The Crystallization and Melting Behavior of Neodymium-Based Butadiene Rubber Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Testing and Characterization
3. Results
3.1. Crystallization Behavior of Nd-BR
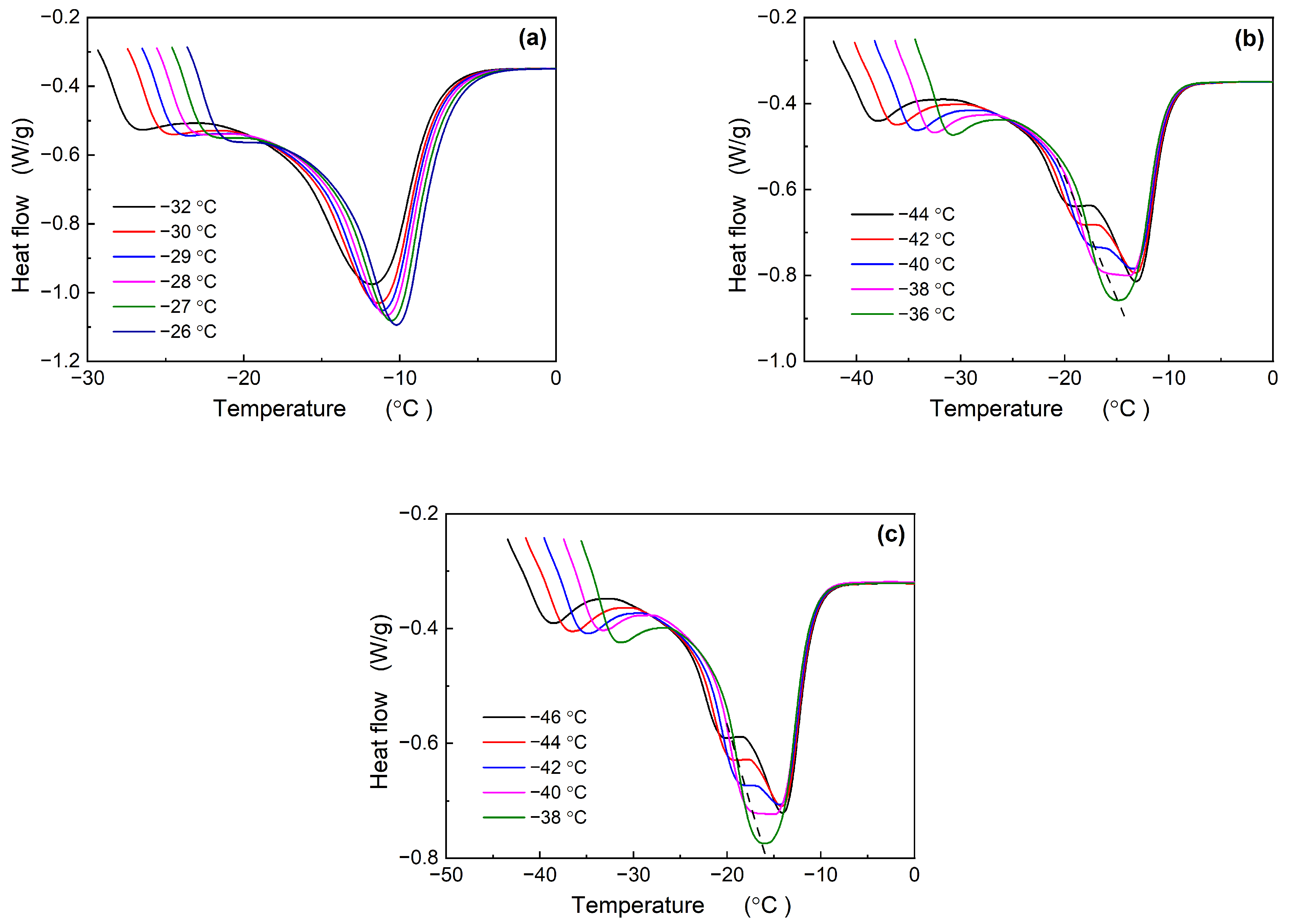
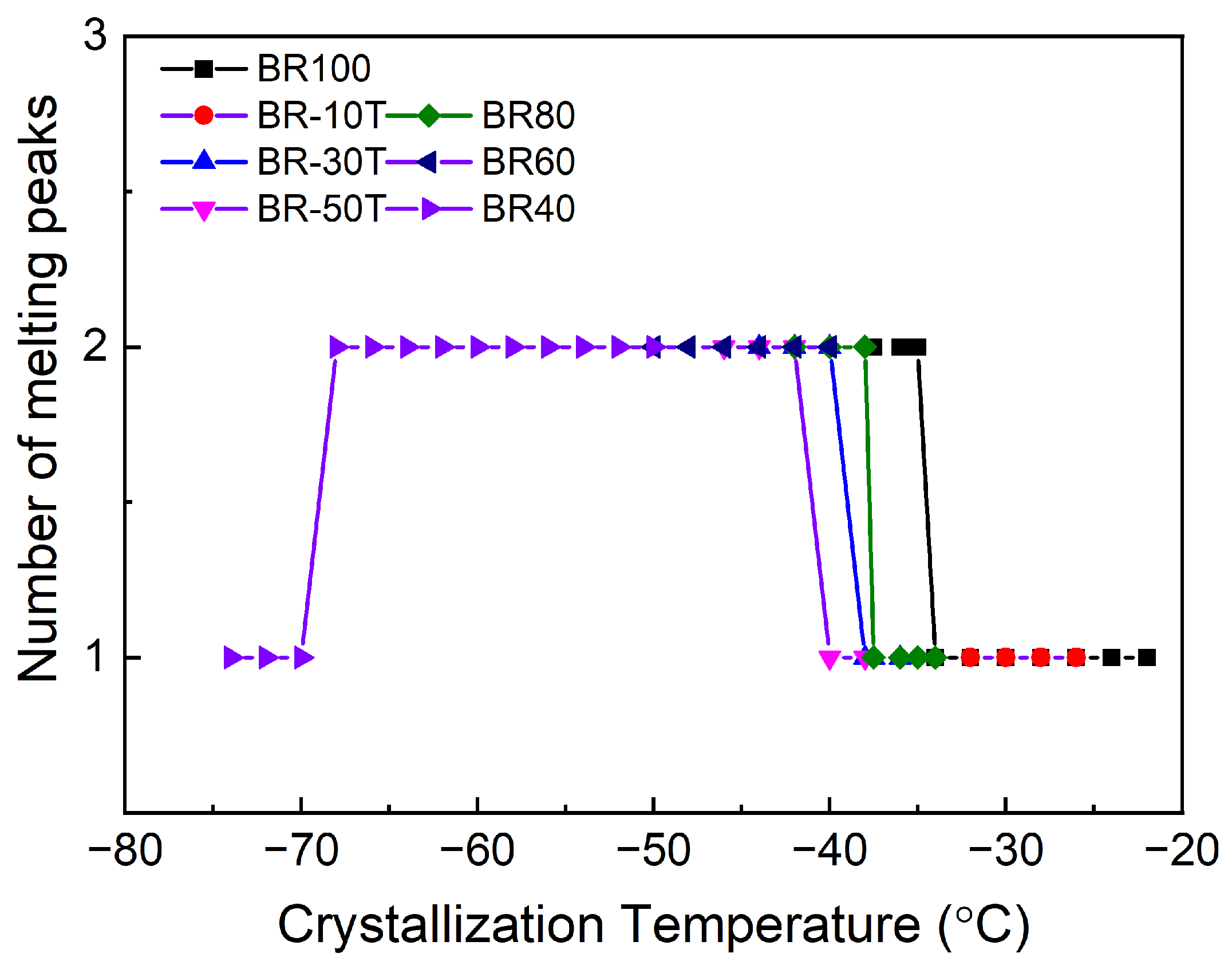
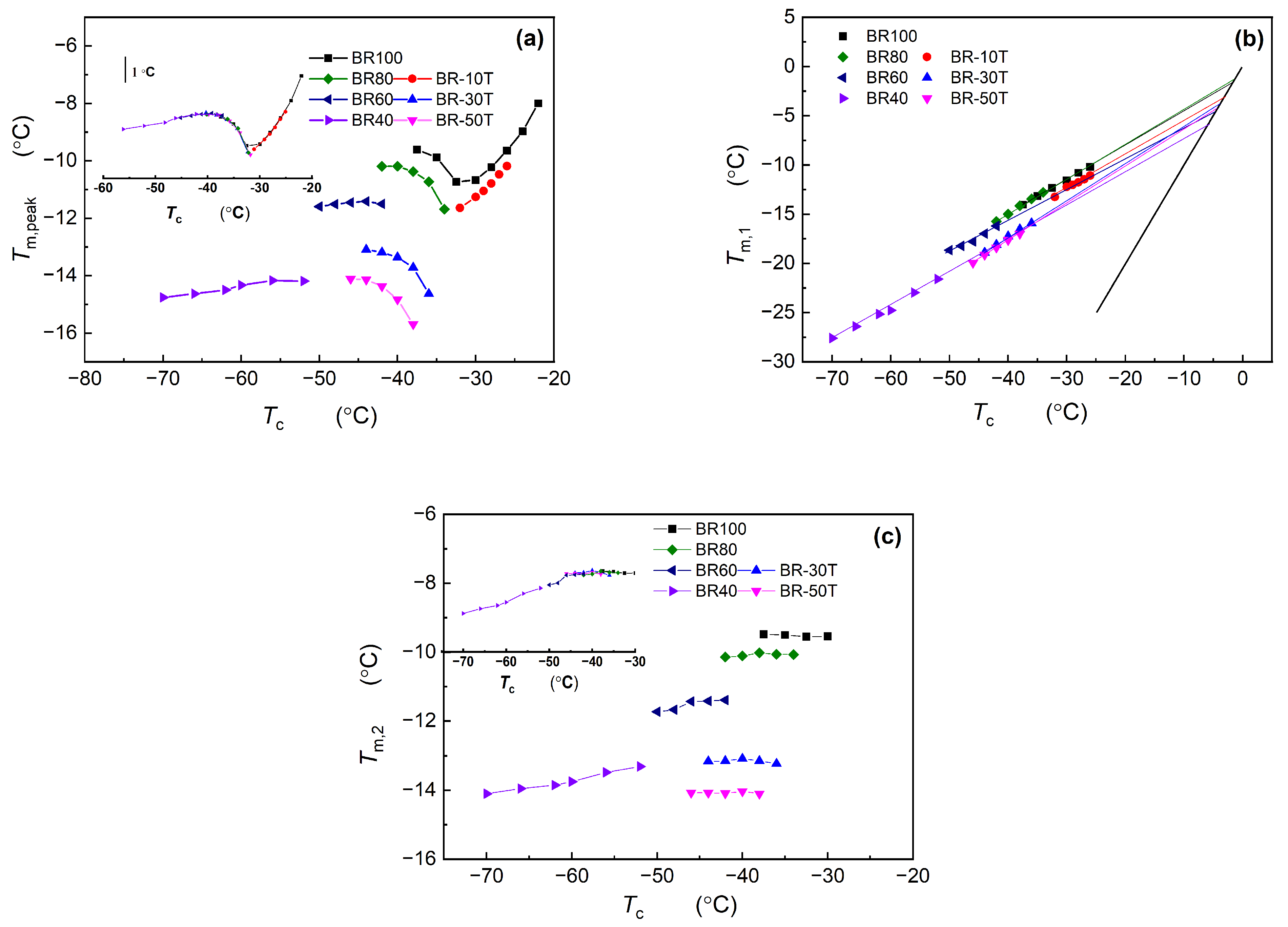
3.2. Analysis of Melting Behavior of Nd-BR and Its Composites
3.2.1. Calculation and Analysis of the Equilibrium Melting Point of Nd-BR
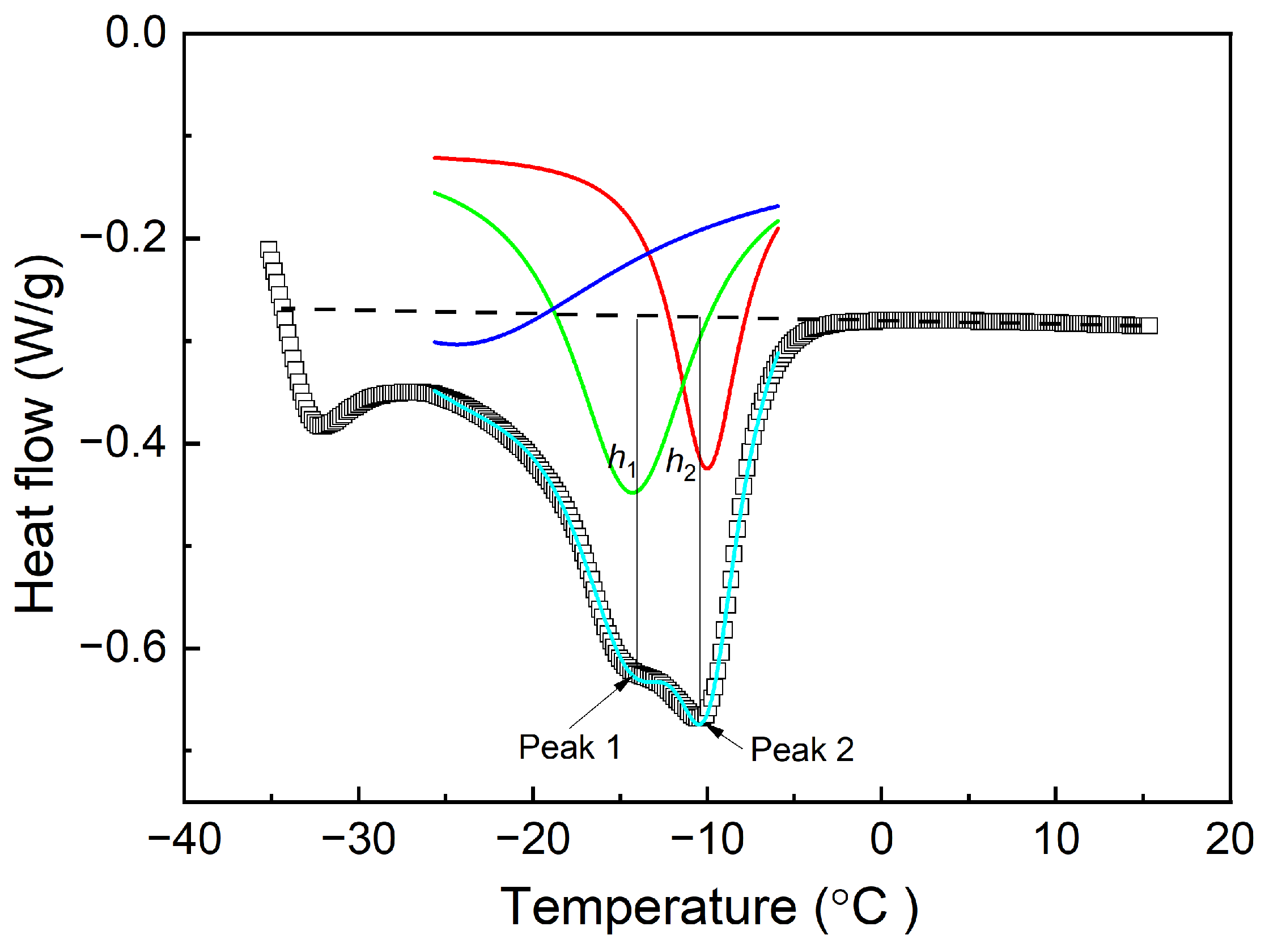
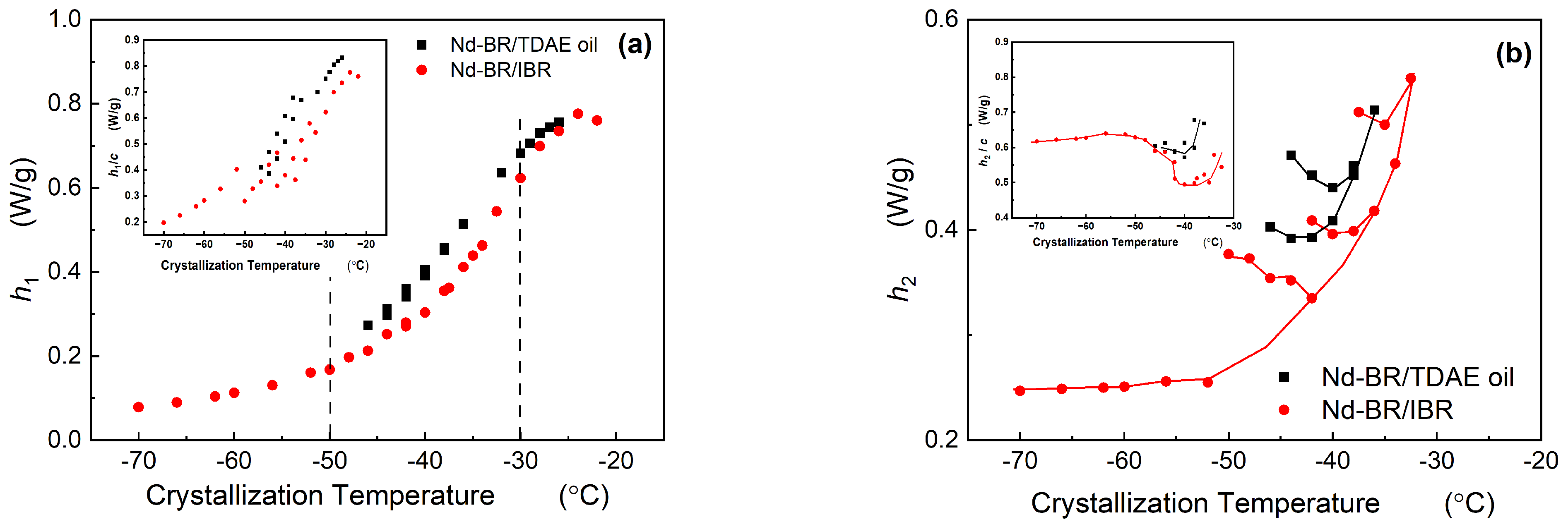
3.2.2. Height of Melting Peak
3.3. Analysis of the Impact of Diluents on Nd-BR Crystallization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, X.; Hu, Y.; Dai, Q. Synthesis of syndiotactic cis-1,4-polypentadiene by using ternary neodymium-based catalyst. Polymer 2013, 54, 2973–2978. [Google Scholar] [CrossRef]
- Méndez-Hernández, M.L.; Rivera-Armenta, J.L.; Páramo-García, U. Synthesis of high cis-1,4-BR with neodymium for the manufacture of Tires. Int. J. Polym. Sci. 2016, 2016, 7239540. [Google Scholar] [CrossRef]
- Cai, X.M. Progress in production technology and market prospect of rare-earth catalyst-based polybutadiene rubber in China. Shang. Chem. Ind. 2016, 41, 21. [Google Scholar]
- Chatarsa, C.; Prasassarakich, P.; Rempel, G.L. The influence of Ni/Nd-based Ziegler–Natta catalyst on microstructure configurations and properties of butadiene rubber. J. Appl. Polym. Sci. 2015, 132, 41834. [Google Scholar] [CrossRef]
- Jain, R.K.; Simha, R. Equation of state of cis-1,4-polybutadiene melt. Polym. Eng. Sci. 1979, 19, 845. [Google Scholar] [CrossRef]
- Mitchell, J.C. Mechanical history effects in the crystallization of cis-l,4-polybutadiene. Polymer 1967, 8, 369–379. [Google Scholar] [CrossRef]
- Feio, G.; Cohen-Addad, J.P. NMR approach to the kinetics of polymer crystallization. 1. cis-1,4-Polybutadiene. J. Polym. Sci. B Polym. Phys. 1988, 26, 389–412. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, G.; Zhou, E.; Yu, F.; Qian, B. Effects of molecular weight and temperature on crystallization morphology of cis-1.4 polybutadiene. J. Chin. Elect. Microsc. Soc. 1984, 2, 51–57. (In Chinese) [Google Scholar]
- Lorenzo, M.L.D. The melting process and the rigid amorphous fraction of cis-1,4-polybutadiene. Polymer 2009, 50, 578–584. [Google Scholar] [CrossRef]
- Lorenzo, M.L.D. Thermal properties and three-phase structure of cis-1,4-polybutadiene. Open Macromol. J. 2010, 4, 15–21. [Google Scholar] [CrossRef]
- Zhang, X.H.; Yang, H.M.; Song, Y.H.; Zheng, Y. Rheological behaviors of randomly crosslinked low density polyethylene and its gel network. Polymer 2012, 53, 3035–3042. [Google Scholar] [CrossRef]
- Zhang, X.H.; Yang, H.M.; Song, Y.H.; Zheng, Q. Influence of crosslinking on physical properties of low density polyethylene. Chin. J. Polym. Sci. 2012, 30, 837–844. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yang, H.M.; Sonf, Y.H.; Zheng, Q. Influence of crosslinking on crystallization, rheological, and mechanical behaviors of high density polyethylene/ethylene-vinyl acetate copolymer blends. Polym. Eng. Sci. 2014, 54, 2848–2858. [Google Scholar] [CrossRef]
- Salim, H.; Nadji, M.M.; Pieter, S.; Mikhael, B.; Alain, D.; Ahmed, B. Morphology, rheology and crystallization in relation to the viscosity ratio of polystyrene/polypropylene polymer Blends. Materials 2020, 13, 926. [Google Scholar] [CrossRef]
- Fenni, S.E.; Müller, A.J.; Cavallo, D. Understanding polymer nucleation by studying droplets crystallization in immiscible polymer blends. Polymer 2023, 264, 125514. [Google Scholar] [CrossRef]
- Ikehara, T.; Nishi, T. Interpenetrated spherulites of poly(butylene succinate) poly(vinylidene chloride-co-vinyl chloride) blends. An optical microscopic study. Polym. J. 2000, 32, 683–687. [Google Scholar] [CrossRef]
- Ikehara, T.; Kimura, H.; Qiu, Z. Penetrating spherulitic growth in poly(butylene adipate-co-butylene succinate)/poly(ethylene oxide) blends. Macromolecules 2005, 38, 5104–5108. [Google Scholar] [CrossRef]
- Qiu, Z.; Yan, C.; Lu, J.; Yang, W. Various crystalline morphology of poly(butylene succinate-co-butylene adipate) in its miscible blends with poly(vinylidene fluoride). J. Phys. Chem. B 2007, 111, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Ikehara, T.; Nishi, T. Unique morphology of poly(ethylene succinate)/poly(ethylene oxide) blends. Macromolecules 2002, 35, 8251–8254. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F., Jr. Spherulitic crystallization from the melt. I. Fractionation and impurity segregation and their influence on crystalline morphology. J. Appl. Phys. 1964, 35, 1270–1286. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, X.F.; Zhu, W.B.; Xie, X.Z.; Ji, H.; Bi, J.F. Effect of Dilution on the Crystallization Kinetics of Neodym-ium-Based Rare Earth Polybutadiene Rubber. Polymers 2024, 16, 35. [Google Scholar] [CrossRef] [PubMed]


| Tc,peak (°C) | Temperature Range of Crystallization (°C) | Tm0 (°C) | Ea,t(1/2) (kJ/mol) | |
|---|---|---|---|---|
| BR100 | −37.44 | −37.5~−24 | −0.135 | −264.3 |
| BR80 | −45.22 | −42~−34 | −0.0735 | −232.8 |
| BR60 | −52.72 | −50~−42 | −4.922 | −125.11 |
| BR40 | −68.67 | −70~−52 | −6.579 | 42.9 |
| BR-10T | −41.04 | −32~−26 | −3.194 | −191.4 |
| BR-30T | −49.98 | −44~−36 | −3.572 | −93.9 |
| BR-50T | −55.23 | −46~−38 | −4.579 | −50.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, X.; Ji, H.; Wei, Y.; Xie, X.; Zhu, W.; Bi, J. The Crystallization and Melting Behavior of Neodymium-Based Butadiene Rubber Blends. Polymers 2024, 16, 342. https://doi.org/10.3390/polym16030342
Li X, Zhang X, Ji H, Wei Y, Xie X, Zhu W, Bi J. The Crystallization and Melting Behavior of Neodymium-Based Butadiene Rubber Blends. Polymers. 2024; 16(3):342. https://doi.org/10.3390/polym16030342
Chicago/Turabian StyleLi, Xiaofan, Xiaohu Zhang, Huan Ji, Yanxing Wei, Xinzheng Xie, Wenbin Zhu, and Jifu Bi. 2024. "The Crystallization and Melting Behavior of Neodymium-Based Butadiene Rubber Blends" Polymers 16, no. 3: 342. https://doi.org/10.3390/polym16030342




