Ultrasensitive Detection of PSA Using Antibodies in Crowding Polyelectrolyte Multilayers on a Silicon Nanowire Field-Effect Transistor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silicon Surface Modification by Polyelectrolytes and Adsorption of Antibodies in the Matrix of Polymers
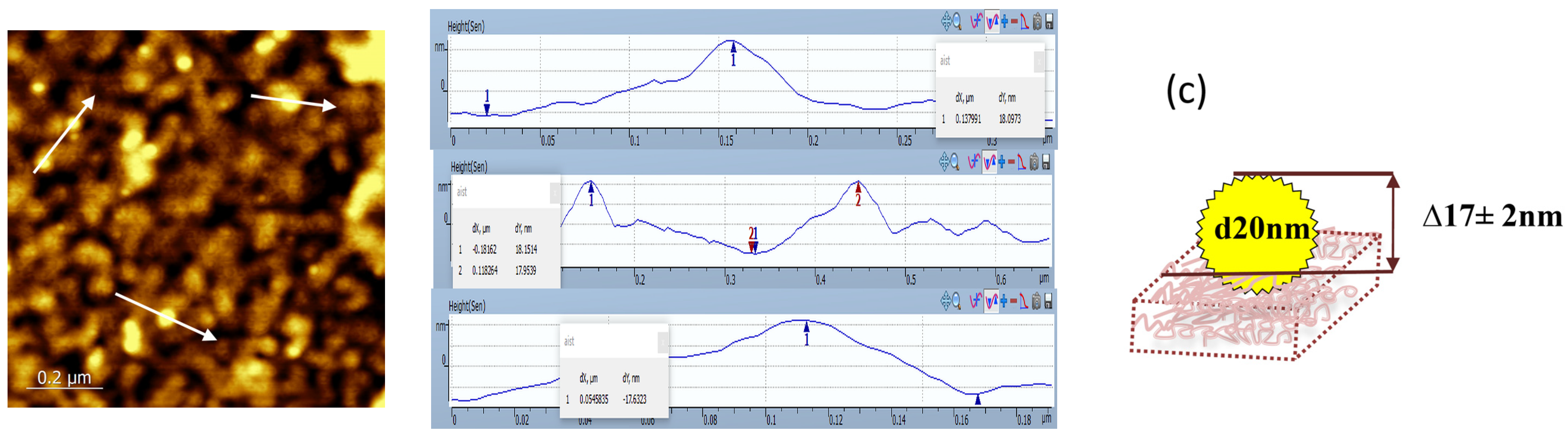
2.3. Characterization of a Silicon Surface Covered by Multilayers of Polyelectrolytes and Antibodies
2.4. Fabrication of NW FETs and Electrical Measurements
2.5. Determination of PSA by the NWFETs
3. Results and Discussion
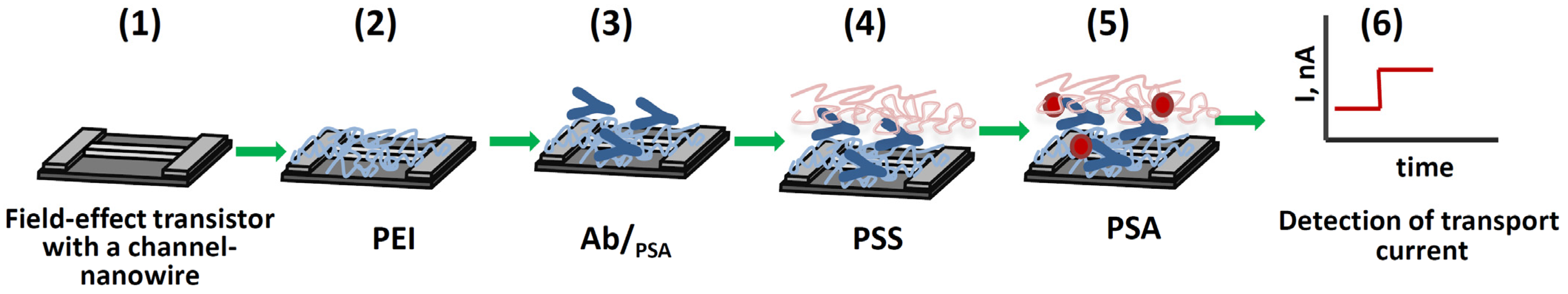
3.1. Principle of Immunosensor Based on Field-Effect Transistors with Nanowire Channel Coated by Layers of Oppositely Charged Polyelectrolytes
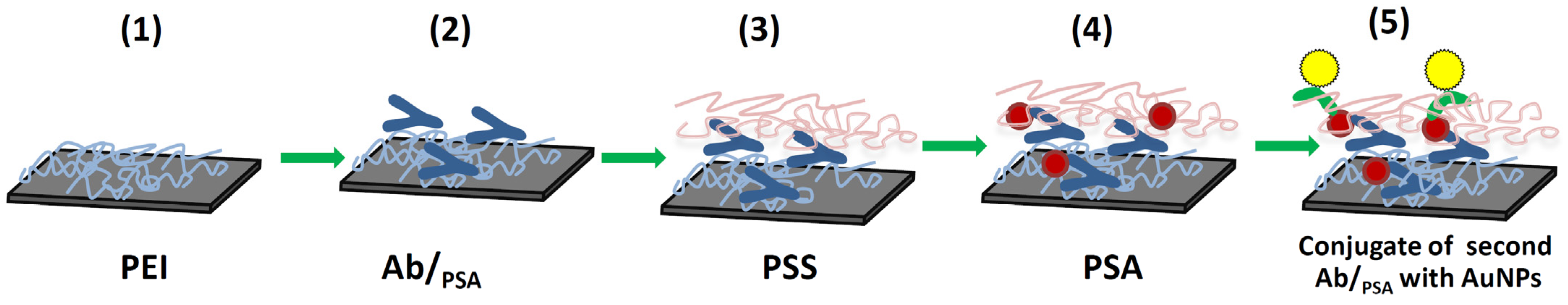
3.2. Modification of Silicon Surface by Antibodies Adsorbed in a Matrix of Charged Polyelectrolytes and Study of the Formation of Immune Complexes
3.3. Characteristics of NWFETs with Antibodies Adsorbed in the Multilayers of Polyelectrolytes
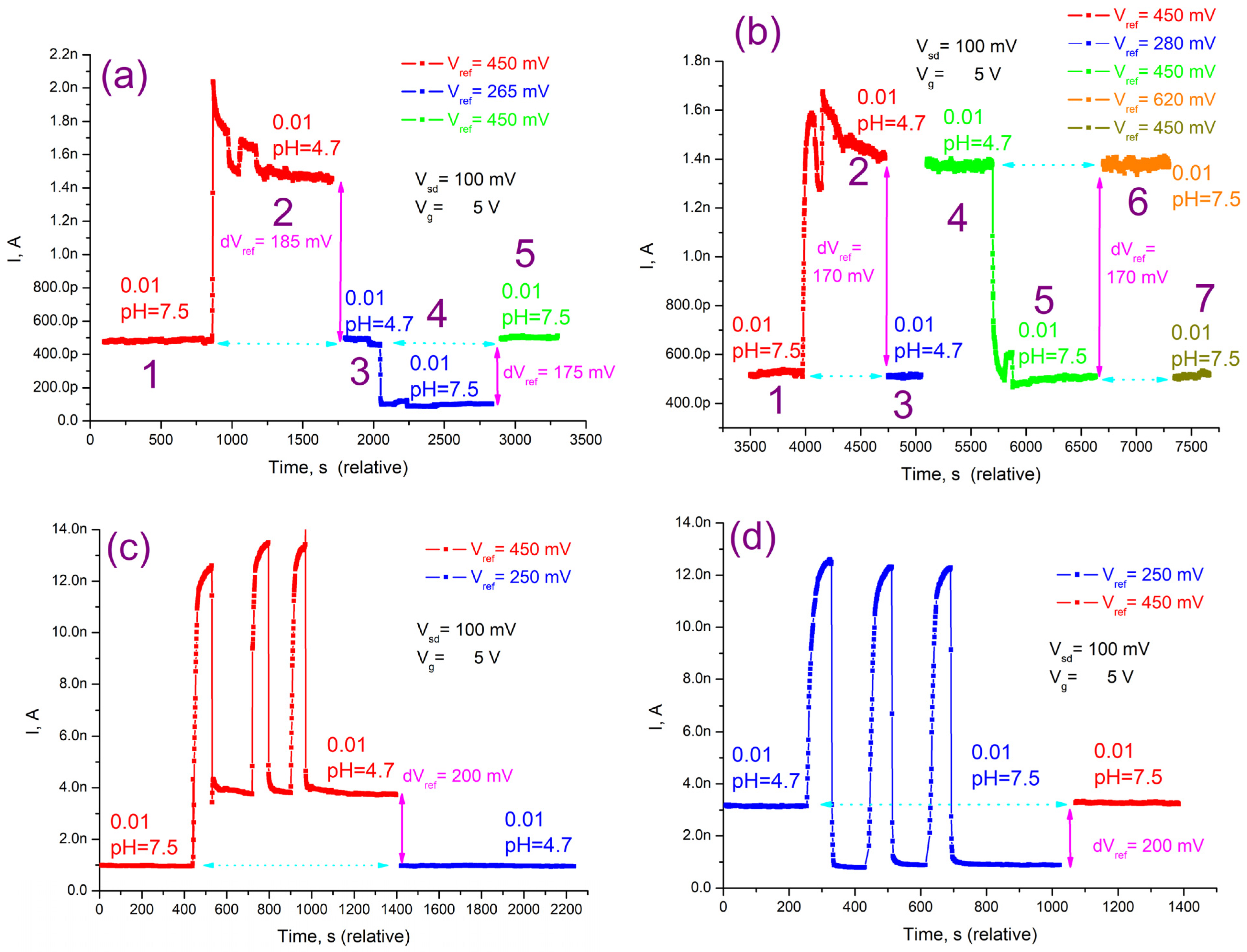
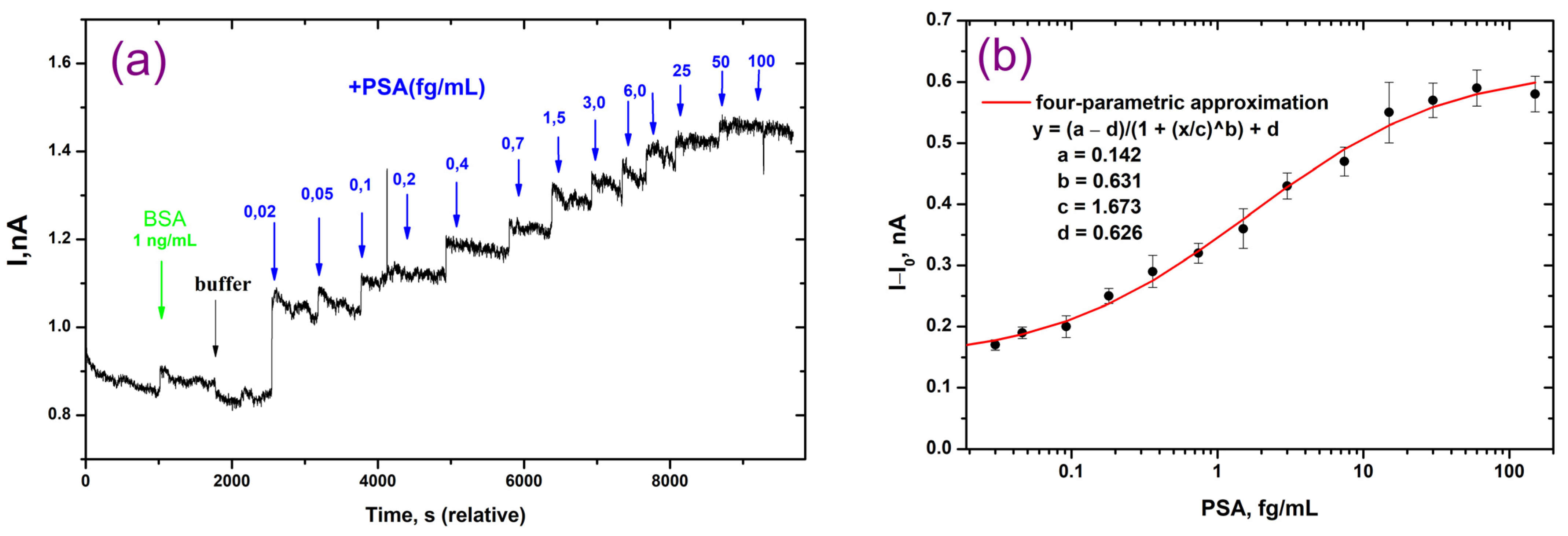
3.4. Detection of PSA on the NWFETs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. Trends Analyt. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Salfi, J.; Savelyev, I.G.; Blumin, M.; Nair, S.V.; Ruda, H.E. Direct observation of single-charge detection capability of nanowire field-effect transistors. Nat. Nanotech. 2010, 5, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Clement, N.; Nishiguchi, K.; Dufreche, J.F.; Guerin, D.; Fujiwara, A.; Vuillaume, D. A silicon nanowire ion-sensitive field-effect transistor with the elementary charge sensitivity. Appl. Phys. Lett. 2011, 98, 014104. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, S.; Koo, K.-I.; Kim, K. Nanowire-Based Sensors for Biological and Medical Applications. IEEE Trans. NanoBiosci. 2016, 15, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Panahi, A.; Sadighbayan, D.; Forouhi, S.; Ghafar-Zadeh, E. Recent Advances of Field-Effect Transistor Technology for Infectious Diseases. Biosensors 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.A.; Ivanov, Y.D.; Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Kozlov, A.F.; Archakov, A.I.; Popov, V.P.; Fomin, B.I.; Latyshev, A.V. A SOI-nanowire biosensor for the multiple detection of D-NFATc1 protein in the serum. Anal. Methods 2015, 7, 8078–8085. [Google Scholar] [CrossRef]
- Presnova, G.; Presnov, D.; Krupenin, V.; Grigorenko, V.; Trifonov, A.; Andreeva, I.; Ignatenko, O.; Egorov, A.; Rubtsova, M. Biosensor based on a silicon nanowire field-effect transistor functionalized by gold nanoparticles for the highly sensitive determination of prostate specific antigen. Biosens. Bioelectron. 2017, 88, 283–289. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, J.; Liu, J.; Li, X.; Sun, S.; Luan, X.; Zhao, Y.; Wei, S.; Li, M.; Zhang, Q.; et al. Si nanowire Bio-FET for electrical and label-free detection of cancer cell-derived exosomes. Microsyst. Nanoeng. 2022, 8, 57. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Kim, K.; Park, C.; Kwon, D.; Kim, D.; Meyyappan, M.; Jeon, S.; Lee, J.S. Silicon nanowire biosensors for detection of cardiac troponin I (cTnI) with high sensitivity. Biosens. Bioelectron. 2016, 77, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, F.M.; Uhlig, M.R.; Garcia, R. Molecular Recognition by Silicon Nanowire Field-Effect Transistor and Single-Molecule Force Spectroscopy. Micromachines 2022, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chen, S.; Solomon, P.; Zhang, Z. Ion sensing with single charge resolution using sub–10-nm electrical double layer–gated silicon nanowire transistors. Sci. Adv. 2021, 7, eabj6711. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, A.; Awwad, F.; Gelovani, J.G.; Qamhieh, N.; Ayesh, A.I. COVID-19 Detection via Silicon Nanowire Field-Effect Transistor: Setup and Modeling of Its Function. Nanomaterials 2022, 12, 2638. [Google Scholar] [CrossRef] [PubMed]
- Abidin, W.A.B.Z.; Nor, N.M.M.; Arshad, M.K.M.; Fathil, M.F.M.; Parmin, N.A.; Sisin, N.A.H.T.; Ibau, C.; Azlan, A.S. Femtomolar Dengue Virus Type-2 DNA Detection in Back-gated Silicon Nanowire Field-effect Transistor Biosensor. Curr. Nanosci. 2022, 18, 139–146. [Google Scholar] [CrossRef]
- Zafar, S.; D’Emic, C.; Jagtiani, A.; Kratschmer, E.; Miao, X.; Zhu, Y.; Mo, R.; Sosa, N.; Hamann, H.; Shahidi, G.; et al. Silicon Nanowire Field Effect Transistor Sensors with Minimal Sensor-to-Sensor Variations and Enhanced Sensing Characteristics. ACS Nano 2018, 12, 6577–6587. [Google Scholar] [CrossRef]
- Kesler, V.; Murmann, B.; Soh, H.T. Going beyond the Debye Length: Overcoming Charge Screening Limitations in Next-Generation Bioelectronic Sensors. ACS Nano 2020, 14, 16194–16201. [Google Scholar] [CrossRef] [PubMed]
- Presnov, D.E.; Bozhev, I.V.; Miakonkikh, A.V.; Simakin, S.G.; Trifonov, A.S.; Krupenin, V.A. Local sensor based on nanowire field effect transistor from inhomogeneously doped silicon on insulator. J. Appl. Phys. 2018, 123, 054503. [Google Scholar] [CrossRef]
- Rajan, N.K.; Routenberg, D.A.; Reed, M.A. Optimal signal-to-noise ratio for silicon nanowire biochemical sensors. Appl. Phys. Lett. 2018, 98, 264107. [Google Scholar] [CrossRef]
- De Vico, L.; Sorensen, M.H.; Iversen, L.; Rogers, D.M.; Sorensen, B.S.; Brandbyge, M.; Nygard, J.; Martinez, L.; Jensen, J.H. Quantifying signal changes in nano-wire based biosensors. Nanoscale 2011, 3, 706–717. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; To, S.; You, L.; Sun, Y. Effect of nanowire number, diameter, and doping density on nano-FET biosensor sensitivity. ACS Nano 2011, 5, 6661–6668. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Wagner, R.; Sigworth, R.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the Debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Awsiuk, K.; Budkowski, A.; Psarouli, A.; Petrou, P.; Bernasik, A.; Kakabakos, S.; Rysz, J.; Raptis, I. Protein adsorption and covalent bonding to silicon nitride surfaces modified with organo-silanes: Comparison using AFM, angle-resolved XPS and multivariate ToF-SIMS analysis. Colloids Surf. B Biointerfaces 2013, 110, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ghobaei Namhil, Z.; Kemp, C.; Verrelli, E.; Iles, A.; Pamme, N.; Adawi, A.M.; Kemp, N.T. A label-free aptamer-based nanogap capacitive biosensor with greatly diminished electrode polarization effects. Phys. Chem. Chem. Phys. 2019, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Zhou, W.; Jiang, X.; Hong, G.; Fu, T.-M.; Lieber, C.M. General Strategy for Biodetection in High Ionic Strength Solutions Using Transistor-Based Nanoelectronic Sensors. Nano Lett. 2015, 15, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Henzler, K.; Welsch, N.; Ballauff, M.; Borisov, O. Proteins and polyelectrolytes: A charged relationship. Curr. Opin. Colloid Interface Sci. 2012, 17, 90–96. [Google Scholar] [CrossRef]
- Lee, A.A.; Kostinski, S.V.; Brenner, M.P. Controlling Polyelectrolyte Adsorption onto Carbon Nanotubes by Tuning Ion–Image Interactions. J. Phys. Chem. B 2018, 122, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Stekolshchikova, A.A.; Radaev, A.V.; Orlova, O.Y.; Nikolaev, K.G.; Skorb, E.V. Thin and Flexible Ion Sensors Based on Polyelectrolyte Multilayers Assembled onto the Carbon Adhesive Tape. ACS Omega 2019, 4, 15421–15427. [Google Scholar] [CrossRef]
- Zhao, H.; Ibrahimova, V.; Garanger, E.; Lecommandoux, S. Dynamic Spatial Formation and Distribution of Intrinsically Disordered Protein Droplets in Macromolecularly Crowded Protocells. Angew. Chem. Int. Ed. 2020, 59, 11028–11036. [Google Scholar] [CrossRef] [PubMed]
- Baldina, A.A.; Nikolaev, K.G.; Ivanov, A.S.; Nikitina, A.A.; Rubtsova, M.Y.; Vorovitch, M.F.; Ishmukhametov, A.A.; Egorov, A.M.; Skorb, E.V. Immunochemical Biosensor for Single Virus Particle Detection Based on Crowding Molecular Polyelectrolyte System. J. Appl. Polym. Sci. 2022, 139, 52360. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Green, C.P.; Lioe, H.; Cleveland, J.P.; Proksch, R.; Mulvaney, P.; Sader, J.E. Normal and torsional spring constants of atomic force microscope cantilevers. Rev. Sci. Instr. 2004, 75, 1988–1996. [Google Scholar] [CrossRef]
- Tsiniaikin, I.I.; Presnova, G.V.; Bozhev, I.V.; Skorik, A.A.; Rubtsova, M.Y.; Kamalov, A.A.; Matskeplishvili, S.T.; Snigirev, O.V.; Krupenin, V.A.; Presnov, D.E. A sensor system based on a field-effect transistor with a nanowire channel for the quantitative determination of thyroid-stimulating hormone. Mosc. Univ. Phys. Bull. 2020, 75, 645–656. [Google Scholar] [CrossRef]
- Presnova, G.V.; Tcinyaykin, I.I.; Bozhev, I.V.; Rubtsova, M.Y.; Shorokhov, V.V.; Trifonov, A.S.; Ulyashova, M.M.; Krupenin, V.A.; Presnov, D.E. Thyroglobulin detection by biosensor based on two independent Si NWFETs. Proc. SPIE-Int. Soc. Opt. Eng. 2019, 11022, 110220Z. [Google Scholar] [CrossRef]
- Soranno, A.; Koenig, I.; Borgia, M.B.; Hofmann, H.; Zosel, F.; Nettels, D.; Schuler, B. Single-molecule spectroscopy reveals polymer effects of disordered proteins in crowded environments. Proc. Natl. Acad. Sci. USA 2014, 111, 4874–4879. [Google Scholar] [CrossRef]
- Filoti, D.I.; Shire, S.J.; Yadav, S.; Laue, T.M. Comparative study of analytical techniques for determining protein charge. J. Pharm. Sci. 2015, 104, 2123–2131. [Google Scholar] [CrossRef]
- Füzik, T.; Formanov, P.; Ružek, D.; Yoshii, K.; Niedrig, M.; Plevka, P. Structure of tick-borne encephalitis virus and its neutralization by a monoclonal antibody. Nat. Commun. 2018, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Straeten, A.V.; Bratek-Skicki, A.; Jonas, A.M.; Fustin, C.-A.; Dupont-Gillain, C. Integrating Proteins in Layer-by-Layer Assemblies Independently of their Electrical Charge. ACS Nano 2018, 12, 8372–8381. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, A.S.; Presnov, D.E.; Bozhev, I.V.; Evplov, D.A.; Desmaris, V.; Krupenin, V.A. Non-contact scanning probe technique for electric field measurements based on nanowire field-effect transistor. Ultramicroscopy 2017, 179, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Van Hal, R.E.G.; Eijkel, J.C.T.; Bergveld, P. A novel description of ISFET sensitivity with the buffer capacity and double-layer capacitance as key parameters. Sens. Actuators B 1995, 24, 201–205. [Google Scholar] [CrossRef]
- Gao, A.; Lu, N.; Dai, P.; Fan, C.; Wang, Y.; Li, T. Direct ultrasensitive electrical detection of prostate cancer biomarkers with CMOS-compatible n- and p-type silicon nanowire sensor arrays. Nanoscale 2014, 6, 13036–13042. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, J.; Miao, B.; Zhao, L.; Liu, X.; Wu, D.; Li, J. Highly sensitive AlGaN/GaN HEMT biosensors using an ethanolamine modification strategy for bioassay applications. RSC Adv. 2019, 9, 15341–15349. [Google Scholar] [CrossRef]
- Mwanza, D.; Adeniyi, O.; Tesfalidet, S.; Nyokong, T.; Mashazi, P. Capacitive Label-Free Ultrasensitive Detection of PSA on a Covalently Attached Monoclonal Anti-PSA Antibody Gold Surface. J. Electroanal. Chem. 2022, 927, 116983. [Google Scholar] [CrossRef]
- Liu, Y. Highly sensitive sensing detection of prostate-specific antigen based on point-of-care electrochemical immunosensor. Int. J. Electrochem. Sci. 2023, 18, 100028. [Google Scholar] [CrossRef]
- Ma, K.; Zheng, Y.; An, L.; Liu, J. Ultrasensitive Immunosensor for Prostate-Specific Antigen Based on Enhanced Electrochemiluminescence by Vertically Ordered Mesoporous Silica-Nanochannel Film. Front. Chem. 2022, 10, 851178. [Google Scholar] [CrossRef] [PubMed]
- Mandal, N.; Pakira, V.; Samanta, N.; Das, N.; Chakraborty, S.; Pramanick, B.; RoyChaudhuri, C. PSA Detection using Label Free Graphene FET with Coplanar Electrodes Based Microfluidic Point of Care Diagnostic Device. Talanta 2021, 222, 121581. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, X.; Lee, Y.K.; Zhang, A. Direct Label-free Protein Detection in High Ionic Strength Solution and Human Plasma Using Dual-Gate Nanoribbon-based Ion-Sensitive Field-Effect Transistor Biosensor. Biosens. Bioelectron. 2018, 117, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Rani, D.; Pachauri, V.; Madaboosi, N.; Jolly, P.; Vu, X.T.; Estrela, P.; Chu, V.; Conde, J.P.; Ingebrandt, S. Top-Down Fabricated Silicon Nanowire Arrays for Field-Effect Detection of Prostate-Specific Antigen. ACS Omega 2018, 3, 8471–8482. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sachdeva, A.; Peeters, M.; McClements, J. Point-of-Care Prostate Specific Antigen Testing: Examining Translational Progress toward Clinical Implementation. ACS Sens. 2023, 8, 3643–3658. [Google Scholar] [CrossRef]


| Components of the Matrix | Matrix Thickness Measured by SEM, nm | Matrix Thickness Measured by AFM, nm | Surface Roughness Measured by AFM, nm |
|---|---|---|---|
| No matrix (native silicon with a layer of silicon oxide) | 3 ± 0.5 | ND | 0.5 |
| PEI | 6 ± 3 | 10 ± 2 | 3.7 |
| PEI, AB/PSA, PSS | 22 ± 7 | 23 ± 5 | 3.7 |
| (PEI, AB/PSA, PSS) after interacting with PSA | 27 ± 10 | 32 ± 5 | 7.8 |
| (PEI, AB/PSA, PSS) after interacting with PSA and Ab/PSA–AuNPs | 25 ± 3 * | 35 ± 5 | 5.0 |
| Control: (PEI, AB/non-specific, PSS) after interacting with PSA and Ab/PSA–AuNPs | 20 ± 5 * | 37 ± 5 | 5.3 |
| Control: (PEI, AB/non-specific, PSS) after interacting with PSA and Ab/non-specific–AuNPs | 20 ± 5 * | 24 ± 5 | 3.3 |
| Biosensor Type/Surface Modification | Limit of PSA Detection, fg/mL | Detection Range, fg/mL | Reference |
|---|---|---|---|
| Si NWFETs//matrix of polyelectrolytes PEI and PSS | 0.04 (1.2 aM) | 0.06–60 | This study |
| Si NWFETs//GOPS-SH and AuNPs//the same clone of antibodies to PSA | 23 | 23–5 × 108 | [8] |
| n- and p-type Si NW FET arrays//APTES and glutaraldehyde | 1.0 | 1.0–105 | [41] |
| AlGaN/GaN HEMT//ethanolamine | 1 (in PBS buffer) | 1–108 (1 fg/mL to 100 ng/mL). | [42] |
| Electrochemical capacitive immunosensor/gold electrode modified with a thin film of isophthalic acid | 3.3 (3.3 × 10−6 ng/mL) | 1–108 (1 × 10−5−100 ng/mL) | [43] |
| Immunosensor//screen-printed electrodes//AgNPs/ds- DNA/PEDOT | 17 | 100–108 (0.1 pg/mL–100 ng/mL) | [44] |
| Electrochemical immunosensor with silica nanochannel films//GPTMS | 100 0.1 pg/mL | 103–108 (1 pg/mL to 100 ng/mL) | [45] |
| Graphene FET with coplanar electrodes//glutaraldehyde | 103 (1 pg/mL in serum) | Up to 4 × 107 (up to 4 ng/mL in 100 mM phosphate buffer) | [46] |
| A dual-gate PEG-modified NR-ISFET nanoribbon-based ion-sensitive field-effect transistor (NR-ISFET)//polyethylene glycol | 10 pM (in 100 mM phosphate buffer) | 10 pM to 1 μM (in 100 mM phosphate buffer) | [47] |
| Si NW-Ionsensitive field-effect transistor (Si-NW ISFET)//DNA aptamer | ND * | 103–109 (1 pg/mL to 1 μg/mL) | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presnova, G.V.; Presnov, D.E.; Ulyashova, M.M.; Tsiniaikin, I.I.; Trifonov, A.S.; Skorb, E.V.; Krupenin, V.A.; Snigirev, O.V.; Rubtsova, M.Y. Ultrasensitive Detection of PSA Using Antibodies in Crowding Polyelectrolyte Multilayers on a Silicon Nanowire Field-Effect Transistor. Polymers 2024, 16, 332. https://doi.org/10.3390/polym16030332
Presnova GV, Presnov DE, Ulyashova MM, Tsiniaikin II, Trifonov AS, Skorb EV, Krupenin VA, Snigirev OV, Rubtsova MY. Ultrasensitive Detection of PSA Using Antibodies in Crowding Polyelectrolyte Multilayers on a Silicon Nanowire Field-Effect Transistor. Polymers. 2024; 16(3):332. https://doi.org/10.3390/polym16030332
Chicago/Turabian StylePresnova, Galina V., Denis E. Presnov, Mariya M. Ulyashova, Ilia I. Tsiniaikin, Artem S. Trifonov, Ekaterina V. Skorb, Vladimir A. Krupenin, Oleg V. Snigirev, and Maya Yu. Rubtsova. 2024. "Ultrasensitive Detection of PSA Using Antibodies in Crowding Polyelectrolyte Multilayers on a Silicon Nanowire Field-Effect Transistor" Polymers 16, no. 3: 332. https://doi.org/10.3390/polym16030332
APA StylePresnova, G. V., Presnov, D. E., Ulyashova, M. M., Tsiniaikin, I. I., Trifonov, A. S., Skorb, E. V., Krupenin, V. A., Snigirev, O. V., & Rubtsova, M. Y. (2024). Ultrasensitive Detection of PSA Using Antibodies in Crowding Polyelectrolyte Multilayers on a Silicon Nanowire Field-Effect Transistor. Polymers, 16(3), 332. https://doi.org/10.3390/polym16030332






