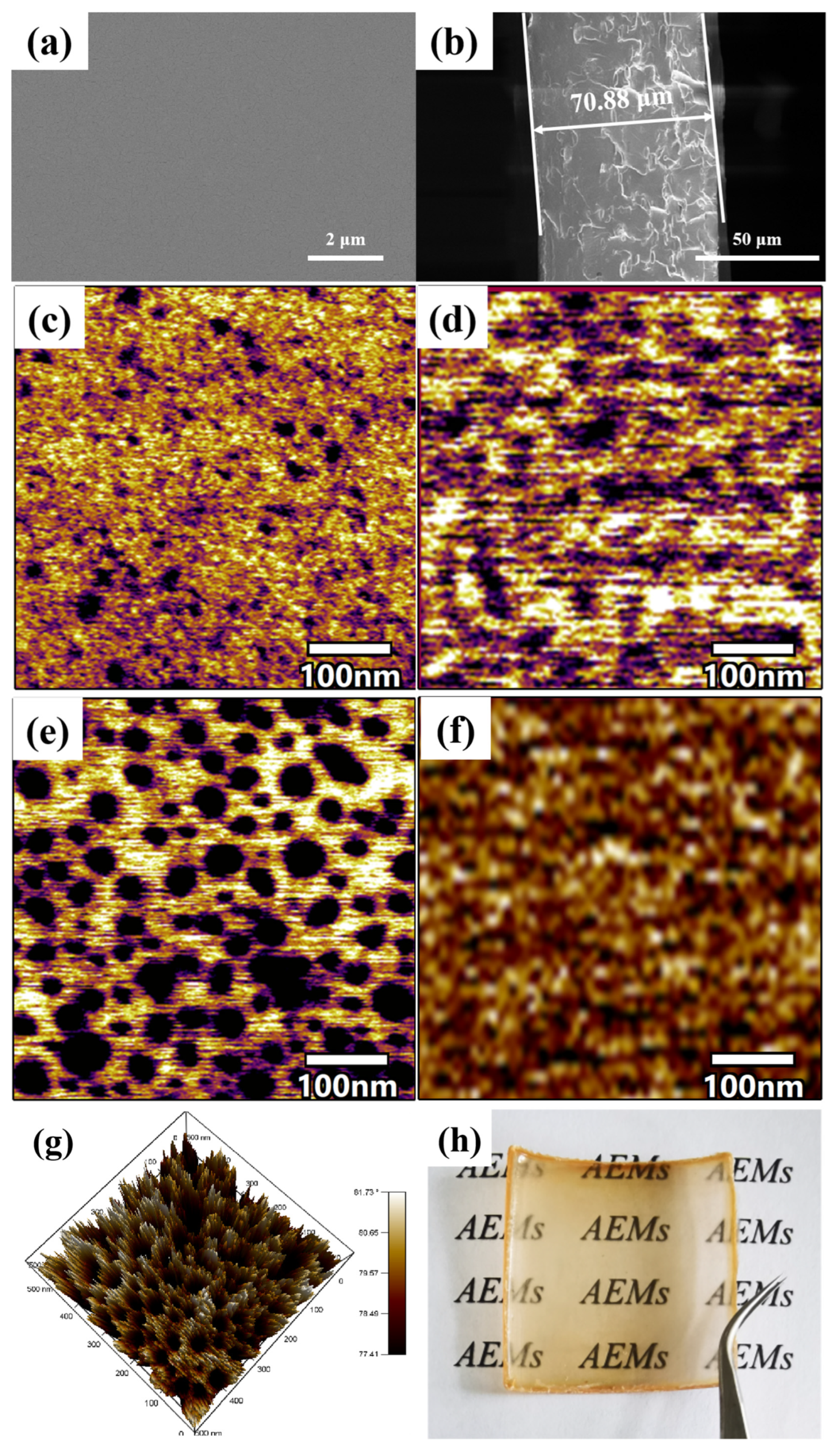
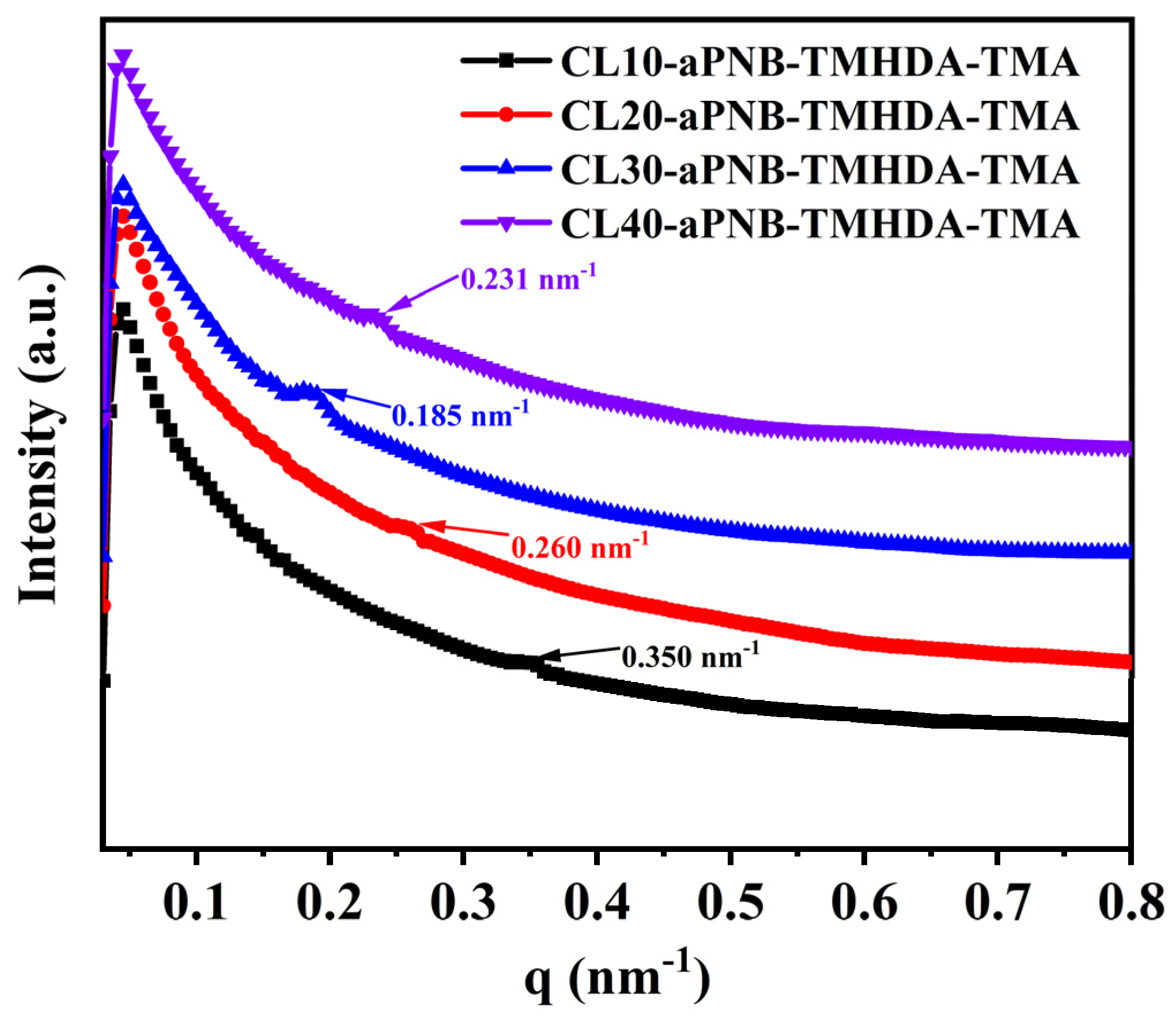
3.3. Morphology Characterization
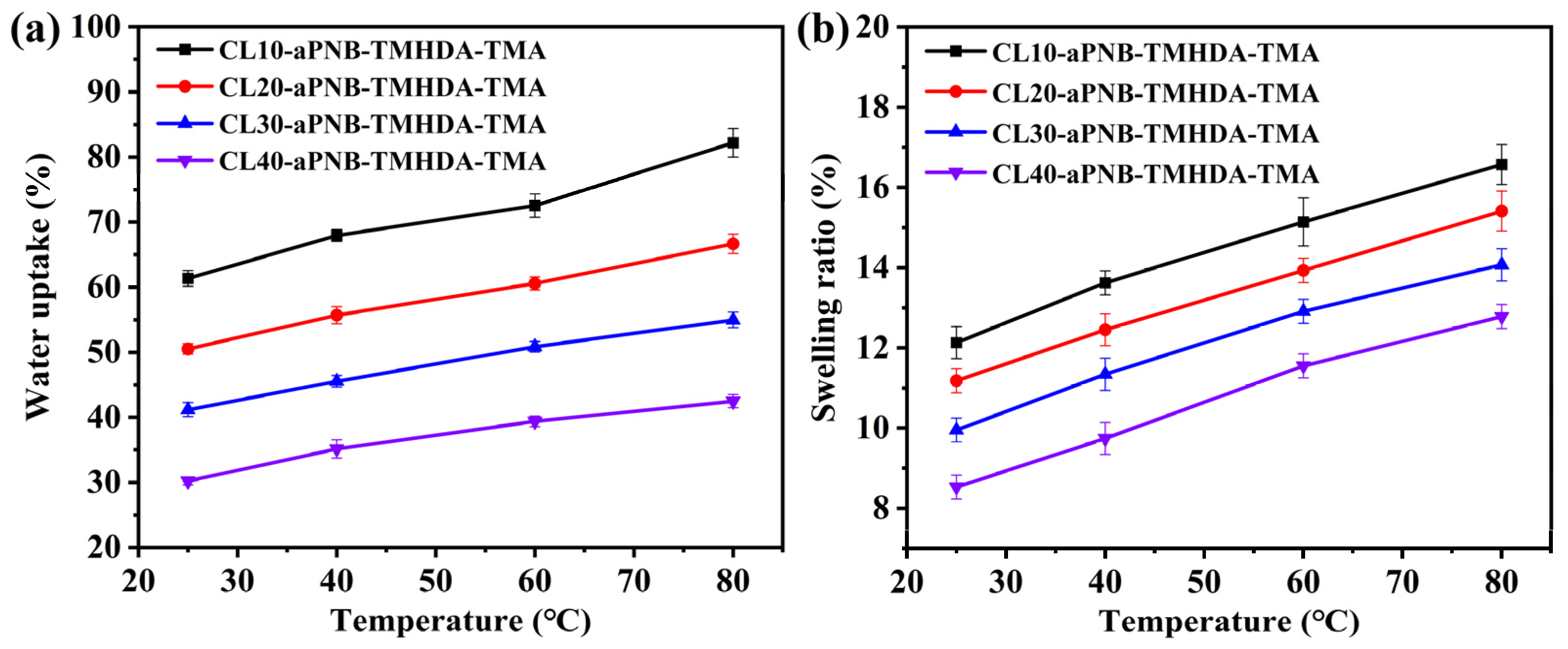
3.4. Ion Exchange Capacity, Water Uptake, Swelling Ratio, and Hydration Number
3.5. Ionic Conductivity
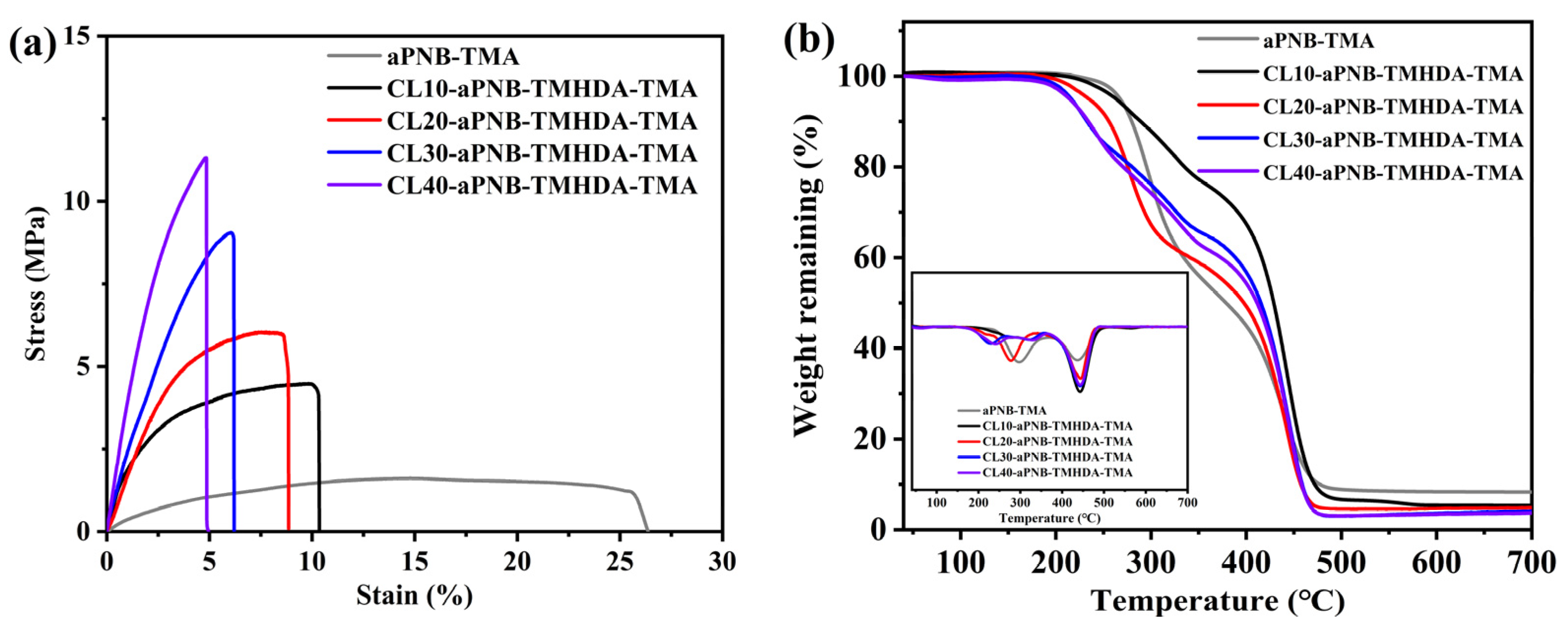
3.6. Mechanical Properties and Thermal Stability
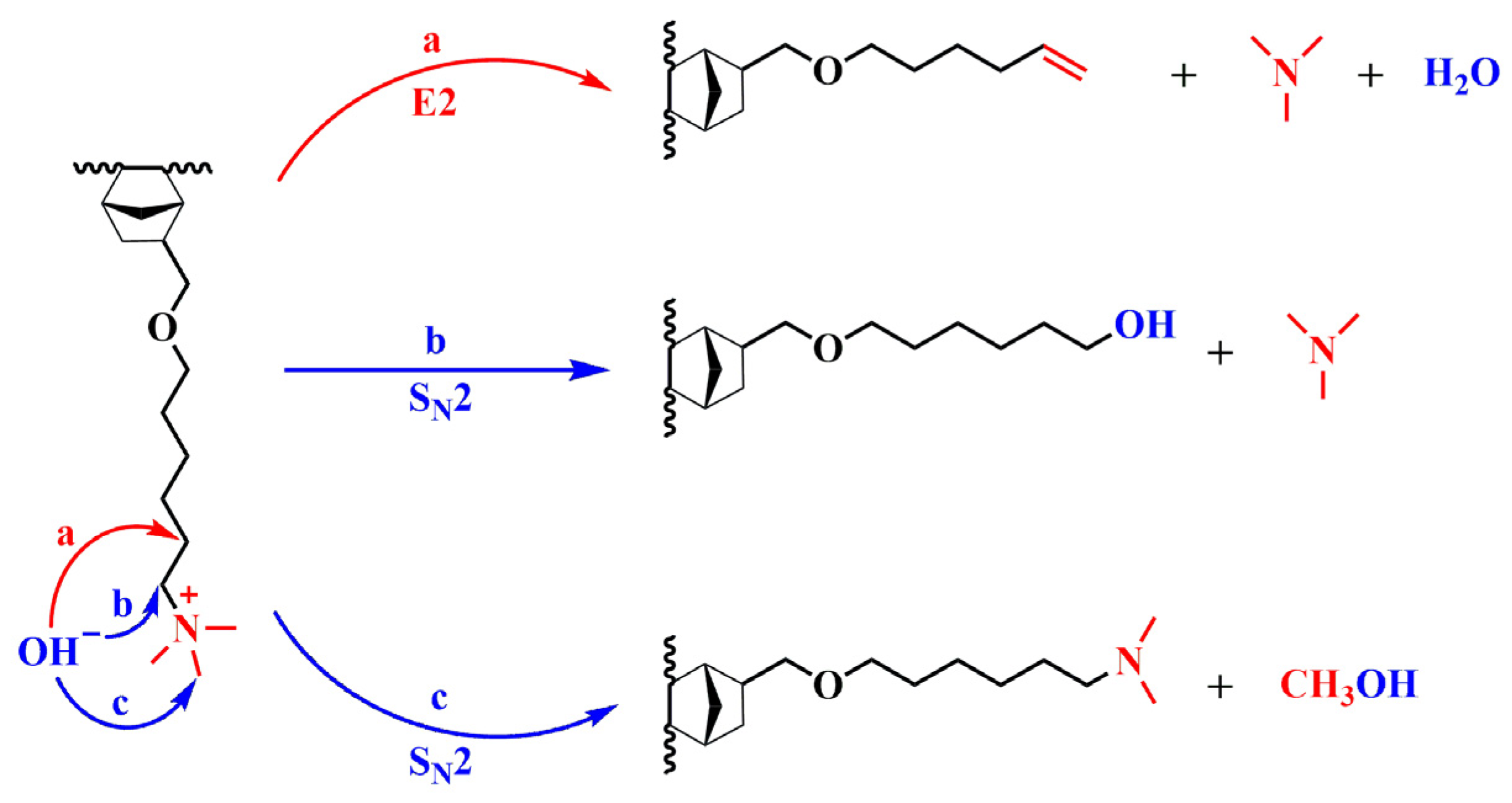
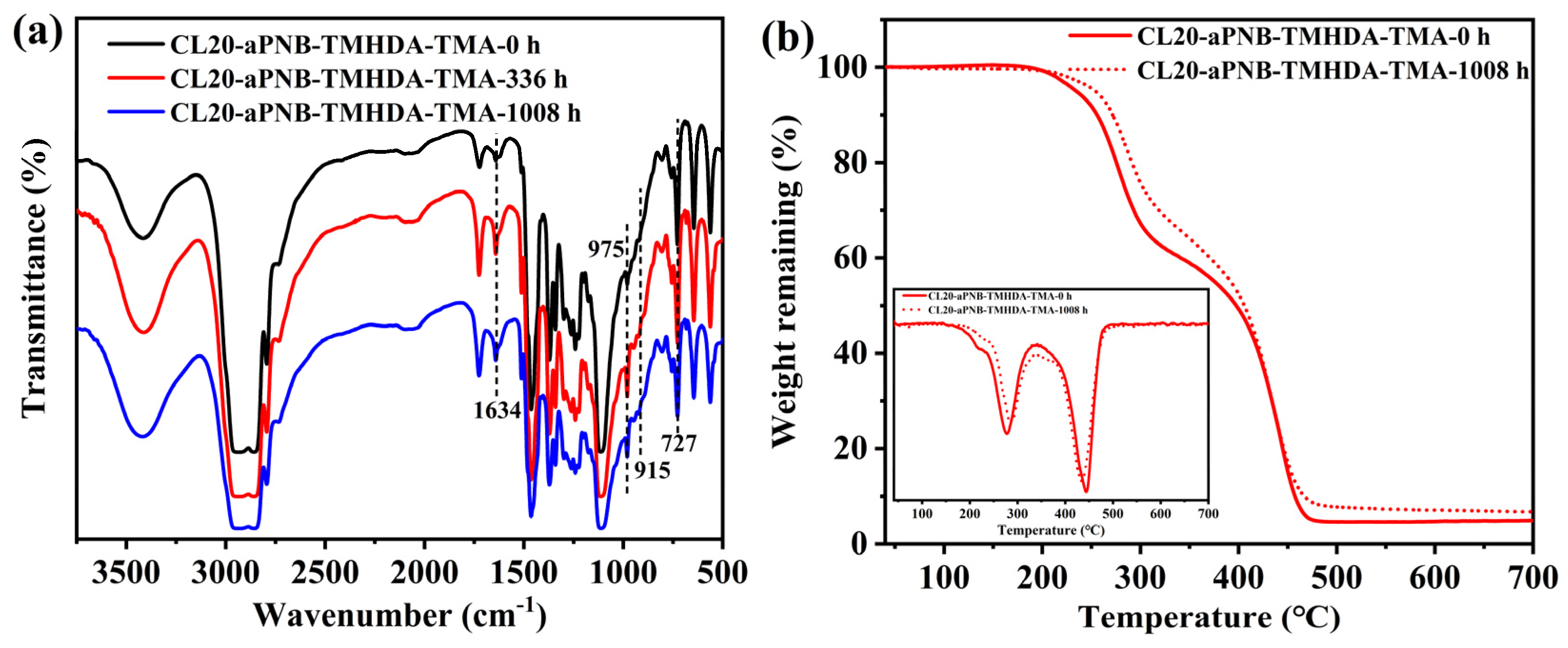
3.7. Alkaline and Oxidative Stability
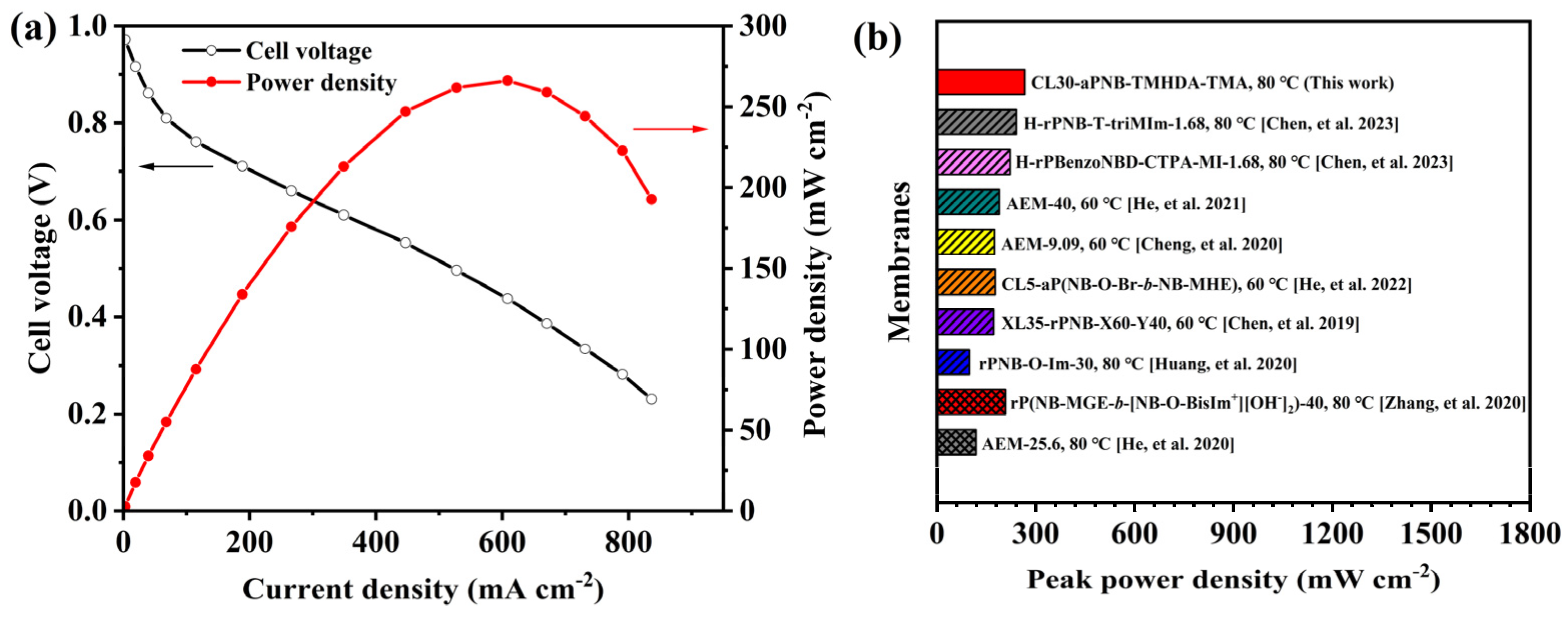
3.8. Single Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Peltier, C.R.; Zeng, R.; Schimmenti, R.; Li, Q.H.; Huang, X.; Yan, Z.F.; Potsi, G.; Selhorst, R.; Lu, X.Y.; et al. Electrocatalysis in Alkaline Media and Alkaline Membrane-Based Energy Technologies. Chem. Rev. 2022, 122, 6117–6321. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.D.; Zhang, J.F.; Liu, X.; Huang, T.; Jiang, H.F.; Yin, Y.; Qin, Y.Z.; Guiver, M.D. Toward alkaline-stable anion exchange membranes in fuel cells: Cycloaliphatic quaternary ammonium-based anion conductors. Electrochem. Energy Rev. 2022, 5, 348–400. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, T.; Setzler, B.P.; Abbasi, R.; Wang, J.H.; Yan, Y.S. A High-Performance Gas-Fed Direct Ammonia Hydroxide Exchange Membrane Fuel Cell. ACS Energy Lett. 2021, 6, 1996–2002. [Google Scholar] [CrossRef]
- Cao, H.X.; Pan, J.; Zhu, H.R.; Sun, Z.; Wang, B.W.; Zhao, J.L.; Yan, F. Interaction Regulation Between Ionomer Binder and Catalyst: Active Triple-Phase Boundary and High Performance Catalyst Layer for Anion Exchange Membrane Fuel Cells. Adv. Sci. 2021, 8, 2101744. [Google Scholar] [CrossRef]
- Zion, N.; Douglin, J.C.; Cullen, D.A.; Zelenay, P.; Dekel, D.R.; Elbaz, L. Porphyrin Aerogel Catalysts for Oxygen Reduction Reaction in Anion-Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2021, 31, 2100963. [Google Scholar] [CrossRef]
- Pan, Z.F.; An, L.; Zhao, T.S.; Tang, Z.K. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141–175. [Google Scholar] [CrossRef]
- Du, X.M.; Wang, Z.; Zhang, H.Y.; Liu, W.C.; Xu, J.M. Constructing micro-phase separation structure by multi-arm side chains to improve the property of anion exchange membrane. Int. J. Hydrogen Energy 2020, 45, 17916–17926. [Google Scholar] [CrossRef]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Dekel, D.R.; Willdorf, S.; Ash, U.; Amar, M.; Pusara, S.; Dhara, S.; Srebnik, S.; Diesendruck, C.E. The critical relation between chemical stability of cations and water in anion exchange membrane fuel cells environment. J. Power Sources 2018, 375, 351–360. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Q.G.; Wu, H.Y.; Deng, X.L.; Yang, Q.; Liu, P.; Hong, Y.Z.; Zhu, A.M.; Liu, Q.L. Dual hydrophobic modifications toward anion exchange membranes with both high ion conductivity and excellent dimensional stability. J. Membr. Sci. 2020, 595, 117521. [Google Scholar] [CrossRef]
- Han, J.J.; Liu, C.F.; Deng, C.W.; Zhang, Y.Y.; Song, W.F.; Zheng, X.M.; Liu, X.; Zhang, Y.M.; Yang, X.H.; Ren, Z.D.; et al. Mechanically robust and highly conductive semi-interpenetrating network anion exchange membranes for fuel cell applications. J. Power Sources 2022, 548, 232097. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, Y.Y.; Yang, Y.K.; Wu, C.; Fang, H.G.; Yang, S.Z.; Wei, H.B.; Ding, Y.S. Dimensionally and oxidatively stable anion exchange membranes based on bication cross-linked poly(meta-terphenylene alkylene)s. Polymer 2021, 216, 123433. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zhao, C.H.; Huang, S.M.; Gan, Y.L.; Xiong, L.; Zhou, J.P.; Liang, H.B. Bis-pyridinium crosslinked poly(ether ether ketone) anion exchange membranes with enhancement of hydroxide conductivity and alkaline stability. Int. J. Hydrogen Energy 2022, 47, 6097–6110. [Google Scholar] [CrossRef]
- Li, Q.; He, X.H.; Huang, L.; Lu, Y.; Zou, S.Y.; Ye, J.; Huang, L.M.; Yu, N.Q.; Fu, Z.H.; Zang, X.J.; et al. Porous PTFE supported bis(siloxane imidazole) functionalized Norbornyl copolymer composite anion exchange membrane for alkaline fuel cells. J. Appl. Polym. Sci. 2023, 140, e54495. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, X.D.; Peng, X.; Zimudzi, T.J.; Saikia, N.; Kwasny, M.T.; Song, S.F.; Kushner, D.I.; Fu, Z.S.; Tew, G.N.; et al. Poly(olefin)-Based Anion Exchange Membranes Prepared Using Ziegler-Natta Polymerization. Macromolecules 2019, 52, 4030–4041. [Google Scholar] [CrossRef]
- Wang, L.Q.; Peng, X.; Mustain, W.E.; Varcoe, J.R. Radiation-grafted anion-exchange membranes: The switch from low- to high-density polyethylene leads to remarkably enhanced fuel cell performance. Energy Environ. Sci. 2019, 12, 1575–1579. [Google Scholar] [CrossRef]
- Mayadevi, T.S.; Min, K.; Choi, O.; Chae, J.E.; Kim, H.J.; Choi, C.H.; Kang, H.S.; Park, C.H.; Kim, T.H. PPOs having piperidinium-based conducting head groups with extra molecular interaction sites as new anion exchange membranes. Int. J. Hydrogen Energy 2022, 47, 16222–16234. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Song, Y.E.; Jung, B.; Kim, J.R.; Kim, N.; Paik, H.J. Partially crosslinked comb-shaped PPO-based anion exchange membrane grafted with long alkyl chains: Synthesis, characterization and microbial fuel cell performance. Int. J. Hydrogen Energy 2020, 45, 27346–27358. [Google Scholar] [CrossRef]
- Yang, Z.J.; Zhang, M.H.; Zhao, Z.X.; Lan, W.T.; Zhang, X.; Fan, M.M. Highly alkaline stable fully-interpenetrating network poly(styrene-co-4-vinyl pyridine)/polyquaternium-10 anion exchange membrane without aryl ether linkages. Int. J. Hydrogen Energy 2022, 47, 16580–16596. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Van der Bruggen, B.; Yaroslavtsev, A.B. Ion exchange membranes based on radiation-induced grafted functionalized polystyrene for high-performance reverse electrodialysis. J. Power Sources 2021, 511, 230460. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N.T.; Wang, J.A.; Ma, L.L.; Bai, L.; Zhang, A.R.; Chen, Y.F.; Hao, C.; Yan, X.M.; Zhang, F.X.; et al. Stable alkoxy chain enhanced anion exchange membrane and its fuel cell. J. Membr. Sci. 2022, 644, 120179. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.L.; Li, N.; Hu, Z.X.; Chen, S.W. Homologous flexible multi-cationic cross-linkers modified poly(aryl ether sulfone) anion exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2022, 47, 17329–17340. [Google Scholar] [CrossRef]
- Liu, D.; Lin, L.M.; Xie, Y.J.; Pang, J.H.; Jiang, Z.H. Anion exchange membrane based on poly(arylene ether ketone) containing long alkyl densely quaternized carbazole derivative pendant. J. Membr. Sci. 2021, 623, 119079. [Google Scholar] [CrossRef]
- Yang, K.; Xu, J.M.; Shui, T.N.; Zhang, Z.G.; Wang, H.; Liu, Q.; Chen, W.B.; Shen, H.C.; Zhang, H.Y.; Wang, Z.; et al. Cross-linked poly (aryl ether ketone) anion exchange membrane with high ion conductivity by two different functional imidazole side chain. React. Funct. Polym. 2020, 151, 104551. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.Q.; Liu, M.; Liu, L.; Li, N.W. The effect of -NH- on quaternized polybenzimidazole anion exchange membranes for alkaline fuel cells. J. Membr. Sci. 2021, 626, 119178. [Google Scholar] [CrossRef]
- Tang, W.Q.; Yang, Y.F.; Liu, X.L.; Dong, J.H.; Li, H.H.; Yang, J.S. Long side-chain quaternary ammonium group functionalized polybenzimidazole based anion exchange membranes and their applications. Electrochim. Acta 2021, 391, 138919. [Google Scholar] [CrossRef]
- Cao, D.F.; Yang, F.; Sheng, W.B.; Zhou, Y.F.; Zhou, X.X.; Lu, Y.G.; Nie, F.M.; Li, N.W.; Pan, L.; Li, Y.S. Polynorbornene-based anion exchange membranes with hydrophobic large steric hindrance arylene substituent. J. Membr. Sci. 2022, 641, 119938. [Google Scholar] [CrossRef]
- He, X.H.; Zou, J.H.; Guo, Y.; Wang, K.; Wu, B.; Wen, Y.F.; Zang, X.J.; Chen, D.F. Synthesis of halogenated benzonorbornadiene monomer and preparation of self-crosslinking bisimidazole cationic functionalized benzonorbornadiene triblock copolymer anion exchange membrane. Polymer 2021, 218, 123535. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Kohl, P.A. Anionic multiblock copolymer membrane based on vinyl addition polymerization of norbornenes: Applications in anion-exchange membrane fuel cells. J. Membr. Sci. 2019, 570, 394–402. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Kohl, P.A. Highly Conductive Anion-Exchange Membranes Based on Cross-Linked Poly(norbornene): Vinyl Addition Polymerization. ACS Appl. Energ. Mater. 2019, 2, 2447–2457. [Google Scholar] [CrossRef]
- Huang, S.M.; He, X.H.; Cheng, C.W.; Zhang, F.; Guo, Y.; Chen, D.F. Facile self-crosslinking to improve mechanical and durability of polynorbornene for alkaline anion exchange membranes. Int. J. Hydrogen Energy 2020, 45, 13068–13079. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Hassan, N.U.; Peng, X.; Gu, T.L.; Brooks-Starks, A.H.; Bahar, B.; Mustain, W.E.; Kohl, P.A. The Importance of Water Transport in High Conductivity and High-Power Alkaline Fuel Cells. J. Electrochem. Soc. 2019, 167, 054501. [Google Scholar] [CrossRef]
- Chen, W.T.; Mandal, M.; Huang, G.; Wu, X.M.; He, G.H.; Kohl, P.A. Highly Conducting Anion-Exchange Membranes Based on Cross-Linked Poly(norbornene): Ring Opening Metathesis Polymerization. ACS Appl. Energ. Mater. 2019, 2, 2458–2468. [Google Scholar] [CrossRef]
- He, X.H.; Zou, J.H.; Wen, Y.F.; Wu, B.; Zang, X.J.; Deng, J.H.; Qin, Z.W.; Yang, G.X.; Xu, J.; Chen, D.F. Preparation and performance of bisimidazole cationic crosslinked addition-type polynorbornene-based anion exchange membrane. Int. J. Hydrogen Energy 2022, 47, 69–80. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, C.; Zhou, Y.Y.; Cheng, S.; Yang, S.Z.; Wei, H.B.; Ding, Y.S.; Wu, Y.C. Highly stable poly(p-quaterphenylene alkylene)-based anion exchange membranes. J. Membr. Sci. 2022, 647, 120342. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Biancolli, A.L.G.; Lanfredi, A.J.C.; Rodrigues, O.; Fonseca, F.C.; Santiago, E.I. Enhancing the durability and performance of radiation-induced grafted low-density polyethylene-based anion-exchange membranes by controlling irradiation conditions. J. Membr. Sci. 2022, 659, 120804. [Google Scholar] [CrossRef]
- Suhag, S.; Kumar, P.; Mandal, J.R.; Shahi, V.K. Macromolecular modification of partial fluorinated polymer by side-chain grafting of multi-cationic groups for alkaline membrane fuel cells. Int. J. Hydrogen Energy 2024, 73, 32–42. [Google Scholar] [CrossRef]
- Lin, Y.F.; He, B.; Zhou, Q.; Tang, S.K. High-strength anion-exchange fuel cell membranes based on imidazole-functionalized poly-ether-ether-ketone materials. Int. J. Hydrogen Energy 2024, 62, 760–768. [Google Scholar] [CrossRef]
- Mothupi, M.L.; Msomi, P.F. Quaternized Polyethersulfone (QPES) Membrane with Imidazole Functionalized Graphene Oxide (ImGO) for Alkaline Anion Exchange Fuel Cell Application. Sustainability 2023, 15, 2209. [Google Scholar] [CrossRef]
- Zhang, N.X.; Li, X.; Li, P.; Tang, S.K. Guanidinium cationic covalent organic nanosheets-based anion exchange composite membrane for fuel cells. Int. J. Hydrogen Energy 2023, 48, 25972–25983. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, L.; Zheng, J.F.; Zhang, Q.F.; Qin, G.R.; Li, S.H.; Zhang, S.B. Design, synthesis and characterization of anion exchange membranes containing guanidinium salts with ultrahigh dimensional stability. J. Membr. Sci. 2022, 643, 120008. [Google Scholar] [CrossRef]
- Zhu, T.Y.; Sha, Y.; Firouzjaie, H.A.; Peng, X.; Cha, Y.J.; Dissanayake, D.; Smith, M.D.; Vannucci, A.K.; Mustain, W.E.; Tang, C.B. Rational Synthesis of Metallo-Cations Toward Redox- and Alkaline-Stable Metallo-Polyelectrolytes. J. Am. Chem. Soc. 2020, 142, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Liu, S.; Yan, J.; Zhong, F.L.; Jia, N.F.; Yan, Y.; Zhang, Q.Y. Metallo-Polyelectrolyte-Based Robust Anion Exchange Membranes via Acetalation of a Commodity Polymer. Macromolecules 2021, 54, 9145–9154. [Google Scholar] [CrossRef]
- Mahmoud, A.M.A.; Miyatake, K. Highly conductive and alkaline stable partially fluorinated anion exchange membranes for alkaline fuel cells: Effect of ammonium head groups. J. Membr. Sci. 2022, 643, 120072. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Functionalizing Polystyrene with N-Alicyclic Piperidine-Based Cations via Friedel-Crafts Alkylation for Highly Alkali-Stable Anion-Exchange Membranes. Macromolecules 2020, 53, 4722–4732. [Google Scholar] [CrossRef]
- Liu, L.; Chu, X.M.; Liao, J.Y.; Huang, Y.D.; Li, Y.; Ge, Z.Y.; Hickner, M.A.; Li, N.W. Tuning the properties of poly(2,6-dimethyl-1,4-phenylene oxide) anion exchange membranes and their performance in H2/O2 fuel cells fuel cells. Energy Environ. Sci. 2018, 11, 435–446. [Google Scholar] [CrossRef]
- Li, X.F.; Yang, K.; Li, Z.Q.; Guo, J.; Zheng, J.F.; Li, S.H.; Zhang, S.B.; Sherazi, T.A. The effect of side chain length on the morphology and transport properties of fluorene-based anion exchange membranes. Int. J. Hydrogen Energy 2022, 47, 15044–15055. [Google Scholar] [CrossRef]
- Liu, Y.J.; Gao, W.T.; Zhu, A.M.; Zhang, Q.G.; Liu, Q.L. High-performance di-piperidinium-crosslinked poly(p-terphenyl piperi-dinium) anion exchange membranes. J. Membr. Sci. 2023, 687, 122045. [Google Scholar] [CrossRef]
- Clemens, A.L.; Jayathilake, B.S.; Karnes, J.J.; Schwartz, J.J.; Baker, S.E.; Duoss, E.B.; Oakdale, J.S. Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships. Polymers 2023, 15, 1534. [Google Scholar] [CrossRef]
- Hao, J.K.; Gao, X.Q.; Jiang, Y.Y.; Zhang, H.J.; Luo, J.S.; Shao, Z.G.; Yi, B.L. Crosslinked high-performance anion exchange membranes based on poly (styrene-b-(ethylene-co-butylene)-b-styrene). J. Membr. Sci. 2018, 551, 66–75. [Google Scholar] [CrossRef]
- Li, H.H.; Dong, J.H.; Cao, X.R.; Ren, X.R.; Hao, Z.; Yang, J.S. Diamine crossklinked anion exchange membranes based on poly(vinyl benzyl methylpyrrolidinium). Polymer 2021, 212, 123156. [Google Scholar] [CrossRef]
- Cao, D.F.; Nie, F.M.; Liu, M.; Sun, X.W.; Wang, B.B.; Wang, F.; Li, N.W.; Wang, B.; Ma, Z.; Pan, L.; et al. Crosslinked anion exchange membranes prepared from highly reactive polyethylene and polypropylene intermediates. J. Membr. Sci. 2022, 661, 120921. [Google Scholar] [CrossRef]
- Al Munsur, A.; Hossain, I.; Nam, S.Y.; Chae, J.E.; Kim, T.H. Quaternary ammonium-functionalized hexyl bis(quaternary ammonium)-mediated partially crosslinked SEBSs as highly conductive and stable anion exchange membranes. Int. J. Hydrogen Energy 2020, 45, 15658–15671. [Google Scholar] [CrossRef]
- Sung, S.; Mayadevi, T.S.; Min, K.; Lee, J.; Chae, J.E.; Kim, T.H. Crosslinked PPO-based anion exchange membranes: The effect of crystallinity versus hydrophilicity by oxygen-containing crosslinker chain length. J. Membr. Sci. 2021, 619, 118774. [Google Scholar] [CrossRef]
- Long, C.A.; Wang, Z.H.; Zhu, H. High chemical stability anion exchange membrane based on poly(aryl piperidinium): Effect of monomer configuration on membrane properties. Int. J. Hydrogen Energy 2021, 46, 18524–18533. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Gou, W.W.; Chen, J.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Crosslinked naphthalene-based triblock polymer anion exchange membranes for fuel cells. J. Membr. Sci. 2021, 636, 119569. [Google Scholar] [CrossRef]
- Yang, K.; Chu, X.M.; Zhang, X.J.; Li, X.F.; Zheng, J.F.; Li, S.H.; Li, N.W.; Sherazi, T.A.; Zhang, S.B. The effect of polymer backbones and cation functional groups on properties of anion exchange membranes for fuel cells. J. Membr. Sci. 2020, 603, 118025. [Google Scholar] [CrossRef]
- He, X.H.; Cheng, C.W.; Huang, S.M.; Zhang, F.; Duan, Y.P.; Zhu, C.Y.; Guo, Y.; Wang, K.; Chen, D.F. Alkaline anion exchange membranes with imidazolium-terminated flexible side-chain cross-linked topological structure based on ROMP-type norbornene copolymers. Polymer 2020, 195, 122412. [Google Scholar] [CrossRef]
- Zhang, F.; He, X.H.; Cheng, C.W.; Huang, S.M.; Duan, Y.P.; Zhu, C.Y.; Guo, Y.; Wang, K.; Chen, D.F. Bis-imidazolium functionalized self-crosslinking block polynorbornene anion exchange membrane. Int. J. Hydrogen Energy 2020, 45, 13090–13100. [Google Scholar] [CrossRef]
- Cheng, C.W.; He, X.H.; Huang, S.M.; Zhang, F.; Guo, Y.; Wen, Y.F.; Wu, B.; Chen, D.F. Novel self-cross-linked multi-imidazolium cations long flexible side chains triblock copolymer anion exchange membrane based on ROMP-type polybenzonorbornadiene. Int. J. Hydrogen Energy 2020, 45, 19676–19690. [Google Scholar] [CrossRef]
- Chen, S.X.; Zhang, A.Q.; He, X.H.; Chen, D.F. Multi-imidazolium cationic clusters cross-linked hydrogenated benzonorbornadiene diblock copolymer anion exchange membranes for improving conductivity and mitigating swelling. Polymer 2023, 287, 126429. [Google Scholar] [CrossRef]
- Chen, S.X.; Zhang, A.Q.; He, X.H.; Chen, D.F. Hydrogenated diblock copoly(norbornene)s bearing triimidazolium anion exchange membranes with enhanced alkaline stability for fuel cells. Int. J. Hydrogen Energy 2024, 50, 1282–1292. [Google Scholar] [CrossRef]
- Hassan, N.U.; Mandal, M.; Huang, G.R.; Firouzjaie, H.A.; Kohl, P.A.; Mustain, W.E. Mustain, Achieving High-Performance and 2000 h Stability in Anion Exchange Membrane Fuel Cells by Manipulating Ionomer Properties and Electrode Optimization. Adv. Energy Mater. 2020, 10, 2001986. [Google Scholar] [CrossRef]
- Huang, G.; Mandal, M.; Peng, X.; Yang-Neyerlin, A.C.; Pivovar, B.S.; Mustain, W.E.; Kohl, P.A. Composite Poly(norbornene) Anion Conducting Membranes for Achieving Durability, Water Management and High Power (3.4 W/cm2) in Hydrogen/Oxygen Alkaline Fuel Cells. J. Electrochem. Soc. 2019, 166, F637–F644. [Google Scholar] [CrossRef]
- Guo, M.L.; Ban, T.; Wang, Y.J.; Wang, Y.N.; Zhang, Y.Y.; Zhang, J.S.; Zhu, X.L. Exploring highly soluble ether-free polybenzimidazole as anion exchange membranes with long term durability. J. Membr. Sci. 2022, 647, 120299. [Google Scholar] [CrossRef]
- Li, L.; Lin, C.X.; Wang, X.Q.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Highly conductive anion exchange membranes with long flexible multication spacer. J. Membr. Sci. 2018, 553, 209–217. [Google Scholar] [CrossRef]





| AEMs | THF | CHCl3 | Toluene | Chlorobenzene | CH2Cl2 | DMSO | GF (wt%) |
|---|---|---|---|---|---|---|---|
| a | + | + | + | + | ± | − | 0 |
| b | − | − | − | − | − | − | 75.37 ± 1.1 |
| c | − | − | − | − | − | − | 81.71 ± 0.8 |
| d | − | − | − | − | − | − | 86.25 ± 0.5 |
| e | − | − | − | − | − | − | 90.12 ± 0.9 |
| AEMs | IEC (mmol g−1) | WU c (%) | SR c (%) | λ | |
|---|---|---|---|---|---|
| Cal. a | Exp. b | ||||
| aPNB-TMA | 1.68 | 1.66 ± 0.03 | 83.43 ± 2.8 | 17.17 ± 0.8 | 27.92 |
| CL10-aPNB-TMHDA-TMA | 1.66 | 1.61 ± 0.05 | 61.33 ± 1.2 | 12.13 ± 0.4 | 21.16 |
| CL20-aPNB-TMHDA-TMA | 1.65 | 1.61 ± 0.04 | 50.52 ± 0.8 | 11.18 ± 0.3 | 17.43 |
| CL30-aPNB-TMHDA-TMA | 1.63 | 1.60 ± 0.03 | 41.17 ± 1.1 | 9.95 ± 0.3 | 14.30 |
| CL40-aPNB-TMHDA-TMA | 1.62 | 1.59 ± 0.04 | 30.21 ± 0.6 | 8.53 ± 0.3 | 10.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; He, X.; Feng, L.; Ye, J.; Zhang, W.; Huang, L.; Chen, D. Diamine Crosslinked Addition-Type Diblock Poly(Norbornene)s-Based Anion Exchange Membranes with High Conductivity and Stability for Fuel Cell Applications. Polymers 2024, 16, 3534. https://doi.org/10.3390/polym16243534
Li Q, He X, Feng L, Ye J, Zhang W, Huang L, Chen D. Diamine Crosslinked Addition-Type Diblock Poly(Norbornene)s-Based Anion Exchange Membranes with High Conductivity and Stability for Fuel Cell Applications. Polymers. 2024; 16(24):3534. https://doi.org/10.3390/polym16243534
Chicago/Turabian StyleLi, Quan, Xiaohui He, Ling Feng, Jia Ye, Wenjun Zhang, Longming Huang, and Defu Chen. 2024. "Diamine Crosslinked Addition-Type Diblock Poly(Norbornene)s-Based Anion Exchange Membranes with High Conductivity and Stability for Fuel Cell Applications" Polymers 16, no. 24: 3534. https://doi.org/10.3390/polym16243534
APA StyleLi, Q., He, X., Feng, L., Ye, J., Zhang, W., Huang, L., & Chen, D. (2024). Diamine Crosslinked Addition-Type Diblock Poly(Norbornene)s-Based Anion Exchange Membranes with High Conductivity and Stability for Fuel Cell Applications. Polymers, 16(24), 3534. https://doi.org/10.3390/polym16243534







