Controlled Alignment of Carbon Black Nanoparticles in Electrospun Carbon Nanofibers for Flexible Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Pre-Treatment and Electrospinning Precursor Solution
2.3. Electrospinning Process and Thermal Treatments
2.4. Characterizations
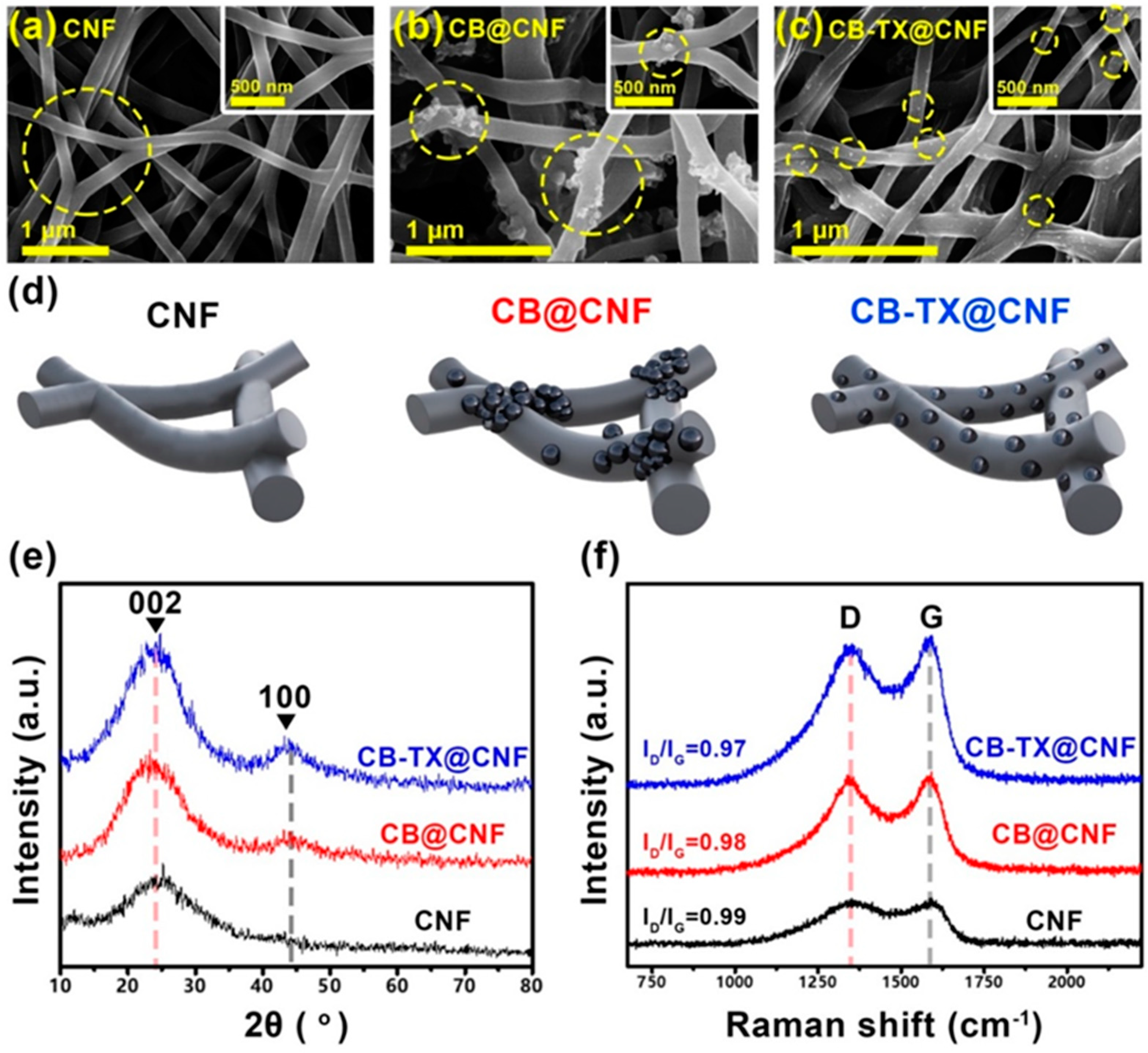
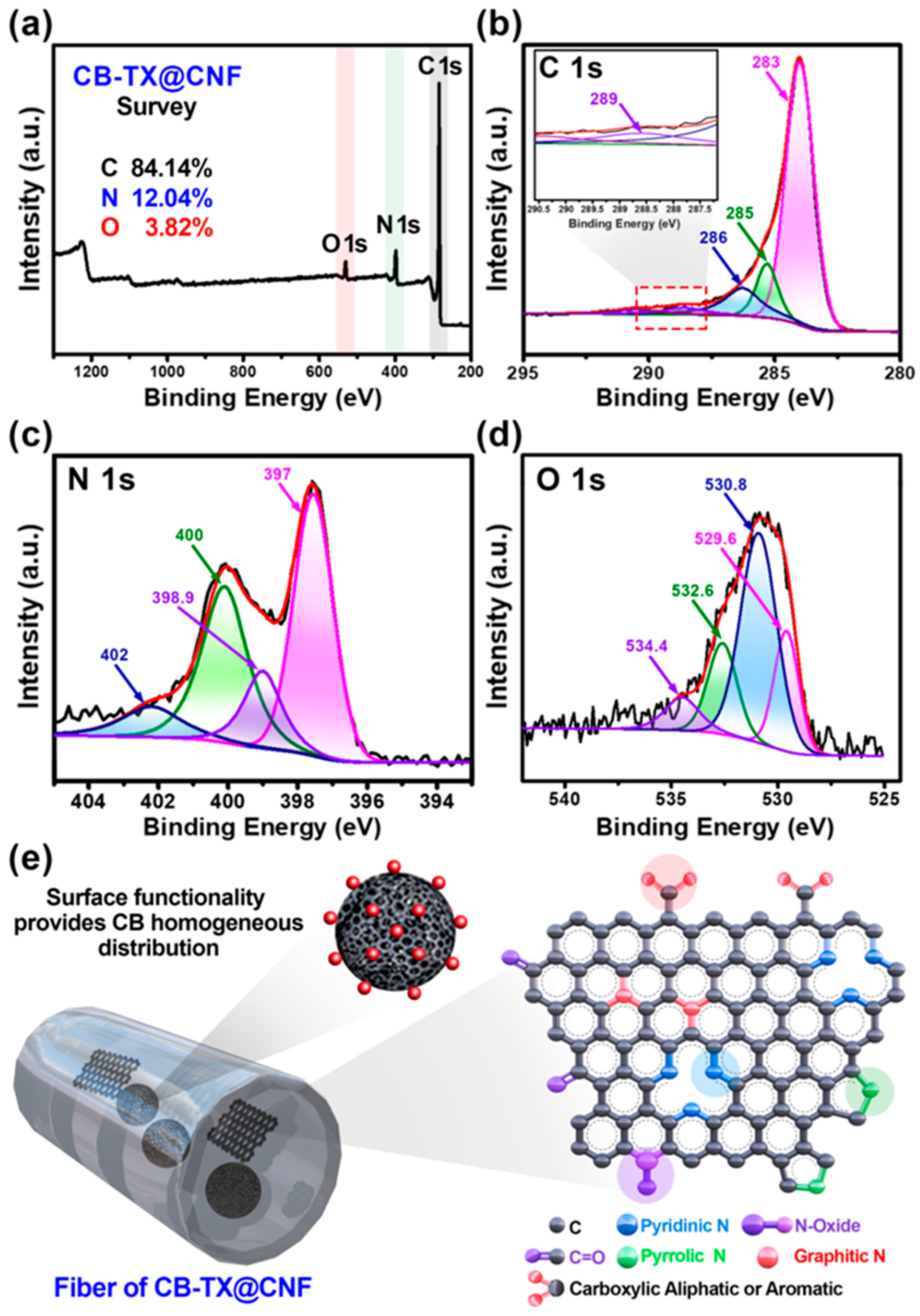
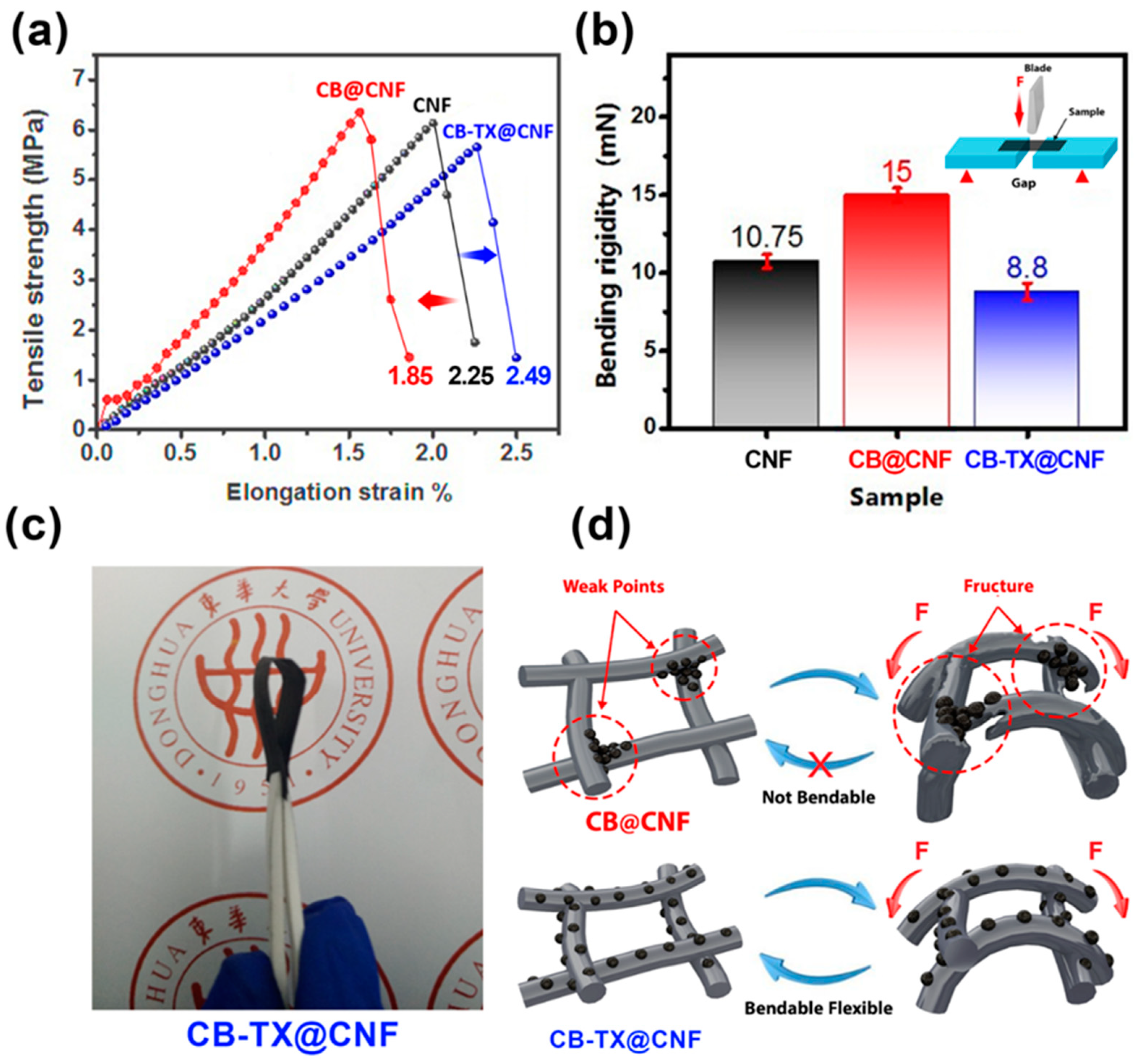
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Y.; Ha, H.; Choi, C.; Yoon, H.; Matteini, P.; Cheong, J.Y.; Hwang, B. Review of Flexible Supercapacitors Using Carbon Nanotube-Based Electrodes. Appl. Sci. 2023, 13, 3290. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Yu, J.; Tian, F.; Luo, X.; Hao, Y.; Huang, J.; Wang, F.; Zhou, W.; Xu, J. Flexible Supercapacitors Based on Stretchable Conducting Polymer Electrodes. Polymers 2023, 15, 1856. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Z.L.; Fukuda, K.; Someya, T. Flexible self-charging power sources. Nat. Rev. Mater. 2022, 7, 870–886. [Google Scholar] [CrossRef]
- Mamun, A.; Kiari, M.; Sabantina, L. A Recent Review of Electrospun Porous Carbon Nanofiber Mats for Energy Storage and Generation Applications. Membranes 2023, 13, 830. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent progress of the preparation and application of electrospun porous nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Gong, W.; Xu, F.; Song, X.; He, X.; Fan, M. Electrospun Carbon Nanofibers and Their Applications in Several Areas. ACS Omega 2023, 8, 22316–22330. [Google Scholar] [CrossRef]
- Nie, G.; Zhao, X.; Luan, Y.; Jiang, J.; Kou, Z.; Wang, J. Key issues facing electrospun carbon nanofibers in energy applications: On-going approaches and challenges. Nanoscale 2020, 12, 13225–13248. [Google Scholar] [CrossRef]
- Calvo, V.; Paleo, A.J.; González-Domínguez, J.M.; Muñoz, E.; Krause, B.; Pötschke, P.; Maser, W.K.; Benito, A.M. The aqueous processing of carbon nanofibers via cellulose nanocrystals as a green path towards e-textiles with n-type thermoelectric behaviour. Carbon 2024, 217, 118640. [Google Scholar] [CrossRef]
- Liu, R.; Hou, L.; Yue, G.; Li, H.; Zhang, J.; Liu, J.; Miao, B.; Wang, N.; Bai, J.; Cui, Z. Progress of fabrication and applications of electrospun hierarchically porous nanofibers. Adv. Fiber Mater. 2022, 4, 604–630. [Google Scholar] [CrossRef]
- Jiang, H.; Yao, M.; Chen, J.; Zhang, M.; Hong, W. Advances in biomass-based nanofibers prepared by electrospinning for energy storage devices. Fuel 2024, 355, 129534. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, X.; Liu, X.; Yu, J.; Ding, B. Strategies in precursors and post treatments to strengthen carbon nanofibers. Adv. Fiber Mater. 2020, 2, 46–63. [Google Scholar] [CrossRef]
- Huang, L.; Guan, Q.; Cheng, J.; Li, C.; Ni, W.; Wang, Z.; Zhang, Y.; Wang, B. Free-standing N-doped carbon nanofibers/carbon nanotubes hybrid film for flexible, robust half and full lithium-ion batteries. Chem. Eng. J. 2018, 334, 682–690. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kim, J.-H.; Kim, I.-G.; Park, J.-H.; Jung, J.-W.; Kim, H.-S.; Kim, I.-D. Reduced graphene-oxide-encapsulated MoS2/carbon nanofiber composite electrode for high-performance Na-ion batteries. Nanomaterials 2021, 11, 2691. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Martinez, D.; Kurniawan, D.; Chiang, W.H.; Bartolo, P. Carbon nanomaterials for electro-active structures: A review. Polymers 2020, 12, 2946. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, X.; He, Z.; Liu, J.; Zhang, X.; Xu, J.; Lei, C. Amine-terminated highly cross-linked polyphosphazene-functionalized carbon nanotube-reinforced lignin-based electrospun carbon nanofibers. ACS Sustain. Chem. Eng. 2020, 8, 1840–1849. [Google Scholar] [CrossRef]
- Hwang, H.; Byun, S.; Yuk, S.; Kim, S.; Song, S.H.; Lee, D. High-rate electrospun Ti3C2Tx MXene/carbon nanofiber electrodes for flexible supercapacitors. Appl. Surf. Sci. 2021, 556, 149710. [Google Scholar] [CrossRef]
- Pathak, I.; Acharya, D.; Chhetri, K.; Lohani, P.C.; Subedi, S.; Muthurasu, A.; Kim, T.; Ko, T.H.; Dahal, B.; Kim, H.Y. Ti3C2Tx MXene embedded metal–organic framework-based porous electrospun carbon nanofibers as a freestanding electrode for supercapacitors. J. Mater. Chem. A 2023, 10, 5001–5014. [Google Scholar] [CrossRef]
- Levitt, A.S.; Alhabeb, M.; Hatter, C.B.; Sarycheva, A.; Dion, G.; Gogotsi, Y. Electrospun MXene/carbon nanofibers as supercapacitor electrodes. J. Mater. Chem. A 2019, 1, 269–277. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Orha, C.; Pop, A. Enhanced electrochemical response of diclofenac at a fullerene–carbon nanofiber paste electrode. Sensors 2019, 19, 1332. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, J.; Li, Y.; Wang, L.; Dong, Y.; Jiang, Z.; Fan, C.; Cao, Y.; Sheng, R.; Liu, A. Graphene quantum dot reinforced electrospun carbon nanofiber fabrics with high surface area for ultrahigh rate supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 11669–11678. [Google Scholar] [CrossRef]
- Gaminian, H.; Montazer, M. Carbon black enhanced conductivity, carbon yield and dye adsorption of sustainable cellulose derived carbon nanofibers. Cellulose 2018, 25, 5227–5240. [Google Scholar] [CrossRef]
- Albright, T.; Hobeck, J. Characterization of Carbon-Black-Based Nanocomposite Mixtures of Varying Dispersion for Improving Stochastic Model Fidelity. Nanomaterials 2023, 13, 916. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, S.; Xu, S. Preparation and characterization of water-dispersible carbon black grafted with polyacrylic acid by high-energy electron beam irradiation. J. Mater. Sci. 2018, 53, 6106–6115. [Google Scholar] [CrossRef]
- Bo, Y.; Cui, J.; Cai, Y.; Xu, S. Preparation and characterization of poly (methyl methacrylate) and poly (maleic anhydride-co-diallyl phthalate) grafted carbon black through γ-ray irradiation. Radiat. Phys. Chem. 2016, 119, 236–246. [Google Scholar] [CrossRef]
- Ma, X.; Song, X.; Yu, Z.; Li, S.; Wang, X.; Zhao, L.; Zhao, L.; Xiao, Z.; Qi, C.; Ning, G. S-doping coupled with pore-structure modulation to conducting carbon black: Toward high mass loading electrical double-layer capacitor. Carbon 2019, 149, 646–654. [Google Scholar] [CrossRef]
- ISO 1798:2008; Flexible Cellular Polymeric Materials—Determination of Tensile Strength and Elongation at Break. ISO: Geneva, Switzerland, 2008.
- ASTM D 2923-95; Standard Test Method for Rigidity of Polyolefin Film and Sheeting. ASTM International: West Conshohocken, PA, USA, 2001.
- Sun, L.; Miyagi, D.; Cai, Y.; Ullah, A.; Haider, M.K.; Zhu, C.; Gopiraman, M.; Kim, I.S. Rational construction of hierarchical nanocomposites by growing dense polyaniline nanoarrays on carbon black-functionalized carbon nanofiber backbone for freestanding supercapacitor electrodes. J. Energy Storage 2023, 61, 106738. [Google Scholar] [CrossRef]
- Shukla, S.; Bhattacharjee, S.; Weber, A.; Secanell, M. Experimental and theoretical analysis of ink dispersion stability for polymer electrolyte fuel cell applications. J. Electrochem. Soc. 2017, 164, F600. [Google Scholar] [CrossRef]
- Aykut, Y.; Pourdeyhimi, B.; Khan, S.A. Effects of surfactants on the microstructures of electrospun polyacrylonitrile nanofibers and their carbonized analogs. J. Appl. Polym. Sci. 2013, 130, 3726–3735. [Google Scholar] [CrossRef]
- Suhdi, S.; Wang, S.C. The production of carbon nanofiber on rubber fruit shell-derived activated carbon by chemical activation and hydrothermal process with low temperature. Nanomaterials 2021, 11, 2038. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Afzal, M.A.F.; Srivastava, S.; Patil, S.; Sharma, A. Enhanced electrical conductivity of suspended carbon nanofibers: Effect of hollow structure and improved graphitization. Carbon 2016, 108, 135–145. [Google Scholar]
- Wang, L.; Wang, J.; Ng, D.H.; Li, S.; Zou, B.; Cui, Y.; Liu, X.; El-Khodary, S.A.; Qiu, J.; Lian, J. Operando mechanistic and dynamic studies of N/P co-doped hard carbon nanofibers for efficient sodium storage. Chem. Commun. 2021, 75, 9610–9613. [Google Scholar] [CrossRef]
- Maitra, T.; Sharma, S.; Srivastava, A.; Cho, Y.-K.; Madou, M.; Sharma, A. Improved graphitization and electrical conductivity of suspended carbon nanofibers derived from carbon nanotube/polyacrylonitrile composites by directed electrospinning. Carbon 2012, 50, 1753–1761. [Google Scholar] [CrossRef]
- Li, Q.; Xie, W.; Liu, D.; Wang, Q.; He, D. Nitrogen and oxygen co-doped carbon nanofibers with rich sub-nanoscale pores as self-supported electrode material of high-performance supercapacitors. Electrochim. Acta 2016, 222, 1445–1454. [Google Scholar] [CrossRef]
- El-Khodary, S.A.; Subburam, G.; Zou, B.-B.; Wang, J.; Qiu, J.-X.; Liu, X.-H.; Ng, D.H.; Wang, S.; Lian, J.-B. Mesoporous silica anchored on reduced graphene oxide nanocomposite as anode for superior lithium-ion capacitor. Rare Met. 2022, 41, 368–377. [Google Scholar] [CrossRef]
- Yang, T.; Tian, X.; Song, Y.; Wu, S.; Wu, J.; Liu, Z. Oxygen-doped carbon nanofiber nonwovens as an effective interlayer towards accelerating electrochemical kinetics for lithium-sulfur battery. Appl. Surf. Sci. 2023, 611, 155690. [Google Scholar] [CrossRef]
- Aboalhassan, A.A.; Yan, J.; Zhao, Y.; Dong, K.; Wang, X.; Yu, J.; Ding, B. Self-assembled porous-silica within N-doped carbon nanofibers as ultra-flexible anodes for soft lithium batteries. Iscience 2019, 16, 122–132. [Google Scholar] [CrossRef]
- Pandey, K.; Jeong, H.K. Silicon-Carbon nanofiber composite film for supercapacitor applications. Chem. Phys. Lett. 2024, 834, 140972. [Google Scholar] [CrossRef]
- Senthilkumar, S.H.; Ramasubramanian, B.; Rao, R.P.; Chellappan, V.; Ramakrishna, S. Advances in Electrospun Materials and Methods for Li-Ion Batteries. Polymers 2023, 15, 1622. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboalhassan, A.; Babar, A.A.; Iqbal, N.; Yan, J.; El-Newehy, M.; Yu, J.; Ding, B. Controlled Alignment of Carbon Black Nanoparticles in Electrospun Carbon Nanofibers for Flexible Films. Polymers 2024, 16, 327. https://doi.org/10.3390/polym16030327
Aboalhassan A, Babar AA, Iqbal N, Yan J, El-Newehy M, Yu J, Ding B. Controlled Alignment of Carbon Black Nanoparticles in Electrospun Carbon Nanofibers for Flexible Films. Polymers. 2024; 16(3):327. https://doi.org/10.3390/polym16030327
Chicago/Turabian StyleAboalhassan, Ahmed, Aijaz Ahmed Babar, Nousheen Iqbal, Jianhua Yan, Mohamed El-Newehy, Jianyong Yu, and Bin Ding. 2024. "Controlled Alignment of Carbon Black Nanoparticles in Electrospun Carbon Nanofibers for Flexible Films" Polymers 16, no. 3: 327. https://doi.org/10.3390/polym16030327





