Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review
Abstract
:1. Introduction
2. Methods for Preparing Chitosan-Based Hydrogel
3. Chitosan-Based Hydrogel for Skin Injury Repair
3.1. Intelligent Chitosan-Based Hydrogel
3.2. Self-Healing Chitosan-Based Hydrogel
3.3. Drug-Loaded Chitosan-Based Hydrogels
3.3.1. Loading Metal Ion Chitosan Hydrogel
3.3.2. Chitosan-Based Hydrogel Loaded with Flavonoids
3.3.3. Chitosan-Based Hydrogels Loaded with Phenolic Acids
3.3.4. Chitosan-Based Hydrogel Carrying Plant Essential Oil
3.3.5. Chitosan-Based Hydrogel Carrying Polypeptide
3.3.6. Chitosan-Based Hydrogel Carrying Other Therapeutic Components
4. Process of Skin Repair
4.1. Hemostasis Stage
4.2. Inflammatory Stage
4.3. Proliferation Stage
4.4. Remodeling Phase
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern wound dressings: Hydrogel dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yui, T.; Okuyama, K. Three d structures of chitosan. Int. J. Biol. Macromol. 2004, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Pella, M.; Lima-Tenorio, M.K.; Tenorio-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.N.; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1e–32e. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review. J. Mat. Chem. B 2020, 8, 10050–10064. [Google Scholar] [CrossRef]
- Kim, G.O.; Kim, N.; Kim, D.Y.; Kwon, J.S.; Min, B.H. An electrostatically crosslinked chitosan hydrogel as a drug carrier. Molecules 2012, 17, 13704–13711. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Feng, Z.; Peng, K.; Wei, A.; Wang, Y.; Tong, Z.; Cheng, B. Preparation of a chitosan/carboxymethyl chitosan/agnps polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos. Pt. B Eng. 2020, 197, 108139. [Google Scholar] [CrossRef]
- Mirzaei, E.B.; Ramazani, A.S.A.; Shafiee, M.; Danaei, M. Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int. J. Polym. Mater. Polym. Biomat. 2013, 62, 605–611. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudlacik-Kramarczyk, S.; Glab, M.; Kedzierska, M.; Jaromin, A.; Mierzwinski, D.; Tyliszczak, B. Physicochemical investigations of chitosan-based hydrogels containing aloe vera designed for biomedical use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Liu, J.H. Fabrication and characterization of poly (vinyl alcohol)/chitosan hydrogel thin films via uv irradiation. Eur. Polym. J. 2013, 49, 4201–4211. [Google Scholar] [CrossRef]
- Pita-Lopez, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodriguez-Rodriguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Schuetz, Y.B.; Gurny, R.; Jordan, O. A novel thermoresponsive hydrogel based on chitosan. Eur. J. Pharm. Biopharm. 2008, 68, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, S.; Han, J.; Lin, H.; Zhang, X. Design and fabrication of a chitosan hydrogel with gradient structures via a step-by-step cross-linking process. Carbohydr. Polym. 2017, 176, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Park, H.; Park, K.; Kim, D. Preparation and swelling behavior of chitosan-based superporous hydrogels for gastric retention application. J. Biomed. Mater. Res. Part A 2006, 76, 144–150. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/pva hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef]
- Burn injury. Nat. Rev. Dis. Primers 2020, 6, 12. [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Peppa, M.; Stavroulakis, P.; Raptis, S.A. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009, 17, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo, A.B.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Kottner, J.; Black, J.; Call, E.; Gefen, A.; Santamaria, N. Microclimate: A critical review in the context of pressure ulcer prevention. Clin. Biomech. 2018, 59, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Vangilder, C.; Lachenbruch, C.; Algrim-Boyle, C.; Meyer, S. The international pressure ulcer prevalence survey: 2006–2015: A 10-year pressure injury prevalence and demographic trend analysis by care setting. J. Wound Ostomy Cont. Nurs. 2017, 44, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Li, X.; Tong, A.; Guo, G. Multi-functional chitosan-based smart hydrogels mediated biomedical application. Expert Opin. Drug Deliv. 2019, 16, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent development of functional chitosan-based hydrogels for pharmaceutical and biomedical applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef]
- Arafa, A.A.; Nada, A.A.; Ibrahim, A.Y.; Sajkiewicz, P.; Zahran, M.K.; Hakeim, O.A. Preparation and characterization of smart therapeutic ph-sensitive wound dressing from red cabbage extract and chitosan hydrogel. Int. J. Biol. Macromol. 2021, 182, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Tong, Y.; Zhang, S.; He, R.; Xiao, L.; Iqbal, Z.; Zhang, Y.; Gao, J.; Zhang, L.; Jiang, L.; et al. Flexible bicolorimetric polyacrylamide/chitosan hydrogels for smart real—Time monitoring and promotion of wound healing. Adv. Funct. Mater. 2021, 31, 2102599. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fu, X.; Zhou, Y.; Lei, L.; Wang, J.; Zeng, W.; Yang, Z. A hydrogel system for drug loading toward the synergistic application of reductive/heat-sensitive drugs. J. Control. Release 2023, 362, 409–424. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, C.; Cui, Z.; Liang, Y.; et al. Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds. Chem. Eng. J. 2020, 396, 125194. [Google Scholar] [CrossRef]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart hydrogel-based dvdms/bfgf nanohybrids for antibacterial phototherapy with multiple damaging sites and accelerated wound healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Xu, F.; Han, Z.; Wei, D.; Jia, D.; Zhou, Y. Tough magnetic chitosan hydrogel nanocomposites for remotely stimulated drug release. Biomacromolecules 2018, 19, 3351–3360. [Google Scholar] [CrossRef]
- Zheng, F.; Li, R.; He, Q.; Koral, K.; Tao, J.; Fan, L.; Xiang, R.; Ma, J.; Wang, N.; Yin, Y.; et al. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Mater. Sci. Eng. C 2020, 109, 110560. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, X.; Zheng, S.; Wang, Y.; Li, Z.; Zhang, H.; Nie, L.; Zhang, Y.; Zhao, Y.; Yang, X. Bio-adhesive catechol-modified chitosan wound healing hydrogel dressings through glow discharge plasma technique. Chem. Eng. J. 2022, 427, 130843. [Google Scholar] [CrossRef]
- Blacklow, S.O.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 2019, 5, eaaw3963. [Google Scholar] [CrossRef]
- Odinokov, A.V.; Dzhons, D.Y.; Budruev, A.V.; Mochalova, A.E.; Smirnova, L.A. Chitosan modified with terephthaloyl diazide as a drug delivery system. Russ. Chem. Bull. 2016, 65, 1122–1130. [Google Scholar] [CrossRef]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M. Peg-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Han, B.; Jiang, Z.; Yang, Y.; Peng, Y.; Liu, W. Synthesis of a chitosan-based photo-sensitive hydrogel and its biocompatibility and biodegradability. Carbohydr. Polym. 2017, 166, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Lam, K.Y. A multiphysics model of photo-sensitive hydrogels in response to light-thermo-pH-salt coupled stimuli for biomedical applications. Bioelectrochemistry 2020, 135, 107584. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Y.; Wang, X.; Huang, L.; Chen, Y.; Bao, C. Glucose-sensitive delivery of metronidazole by using a photo-crosslinked chitosan hydrogel film to inhibit porphyromonas gingivalis proliferation. Int. J. Biol. Macromol. 2019, 122, 19–28. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J. Mat. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef]
- Yan, H.; Jin, B. Equilibrium swelling of a polyampholytic pH-sensitive hydrogel. Eur. Phys. J. E 2013, 36, 27. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Wu, J.; Su, Z.G.; Ma, G.H. A thermo- and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int. J. Pharm. 2006, 315, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ke, T.; Ling, Q.; Zhao, L.; Gu, H. Rapid self-healing and self-adhesive chitosan-based hydrogels by host-guest interaction and dynamic covalent bond as flexible sensor. Carbohydr. Polym. 2021, 273, 118533. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Tian, M. Advances in multifunctional chitosan-based self-healing hydrogels for biomedical applications. J. Mat. Chem. B 2021, 9, 7955–7971. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic covalent constructed self—Healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Deng, L.; Wang, B.; Li, W.; Han, Z.; Chen, S.; Wang, H. Bacterial cellulose reinforced chitosan-based hydrogel with highly efficient self-healing and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 2022, 217, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yan, Y.; Huang, J.; Liang, Q.; Li, J.; Wang, B.; Ma, B.; Bianco, A.; Ge, S.; Shao, J. A multifunctional chitosan-based hydrogel with self-healing, antibacterial, and immunomodulatory effects as wound dressing. Int. J. Biol. Macromol. 2023, 231, 123149. [Google Scholar] [CrossRef]
- Robson, M.C. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg. Clin. North Am. 1997, 77, 637–650. [Google Scholar] [CrossRef]
- Wang, C.; Huang, X.; Deng, W.; Chang, C.; Hang, R.; Tang, B. A nano-silver composite based on the ion-exchange response for the intelligent antibacterial applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 41, 134–141. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Tamayo, L.; Azocar, M.; Kogan, M.; Riveros, A.; Paez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 69, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, C.H.; Padma, M.; Samadanam, I.D.; Mareeswaran, R.; Suyavaran, A.; Kumar, M.S.; Premkumar, K.; Thirunavukkarasu, C. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloid Surf. B Biointerfaces 2013, 102, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Odularu, A.T.; Ajibade, P.A.; Mbese, J.Z.; Oyedeji, O.O. Developments in platinum-group metals as dual antibacterial and anticancer agents. J. Chem. 2019, 2019, 5459461. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Nesovic, K.; Jankovic, A.; Radetic, T.; Vukasinovic-Sekulic, M.; Kojic, V.; Zivkovic, L.; Peric-Grujic, A.; Rhee, K.Y.; Miskovic-Stankovic, V. Chitosan-based hydrogel wound dressings with electrochemically incorporated silver nanoparticles—In vitro study. Eur. Polym. J. 2019, 121, 109257. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of apigenin loaded gellan gum-chitosan hydrogels (ggch-hgs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhao, Y.; Chen, X.; Zheng, Y.; Liu, W.; Liu, X. Taxifolin, a novel food, attenuates acute alcohol-induced liver injury in mice through regulating the nf-κb-mediated inflammation and pi3k/akt signalling pathways. Pharm. Biol. 2021, 59, 866–877. [Google Scholar] [CrossRef]
- Ding, Q.; Ding, C.; Liu, X.; Zheng, Y.; Zhao, Y.; Zhang, S.; Sun, S.; Peng, Z.; Liu, W. Preparation of nanocomposite membranes loaded with taxifolin liposome and its mechanism of wound healing in diabetic mice. Int. J. Biol. Macromol. 2023, 241, 124537. [Google Scholar] [CrossRef]
- Ding, C.; Liu, Z.; Zhao, T.; Sun, S.; Liu, X.; Zhang, J.; Ma, L.; Yang, M. A temperature-sensitive hydrogel loaded with taxifolin promotes skin repair by modulating mapk-mediated autophagic pathway. J. Mater. Sci. 2023, 58, 14831–14845. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650. [Google Scholar]
- Zhou, C.; Xu, R.; Han, X.; Tong, L.; Xiong, L.; Liang, J.; Sun, Y.; Zhang, X.; Fan, Y. Protocatechuic acid-mediated injectable antioxidant hydrogels facilitate wound healing. Compos. Pt. B Eng. 2023, 250, 110451. [Google Scholar] [CrossRef]
- Pasanphan, W.; Chirachanchai, S. Conjugation of gallic acid onto chitosan: An approach for green and water-based antioxidant. Carbohydr. Polym. 2008, 72, 169–177. [Google Scholar] [CrossRef]
- Sun, X.; Dong, M.; Guo, Z.; Zhang, H.; Wang, J.; Jia, P.; Bu, T.; Liu, Y.; Li, L.; Wang, L. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int. J. Biol. Macromol. 2021, 167, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn. Trauma 2021, 9, tkab41. [Google Scholar] [CrossRef] [PubMed]
- Koosehgol, S.; Ebrahimian-Hosseinabadi, M.; Alizadeh, M.; Zamanian, A. Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 79, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L. The human tri-peptide ghk and tissue remodeling. J. Biomater. Sci.-Polym. Ed. 2008, 19, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. Ghk peptide as a natural modulator of multiple cellular pathways in skin regeneration. Biomed. Res. Int. 2015, 2015, 648108. [Google Scholar] [CrossRef]
- Wang, S.; Feng, C.; Yin, S.; Feng, Z.; Tang, J.; Liu, N.; Yang, F.; Yang, X.; Wang, Y. A novel peptide from the skin of amphibian rana limnocharis with potency to promote skin wound repair. Nat. Prod. Res. 2021, 35, 3514–3518. [Google Scholar] [CrossRef]
- Ouyang, Q.Q.; Hu, Z.; Lin, Z.P.; Quan, W.Y.; Deng, Y.F.; Li, S.D.; Li, P.W.; Chen, Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018, 112, 1191–1198. [Google Scholar] [CrossRef]
- Qianqian, O.; Songzhi, K.; Yongmei, H.; Xianghong, J.; Sidong, L.; Puwang, L.; Hui, L. Preparation of nano-hydroxyapatite/chitosan/tilapia skin peptides hydrogels and its burn wound treatment. Int. J. Biol. Macromol. 2021, 181, 369–377. [Google Scholar] [CrossRef]
- Xiao, Y.; Ge, H.; Zou, S.; Wen, H.; Li, Y.; Fan, L.; Xiao, L. Enzymatic synthesis of n-succinyl chitosan-collagen peptide copolymer and its characterization. Carbohydr. Polym. 2017, 166, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, M.; Yang, X.; Zhang, C.; Zhou, H.; Xie, W.; Fan, L.; Nie, M. Modification of chitosan grafted with collagen peptide by enzyme crosslinking. Carbohydr. Polym. 2019, 206, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Yang, Y.; Du, S.; Yang, X.; Pang, S.; Wang, X.; Yang, S. Preparation of a recombinant collagen-peptide (rhc)-conjugated chitosan thermosensitive hydrogel for wound healing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 119, 111555. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Amiri, D.S.; Fazly, B.B.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-peo nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, M.R.; Dadfarnia, S.; Shabani, A.; Amraei, R.; Hafezi, M.Z. Doxycycline drug delivery using hydrogels of o-carboxymethyl chitosan conjugated with caffeic acid and its composite with polyacrylamide synthesized by electron beam irradiation. Int. J. Biol. Macromol. 2020, 154, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Fasiku, V.O.; Omolo, C.A.; Devnarain, N.; Ibrahim, U.H.; Rambharose, S.; Faya, M.; Mocktar, C.; Singh, S.D.; Govender, T. Chitosan-based hydrogel for the dual delivery of antimicrobial agents against bacterial methicillin-resistant staphylococcus aureus biofilm-infected wounds. ACS Omega 2021, 6, 21994–22010. [Google Scholar] [CrossRef] [PubMed]
- Ilomuanya, M.O.; Enwuru, N.V.; Adenokun, E.; Fatunmbi, A.; Adeluola, A.; Igwilo, C.I. Chitosan-based microparticle encapsulated acinetobacter baumannii phage cocktail in hydrogel matrix for the management of multidrug resistant chronic wound infection. Turk. J. Pharm. Sci. 2022, 19, 187–195. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Hoshi, R.; Murata, S.; Matsuo, R.; Myronovych, A.; Hashimoto, I.; Ikeda, H.; Ohkohchi, N. Freeze-dried platelets promote hepatocyte proliferation in mice. Cryobiology 2007, 55, 255–260. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Leggat, P.A.; Smith, D.R.; Kedjarune, U. Surgical applications of cyanoacrylate adhesives: A review of toxicity. ANZ J. Surg. 2007, 77, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Zeng, Y.; Zaldivar-Silva, D.; Aguero, L.; Wang, S. Chitosan-based hemostatic hydrogels: The concept, mechanism, application, and prospects. Molecules 2023, 28, 1473. [Google Scholar] [CrossRef] [PubMed]
- Kozen, B.G.; Kircher, S.J.; Henao, J.; Godinez, F.S.; Johnson, A.S. An alternative hemostatic dressing: Comparison of celox, hemcon, and quikclot. Acad. Emerg. Med. 2008, 15, 74–81. [Google Scholar] [CrossRef]
- Pusateri, A.E.; Mccarthy, S.J.; Gregory, K.W.; Harris, R.A.; Cardenas, L.; Mcmanus, A.T.; Goodwin, C.J. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma 2003, 54, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wang, S.; Jiang, Z.; Chi, J.; Yu, S.; Li, H.; Zhang, Y.; Li, L.; Zhou, C.; Liu, W.; et al. Hemostatic performance of chitosan-based hydrogel and its study on biodistribution and biodegradability in rats. Carbohydr. Polym. 2021, 264, 117965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable self-healing adhesive chitosan hydrogel with antioxidative, antibacterial, and hemostatic activities for rapid hemostasis and skin wound healing. Acs Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Lan, Y.; Zuo, Q.; Li, C.; Zhang, Y.; Guo, R.; Xue, W. Acceleration of skin regeneration in full-thickness burns by incorporation of bfgf-loaded alginate microspheres into a cmcs-pva hydrogel. J. Tissue Eng. Regen. Med. 2017, 11, 1562–1573. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M. Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Brasil. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Zhai, X.; Ji, S.; Ye, J.; Zhu, Z.; Teng, C.; Dong, W.; Wei, W. A multi-functional double cross-linked chitosan hydrogel with tunable mechanical and antibacterial properties for skin wound dressing. Carbohydr. Polym. 2023, 322, 121344. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Chen, X.; Cao, X.; Jiang, H.; Che, X.; Xu, X.; Ma, B.; Zhang, J.; Huang, T. Sikvav-modified chitosan hydrogel as a skinsubstitutes for wound closure in mice. Molecules 2018, 23, 2611. [Google Scholar] [CrossRef]
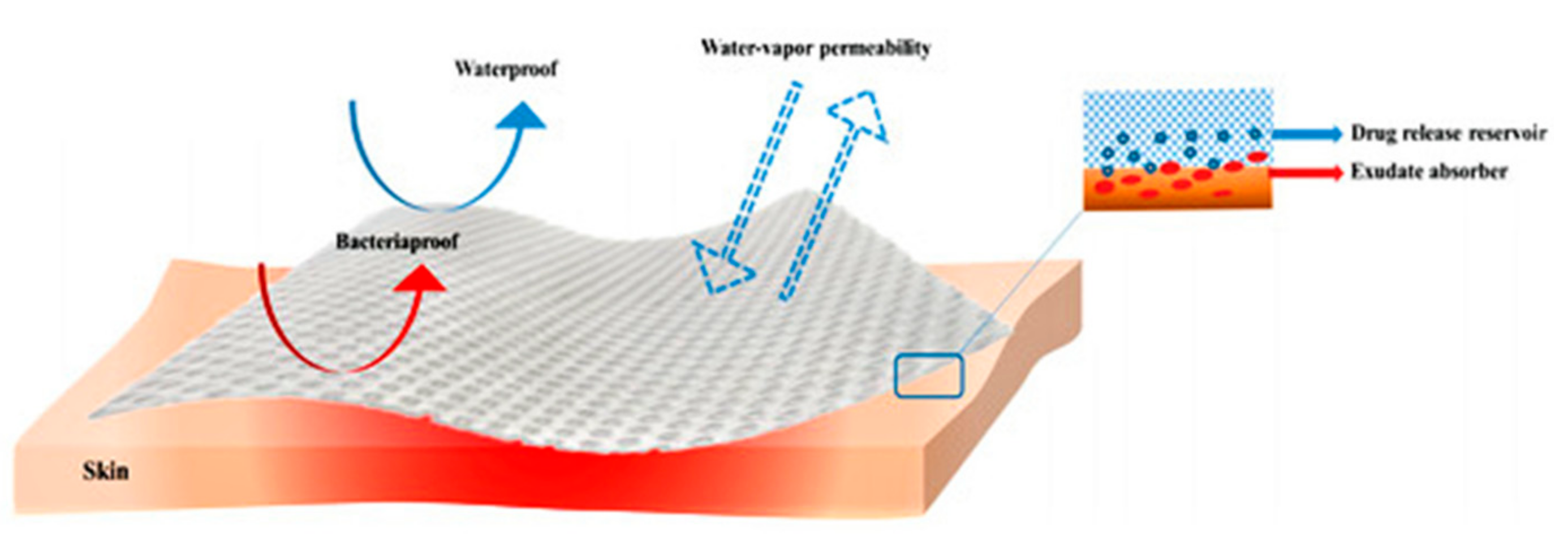

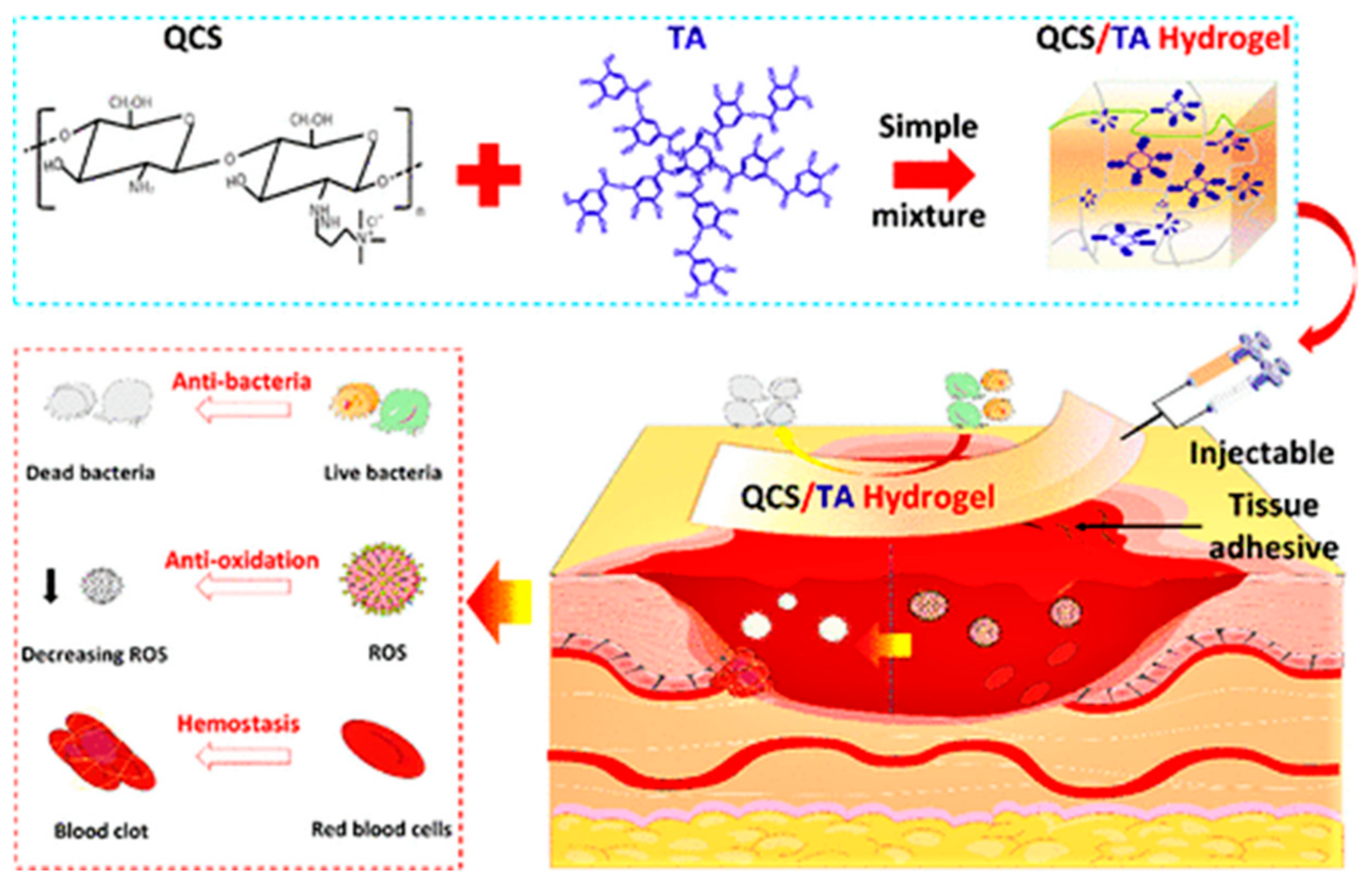
| Cross-Linking Type | Method | Mechanism | Refs. |
|---|---|---|---|
| Physical cross-linking | Electrostatic interaction | Electrostatic interactions occur through an interaction between anionic molecules and the amino groups of chitosan to gelate them. | [9] |
| Metal ion coordination | Metal ion coordination is used to synthesize gels through intermolecular coordination bonds, forming more stable hydrogels. | [10] | |
| Hydrophobic interaction | Chitosan can undergo gelation through hydrophobic interactions, and this interacting biopolymer system can advantageously avoid the potential side effects of in situ polymerization associated with monomer or initiator toxicity. | [11] | |
| Chemical cross-linking | Cross-linking agent initiated cross-linking method | Initiators are substances that can cause monomers to undergo polymerization, which can cause chitosan and its derivative molecules to combine through covalent bonds to form a reticulated structure and improve the strength, elasticity, and other properties of the material. | [12] |
| Radiation cross-linking | The radiation cross-linking method uses the action of a radiation source with a substance to ionize and excite the generation of activated atoms and molecules, causing cross-linking between the substances. | [13] |
| Type | Added Ingredients | Action Mechanism | Refs. |
|---|---|---|---|
| pH - sensitive | Red cabbage extract (RCE)/ chitosan (cs)/Methylenebisacrylamide (MBAA) | The colorimetric properties of RCE-loaded chitosan hydrogels show that RCE undergoes visual color changes in both acidic and alkaline media. Monitoring wound pH changes can protect, heal, and indicate the healing process | [31] |
| Polyacrylamide-quaternary ammonium/chitosan-carbon quantum dots (CQDs) phenol red hydrogel | Hybridization of CQD and pH indicator (phenol red) with the hydrogel resulted in a highly responsive, reversible, and accurate indication of pH variability to reflect dynamic wound states in both UV and visible backgrounds. | [32] | |
| Heat - sensitive | Curcumin/Carboxymethyl/cellulose | Cur-micellar-loaded hydrogels promote tissue regenerative capacity with enhanced fibroblasts, keratin-forming cells, and collagen deposition to stimulate epidermal junctions. Interestingly, chitosan-CMC-g-PF127 injectable hydrogel exhibited rapid wound repair potential by increasing cell migration and proliferation at the site of injury and providing a continuous drug delivery platform to the hydrophobic fraction. | [33] |
| Synthesis by free radical-mediated polymerization of tannic acid-assisted New-gel hydrogels. | The new gel has excellent chemical/physical properties and can effectively load and release drugs and maintain drug activity. At the same time, New-gel has excellent oxygen loading capacity, which provides significant practical therapeutic benefits for diabetic wound repair. | [34] | |
| Photo - responsive | Prussian blue nanoparticles (PBNPs)/Glycidyl trimethylammonium chloride (GTAC)/ Glycidyl methacrylate (GMA) | The positively charged QC in the hydrogel can capture bacteria through electrostatic attraction, change the potential of the bacterial membrane, destroy the bacterial membrane, and ultimately reduce the activity of the bacteria or even kill them. At the same time, the heat therapy generated by PBNPs under near-infrared (NIR) light irradiation can effectively and quickly kill these weak bacteria at mild temperatures (<55 °C). | [35] |
| Porphyrin photosensitizer/polylactic acid-glycolic acid (PLGA)/basic fibroblast growth factor (bFGF)/carboxymethyl chitosan (CMCS) | The hydrogel block helps with repeated photodynamic stimulation and inhibits bacterial growth, while the aFGF content promotes wound healing. | [36] | |
| Magnetic field corresponding hydrogel | Magnetite precursor/chitosan | The direct remote control of drug release behavior using low-frequency alternating magnetic field (LAMF) also avoids potential adverse thermal effects. | [37] |
| Electric field responsive hydrogel | Chitosan (CS)/Hydroxyethyl cellulose oxide (OHEC)/Reduced graphene oxide (RGO)/Salicylide liposomes | CS/OHEC/rGO/asiaticoside liposome hydrogel was prepared by dispersing RGO and asiaticoside into the hydrogel. This hydrogel serves as a filler for hollow nerve conduits, leveraging the benefits of OHEC and CS to enhance the mechanical and degradation properties of CS. Moreover, the hydrogel incorporates conductive rGO, which facilitates electric stimulation and scar inhibition, thereby promoting peripheral nerve regeneration. | [38] |
| Plasma-activated hydrogel | Gallic acid-modified chitosan-based (CS-GA) | Through self-cross-linking reaction exposed to oxygen, CS-GA solution may become bioadhesive hydrogel with high biocompatibility and blood compatibility, which is helpful for wound healing and hemostasis. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. https://doi.org/10.3390/polym16030344
Che X, Zhao T, Hu J, Yang K, Ma N, Li A, Sun Q, Ding C, Ding Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers. 2024; 16(3):344. https://doi.org/10.3390/polym16030344
Chicago/Turabian StyleChe, Xueyan, Ting Zhao, Jing Hu, Kaicheng Yang, Nan Ma, Anning Li, Qi Sun, Chuanbo Ding, and Qiteng Ding. 2024. "Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review" Polymers 16, no. 3: 344. https://doi.org/10.3390/polym16030344
APA StyleChe, X., Zhao, T., Hu, J., Yang, K., Ma, N., Li, A., Sun, Q., Ding, C., & Ding, Q. (2024). Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers, 16(3), 344. https://doi.org/10.3390/polym16030344





