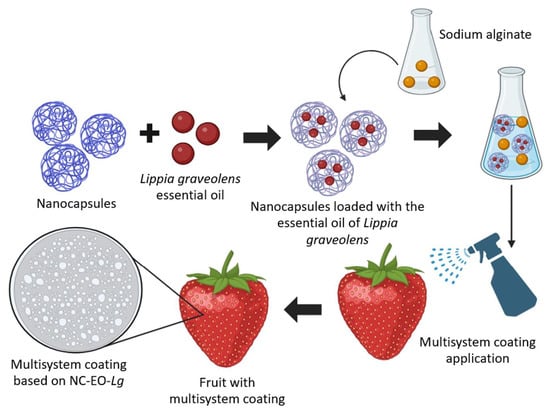
Enhancement of Strawberry Shelf Life via a Multisystem Coating Based on Lippia graveolens Essential Oil Loaded in Polymeric Nanocapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Physicochemical Characterization of Essential Oil (EO)
2.2.1. Isolation of EO
2.2.2. Physical Characterization of EO
2.2.3. Chemical Composition of EO Using Gas Chromatography–Mass Spectrometry (GC-MS) and GC with Flame Ionization Detection (GC-FID)
2.3. Preparation and Characterization of the Nanocapsules
2.4. Preparation of the Coatings
2.5. Physical Characterization of the Coatings
2.5.1. Optical Evaluation
2.5.2. Mechanical Evaluation
2.5.3. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.5.4. Water Vapor Barrier Properties
2.6. Application of the Coatings on Strawberries
2.6.1. Weight Loss Percentage and Texture Analyses: Penetration Test
2.6.2. Visual Microbiological Damage
2.7. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Essential Oil of Lippia graveolens (EO-Lg)
3.2. Physical Properties of the EO-Lg
3.3. Chemical Composition of the EO Using Gas Chromatography–Mass Spectrometry (GC-MS) and GC with Flame Ionization Detection (GC-FID)
3.4. Preparation and Characterization of the NC-EO-Lg
3.5. Preparation of the Coating
3.6. Physical Characterization of the Coatings
3.6.1. Optical Evaluation
3.6.2. Mechanical Evaluation
3.6.3. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
3.6.4. Water Vapor Barrier Properties
3.7. Application of Multisystem Coatings in Strawberries
3.7.1. Weight Loss Percentage and Texture Analyses: Penetration Test
3.7.2. Visual Microbiological Damage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. El Estado de la Alimentación y la Agricultura. In Avanzar en la Reducción de la Pérdida y el Desperdicio de Alimentos; FAO: Rome, Italy, 2019. [Google Scholar]
- Curiel, J.A. Application of biotechnological techniques aimed to obtain bioactive compounds from food industry by-products. Biomolecules 2021, 12, 88. [Google Scholar] [CrossRef]
- Pace, B.; Cefola, M. Innovative preservation technology for the fresh fruit and vegetables. Foods 2021, 29, 719. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, R.; Martínez-Zafra, M.T.; Castillejo, N.; Guillamón-Frutos, A.; Artés-Hernández, F. Real-time monitoring system for shelf life estimation of fruit and vegetables. Sensors 2020, 20, 1860. [Google Scholar] [CrossRef]
- Mendoza-Barboza, C.R.; Borges, C.D.; Kringel, A.L.; da-Silveira, R.P.; da-Silva, F.A.; Schulz, G.A.S. Application of microemulsions as coating in fresh cut strawberries. J. Food Sci. Technol. 2020, 57, 2764–2770. [Google Scholar]
- Trinetta, V.; McDaniel, A.; Batziakas, K.; Yucel, U.; Nwadike, L.; Pliakoni, E. Antifungal packaging film to maintain quality and control postharvest diseases in strawberries. Antibiotics 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Salsabiela, S.; Sukma-Sekarina, A.; Bagus, H.; Audiensi, A.; Azizah, F.; Heristika, W.; Susanto, E.; Munawaroh, H.S.H.; Show, P.L.; Ningrum, A. Development of edible coating from gelatin composites with the addition of black tea extract (Camellia sinensis) on minimally processed watermelon (Citrullus lanatus). Polymers 2022, 28, 2628. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, M.; Di-Stasio, M.; Sorrentino, A.; La-Cara, F.; Volpe, M.G. Active edible polysaccharide-based coating for preservation of fresh figs (Ficus carica L.). Foods 2020, 3, 1793. [Google Scholar] [CrossRef]
- Nor, S.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef]
- Salas-Méndez, E.; Pinheiro, A.C.; Ballesteros, L.F.; Silva, P.; Rodríguez-García, R.; Jasso-de-Rodríguez, D. Application of edible nanolaminate coatings with antimicrobial extract of Flourensia cernua to extend the shelf-life of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2019, 150, 19–27. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of chitosan and alginate-based coatings enriched with cinnamon essential oil microcapsules to improve the postharvest quality of mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef]
- Tavares, L.S.; de-Souza, V.C.; Schmitz-Nunes, V.; Nascimento-Silva, O.; de-Souza, G.T.; Farinazzo-Marques, L.; Capriles-Goliatt, P.V.Z.; Facio-Viccini, L.; Franco, O.L.; de-Oliveira-Santos, M. Antimicrobial peptide selection from Lippia spp leaf transcriptomes. Peptides 2020, 129, 170317. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Heredia, J.B. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α- amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Int. Food Res. J. 2019, 116, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Matadamas-Ortiz, A.; Hernández-Hernández, E.; Castaño-Tostado, E.; Amaro-Reyes, A.; García-Almendárez, B.E.; Velazquez, G.; Regalado-González, C. Long-term refrigerated storage of beef using an active edible film reinforced with mesoporous silica nanoparticles containing oregano essential oil (Lippia graveolens Kunth). Int. J. Mol. Sci. 2022, 24, 92. [Google Scholar] [CrossRef]
- Rodríguez-García, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Moctezuma, E.; Gutierrez-Pacheco, M.M.; Tapia-Rodriguez, M.R.; Ortega-Ramirez, L.A.; Ayala-Zavala, J.F. Oregano (Lippia graveolens) essential oil added within pectin edible coatings prevents fungal decay and increases the antioxidant capacity of treated tomatoes. J. Sci. Food Agric. 2016, 11, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 14, 989. [Google Scholar] [CrossRef]
- Herrera-Rodríguez, S.E.; López-Rivera, R.J.; García-Márquez, E.; Estarrón-Espinosa, M.; Espinosa-Andrews, H. Mexican oregano (Lippia graveolens) essential oil-in-water emulsions: Impact of emulsifier type on the antifungal activity of Candida albicans. Food Sci. Biotechnol. 2019, 28, 441–448. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da-Rocha, S.R.P.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Elaissari, A. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 15, 67–78. [Google Scholar] [CrossRef]
- Odetayo, T.; Tesfay, S.; Ngobese, N.Z. Nanotechnology-enhanced edible coating application on climacteric fruits. Food Sci. Nutr. 2022, 20, 2149–2167. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, W.; Yang, K.; Li, C. Effects of sodium alginate edible coating with cinnamon essential oil nanocapsules and nisin on quality and shelf life of beef slices during refrigeration. J. Food Prot. 2022, 1, 896–905. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, B.J. Cubierta Biopolimérica de Nanoingredientes a Base de Thymus vulgaris con Potencial Aplicación en Productos Hortofrutícolas. Master’s Thesis, Universidad Autónoma de Nuevo León, Monterrey Nuevo León, Mexico, 2020. [Google Scholar]
- Piña-Barrera, A.M.; Álvarez-Román, R.; Báez-González, J.G.; Amaya-Guerra, C.A.; Rivas-Morales, C.; Gallardo-Rivera, C.T.; Galindo-Rodríguez, S.A. Application of a multisystem coating based on polymeric nanocapsules containing essential oil of Thymus vulgaris L. to increase the shelf life of table grapes (Vitis vinifera L.). IEEE Trans. Nanotechnol. 2019, 18, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Samba, N.; Aitfella-Lahlou, R.; Nelo, M.; Silva, L.; Coca, R.; Rocha, P.; López-Rodilla, J.M. Chemical composition and antibacterial activity of Lippia multiflora moldenke essential oil from different regions of Angola. Molecules 2020, 26, 155. [Google Scholar] [CrossRef] [PubMed]
- Permanent Commission of Pharmacopoeia of the United Mexican States. Farmacopea de los Estados Unidos Mexicanos, 10th ed.; Secretaría de Salud: Mexico City, Mexico, 2011.
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Ye, J.; Ma, D.; Qin, W.; Liu, Y. Physical and antibacterial properties of sodium alginate-sodium carboxymethylcellulose films containing Lactococcus lactis. Molecules 2018, 23, 2645. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Al-Harrasi, A.; Al-Azri, M.S.; Ullah, S.; Bekhit, A.E.A.; Pratap-Singh, A.; Chatli, M.K.; Anwer, M.K.; Aldawsari, M.F. Preparation and physiochemical characterization of bitter orange oil loaded sodium alginate and casein based edible films. Polymers 2022, 15, 3855. [Google Scholar] [CrossRef]
- ASTM E-96-95; Standard Test Method for Water Vapor Transmission of Materials. ASTM International: West, Conshohocken, PA, USA, 1995.
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Lotti, N. Characterization of active edible films based on citral essential oil, alginate and pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef]
- Dilworth, L.; Riley, C.K.; Stennett, D. Plant Constituents: Carbohydrates, oils, resins, balsams, and plant hormones. In Pharmacognosy Fundamentals, Applications and Strategie; Academic Press: Cambridge, MA, USA, 2017; pp. 61–80. [Google Scholar]
- Hernández-Hernández, E.; Regalado-González, C.; Vázquez-Landaverde, P.; Guerrero-Legarreta, I.; García-Almendárez, B.E. Microencapsulation, chemical characterization, and antimicrobial activity of Mexican (Lippia graveolens H.B.K.) and European (Origanum vulgare L.) oregano essential oils. Sci. World J. 2014, 64, 1814. [Google Scholar]
- Lugo-Estrada, L.; Galindo-Rodríguez, S.A.; Pérez-López, L.A.; Waksman-deTorres, N.; Álvarez-Román, R. Headspace–solid-phase microextraction gas chromatography method to quantify Thymus vulgaris essential oil in polymeric nanoparticles. Pharmacogn. Mag. 2019, 15, 473–478. [Google Scholar]
- Torrenegra-Alarcón, M.E.; Matiz-Melo, G.E.; González, J.G.; León-Méndez, G. In vitro antibacterial activity of the essential oil against microorganisms involved in acné. Rev. Cuba. Farm 2015, 49, 512–523. [Google Scholar]
- Domínguez, X. Métodos de Investigación Fitoquímica; Limusa S.A.: Ciudad de México, Mexico, 1985. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 1–804. [Google Scholar]
- Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Chávez-González, M.L.; Adame-Gallegos, J.R.; Nevárez-Moorillón, G.V. Mexican oregano (Lippia berlandieri Schauer and Poliomintha longiflora Gray) essential oils induce cell death by apoptosis in Leishmania (leishmania) mexicana promastigotes. Molecules 2022, 15, 5183. [Google Scholar] [CrossRef]
- Medina-Romero, Y.M.; Rodriguez-Canales, M.; Rodriguez-Monroy, M.A.; Hernandez-Hernandez, A.B.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Cruz-Sanchez, T.; Garcia-Tova, C.G.; Canales-Martinez, M.M. Effect of the essential oils of Bursera morelensis and Lippia graveolens and five pure compounds on the mycelium, spore production, and germination of species of fusarium. J. Fungi 2022, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Capatina, L.; Napoli, E.M.; Ruberto, G.; Hritcu, L. Origanum vulgare ssp. hirtum (Lamiaceae) essential oil prevents behavioral and oxidative stress changes in the scopolamine zebrafish model. Molecules 2021, 23, 7085. [Google Scholar] [CrossRef]
- Sharififard, M.; Alizadeh, I.; Jahanifard, E.; Wang, C.; Azemi, M.E. Chemical composition and repellency of Origanum vulgare essential oil against cimex lectularius under laboratory conditions. J. Arthropod. Borne Dis. 2018, 25, 387–397. [Google Scholar] [CrossRef]
- Ribeiro, F.P.; Santana-de-Oliveira, M.; de-Oliveira-Feitosa, A.; Santana-Barbosa- Marinho, P.; Moacir-do-Rosario-Marinho, A.; de-Aguiar-Andrade, E.H.; Favacho-Ribeiro, A. Chemical composition and antibacterial activity of the Lippia origanoides Kunth essential oil from the Carajás National Forest, Brazil. Evid. Based Complement. Alternat. Med. 2021, 19, 9930336. [Google Scholar] [CrossRef]
- Amiri, H. Essential oils composition and antioxidant properties of three thymus species. Evid.-Based Complement. Altern. Med. 2012, 2012, 728065. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Encapsulation of alendronate sodium by nanoprecipitation and double emulsion: From preparation to in vitro studies. Ind. Crops. Prod. 2015, 72, 24–33. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. Food Sci. Technol. 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Antonioli, G.; Fontanella, G.; Echeverrigaray, S.; Longaray-Delamare, A.P.; Fernandes-Pauletti, G.; Barcellos, T. Poly(lactic acid) nanocapsules containing lemongrass essential oil for postharvest decay control: In vitro and in vivo evaluation against phytopathogenic fungi. Food Chem. 2020, 1, 326. [Google Scholar] [CrossRef]
- Chávez-Magdaleno, M.E.; González-Estrada, R.R.; Ramos-Guerrero, A.; Plascencia-Jatomea, M.; Gutiérrez-Martínez, P. Effect of pepper tree (Schinus molle) essential oil-loaded chitosan bio-nanocomposites on postharvest control of Colletotrichum gloeosporioides and quality evaluations in avocado (Persea americana) cv. Hass. Food Sci. Biotechnol. 2018, 12, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Rodríguez, S.A.; Allemann, E.; Fessi, H.; Doelker, E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-difussion, and nanoprecipitation methods. Pharm. Res. 2004, 21, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.O.; Oliveira, E.F.; Paula, H.C.; de-Paula, R.C. Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 1, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.; Rodrigues, T.H.S.; Pereira, R.; Silva, L.M.A.; Cáceres, C.A.; Azeredo, H.M.; de-Canuto, K.M. Production and physico-chemical characterization of nanocapsules of the essential oil from Lippia sidoides Cham. Ind Crops Prod. 2016, 86, 279–288. [Google Scholar] [CrossRef]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/microspheres: Preparation, physicochemical characterization, and storage stability. Ind. Crops Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Salas-Cedillo, H.I. Desarrollo de un Potencial Insecticida Nanoparticulado de Schinus molle Para el Control de Aedes aegypti. Master’s Thesis, Universidad Autónoma de Nuevo León, Monterrey Nuevo León, Mexico, 2016. [Google Scholar]
- Cartaxo, A.L.; Costa-Pinto, A.R.; Martins, A.; Faria, S.; Gonçalves, V.M.F.; Tiritan, M.E.; Ferreira, H.; Neves, N.M. Influence of PDLA nanoparticles size on drug release and interaction with cells. J. Biomed. Mater. Res. A. 2019, 107, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Solano-Doblado, L.G.; Alamilla-Beltrán, L.; Jiménez-Martínez, C. Películas y recubrimientos comestibles funcionalizados. Rev. Espec. Cienc. Químico-Biológicas 2018, 21, 30–42. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Jawad, M.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. Physicochemical characterization and antioxidant properties of chitosan and sodium alginate based films incorporated with ficus extract. Polymers 2023, 28, 1215. [Google Scholar] [CrossRef]
- Yadav, R.K.; Nandy, B.C.; Maity, S.; Sarkar, S.; Saha, S. Phytochemistry, pharmacology, toxicology, and clinical trial of Ficus racemosa. Pharmacogn. Rev. 2015, 9, 73–80. [Google Scholar]
- Ma, Y.; Chen, S.; Liu, P.; He, Y.; Chen, F.; Cai, Y.; Yang, X. Gelatin improves the performance of oregano essential oil nanoparticle composite films-application to the preservation of mullet. Foods 2023, 29, 2542. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Moisture sensitivity, optical, mechanical and structural properties of whey protein-based edible films incorporated with rapeseed oil. Food Technol. Biotechnol. 2016, 54, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Gómez-Hoyos, C.; Zuluaga-Gallego, R.; López-Córdoba, A. Alginate-edible coatings for application on wild andean blueberries (Vaccinium meridionale Swartz): Effect of the addition of nanofibrils isolated from cocoa by-products. Polymers 2020, 5, 824. [Google Scholar] [CrossRef]
- Jancy, S.; Shruthy, R.; Preetha, R. Fabrication of packaging film reinforced with cellulose nanoparticles synthesised from jack fruit non-edible part using response surface methodology. Int. J. Biol. Macromol. 2020, 142, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lofland, S.; Hu, X. Thermal conductivity of protein-based materials: A review. Polymers 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Marrón, M.; Mani-López, E.; Palou, E.; López-Malo, A. Effects of alginate-glycerol-citric acid concentrations on selected physical, mechanical, and barrier properties of papaya puree-based edible films and coatings, as evaluated by response surface methodology. LWT 2018, 101, 83–91. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Noronha, C.M.; de-Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll. 2009, 23, 2102–2109. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, P.; Ye, K.; He, Y.; Chen, S.; Yuan, A.; Chen, F.; Yang, W. Preparation, characterization, in vitro release, and antibacterial activity of oregano essential oil chitosan nanoparticles. Foods 2022, 22, 3756. [Google Scholar] [CrossRef]
- González-Sandoval, D.C.; Luna-Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez-Fuentes, H.; Avendaño-Abarca, V.H.; Rojas, R. Formulation and characterization of edible films based on organic mucilage from mexican opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Li, Y.; Yao, M.; Liang, C.; Zhao, H.; Liu, Y.; Zong, Y. Hemicellulose and nano/microfibrils improving the pliability and hydrophobic properties of cellulose film by interstitial filling and forming micro/nanostructure. Polymers 2022, 23, 1297. [Google Scholar] [CrossRef] [PubMed]
- Al-Harrasi, A.; Bhatia, S.; Al-Azri, M.S.; Ullah, S.; Najmi, A.; Albratty, M.; Meraya, A.M.; Mohan, S.; Aldawsari, M.F. Effect of drying temperature on physical, chemical, and antioxidant properties of ginger oil loaded gelatin-sodium alginate edible films. Membranes 2022, 6, 862. [Google Scholar] [CrossRef] [PubMed]
- Martínez, K.; Ortiz, M.; Albis, A.; Gilma-Gutiérrez-Castañeda, C.; Valencia, M.E.; Grande-Tovar, C.D. The Effect of edible chitosan coatings incorporated with Thymus capitatus essential oil on the shelf-life of strawberry (Fragaria ananassa) during cold storage. Biomolecules 2018, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Heristika, W.; Ningrum, A.; Supriyadi-Munawaroh, H.S.H.; Show, P.L. Development of composite edible coating from gelatin-pectin incorporated garlic essential oil on physicochemical characteristics of red chili (Capsicum annnum L.). Gels 2023, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, K.A.; Ngiwngam, K.; Tongdeesoontorn, W. Polysaccharide-Based Active coatings incorporated with bioactive compounds for reducing postharvest losses of fresh fruits. Coatings 2022, 12, 8. [Google Scholar] [CrossRef]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of edible coating enriched with natural antioxidant extract and bergamot essential oil on the shelf life of strawberries. Foods 2023, 20, 488. [Google Scholar] [CrossRef]
- Pirozzi, A.; Del-Grosso, V.; Ferrari, G.; Donsì, F. Edible coatings containing oregano essential oil nanoemulsion for improving postharvest quality and shelf life of tomatoes. Foods 2020, 4, 1605. [Google Scholar] [CrossRef]







| Parameter | Relative Density 1 | Refractive Index (°) 1 | Specific Rotation (g/mL) 2 |
|---|---|---|---|
| Anethole (FEUM) | 0.983 − 0.988 | 1.557 − 1.561 | −0.150 − 0.150 |
| Anethole | 0.987 ± 0.000 | 1.559 ± 0.000 | 0.050 ± 0.000 |
| EO-Lippia graveolens | 0.987 ± 0.000 | 1.503 ± 0.000 | −0.200 ± 0.000 |
| EO-Lippia alba | 0.945 ± 0.005 | 1.462 ± 0.000 | −0.117 ± 0.028 |
| No. 1 | Composition | tR (min) 2 | Abundance | Component Type |
|---|---|---|---|---|
| 1 | α-thujene | 16.51 | 0.19 | Monoterpene hydrocarbon |
| 2 | α-pinene | 17.83 | 0.08 | Monoterpene hydrocarbon |
| 3 | myrcene | 18.37 | 16.93 | Monoterpene hydrocarbon |
| 4 | α-terpinene | 18.92 | 0.08 | Monoterpene hydrocarbon |
| 5 | p-cymene | 20.25 | 7.56 | Monoterpene hydrocarbon |
| 6 | 1,8-sineole | 22.88 | 0.04 | Monoterpene hydrocarbon |
| 7 | γ-terpinene | 26.18 | 0.08 | Monoterpene hydrocarbon |
| 8 | linalool | 26.63 | 0.12 | Oxygenated monoterpene |
| 9 | terpinen-4-ol | 29.29 | 0.10 | Oxygenated monoterpene |
| 10 | thymol methyl ether | 30.94 | 0.06 | Oxygenated monoterpene |
| 11 | thymol | 32.49 | 4.91 | Oxygenated monoterpene |
| 12 | carvacrol | 33.02 | 66.58 | Oxygenated monoterpene |
| 13 | z-caryophyllene | 36.96 | 2.99 | Sesquiterpene hydrocarbon |
| 14 | α-humulene | 38.33 | 0.11 | Sesquiterpene hydrocarbon |
| 15 | butyl hydroxyanisole | 41.49 | 0.07 | Sesquiterpene oxygenated |
| 16 | caryophyllene oxide | 43.52 | 0.03 | Sesquiterpene oxygenated |
| TOTAL | 100.00 |
| Mean Size (nm) 1 | PI 1 | Zeta Potential (mV) 1 | Component | %E 1 | %EE 1 | |
|---|---|---|---|---|---|---|
| NC-EO-Lg | 287 ± 5.11 | 0.10 ± 0.03 | −50.90 ± 1.44 | Myrcene | 0.10 ± 0.04 | 0.24 ± 0.02 |
| p-cymene | 0.17 ± 0.07 | 0.32 ± 0.01 | ||||
| Carvacrol | 27.91 ± 0.59 | 63.80 ± 1.47 |
| Coating | Opacity 1 (UA mm−1) | Adhesion 1 (Dynes cm−2) | Tensile Strength 1 (g cm−2) | Elongation at Break (%) 1 | WVP 1 (10−7 g mm cm−2 Pa−1 h−1) | WVTR 1 (10−3 g cm−2 h−1) |
|---|---|---|---|---|---|---|
| AL | 0.71 ± 0.10 | 4690.86 ± 2.00 | 977.56 ± 3.94 | 90.76 ± 0.71 | 2.79 ± 0.04 | 4.68 ± 0.09 |
| NP-BCO-AL | 0.96 ± 0.26 | 4989.22 ± 1.03 | 575.54 ± 5.27 | 78.28 ± 0.76 | 1.97 ± 0.03 | 3.72 ± 0.08 |
| NC-EO-Lg-AL | 0.60 ± 0.10 | 5802.36 ± 2.15 | 1555.26 ± 4.31 | 182.27 ± 2.14 | 1.01 ± 0.03 | 2.03 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Moreno, B.J.; Galindo-Rodríguez, S.A.; Rivas-Galindo, V.M.; Pérez-López, L.A.; Granados-Guzmán, G.; Álvarez-Román, R. Enhancement of Strawberry Shelf Life via a Multisystem Coating Based on Lippia graveolens Essential Oil Loaded in Polymeric Nanocapsules. Polymers 2024, 16, 335. https://doi.org/10.3390/polym16030335
González-Moreno BJ, Galindo-Rodríguez SA, Rivas-Galindo VM, Pérez-López LA, Granados-Guzmán G, Álvarez-Román R. Enhancement of Strawberry Shelf Life via a Multisystem Coating Based on Lippia graveolens Essential Oil Loaded in Polymeric Nanocapsules. Polymers. 2024; 16(3):335. https://doi.org/10.3390/polym16030335
Chicago/Turabian StyleGonzález-Moreno, Barbara Johana, Sergio Arturo Galindo-Rodríguez, Verónica Mayela Rivas-Galindo, Luis Alejandro Pérez-López, Graciela Granados-Guzmán, and Rocío Álvarez-Román. 2024. "Enhancement of Strawberry Shelf Life via a Multisystem Coating Based on Lippia graveolens Essential Oil Loaded in Polymeric Nanocapsules" Polymers 16, no. 3: 335. https://doi.org/10.3390/polym16030335
APA StyleGonzález-Moreno, B. J., Galindo-Rodríguez, S. A., Rivas-Galindo, V. M., Pérez-López, L. A., Granados-Guzmán, G., & Álvarez-Román, R. (2024). Enhancement of Strawberry Shelf Life via a Multisystem Coating Based on Lippia graveolens Essential Oil Loaded in Polymeric Nanocapsules. Polymers, 16(3), 335. https://doi.org/10.3390/polym16030335