Design and Development of an Edible Coating for a Ready-to-Eat Fish Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Design and Set Up
2.3. Sample Preparation
2.4. Coating Preparation and Application
2.5. Characterisation of Coating
2.6. Characterisation of Product Quality
2.7. Statistical Analysis
3. Results
3.1. Development of a Chitosan Coating
3.2. Development of an Alginate Coating
3.3. Comparing the Performance of a Chitosan, Alginate and Bilayer Coating under Optimal and Abused Storage Conditions
3.4. Effect of the Edible Coatings on the Product Shelf Life
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koirala, P.; Nirmal, N.P.; Woraprayote, W.; Visessanguan, W.; Bhandari, Y.; Karim, N.U.; Nor-Khaizura, M.A.R.; Saricaoğlu, F.T. Nano-engineered edible films and coatings for seafood products. Food Packag. Shelf Life 2023, 38, 101135. [Google Scholar] [CrossRef]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent advances in edible coating of food products and its legislations: A review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Gürdal, A.A.; Çetinkaya, T. Advancements in edible films for aquatic product preservation and packaging. Rev. Aquac. 2023; early view. [Google Scholar] [CrossRef]
- Vicente, F.A.; Ventura, S.P.; Passos, H.; Dias, A.C.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean waste biorefinery as a sustainable cost-effective business model. Chem. Eng. J. 2022, 442, 135937. [Google Scholar] [CrossRef]
- Muñoz, I.; Rodríguez, C.; Gillet, D.M.; Moerschbacher, B. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT Food Sci. Technol. 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Zarandona, I.; López-Caballero, M.E.; Montero, M.P.; Guerrero, P.; de La Caba, K.; Gómez-Guillén, M.C. Horse mackerel (Trachurus trachurus) fillets biopreservation by using gallic acid and chitosan coatings. Food Control 2021, 120, 107511. [Google Scholar] [CrossRef]
- Sørbø, S.; Lerfall, J. Effect of edible coating and modified atmosphere packaging on the microbiological and physicochemical stability of retail maki sushi. J. Food Sci. 2022, 87, 1211–1229. [Google Scholar] [CrossRef] [PubMed]
- Martínez, O.; Salmerón, J.; Epelde, L.; Vicente, M.; de Vega, C. Quality enhancement of smoked sea bass (Dicentrarchus labrax) fillets by adding resveratrol and coating with chitosan and alginate edible films. Food Control 2018, 85, 168–176. [Google Scholar] [CrossRef]
- Bonilla, F.; Chouljenko, A.; Lin, A.; Young, B.M.; Goribidanur, T.S.; Blake, J.C.; Bechtel, P.J.; Sathivel, S. Chitosan and water-soluble chitosan effects on refrigerated catfish fillet quality. Food Biosci. 2019, 31, 100426. [Google Scholar] [CrossRef]
- Carrión-Granda, X.; Fernández-Pan, I.; Jaime, I.; Rovira, J.; Maté, J.I. Improvement of the microbiological quality of ready-to-eat peeled shrimps (Penaeus vannamei) by the use of chitosan coatings. Int. J. Food Microbiol. 2016, 232, 144–149. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef]
- Li, T.; Hu, W.; Li, J.; Zhang, X.; Zhu, J.; Li, X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control 2012, 25, 101–106. [Google Scholar] [CrossRef]
- Arancibia, M.Y.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Chitosan coatings enriched with active shrimp waste for shrimp preservation. Food Control 2015, 54, 259–266. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018, 242, 412–420. [Google Scholar] [CrossRef]
- Azaza, Y.B.; Hamdi, M.; Charmette, C.; Jridi, M.; Li, S.; Nasri, M.; Nasri, R. Development and characterization of active packaging films based on chitosan and sardinella protein isolate: Effects on the quality and the shelf life of shrimps. Food Packag. Shelf Life 2022, 31, 100796. [Google Scholar] [CrossRef]
- Beata Łabowska, M.; Michalak, I.; Detyna, J. Methods of extraction, physicochemical properties of alginates and their applications in biomedical field—A review. Open Chem. 2019, 17, 738–762. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Pajohi-Alamoti, M. The effects of incorporated resveratrol in edible coating based on sodium alginate on the refrigerated trout (Oncorhynchus mykiss) fillets’ sensorial and physicochemical features. Food Sci. Biotechnol. 2020, 29, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bazargani-Gilani, B. Activating sodium alginate-based edible coating using a dietary supplement for increasing the shelf life of rainbow trout fillet during refrigerated storage (4 ± 1 °C). J. Food Saf. 2017, 38, e12395. [Google Scholar] [CrossRef]
- Heydari, R.; Bavandi, S.; Javadian, S.R. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 °C. Food Sci. Nutr. 2015, 3, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wang, L.; Wang, Q.; Lei, J.; Hong, W.; Huang, B.; Zhang, C. Effect of a Sodium Alginate Coating Infused with Tea Polyphenols on the Quality of Fresh Japanese Sea Bass (Lateolabrax japonicas) Fillets. J. Food Sci. 2018, 83, 1695–1700. [Google Scholar] [CrossRef]
- Sáez, M.I.; Suárez, M.D.; Martínez, T.F. Effects of alginate coating enriched with tannins on shelf life of cultured rainbow trout (Oncorhynchus mykiss) fillets. LWT Food Sci. Technol. 2020, 118, 108767. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Guerrero, A.; Ornaghi, M.G.; Kempinski, E.M.B.C.; Sary, C.; Monteschio, J.D.O.; Matumoto-Pintro, P.T.; Ribeiro, R.P.; do Prado, I.N. Quality and sensory acceptability of fish fillet (Oreochromis niloticus) with alginate-based coating containing essential oils. J. Food Sci. Technol. 2018, 55, 4945–4955. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Ageing 2019: Highlights; United Nations: New York, NY, USA, 2019; Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed on 10 January 2023).
- Liu, C.; Ralston, N.V. Chapter Seven—Seafood and health: What you need to know? Adv. Food Nutr. Res. 2021, 97, 275–318. [Google Scholar] [CrossRef] [PubMed]
- The Commission of the European Communities. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs 2005. OJEU 2005, 338, 1–26. [Google Scholar]
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA test by an extractive method applied to ‘paté’. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed; Wiley: Hoboken, NJ, USA, 2016; ISBN 978-1-118-91601-8. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1118146921. [Google Scholar]
- Mendes, R.; Pestana, C.; Goncalves, A. The effect of soluble gas stabilisation on the quality of packed sardine fillets (Sarddina pilchardus) stored in air, VP and MAP. Int. J. Food Sci. Technol. 2008, 43, 2000–2009. [Google Scholar] [CrossRef]
- Anacleto, P.; Teixeira, B.; Marques, P.; Pedro, S.; Nunes, M.L.; Marques, A. Shelf-life of cooked edible crab (Cancer pagurus) stored under refrigerated conditions. LWT Food Sci. Technol. 2011, 44, 1376–1382. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of Coating Thickness on Viscosity of Coating Solution Applied to Fruits and Vegetables by Dipping Method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Socaciu, M.-I.; Semeniuc, C.; Vodnar, D. Edible Films and Coatings for Fresh Fish Packaging: Focus on Quality Changes and Shelf-life Extension. Coatings 2018, 8, 366. [Google Scholar] [CrossRef]
- Wu, Y.; Rhim, J.W.; Weller, C.L.; Hamouz, F.; Cuppett, S.; Schnepf, M. Moisture Loss and Lipid Oxidation for Precooked Beef Patties Stored in Edible Coatings and Films. J. Food Sci. 2000, 65, 300–304. [Google Scholar] [CrossRef]
- Shankar, S.; Danneels, F.; Lacroix, M. Coating with alginate containing a mixture of essential oils and citrus extract in combination with ozonation or gamma irradiation increased the shelf life of Merluccius sp. fillets. Food Packag. Shelf Life 2019, 22, 100434. [Google Scholar] [CrossRef]

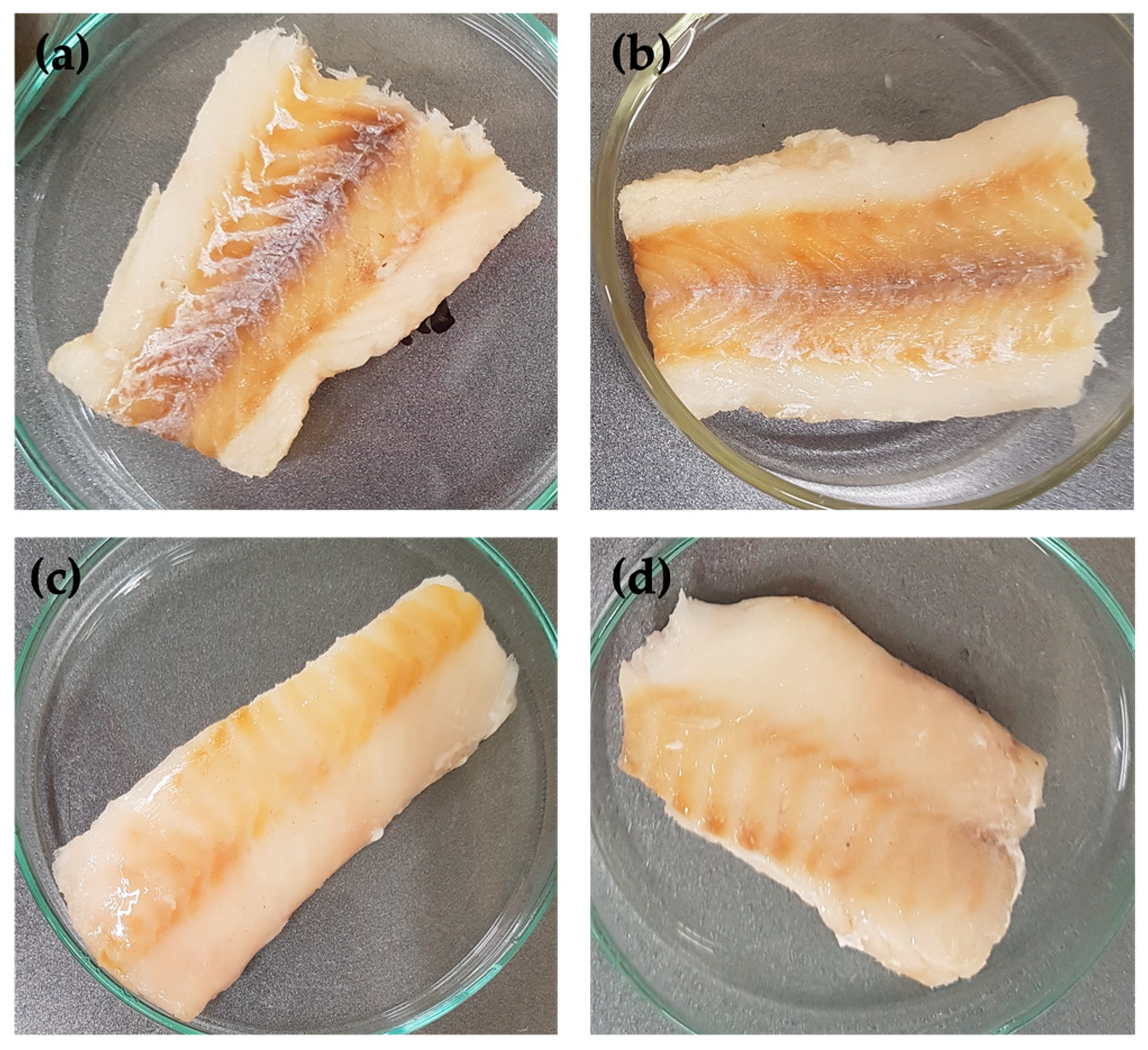



| Experimental Set-Up | Factors | Levels | |||||
|---|---|---|---|---|---|---|---|
| (a) | Chitosan concentration % (w/v) | 1 | 2 | 3 | |||
| Glycerol concentration % (w/w chitosan) | 0 | 15 | 30 | ||||
| (b) | Alginate concentration % (w/v) | 1 | 2 | ||||
| Glycerol concentration % (w/w alginate) | 0 | 15 | |||||
| Crosslinking with calcium chloride | yes | no | |||||
| (c) | Storage temperature | 4 | 14 | ||||
| Alginate coating | no | yes | |||||
| Chitosan coating | no | yes | |||||
| Lipid Oxidation (mg MDA eq./kg Sample) | ||||
| (a) | day 0 | day 2 | day 4 | day 7 |
| Control | 0.16 ± 0.03 a,A | 0.54 ± 0.53 a,A | 0.66 ± 0.27 a,A,B | 1.90 ± 0.82 a,B |
| Alginate | 0.16 ± 0.03 a,A | 0.79 ± 0.22 a,A | 1.56 ± 0.91 a,A | 1.10 ± 0.10 a,B |
| Chitosan | 0.16 ± 0.03 a,A | 0.28 ± 0.17 a,A,B | 0.50 ± 0.12 a,B | 1.81 ± 0.38 a,A,B |
| Chitosan + Alginate | 0.16 ± 0.03 a,A | 0.54 ± 0.07 a,B | 1.15 ± 0.11 a,C | 1.50 ± 0.03 a,D |
| (b) | day 0 | day 7 | day 14 | day 21 |
| Control | 0.16 ± 0.03 a,A | 1.10 ± 0.62 a,A | 1.17 ± 0.39 a,A | 4.38 ± 1.20 a,B |
| Alginate | 0.16 ± 0.03 a,A | 1.55 ± 0.17 a,A,B | 2.21 ± 0.50 b,A,B,C | 4.36 ± 0.96 a,C |
| Chitosan | 0.16 ± 0.03 a,A | 1.05 ± 0.38 a,B | 2.40 ± 0.42 a,b,B | 4.22 ± 1.09 a,C |
| Chitosan + Alginate | 0.16 ± 0.03 a,A | 1.48 ± 0.66 a,A,B | 1.75 ± 0.42 a,b,A,B | 2.07 ± 0.89 a,A,B |
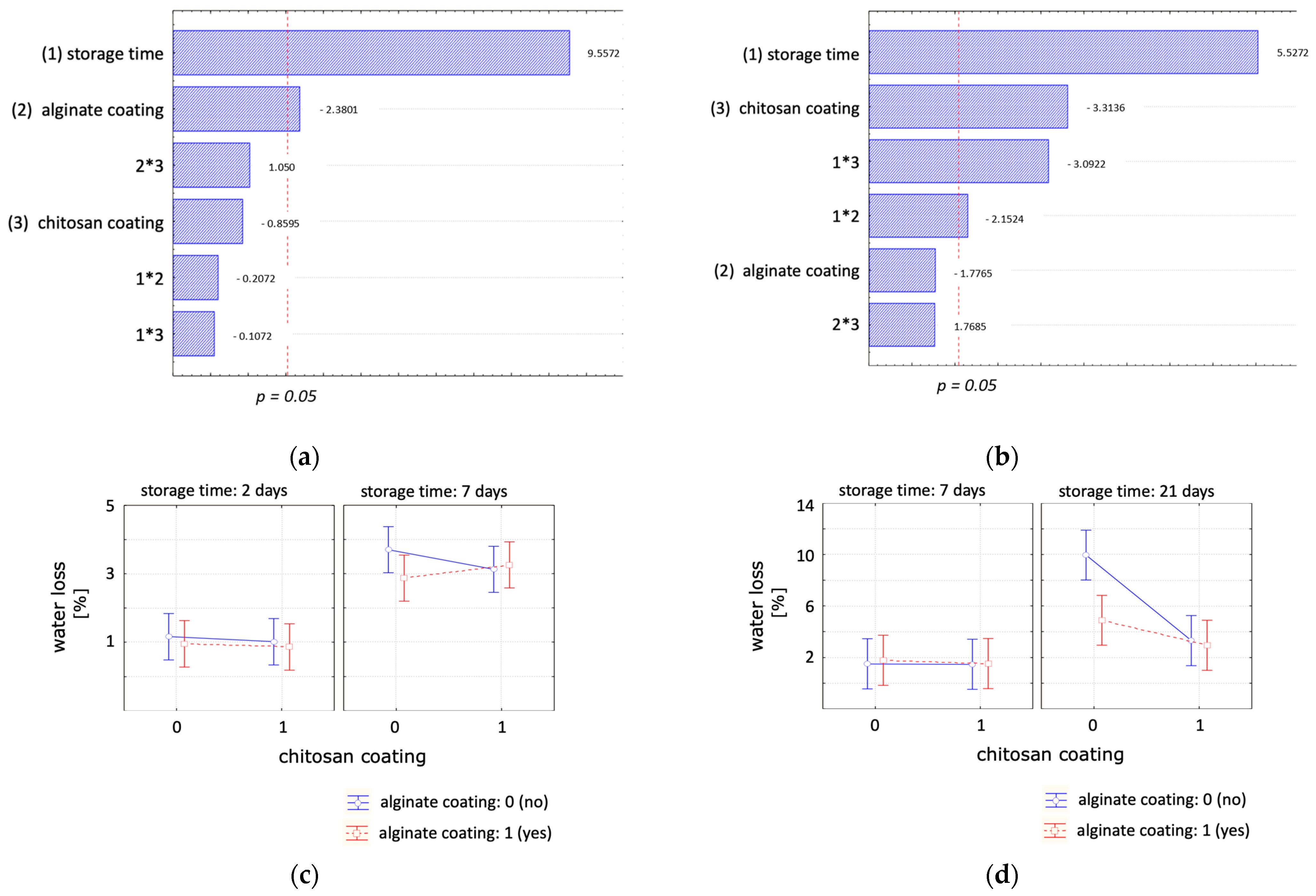
| Water Loss (%) | ||||
| (a) | day 2 | day 4 | day 7 | |
| Control | 1.16 ± 0.35 a,A | 2.70 ± 0.44 a,B | 3.70 ± 0.32 a,B | |
| Alginate | 0.96 ± 0.17 a,A | 1.78 ± 0.36 a,A,B | 2.87 ± 0.54 a,B | |
| Chitosan | 1.02 ± 0.31 a,A | 2.32 ± 0.66 a,A | 3.13 ± 1.00 a,A | |
| Chitosan + Alginate | 0.86 ± 0.06 a,A | 1.59 ± 0.11 a,A | 3.26 ± 0.52 a,B | |
| (b) | day 7 | day 14 | day 21 | |
| Control | 1.50 ± 0.49 a,A | 3.65 ± 0.98 a,A | 9.96 ± 4.02 a,A | |
| Alginate | 1.77 ± 0.25 a,A | 2.63 ± 0.30 a,A,B | 4.88 ± 0.76 a,b,B | |
| Chitosan | 1.46 ± 0.30 a,A | 1.99 ± 0.28 a,A | 3.31 ± 0.50 a,b,B | |
| Chitosan + Alginate | 1.52 ± 0.68 a,A | 2.28 ± 0.51 a,A | 2.94 ± 0.71 b,A | |
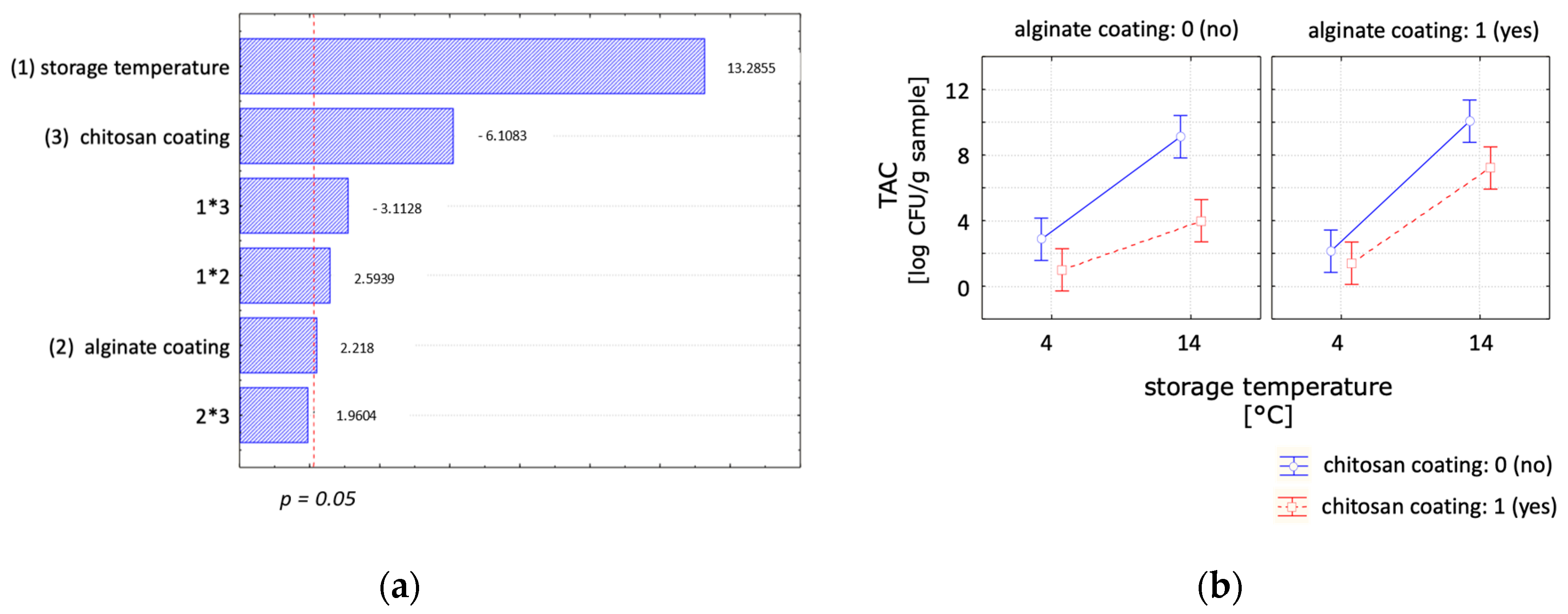
| Total Aerobic Count (log CFU/g Sample) | ||||
| (a) | day 0 | day 2 | day 4 | day 7 |
| Control | <1.06 * ± 0.10 a,A | 4.86 ± 0.74 a,B | 7.19 ± 0.58 a,b,C | 9.11 ± 0.45 a,D |
| Alginate | <1.06 * ± 0.10 a,A | 5.85 ± 0.05 a,B | 7.70 ± 0.58 b,C | 10.06 ± 0.06 a,D |
| Chitosan | <1.06 * ± 0.10 a,A | <1.39 * ± 0.67 b,A | <0.94 * ± 0.34 c,A | 5.07 ± 2.56 b,A |
| Chitosan + Alginate | <1.06 * ± 0.10 a,A | 2.79 * ± 0.74 b,A | 6.00 ± 0.90 a,B | 7.21 * ± 1.05 a,b,B |
| (b) | day 0 | day 7 | day 14 | day 21 |
| Control | <1.06 * ± 0.10 a,A | <2.86 * ± 0.84 a,A | 7.63 ± 0.75 a,B | 9.10 ± 1.04 a,B |
| Alginate | <1.06 * ± 0.10 a,A | <2.13 * ± 0.23 a,b,B | >8.48 * ± 0.00 a,C | 10.35 ± 0.41 a,D |
| Chitosan | <1.06 * ± 0.10 a,A | <1.00 * ± 0.00 b,A | <1.00 * ± 0.00 b,A | <1.79 * ± 2.29 b,A |
| Chitosan + Alginate | <1.06 * ± 0.10 a,A | <1.40 * ± 0.70 a,b,A | 4.85 * ± 2.81 a,A | 10.06 ± 0.00 a,B |
| Day 0 | Day 2 | Day 4 | Day 7 | Day 14 | Day 21 | Day 28 | ||
|---|---|---|---|---|---|---|---|---|
| 4 °C | Alginate | 1 | 0.84 | 0 | 0 | 0 | ||
| Chitosan | 1 | 0.89 | 0.84 | 0.65 | 0 | |||
| Chitosan-Alginate | 1 | 0.87 | 0.61 | 0 | 0 | |||
| Control | 1 | 0.80 | 0 | 0 | 0 | |||
| 14 °C | Alginate | 1 | 0.56 | 0 | 0 | 0 | 0 | 0 |
| Chitosan | 1 | 0.92 | 0.86 | 0.67 | 0 | 0 | 0 | |
| Chitosan-Alginate | 1 | 0.85 | 0.50 | 0 | 0 | 0 | 0 | |
| Control | 1 | 0.67 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 0.8 | 0.6 | 0.4 | 0.2 | 0 | |||
| colour scale from 1 to 0 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bremenkamp, I.; Sousa-Gallagher, M.J. Design and Development of an Edible Coating for a Ready-to-Eat Fish Product. Polymers 2024, 16, 346. https://doi.org/10.3390/polym16030346
Bremenkamp I, Sousa-Gallagher MJ. Design and Development of an Edible Coating for a Ready-to-Eat Fish Product. Polymers. 2024; 16(3):346. https://doi.org/10.3390/polym16030346
Chicago/Turabian StyleBremenkamp, Ina, and Maria J. Sousa-Gallagher. 2024. "Design and Development of an Edible Coating for a Ready-to-Eat Fish Product" Polymers 16, no. 3: 346. https://doi.org/10.3390/polym16030346
APA StyleBremenkamp, I., & Sousa-Gallagher, M. J. (2024). Design and Development of an Edible Coating for a Ready-to-Eat Fish Product. Polymers, 16(3), 346. https://doi.org/10.3390/polym16030346







