Excellent Oxygen Barrier Property of Unfilled Natural Rubber/trans-Butadiene-co-Isoprene Rubber Vulcanizates under the Synergistic Effect of Crosslinking Density and Crystallization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Samples Preparation
2.3. Characterization and Measurements
3. Results and Discussion
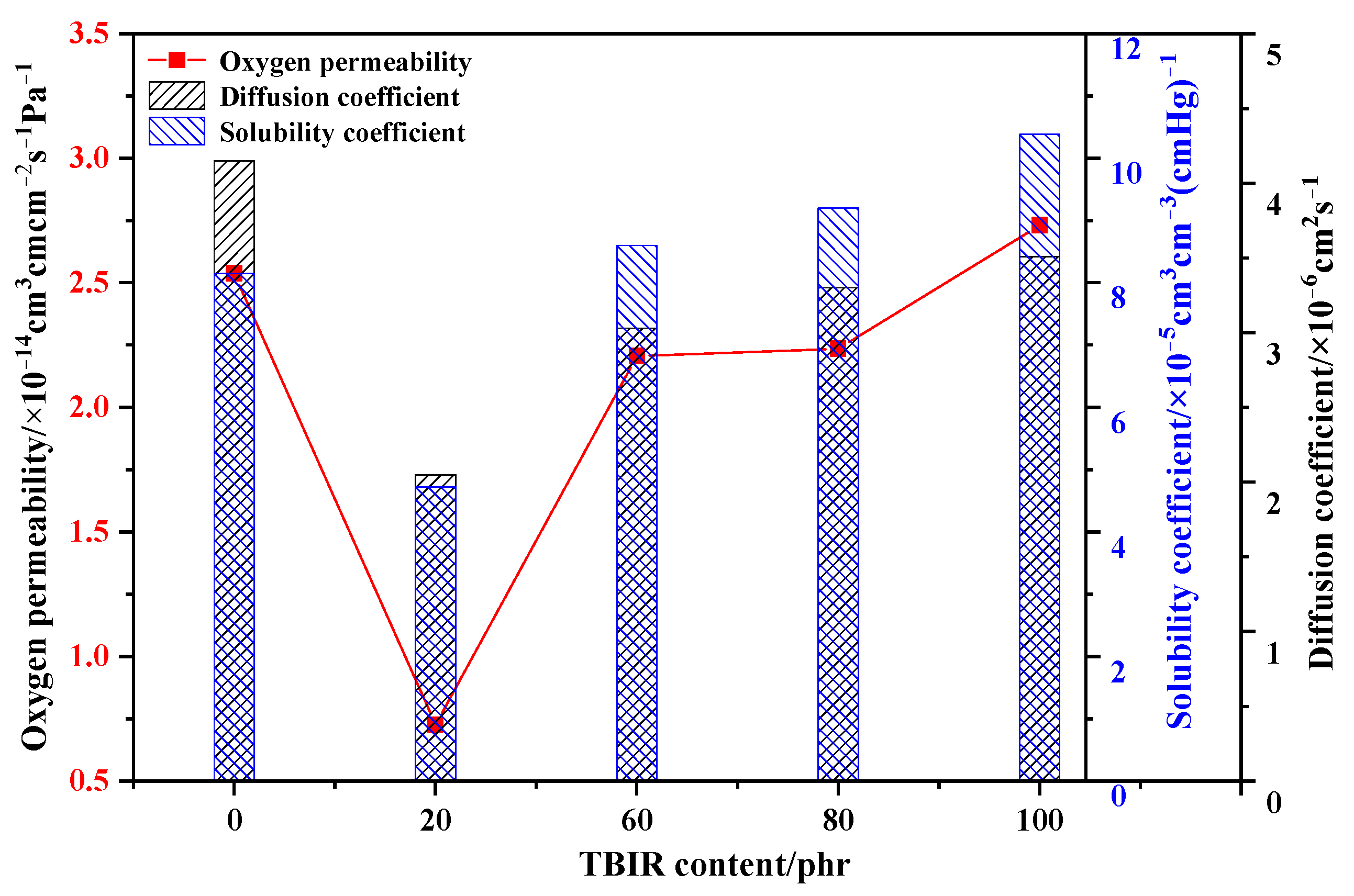
3.1. Oxygen Barrier Property
3.2. DMA
3.3. Curing Characteristics and Crosslinking Density
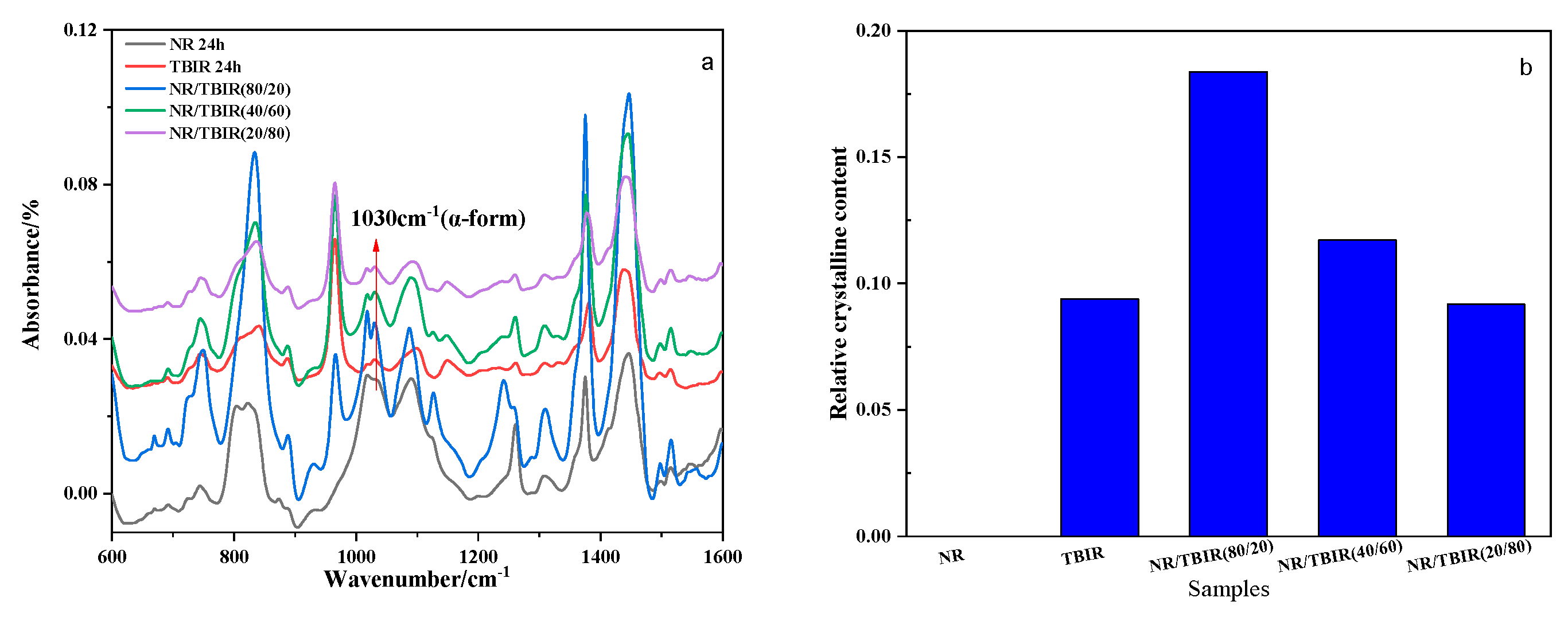
3.4. Crystallinity of TBIR
3.5. Morphology
3.6. Evolution of Oxygen Barrier
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nie, J.; Huang, X.; Xu, C.; Ding, J.; Chen, Y. Antioxidant effects on curing/processing and thermo-oxidative aging of filled nitrile rubber. Mater. Chem. Phys. 2020, 253, 123403. [Google Scholar] [CrossRef]
- Ning, N.; Ma, Q.; Zhang, Y.; Zhang, L.; Wu, H.; Tian, M. Enhanced thermo-oxidative aging resistance of EPDM at high temperature by using synergistic antioxidants. Polym. Degrad. Stab. 2014, 102, 1–8. [Google Scholar] [CrossRef]
- He, S.; Bai, F.; Liu, S.; Ma, H.; Hu, J.; Chen, L.; Lin, J.; Wei, G.; Du, X. Aging properties of styrene-butadiene rubber nanocomposites filled with carbon black and rectorite. Polym. Test. 2017, 64, 92–100. [Google Scholar] [CrossRef]
- Menichetti, S.; Viglianisi, C.; Liguori, F.; Cogliati, C.; Boragno, L.; Stagnaro, P.; Losio, S.; Sacchi, M.C. Ethylene-based copolymers with tunable content of polymerizable hindered phenols as nonreleasing macromolecular additives. J. Polym. Sci. Pol. Chem. 2008, 46, 6393–6406. [Google Scholar] [CrossRef]
- El-Wakil, A.E.-A.; Barakat, M.A. Study of the effect of natural rubber-graft-o-aminophenol on the thermal stability and mechanical properties of nitrile rubber. J. Appl. Polym. Sci. 2011, 119, 2461–2467. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, Y.; Yang, W.; Zhang, Z.; Zhang, L.; Wu, S. A combined experimental and molecular simulation study of factors influencing the selection of antioxidants in butadiene rubber. J. Phys. Chem. B 2017, 121, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Wu, Y.; Yang, J.; Zhang, L. Structure and properties of strain-induced crystallization rubber–clay nanocomposites by co-coagulating the rubber latex and clay aqueous suspension. J. Appl. Polym. Sci. 2005, 96, 318–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, S.; Zhang, Y.; Cheng, H. Gas barrier properties and mechanism of kaolin/styrene–butadiene rubber nanocomposites. Appl. Clay Sci. 2015, 111, 37–43. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Wang, S.; Li, Y.; Zhou, Y. Highly ordered structured montmorillonite/brominated butyl rubber nanocomposites: Dramatic enhancement of the gas barrier properties by an external magnetic field. J. Membr. Sci. 2018, 546, 22–30. [Google Scholar] [CrossRef]
- Tang, M.; Xing, W.; Wu, J.; Huang, G.; Xiang, K.; Guo, L.; Li, G. Graphene as a prominent antioxidant for diolefin elastomers. J. Mater. Chem. A 2015, 3, 5942–5948. [Google Scholar] [CrossRef]
- Raju, A.T.; Dash, B.; Dey, P.; Nair, S.; Naskar, K. Evaluation of air permeability characteristics on the hybridization of carbon black with graphene nanoplatelets in bromobutyl rubber/epoxidized natural rubber composites for inner-liner applications. Polym. Adv. Technol. 2020, 31, 2390–2402. [Google Scholar] [CrossRef]
- Wu, J.; Huang, G.; Li, H.; Wu, S.; Liu, Y.; Zheng, J. Enhanced mechanical and gas barrier properties of rubber nanocomposites with surface functionalized graphene oxide at low content. Polymer 2013, 54, 1930–1937. [Google Scholar] [CrossRef]
- Scherillo, G.; Lavorgna, M.; Buonocore, G.G.; Zhan, Y.H.; Xia, H.S.; Mensitieri, G.; Ambrosio, L. Tailoring assembly of reduced graphene oxide nanosheets to control gas barrier properties of natural rubber nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, H.; Li, C.; Xiong, Y.; Guo, S. Constructing oriented two-dimensional large-sized modified graphene oxide barrier walls in brominated butyl rubber to achieve excellent gas barrier properties. ACS Appl. Mater. Interfaces 2020, 12, 3976–3983. [Google Scholar] [CrossRef] [PubMed]
- Khanlari, S.; Kokabi, M. Thermal stability; aging properties, and flame resistance of NR-based nanocomposite. J. Appl. Polym. Sci. 2011, 119, 855–862. [Google Scholar] [CrossRef]
- Bu, J.; Huang, X.; Li, S.; Jiang, P. Significantly enhancing the thermal oxidative stability while remaining the excellent electrical insulating property of low density polyethylene by addition of antioxidant functionalized graphene oxide. Carbon 2016, 106, 218–227. [Google Scholar] [CrossRef]
- Li, T.; Chen, S.; Wan, S.; Cai, S.; He, X. The effect of functional zirconium phosphate on aging resistance of nitrile butadiene rubber composites. Polym. Compos. 2020, 41, 1867–1877. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wu, Y.; Yang, J.; Zhang, L. Preparation and properties of natural rubber/rectorite nanocomposites. Eur. Polym. J. 2005, 41, 2776–2783. [Google Scholar] [CrossRef]
- Meera, A.P.; Selvin, T.P.; Thomas, S. Effect of organoclay on the gas barrier properties of natural rubber nanocomposites. Polym. Compos. 2012, 33, 524–531. [Google Scholar]
- Liang, Y.; Cao, W.; Li, Z.; Wang, Y.; Wu, Y.; Zhang, L. A new strategy to improve the gas barrier property of isobutylene–isoprene rubber/clay nanocomposites. Polym. Test. 2008, 27, 270–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, Q.; Lu, Y. Gas barrier properties of natural rubber/kaolin composites prepared by melt blending. Appl. Clay Sci. 2010, 50, 255–259. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Shen, H.; Gong, J.; Xiao, Z. A comprehensive review on non-pneumatic tyre research. Mater. Des. 2023, 227, 111742. [Google Scholar] [CrossRef]
- Maria, H.J.; Thomas, M.G.; Morreale, M.; La Mantia, F.P.; Nzihou, A.; Joseph, K.; Rouxel, D.; Fernandes, S.C.M.; Kalarikkal, N.; Thomas, S. Gas barrier, rheological and mechanical properties of immiscible natural rubber/acrylonitrile butadiene rubber/organoclay (NR/NBR/Organoclay) blend nanocomposites. Materials 2020, 13, 2654. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhao, X.; Zhao, X.; Wu, X.; Li, X.; Zhang, L. Bromination modification of butyl rubber and its structure, properties, and application. Ind. Eng. Chem. Res. 2019, 58, 16645–16653. [Google Scholar] [CrossRef]
- Rattanasom, N.; Prasertsri, S.; Suchiva, K. Mechanical properties; thermal stability; gas permeability, and phase morphology in natural rubber/bromobutyl rubber blends. J. Appl. Polym. Sci. 2009, 113, 3985–3992. [Google Scholar] [CrossRef]
- Smitthipong, W.; Tantatherdtam, R.; Rungsanthien, K.; Suwanruji, P.; Klanarong, S.; Radabutra, S.; Thanawan, S.; Vallat, M.F.; Nardin, M.; Mougin, K.; et al. Effect of non-rubber components on properties of sulphur crosslinked natural rubbers. Adv. Mater. Res. 2013, 844, 345–348. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, C.; Luo, Y.; Chen, L.; Zhou, Y.; He, C.; Fang, P.; Peng, X.; Huang, Z. Effect of aluminum hydroxide on low-molecular-weight siloxane distribution and microstructure of high-temperature vulcanized silicone rubber. J. Appl. Polym. Sci. 2018, 135, 45803. [Google Scholar] [CrossRef]
- Zhang, C.; An, X.; Tang, Z.; Fang, S.; Guo, B.; Zhang, L.; Liu, F.; Liu, J.; Chen, Z. Creation of tortuosity in unfilled rubber via heterogeneous cross-linking toward improved barrier property. Macromolecules 2021, 54, 11522–11532. [Google Scholar] [CrossRef]
- De Keer, L.; Kilic, K.I.; Steenberge, P.H.M.V.; Daelemans, L.; Kodura, D.; Frisch, H.; De Clerck, K.; Reyniers, M.F.; Barner-Kowollik, C.; Dauskardt, R.H.; et al. Computational prediction of the molecular configuration of three-dimensional network polymers. Nat. Mater. 2021, 20, 1422–1430. [Google Scholar] [CrossRef]
- Shao, H.; Guo, Q.; He, A. Strategy for the NR/BR blends with improved thermo-oxidative resistance. Polym. Degrad. Stab. 2021, 191, 109665. [Google Scholar] [CrossRef]
- Shao, H.; Guo, Q.; He, A. Evolution of crosslinking networks structure and thermo-oxidative aging behavior of unfilled NR/BR blends with TBIR as extra functional compatibilizer. Polym. Test. 2022, 115, 107715. [Google Scholar] [CrossRef]
- Cai, F.; You, G.; Zhao, X.; Hu, H.; Wu, S. The relationship between specific structure and gas permeability of bromobutyl rubber: A combination of experiments and molecular simulations. Macromol. Theory Simul. 2019, 28, 1900025. [Google Scholar] [CrossRef]
- Mark, J.E. ; Polymer Data Handbook; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Golzar, K.; Amjad-Iranagh, S.; Amani, M.; Modarress, H. Molecular simulation study of penetrant gas transport properties into the pure and nanosized silica particles filled polysulfone membranes. J. Membr. Sci. 2014, 451, 117–134. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Chen, L.-H.; Chang, K.-S.; Chen, T.-H.; Lin, Y.-F.; Tung, K.-L. Structural characteristics and transport behavior of triptycene-based PIMs membranes: A combination study using ab initio calculation and molecular simulations. J. Membr. Sci. 2016, 514, 114–124. [Google Scholar] [CrossRef]
- Pan, F.; Peng, F.; Jiang, Z. Diffusion behavior of benzene/cyclohexane molecules in poly(vinyl alcohol)-graphite hybrid membranes by molecular dynamics simulation. Chem. Eng. Sci. 2007, 62, 703–710. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Song, L.; Ren, H.; Wang, R.; He, A. Elastomer nanocomposites with superior dynamic mechanical properties via trans-1,4-poly (butadiene-co-isoprene) incorporation. Compos. Sci. Technol. 2018, 158, 156–163. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Guiver, M.D. Polysulfone/silica nanoparticle mixed-matrix membranes for gas separation. J. Membr. Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Li, X.; Zong, X.; Wang, R.; He, A. The influence of crystalline component with varied chain sequence structure and crystallinity on properties of NR based nanocomposites. Compos. Part A-Appl. Sci. Manuf. 2023, 168, 107462. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Nie, H.; Wang, R.; He, A. Multi-block copolymer as reactive multifunctional compatibilizer for NR/BR blends with desired network structures and dynamical properties: Compatibility, co-vulcanization and filler dispersion. Compos. Part A-Appl. Sci. Manuf. 2019, 116, 197–205. [Google Scholar] [CrossRef]
- Li, W.; Peng, W.; Ren, S.; He, A. Synthesis and Characterization of trans-1,4-Poly(butadiene-co-isoprene) Rubbers (TBIR) with Different Fraction and Chain Sequence Distribution and Its Influence on the Properties of Natural Rubber/TBIR/Carbon Black Composites. Ind. Eng. Chem. Res. 2019, 58, 10609–10617. [Google Scholar] [CrossRef]
- Gavish, M.; Woodward, A.E. Effects of temperature on the infra-red spectrum of partially crystalline trans-1,4-polyisoprene. Polymer 1989, 30, 905–909. [Google Scholar] [CrossRef]
- Shao, H.; Li, Z.; He, A.; Liu, C.; Yao, W. Fabrication of trans-1,4-polyisoprene nanofibers by electrospinning and its crystallization behavior and mechanism. Chin. J. Polym. Sci. 2016, 34, 797–804. [Google Scholar] [CrossRef]
- Zarshad, S.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. A simple model for gas barrier performance of polymer nanocomposites considering filler alignment angle and diffusion direction. Compos. Sci. Technol. 2022, 230, 109397. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, X.; Shi, Y.; Zhang, L. A novel interface agent for preparation of high gas barrier organic montmorillonite/brominated butyl rubber nanocomposites. Macromol. Mater. Eng. 2023, 308, 2200567. [Google Scholar] [CrossRef]
- Yao, K.; Nie, H.; Liang, Y.; Qiu, D.; He, A. Polymorphic crystallization behaviors in cis-1,4-polyisoprene/trans-1,4-polyisoprene blends. Polymer 2015, 80, 259–264. [Google Scholar] [CrossRef]
- Yuk, J.S.; Mo, E.; Kim, S.; Jeong, H.; Gwon, H.; Kim, N.-K.; Kim, Y.-W.; Shin, J. Thermoplastic superelastomers based on poly(isobutylene)-graft-poly(l-lactide) copolymers: Enhanced thermal stability, tunable tensile strength, and gas barrier property. Macromolecules 2020, 53, 2503–2515. [Google Scholar] [CrossRef]
- Zhuang, G.; Wey, M.; Tseng, H. A novel technique using reclaimed tire rubber for gas separation membranes. J. Membr. Sci. 2016, 520, 314–325. [Google Scholar] [CrossRef]
- Andrio, A.; Compañ, V.; Reis-Nunes, R.C.; López, M.L.; Riande, E. Influence of cellulose reinforcers on gas transport through natural rubber. J. Membr. Sci. 2000, 178, 65–74. [Google Scholar] [CrossRef]
- George, S.; Ninan, K.; Thomas, S. Permeation of nitrogen and oxygen gases through styrene-butadiene rubber, natural rubber and styrene-butadiene rubber/natural rubber blend membranes. Eur. Polym. J. 2001, 37, 183–191. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Martin, A.; Oulyadi, H.; Marais, S.; Fatyeyeva, K. Polymer EVA-OH membrane with improved water/gas separation performance: Influence of VAc/VOH repeating units ratio on membrane physical chemical properties. J. Membr. Sci. 2023, 673, 121386. [Google Scholar] [CrossRef]

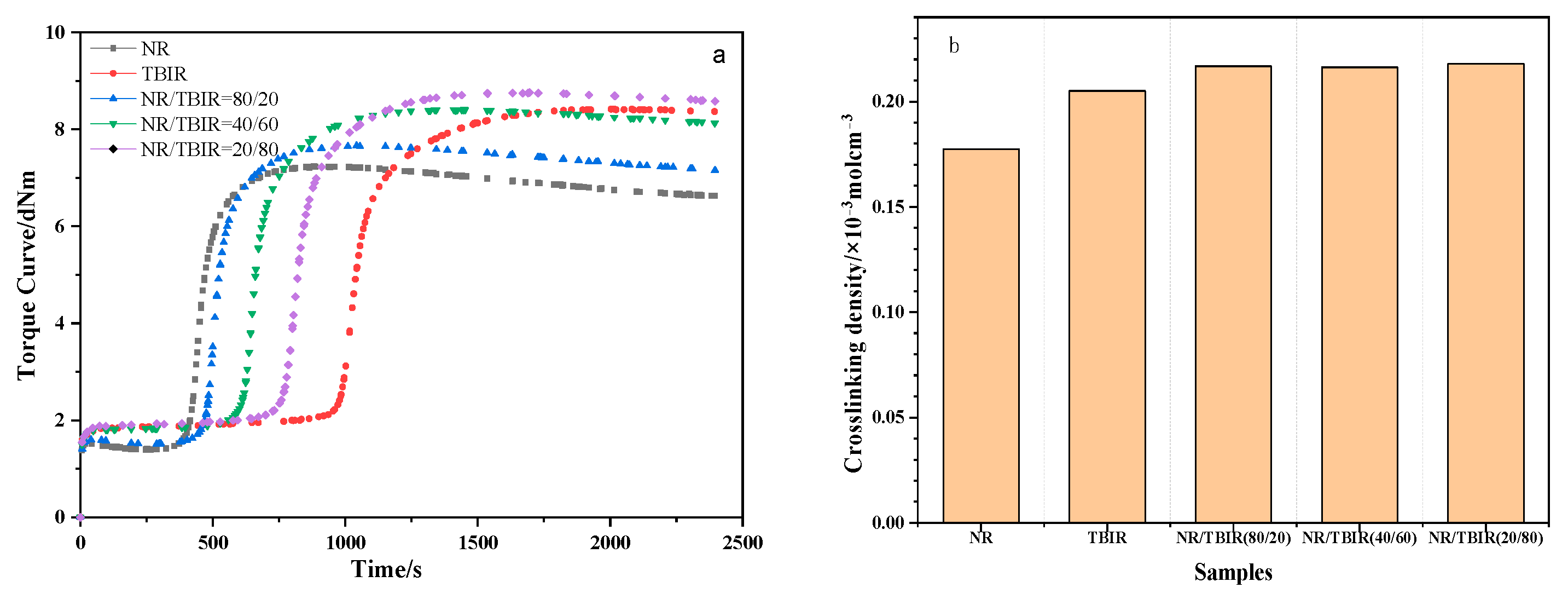





| NR | NR/TBIR (80/20) | NR/TBIR (40/60) | NR/TBIR (20/80) | TBIR | |
|---|---|---|---|---|---|
| ML/dNm | 1.40 | 1.51 | 1.81 | 1.89 | 1.84 |
| MH/dNm | 7.24 | 7.66 | 8.41 | 8.76 | 8.24 |
| t10/min | 6.89 | 7.91 | 10.24 | 12.79 | 16.38 |
| t90/min | 9.69 | 10.96 | 14.38 | 17.49 | 22.07 |
| MH-ML/dNm | 5.84 | 6.15 | 6.60 | 6.87 | 6.58 |
| Crosslinking density/mol/m3 | 178.5 | 216.8 | 216.3 | 217.9 | 211.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, P.; Shao, H.; He, A. Excellent Oxygen Barrier Property of Unfilled Natural Rubber/trans-Butadiene-co-Isoprene Rubber Vulcanizates under the Synergistic Effect of Crosslinking Density and Crystallization. Polymers 2024, 16, 345. https://doi.org/10.3390/polym16030345
Xia P, Shao H, He A. Excellent Oxygen Barrier Property of Unfilled Natural Rubber/trans-Butadiene-co-Isoprene Rubber Vulcanizates under the Synergistic Effect of Crosslinking Density and Crystallization. Polymers. 2024; 16(3):345. https://doi.org/10.3390/polym16030345
Chicago/Turabian StyleXia, Pengcheng, Huafeng Shao, and Aihua He. 2024. "Excellent Oxygen Barrier Property of Unfilled Natural Rubber/trans-Butadiene-co-Isoprene Rubber Vulcanizates under the Synergistic Effect of Crosslinking Density and Crystallization" Polymers 16, no. 3: 345. https://doi.org/10.3390/polym16030345
APA StyleXia, P., Shao, H., & He, A. (2024). Excellent Oxygen Barrier Property of Unfilled Natural Rubber/trans-Butadiene-co-Isoprene Rubber Vulcanizates under the Synergistic Effect of Crosslinking Density and Crystallization. Polymers, 16(3), 345. https://doi.org/10.3390/polym16030345






