Thermodynamic Parameters of Crosslinked Elastomers (BR, SBR and NBR) and Their Blends
Abstract
1. Introduction
2. Physicochemical Models
3. Materials and Methods
3.1. Materials
3.2. Rubber Blends Preparation
3.3. Crosslinking
3.4. Crosslinked Fraction in the Blend and Swelling Tests
3.5. Tensile Tests
3.6. Density of Rubber Blends
4. Results and Discussion
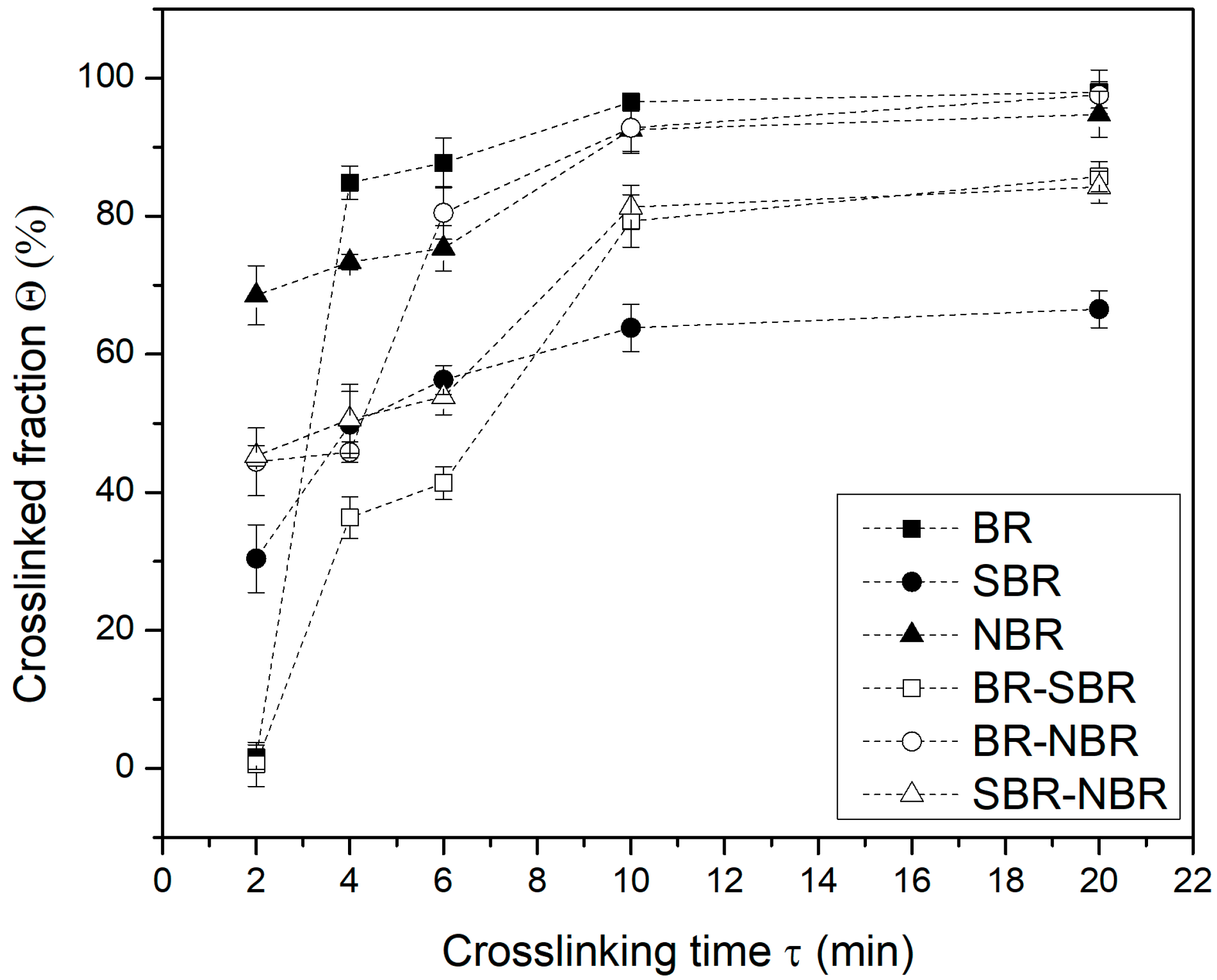
4.1. Crosslinked Fraction and Swelling Ratio
4.2. Tensile Tests of the Elastomers and Their Blends
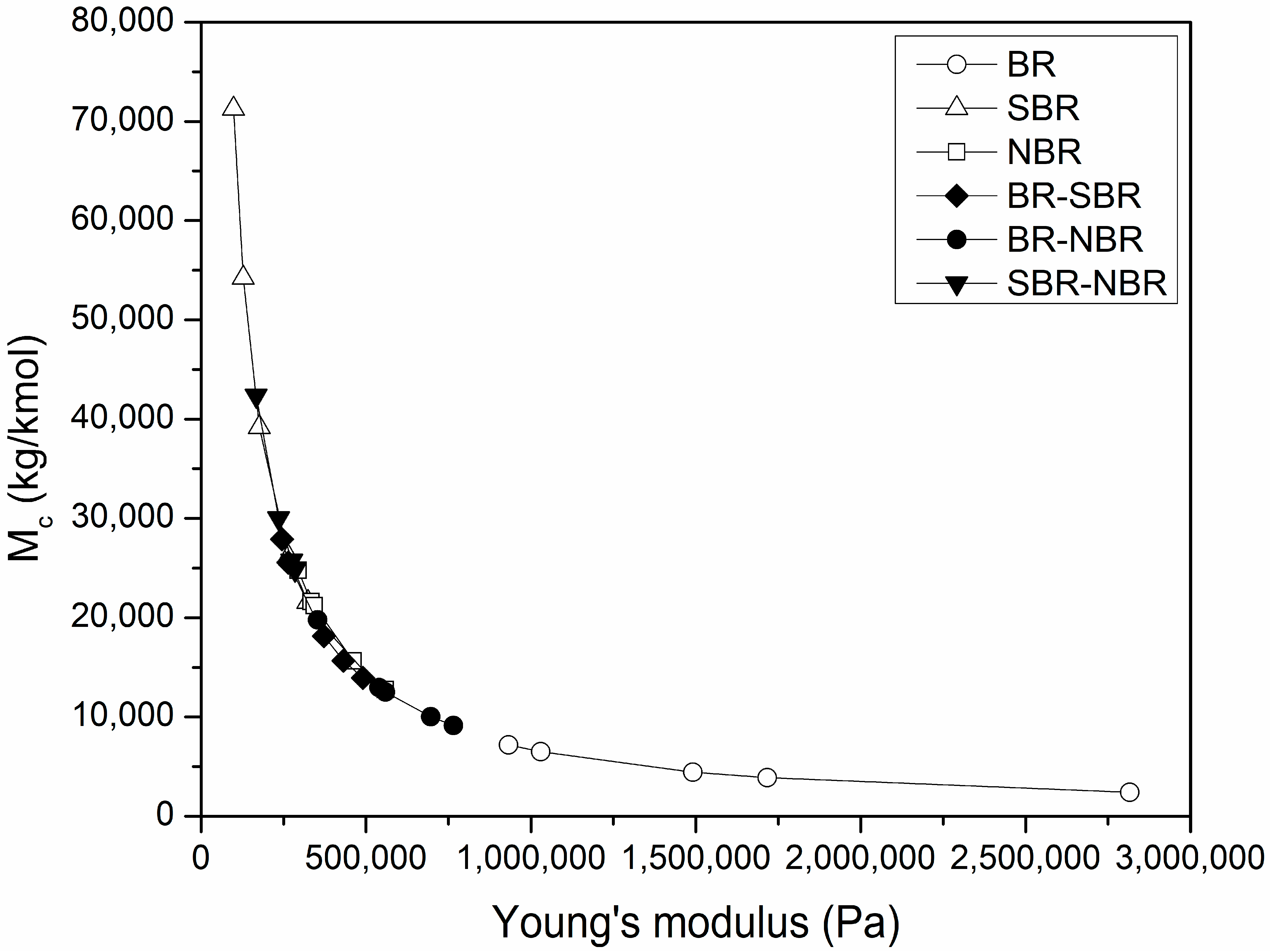
4.3. Average Molecular Weight between Crosslinks
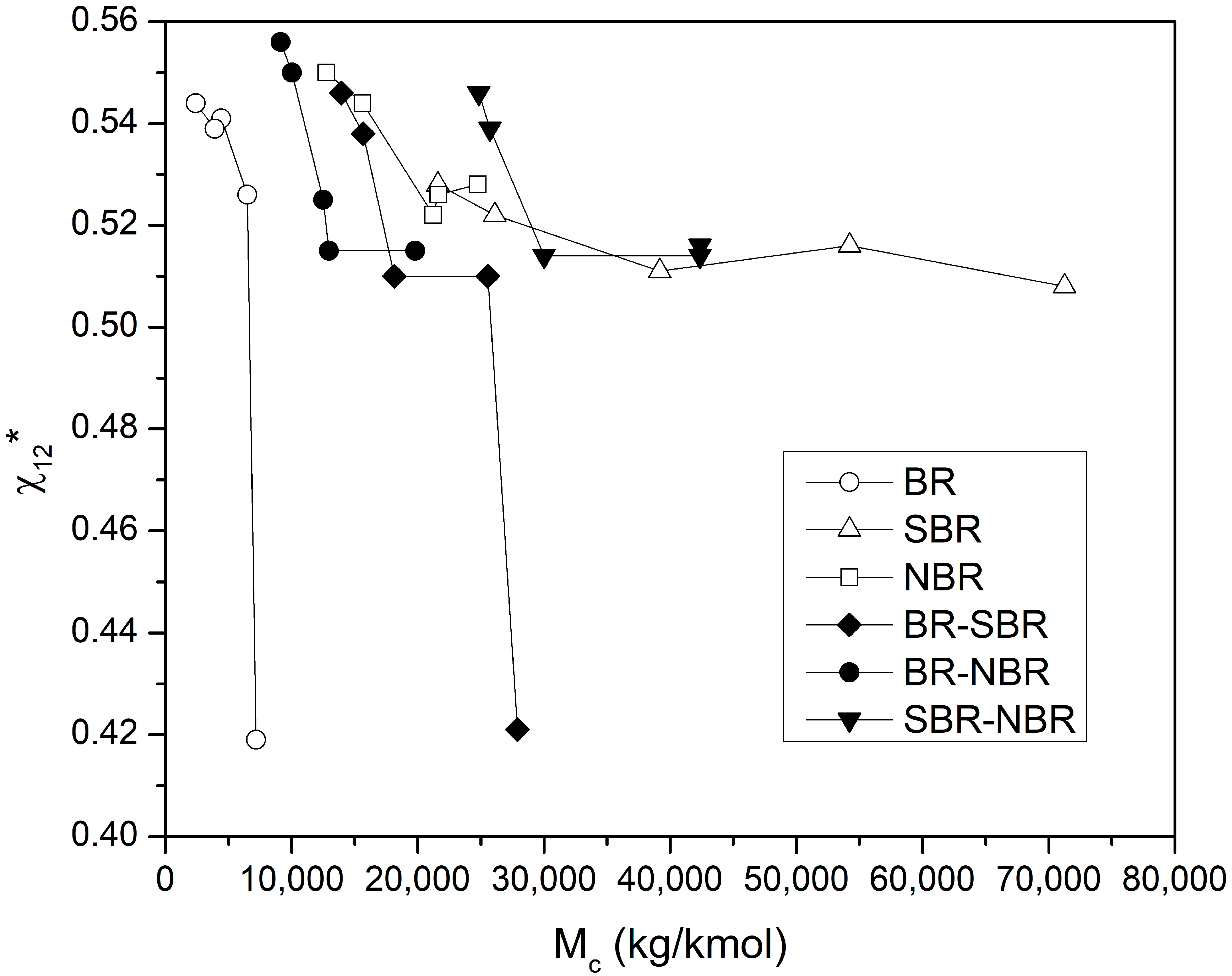
4.4. Determination of the Flory–Huggins Interaction Parameter
4.5. Evaluation of the Thermodynamic Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearce, T.C.; Schiffman, S.S.; Nagle, H.T.; Gardner, J.W. Handbook of Machine Olfaction: Electronic Nose Technology; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ludeelerd, P.; Niamlang, S.; Kunaruksapong, R.; Sirivat, A. Effect of elastomer matrix type on electromechanical response of conductive polypyrrole/elastomer blends. J. Phys. Chem. Solids 2010, 71, 1243–1250. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Bargan, A.; Fifere, N.; Georgescu, M.; Sonmez, M.; Nituica, M.; Alexandrescu, L.; Stefan, A. Mechanical Properties and Equilibrium Swelling Characteristics of Some Polymer Composites Based on Ethylene Propylene Diene Terpolymer (EPDM) Reinforced with Hemp Fibers. Materials 2022, 15, 6838. [Google Scholar] [CrossRef] [PubMed]
- Christova, D.; Velichkova, R.; Goethals, E.J.; Du Prez, F.E. Amphiphilic segmented polymer networks based on poly(2-alkyl-2-oxazoline) and poly(methyl methacrylate). Polymer 2002, 43, 4585–4590. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shrivastava, M. Swelling kinetics of a hydrogel of poly(ethylene glycol) and poly(acrylamide-co-styrene). J. Appl. Polym. Sci. 2002, 85, 1419–1428. [Google Scholar] [CrossRef]
- Chungprempree, J.; Charoenpongpool, S.; Preechawong, J.; Atthi, N.; Nithitanakul, M. Simple preparation of polydimethylsiloxane and polyurethane blend film for marine antibiofouling application. Polymers 2021, 13, 2242. [Google Scholar] [CrossRef]
- Datta, S. Elastomer blends. In Science and Technology of Rubber; Elsevier: Amsterdam, The Netherlands, 2005; pp. 529–554. [Google Scholar]
- Smejda-Krzewicka, A.; Mrozowski, K.; Strzelec, K. Interelastomer Reactions Occurring during the Cross-Linking of Hydrogenated Acrylonitrile-Butadiene (HNBR) and Chloroprene (CR) Rubbers Blends in the Presence of Silver (I) Oxide (Ag2O) and Mechanical Properties of Cured Products. Materials 2023, 16, 4573. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, J.; Han, L.; Luan, J.; Ma, X.; Chen, W. Effect of SEBS Molecular Structure and Formula Composition on the Performance of SEBS/PP TPE for Automotive Interior Skin. Polymers 2023, 15, 2753. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.; Yang, J.; Zhang, Y. Effect of Acetylated SEBS/PP for Potential HVAC Cable Insulation. Materials 2021, 14, 1811. [Google Scholar] [CrossRef] [PubMed]
- Brydson, J.A. Styrene—Butadiene Rubber. In Developments in Rubber Technology—2: Synthetic Rubbers; Springer: Berlin/Heidelberg, Germany, 1981; pp. 21–49. [Google Scholar]
- White, W.C. Butadiene production process overview. Chem. Biol. Interact. 2007, 166, 10–14. [Google Scholar] [CrossRef]
- Sone, T. Industrial synthetic method of the rubbers. 1. Butadiene rubber. Int. Polym. Sci. Technol. 2016, 43, 49–54. [Google Scholar] [CrossRef]
- Fujimoto, K.; Yoshimiya, N. Blends of cis-1, 4-polybutadiene with natural or styrene butadiene rubber. Rubber Chem. Technol. 1968, 41, 669–677. [Google Scholar] [CrossRef]
- Varkey, J.T.; Thomas, S.; Rao, S.S. Rheological behavior of blends of natural rubber and styrene–butadiene rubber latices. J. Appl. Polym. Sci. 1995, 56, 451–460. [Google Scholar] [CrossRef]
- Ismail, H.; Suzaimah, S. Styrene butadiene rubber/epoxidized natural rubber blends: Dynamic properties, curing characteristics and swelling studies. Polym. Test. 2000, 19, 879–888. [Google Scholar] [CrossRef]
- Nair, T.M.; Kumaran, M.G.; Unnikrishnan, G. Mechanical and aging properties of cross-linked ethylene propylene diene rubber/styrene butadiene rubber blends. J. Appl. Polym. Sci. 2004, 93, 2606–2621. [Google Scholar] [CrossRef]
- Higazy, A.A.; Afifi, H.; Khafagy, A.H.; El-Shahawy, M.A.; Mansour, A.M. Ultrasonic studies on polystyrene/styrene butadiene rubber polymer blends filled with glass fiber and talc. Ultrasonics 2006, 44, e1439–e1445. [Google Scholar] [CrossRef]
- Salgueiro, W.; Somoza, A.; Marzocca, A.J.; Torriani, I.; Mansilla, M.A. A SAXS and swelling study of cured natural rubber/styrene–butadiene rubber blends. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2320–2327. [Google Scholar] [CrossRef]
- Alimardani, M.; Abbassi-Sourki, F. New and emerging applications of carboxylated styrene butadiene rubber latex in polymer composites and blends: Review from structure to future prospective. J. Compos. Mater. 2015, 49, 1267–1282. [Google Scholar] [CrossRef]
- Jovanović, S.; Samaržija-Jovanović, S.; Marković, G.; Jovanović, V.; Adamović, T.; Marinović-Cincović, M. Mechanical properties and thermal aging behaviour of polyisoprene/polybutadiene/styrene-butadiene rubber ternary blend reinforced with carbon black. Compos. Part B Eng. 2016, 98, 126–133. [Google Scholar] [CrossRef]
- Habeeb Rahiman, K.; Unnikrishnan, G. The behaviour of styrene butadiene rubber/acrylonitrile butadiene rubber blends in the presence of chlorinated hydrocarbons. J. Polym. Res. 2006, 13, 297–314. [Google Scholar] [CrossRef]
- Jinnai, H.; Yoshida, H.; Kimishima, K.; Funaki, Y.; Hirokawa, Y.; Ribbe, A.E.; Hashimoto, T. Observation of fine structure in bicontinuous phase-separated domains of a polymer blend by laser scanning confocal microscopy. Macromolecules 2001, 34, 5186–5191. [Google Scholar] [CrossRef]
- Noriman, N.Z.; Ismail, H.; Rashid, A.A. Properties of styrene butadiene rubber/recycled acrylonitrile-butadiene rubber (SBR/NBRr) blends: Effect of the addition of trans-polyoctylene rubber. J. Appl. Polym. Sci. 2012, 126, E56–E63. [Google Scholar] [CrossRef]
- Perez, L.D.; Sierra, L.; Lopez, B.L. Effect of the filler characteristics on the miscibility of styrene-butadiene rubber and nitrile-butadiene rubber blends. Polym. Eng. Sci. 2008, 48, 1986–1993. [Google Scholar] [CrossRef]
- Malas, A.; Pal, P.; Das, C.K. Effect of expanded graphite and modified graphite flakes on the physical and thermo-mechanical properties of styrene butadiene rubber/polybutadiene rubber (SBR/BR) blends. Mater. Des. 2014, 55, 664–673. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, X.; Yin, Q.; Jia, H.; Wang, J.; Ji, Q.; Xu, Z. Enhanced compatibility and mechanical properties of carboxylated acrylonitrile butadiene rubber/styrene butadiene rubber by using graphene oxide as reinforcing filler. Compos. Part B Eng. 2017, 111, 243–250. [Google Scholar] [CrossRef]
- Boonrasri, S.; Thipchai, P.; Sae-Oui, P.; Thanakkasaranee, S.; Jantanasakulwong, K.; Rachtanapun, P. Property Improvements of Silica-Filled Styrene Butadiene Rubber/Butadiene Rubber Blend Incorporated with Fatty-Acid-Containing Palm Oil. Polymers 2023, 15, 3429. [Google Scholar] [CrossRef] [PubMed]
- Ramesan, M.T.; Kuriakose, B.; Pradeep, P.; Alex, R.; Varghese, S. Compatibilization of SBR/NBR Blends Using Chemically Modified Styrene Butadiene Rubber: Part I: Effect of Chlorine Content of Compatibilizer. Int. Polym. Process. 2001, 16, 183–191. [Google Scholar] [CrossRef]
- Mendoza, M.; Carrillo, A.; Márquez, A. New distributed optical sensor for detection and localization of liquid hydrocarbons: Part II: Optimization of the elastomer performance. Sens. Actuators A Phys. 2004, 111, 154–165. [Google Scholar] [CrossRef]
- Smejda-Krzewicka, A.; Rybiński, P.; Bradło, D.; Żukowski, W. The Morphology, Mechanical and Dynamic Properties, Fire Hazard and Toxicity of Chloroprene and Butadiene Rubber Composites Cross-Linked with Zinc. Materials 2023, 16, 1240. [Google Scholar] [CrossRef]
- Hess, W.M.; Herd, C.R.; Vegvari, P.C. Characterization of immiscible elastomer blends. Rubber Chem. Technol. 1993, 66, 329–375. [Google Scholar] [CrossRef]
- Mangaraj, D. Elastomer blends. Rubber Chem. Technol. 2002, 75, 365–427. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Munk, P.; Aminabhavi, T.M. Introduction to Macromolecular Science; Wiley Interscience: New York, NY, USA, 1989. [Google Scholar]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- George, S.C.; Thomas, S. Effect of nature and extent of crosslinking on swelling and mechanical behavior of styrene–butadiene rubber membranes. J. Membr. Sci. 1999, 163, 1–17. [Google Scholar] [CrossRef]
- Hedden, R.C.; Saxena, H.; Cohen, C. Mechanical properties and swelling behavior of end-linked poly(diethylsiloxane) networks. Macromolecules 2000, 33, 8676–8684. [Google Scholar] [CrossRef]
- ASTM Standard D638; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- Marzocca, A.J.; Cerveny, S.; Méndez, J.M. Some considerations concerning the dynamic mechanical properties of cured styrene–butadiene rubber/polybutadiene blends. Polym. Int. 2000, 49, 216–222. [Google Scholar] [CrossRef]
- Wang, C. Tear strength of styrene−butadiene−styrene block copolymers. Macromolecules 2001, 34, 9006–9014. [Google Scholar] [CrossRef]
- Nah, C.; Han, S.; Jo, B.W.; Kim, W.D.; Chang, Y. Influences of trans-polyoctylene rubber on the physical properties and phase morphology of natural rubber/acrylonitrile–butadiene rubber blends. J. Appl. Polym. Sci. 2002, 86, 125–134. [Google Scholar] [CrossRef]
- Gardiner, J.B. Curative diffusion between dissimilar elastomers and its influence on adhesion. Rubber Chem. Technol. 1968, 41, 1312–1328. [Google Scholar] [CrossRef]
- Lee, S.H.; Kontopoulou, M.; Park, C.B. Effect of nanosilica on the co-continuous morphology of polypropylene/polyolefin elastomer blends. Polymer 2010, 51, 1147–1155. [Google Scholar] [CrossRef]
- Gopalan Nair, K.; Dufresne, A. Crab shell chitin whisker reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Abu-Abdeen, M.; Elamer, I. Mechanical and swelling properties of thermoplastic elastomer blends. Mater. Des. 2010, 31, 808–815. [Google Scholar] [CrossRef]
- Burrell, H.; Immergut, B. Polymer Handbook; Wiley Interscience: New York, NY, USA, 1975. [Google Scholar]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Paulin, J.A.; Lopez-Aguilar, J.E.; Fouconnier, B.; Vargas, R.O.; Lopez-Serrano, F. Revisiting the Flory–Rehner equation: Taking a closer look at the Flory–Huggins interaction parameter and its functionality with temperature and concentration with NIPA as a case example. Polym. Bull. 2021, 79, 6709–6732. [Google Scholar] [CrossRef]
- Martter, T.D.; Foster, M.D.; Yoo, T.; Xu, S.; Lizzaraga, G.; Quirk, R.P.; Butler, P.D. Nonuniversal behavior of the thermodynamic interaction parameter in blends of star and linear polybutadiene. Macromolecules 2002, 35, 9763–9772. [Google Scholar] [CrossRef]
- Schwahn, D.; Willner, L. Phase Behavior and Flory−Huggins Interaction Parameter of Binary Polybutadiene Copolymer Mixtures with Different Vinyl Content and Molar Volume. Macromolecules 2002, 35, 239–247. [Google Scholar] [CrossRef][Green Version]
- Alberda van Ekenstein, G.; Meyboom, R.; Ten Brinke, G.; Ikkala, O. Determination of the Flory− Huggins interaction parameter of styrene and 4-vinylpyridine using copolymer blends of poly(styrene-co-4-vinylpyridine) and polystyrene. Macromolecules 2000, 33, 3752–3756. [Google Scholar] [CrossRef]
- Okay, O.; Durmaz, S.; Erman, B. Solution cross-linked poly(isobutylene) gels: Synthesis and swelling behavior. Macromolecules 2000, 33, 4822–4827. [Google Scholar] [CrossRef]
- Carbognani, L.; Rogel, E. Solvent swelling of petroleum asphaltenes. Energy Fuels 2002, 16, 1348–1358. [Google Scholar] [CrossRef]
- Paul, D.R.; Ebra-Lima, O.M. Pressure-induced diffusion of organic liquids through highly swollen polymer membranes. J. Appl. Polym. Sci. 1970, 14, 2201–2224. [Google Scholar] [CrossRef]
- Mathai, A.E.; Singh, R.P.; Thomas, S. Transport of substituted benzenes through nitrile rubber/natural rubber blend membranes. J. Membr. Sci. 2002, 202, 35–54. [Google Scholar] [CrossRef]



| Sample | BR (g) | SBR (g) | NBR (g) | DCP (g) |
|---|---|---|---|---|
| P | 60 | 0 | 0 | 0.6 |
| S | 0 | 60 | 0 | 0.6 |
| N | 0 | 0 | 60 | 0.6 |
| PS | 30 | 30 | 0 | 0.6 |
| PN | 30 | 0 | 30 | 0.6 |
| SN | 0 | 30 | 30 | 0.6 |
| Sample | Range of χ12* | Crosslinking Fraction (Θ) | χ Calculated from Equation (5) |
|---|---|---|---|
| BR | 0.447–0.569 | 0.01–0.98 | 0.4369 |
| SBR | 0.510–0.535 | 0.3–0.66 | 0.4039 |
| NBR | 0.524–0.557 | 0.68–0.94 | 0.3449 |
| BR-SBR | 0.434–0.553 | 0.006–0.85 | 0.4204 |
| BR-NBR | 0.516–0.561 | 0.44–0.97 | 0.3909 |
| SBR-NBR | 0.515–0.550 | 0.45–0.84 | 0.3744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Porras, C.; Estrada-Moreno, I.A.; Piñón-Balderrama, C.I.; Flores-Gallardo, S.G.; Márquez-Lucero, A. Thermodynamic Parameters of Crosslinked Elastomers (BR, SBR and NBR) and Their Blends. Polymers 2024, 16, 351. https://doi.org/10.3390/polym16030351
Leyva-Porras C, Estrada-Moreno IA, Piñón-Balderrama CI, Flores-Gallardo SG, Márquez-Lucero A. Thermodynamic Parameters of Crosslinked Elastomers (BR, SBR and NBR) and Their Blends. Polymers. 2024; 16(3):351. https://doi.org/10.3390/polym16030351
Chicago/Turabian StyleLeyva-Porras, César, Iván A. Estrada-Moreno, Claudia I. Piñón-Balderrama, Sergio G. Flores-Gallardo, and Alfredo Márquez-Lucero. 2024. "Thermodynamic Parameters of Crosslinked Elastomers (BR, SBR and NBR) and Their Blends" Polymers 16, no. 3: 351. https://doi.org/10.3390/polym16030351
APA StyleLeyva-Porras, C., Estrada-Moreno, I. A., Piñón-Balderrama, C. I., Flores-Gallardo, S. G., & Márquez-Lucero, A. (2024). Thermodynamic Parameters of Crosslinked Elastomers (BR, SBR and NBR) and Their Blends. Polymers, 16(3), 351. https://doi.org/10.3390/polym16030351








