Giant Response and Selectivity of Hansen Solubility Parameters-Based Graphene-SBS Co-Polymer Matrix Composite Room Temperature Sensor to Organic Vapours
Abstract
:1. Introduction
2. Materials and Methods
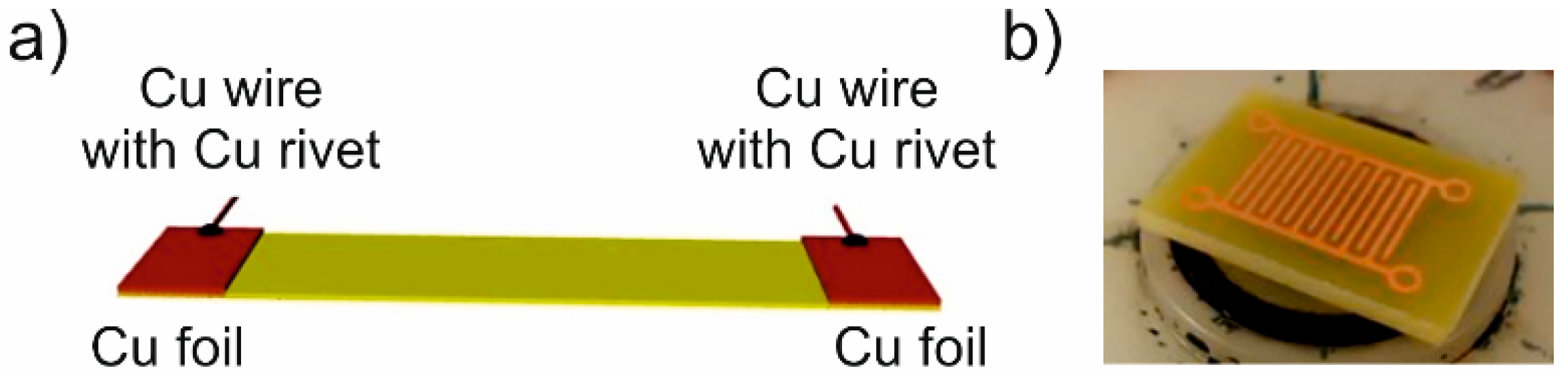
2.1. Materials and Sample Preparation
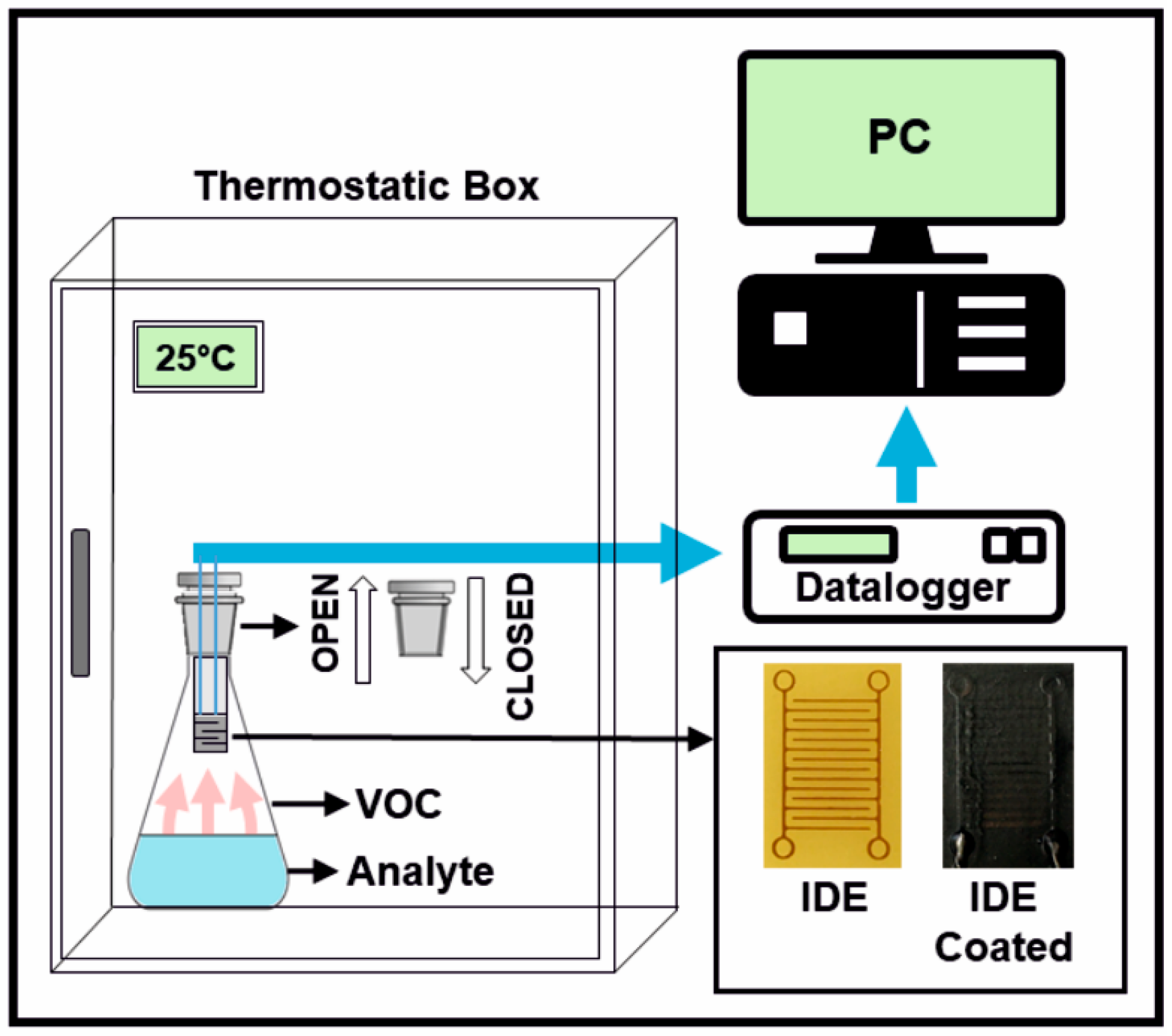
2.2. Characterisation Techniques
3. Results and Discussion
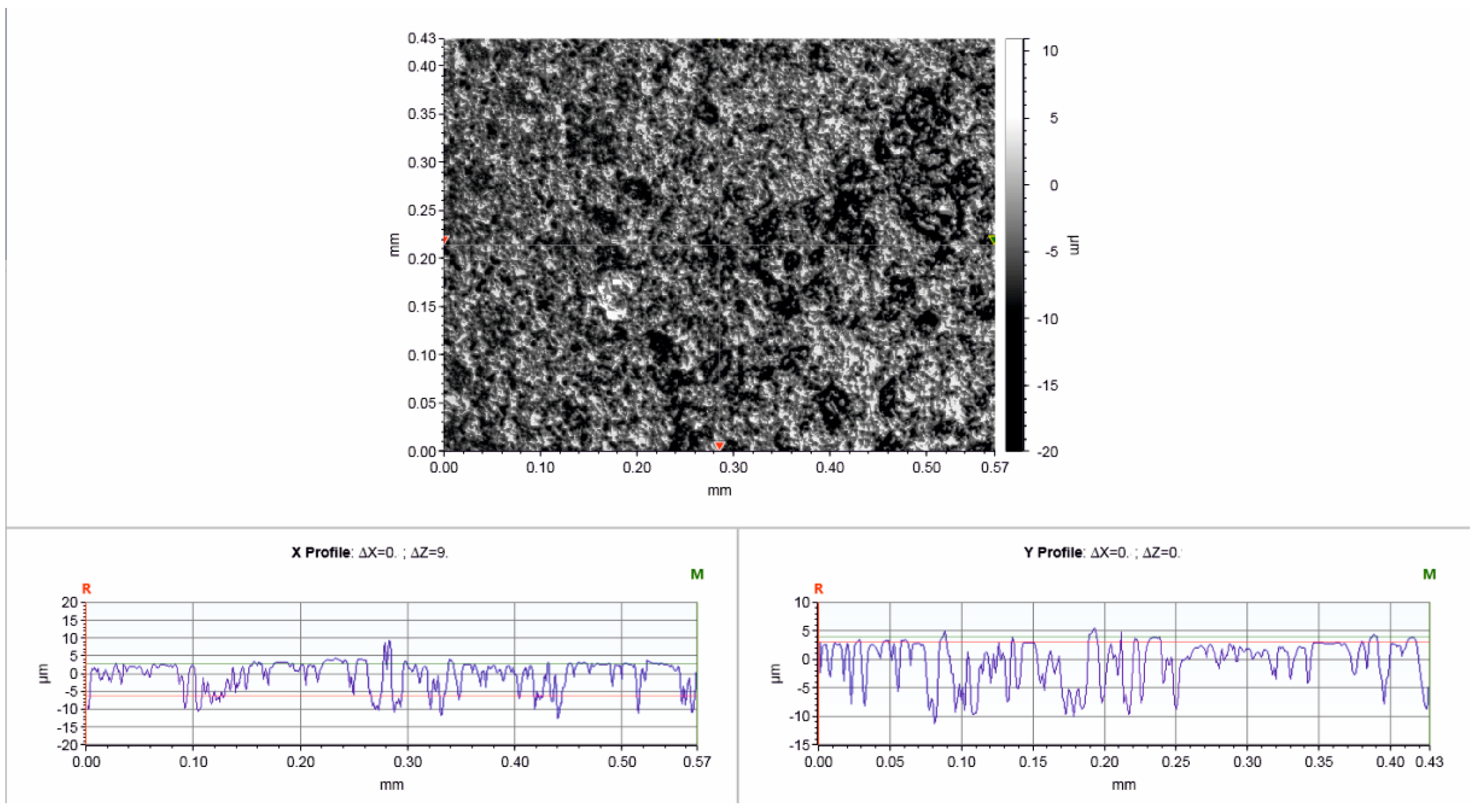
3.1. Material Characterisation
3.2. Electrical Properties of SBS/GNPs Nanocomposite Films
3.3. Selection of the Best SBS/GNPs Composition for Sensor Design
3.4. Response and Selectivity of the Sensor
3.5. Cross-Sensitivity of VOCs to Humidity
3.6. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Aslam, M.; Kalita, H. Graphene derivatives for chemiresistive gas sensors: A review. Mater. Today Commun. 2022, 30, 103182. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A Review on Graphene-Based Gas/Vapor Sensors with Unique Properties and Potential Applications. Nano-Micro Lett. 2016, 8, 95–119. [Google Scholar] [CrossRef]
- Demon, S.Z.N.; Kamisan, A.I.; Abdullah, N.; Noor, S.A.M.; Khim, O.K.; Kasim, N.A.M.; Yahya, M.Z.A.; Manaf, N.A.A.; Azmi, A.F.M.; Halim, N.A. Graphene-based Materials in Gas Sensor Applications: A Review. Sens. Mater. 2020, 32, 2492. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, G.-Y.; Shi, D.-X. Review of graphene-based strain sensors. Chin. Phys. B 2013, 22, 057701. [Google Scholar] [CrossRef]
- Benlikaya, R.; Slobodian, P.; Proisl, K.; Cvelbar, U.; Morozov, I. Ascertaining the factors that influence the vapor sensor response: The entire case of MWCNT network sensor. Sens. Actuators B Chem. 2019, 283, 478–486. [Google Scholar] [CrossRef]
- Santhosh, N.M.; Vasudevan, A.; Jurov, A.; Korent, A.; Slobodian, P.; Zavašnik, J.; Cvelbar, U. Improving sensing properties of entangled carbon nanotube-based gas sensors by atmospheric plasma surface treatment. Microelectron. Eng. 2020, 232, 111403. [Google Scholar] [CrossRef]
- Zhang, Q.; An, C.; Fan, S.; Shi, S.; Zhang, R.; Zhang, J.; Li, Q.; Zhang, D.; Hu, X.; Liu, J. Flexible gas sensor based on graphene/ethyl cellulose nanocomposite with ultra-low strain response for volatile organic compounds rapid detection. Nanotechnology 2018, 29, 285501. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Hao, S.; Li, Y.; Santillo, C.; Zhang, C.; Sorrentino, L.; Lavorgna, M.; Xia, H.; Chen, Z. High sensitivity of multi-sensing materials based on reduced graphene oxide and natural rubber: The synergy between filler segregation and macro-porous morphology. Compos. Sci. Technol. 2021, 205, 108689. [Google Scholar] [CrossRef]
- Rattanabut, C.; Muangrat, W.; Phonyiem, M.; Bungjongpru, W.; Wongwiriyapan, W.; Song, Y.J. Hybrid graphene and poly(methyl methacrylate) for gas sensor application. Mater. Today Proc. 2017, 4, 6397–6403. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Fabiane, M.; Bello, A.; Manyala, N. Graphene: Synthesis, Transfer, and Characterization for Dye-Sensitized Solar Cells Applications. Ind. Eng. Chem. Res. 2013, 52, 14160–14168. [Google Scholar] [CrossRef]
- Zabihi, Z.; Araghi, H.; Rodriguez, P.E.D.S.; Boujakhrout, A.; Villalonga, R. Vapor sensing and interface properties of reduced graphene oxide–poly(methyl methacrylate) nanocomposite. J. Mater. Sci. Mater. Electron. 2019, 30, 2908–2919. [Google Scholar] [CrossRef]
- Pawar, D.; Kanawade, R.; Kumar, A.; Rao, C.N.; Cao, P.; Gaware, S.; Late, D.; Kale, S.N.; Navale, S.T.; Liu, W.J.; et al. High-performance dual cavity-interferometric volatile gas sensor utilizing Graphene/PMMA nanocomposite. Sens. Actuators B Chem. 2020, 312, 127921. [Google Scholar] [CrossRef]
- Slobodian, R.; Olejnik, R.; Matyas, J.; Slobodian, P. The sensing properties of carbon nanotube filled copolymers for VOC vapors detection. In Proceedings of the NANOCON Conference Proceedings-International Conference on Nanomaterials, Brno, Czech Republic, 21–23 October 2020; TANGER Ltd.: Ostrava, Czech Republic, 2020; pp. 63–67. [Google Scholar] [CrossRef]
- Olejnik, R.; Matyas, J.; Slobodian, P.; Riha, P. Using graphene/styrene-isoprene-styrene copolymer composite thin film as a flexible microstrip antenna for the detection of heptane vapors. J. Phys. Conf. Ser. 2018, 987, 012022. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; Taylor & Francis: Abingdon, UK, 2018. [Google Scholar] [CrossRef]
- Alamusi; Hu, N.; Fukunaga, H.; Atobe, S.; Liu, Y.; Li, J. Piezoresistive Strain Sensors Made from Carbon Nanotubes Based Polymer Nanocomposites. Sensors 2011, 11, 10691–10723. [Google Scholar] [CrossRef]
- Pang, H.; Chen, T.; Zhang, G.; Zeng, B.; Li, Z.-M. An electrically conducting polymer/graphene composite with a very low percolation threshold. Mater. Lett. 2010, 64, 2226–2229. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/Polyurethane Nanocomposites for Improved Gas Barrier and Electrical Conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Klüppel, M. The Role of Disorder in Filler Reinforcement of Elastomers on Various Length Scales. In Filler-Reinforced Elastomers Scanning Force Microscopy; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–86. [Google Scholar] [CrossRef]
- Ambrose, D.; Sprake, C.H.S. Thermodynamic properties of organic oxygen compounds XXV. Vapour pressures and normal boiling temperatures of aliphatic alcohols. J. Chem. Thermodyn. 1970, 2, 631–645. [Google Scholar] [CrossRef]
- Ambrose, D.; Sprake, C.H.S.; Townsend, R. Thermodynamic properties of organic oxygen compounds XXXIII. The vapour pressure of acetone. J. Chem. Thermodyn. 1974, 6, 693–700. [Google Scholar] [CrossRef]
- Carruth, G.F.; Kobayashi, R. Vapor pressure of normal paraffins ethane through n-decane from their triple points to about 10 mm mercury. J. Chem. Eng. Data 1973, 18, 115–126. [Google Scholar] [CrossRef]
- Besley, L.M.; Bottomley, G.A. Vapour pressure of toluence from 273.15 to 298.15 K. J. Chem. Thermodyn. 1974, 6, 577–580. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Kruczek, B. Diffusion Coefficient. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Reynier, A.; Dole, P.; Humbel, S.; Feigenbaum, A. Diffusion coefficients of additives in polymers. I. Correlation with geometric parameters. J. Appl. Polym. Sci. 2001, 82, 2422–2433. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Measurement of Multicomponent Solubility Parameters for Graphene Facilitates Solvent Discovery. Langmuir 2010, 26, 3208–3213. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Sherwood, J.; De Bruyn, M.; Budarin, V.L.; Ellis, G.J.; Clark, J.H.; Shuttleworth, P.S. Identification of high performance solvents for the sustainable processing of graphene. Green Chem. 2017, 19, 2550–2560. [Google Scholar] [CrossRef]



| VOC | p0 (kPa) | Maximum Rmax/R0 (%) | Average Rmax/R0 (%) | t90 (s) | t10 (s) |
|---|---|---|---|---|---|
| Ethanol a | 7.870 | 26.8 | 23 | 48 ± 13 | 110 ± 50 |
| Acetone b | 30.868 | 2580 | 1600 | 52 ± 9 | 24 ± 4 |
| Heptane c | 6.476 | 11,600,000 | 9,000,000 | 24 ± 5 | 20 ± 2 |
| Toluene d | 3.8 | 11,500,000 | 10,000,000 | 31 ± 4 | 22 ± 2 |
| Gas (VOC) 1 | Gas (VOC) 2 | |||
|---|---|---|---|---|
| Ethanol | Acetone | Heptane | Toluene | |
| Ethanol | 1 | 0.056 | 0.0000021 | 0.00000111 |
| Acetone | 18 | 1 | 0.0000373 | 0.0000197 |
| Heptane | 476,000 | 26,800 | 1 | 0.528 |
| Toluene | 901,000 | 50,800 | 1.8 | 1 |
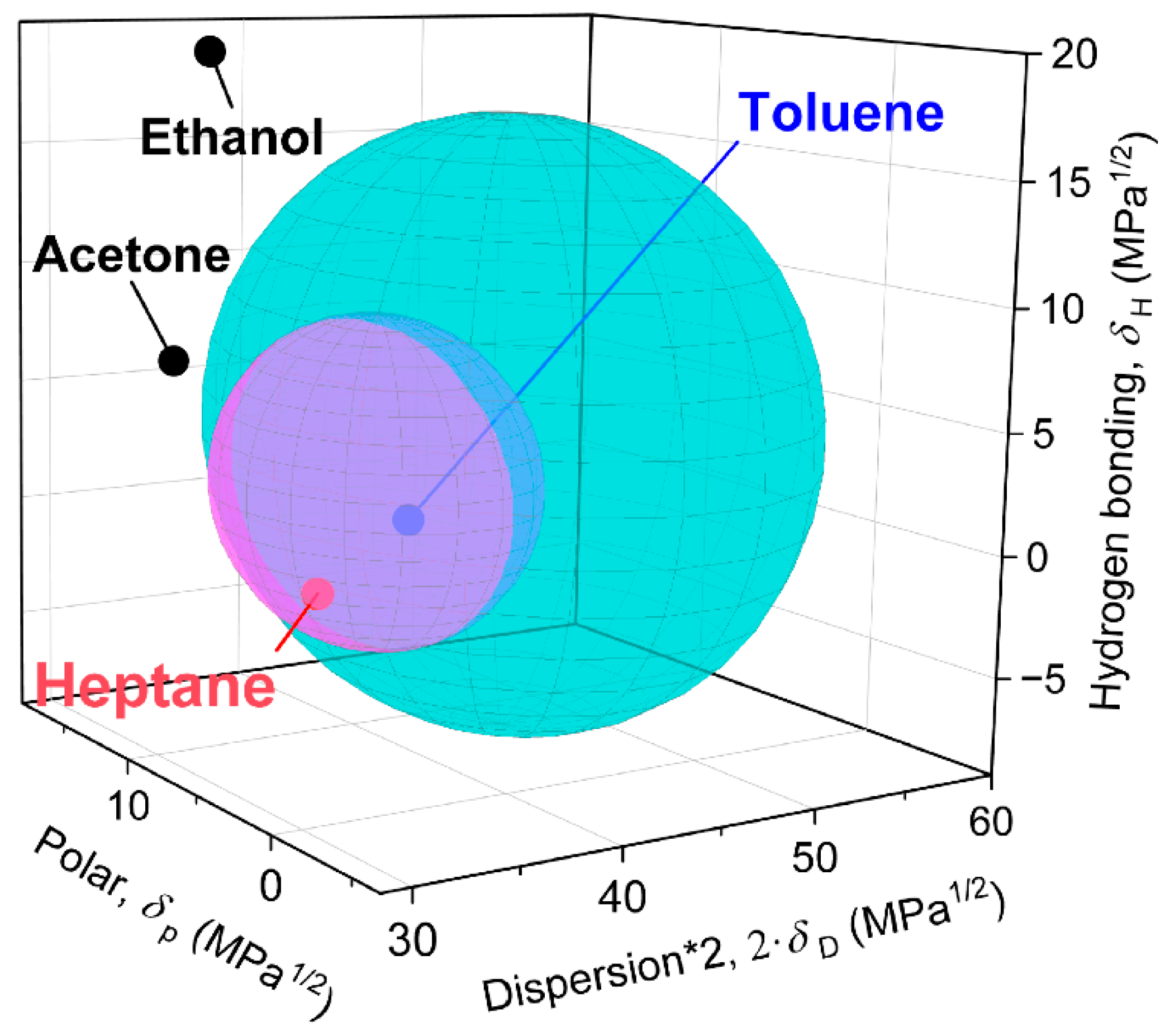
| Polymers and Solvents | HSP Values | |||||
|---|---|---|---|---|---|---|
| δD (MPa1/2) | δp (MPa1/2) | δH (MPa1/2) | r0 (MPa1/2) | ra (MPa1/2) | RED (MPa1/2) | |
| PS | 22.3 | 5.8 | 4.3 | 12.7 | - | - |
| Water | 15.5 | 16.0 | 42.3 | - | 41.63 | 3.28 |
| Ethanol | 15.8 | 8.8 | 19.4 | - | 20.15 | 1.59 |
| Acetone | 15.5 | 10.4 | 7.0 | - | 14.61 | 1.15 |
| Toluene | 18.0 | 1.4 | 2.0 | - | 9.93 | 0.78 |
| Heptane | 15.3 | 0 | 0 | - | 15.75 | 1.24 |
| PB | 17.5 | 2.3 | 3.4 | 6.5 | - | - |
| Water | 15.5 | 16.0 | 42.3 | - | 41.44 | 6.38 |
| Ethanol | 15.8 | 8.8 | 19.4 | - | 17.6 | 2.71 |
| Acetone | 15.5 | 10.4 | 7 | - | 9.72 | 1.5 |
| Toluene | 18 | 1.4 | 2 | - | 1.94 | 0.3 |
| Heptane | 15.3 | 0 | 0 | - | 6.02 | 0.93 |
| Polymers and Solvents | HSP Values | |||||
|---|---|---|---|---|---|---|
| δD (MPa1/2) | δp (MPa1/2) | δH (MPa1/2) | r0 (MPa1/2) | ra (MPa1/2) | RED (MPa1/2) | |
| Graphene | 18.0 | 9.3 | 7.7 | 6.5 | - | - |
| PS | 22.3 | 5.8 | 4.3 | - | 9.89 | 1.52 |
| PB | 17.5 | 2.3 | 3.4 | - | 8.28 | 1.27 |
| Ethanol | 15.8 | 8.8 | 19.4 | - | 12.51 | 1.92 |
| Acetone | 15.5 | 10.4 | 7.0 | - | 5.17 | 0.80 |
| Toluene | 18.0 | 1.4 | 2.0 | - | 9.74 | 1.50 |
| Heptane | 15.3 | 0 | 0 | - | 13.23 | 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slobodian, R.; Olejnik, R.; Dmonte, D.J.; Sevcik, J.; Matyas, J.; Jurca, M.; Pricilla, R.B.; Hanulikova, B.; Slobodian, P.; Kuritka, I. Giant Response and Selectivity of Hansen Solubility Parameters-Based Graphene-SBS Co-Polymer Matrix Composite Room Temperature Sensor to Organic Vapours. Polymers 2024, 16, 309. https://doi.org/10.3390/polym16030309
Slobodian R, Olejnik R, Dmonte DJ, Sevcik J, Matyas J, Jurca M, Pricilla RB, Hanulikova B, Slobodian P, Kuritka I. Giant Response and Selectivity of Hansen Solubility Parameters-Based Graphene-SBS Co-Polymer Matrix Composite Room Temperature Sensor to Organic Vapours. Polymers. 2024; 16(3):309. https://doi.org/10.3390/polym16030309
Chicago/Turabian StyleSlobodian, Rostislav, Robert Olejnik, David John Dmonte, Jakub Sevcik, Jiri Matyas, Marek Jurca, R. Blessy Pricilla, Barbora Hanulikova, Petr Slobodian, and Ivo Kuritka. 2024. "Giant Response and Selectivity of Hansen Solubility Parameters-Based Graphene-SBS Co-Polymer Matrix Composite Room Temperature Sensor to Organic Vapours" Polymers 16, no. 3: 309. https://doi.org/10.3390/polym16030309
APA StyleSlobodian, R., Olejnik, R., Dmonte, D. J., Sevcik, J., Matyas, J., Jurca, M., Pricilla, R. B., Hanulikova, B., Slobodian, P., & Kuritka, I. (2024). Giant Response and Selectivity of Hansen Solubility Parameters-Based Graphene-SBS Co-Polymer Matrix Composite Room Temperature Sensor to Organic Vapours. Polymers, 16(3), 309. https://doi.org/10.3390/polym16030309









