Homogeneous Non-Metallocene Group 4 Metals Ligated with [N,N] Bidentate Ligand(s) for Olefin Polymerization
Abstract
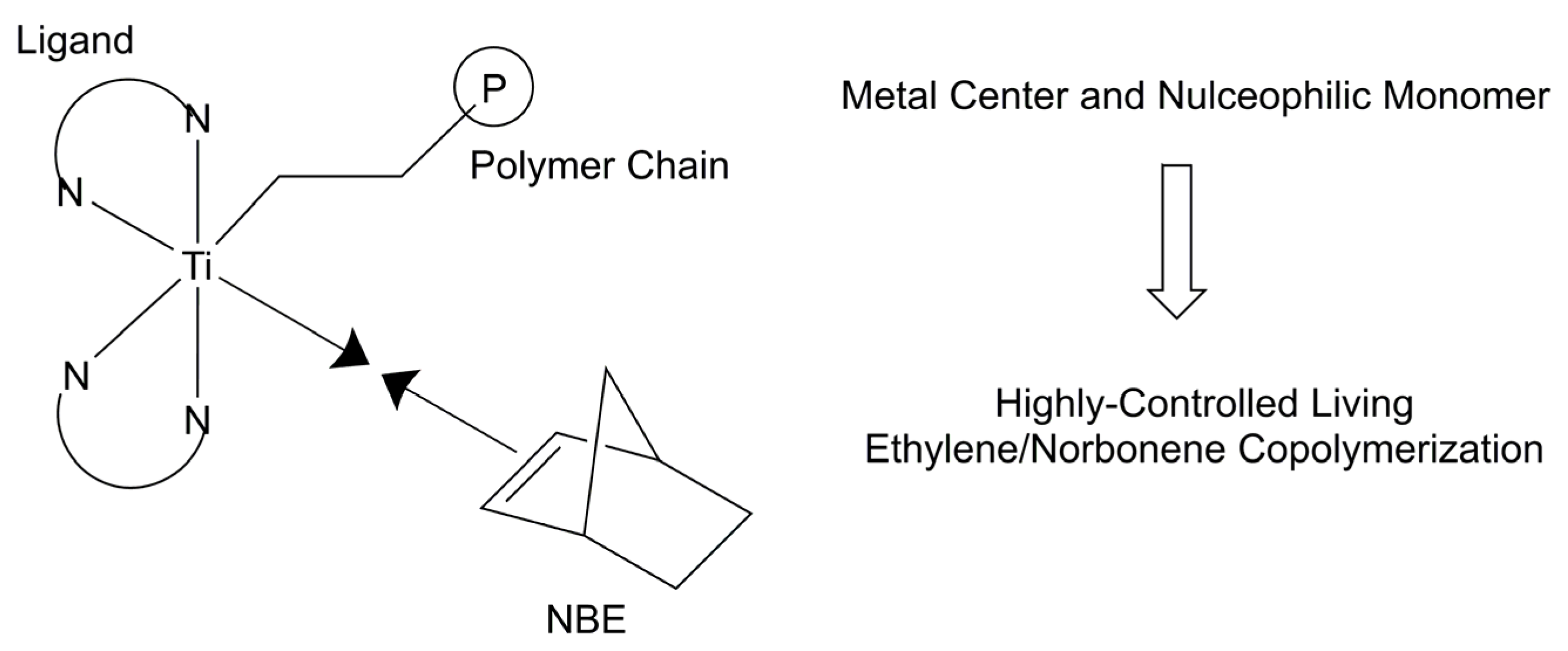
:1. Introduction
2. [N,N]-Ligands Metal Complexes
2.1. Amidinate Ligands Metal Complexes
2.2. Guanidinato Ligand Metal Complexes
2.3. Diamine Ligand Metal Complexes
2.4. N-Heterocyclic Amido Ligand Metal Complexes
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef]
- Mülhaupt, R. Green polymer chemistry and bio-based plastics: Dreams and reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Hogan, J.P. Ethylene polymerization catalysis over chromium oxide. J. Polym. Sci. Part A-1 Polym. Chem. 1970, 8, 2637–2652. [Google Scholar] [CrossRef]
- Sinn, H.; Kaminsky, W.; Vollmer, H.-J.; Woldt, R. “Living polymers” on polymerization with extremely productive Ziegler catalysts. Angew. Chem. Int. Ed. Engl. 1980, 19, 390–392. [Google Scholar] [CrossRef]
- Galli, P.; Vecellio, G. Technology: Driving force behind innovation and growth of polyolefins. Prog. Polym. Sci. 2001, 26, 1287–1336. [Google Scholar] [CrossRef]
- Tan, C.; Zou, C.; Chen, C. An ionic cluster strategy for performance improvements and product morphology control in metal-catalyzed olefin–polar monomer copolymerization. J. Am. Chem. Soc. 2022, 144, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Naga, N. Recent developments in olefin polymerizations with transition metal catalysts. Prog. Polym. Sci. 2001, 26, 1147–1198. [Google Scholar] [CrossRef]
- van der Linden, A.; Schaverien, C.J.; Meijboom, N.; Ganter, C.; Orpen, A.G. Polymerization of α-olefins and butadiene and catalytic cyclotrimerization of 1-alkynes by a new class of group IV catalysts. Control of molecular weight and polymer microstructure via ligand tuning in sterically hindered chelating phenoxide titanium and zirconium species. J. Am. Chem. Soc. 1995, 117, 3008–3021. [Google Scholar]
- Scollard, J.D.; McConville, D.H. Living polymerization of α-olefins by chelating diamide complexes of titanium. J. Am. Chem. Soc. 1996, 118, 10008–10009. [Google Scholar] [CrossRef]
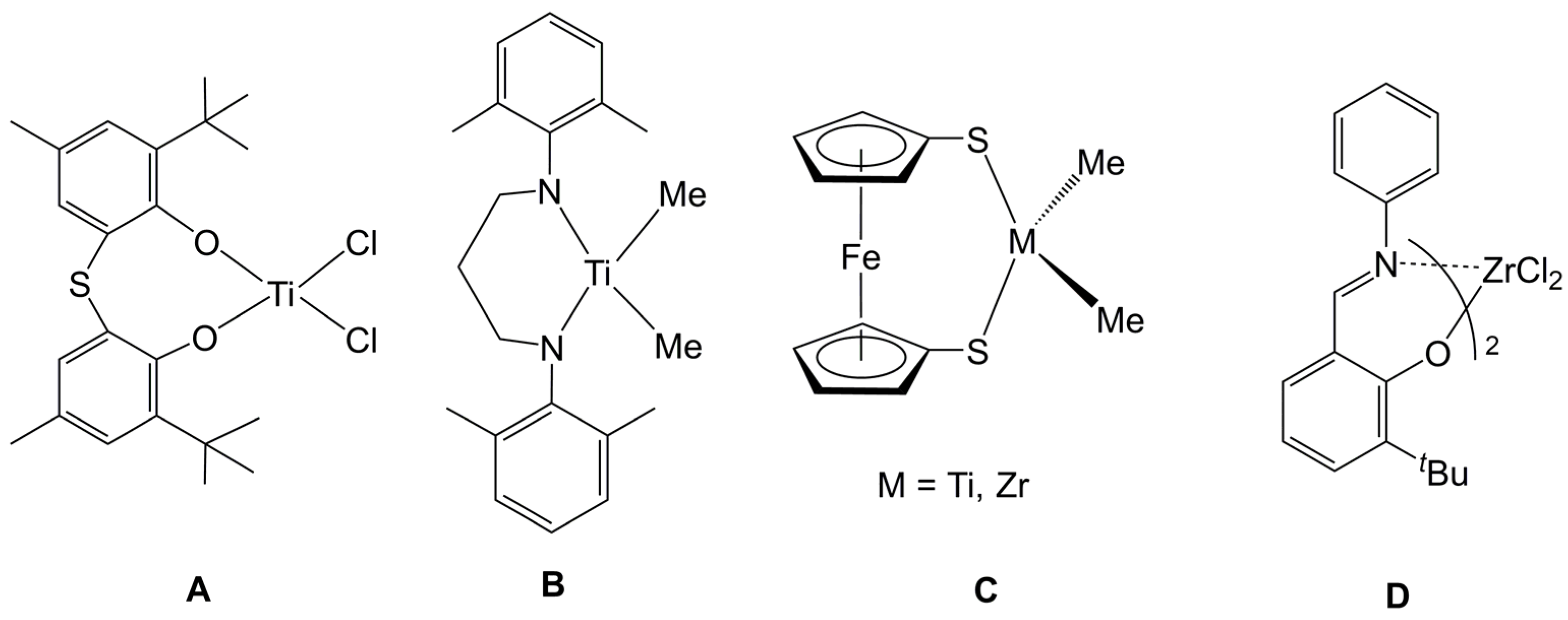
- Gibson, V.C.; Long, N.J.; Martin, J.; Solan, G.A.; Stichbury, J.C. Titanium and zirconium complexes bearing (S,S′)-ferrocenyldithiolate ligands. J. Organomet. Chem. 1999, 590, 115–117. [Google Scholar] [CrossRef]
- Reddy, S.S.; Sivaram, S. Homogeneous metallocene-methylaluminoxane catalyst systems for ethylene polymerization. Prog. Polym. Sci. 1995, 20, 309–367. [Google Scholar] [CrossRef]
- Matsui, S.; Fujita, T. FI catalysts: Super active new ethylene polymerization catalysts. Catal. Today 2001, 66, 63–73. [Google Scholar] [CrossRef]
- Furuyama, R.; Saito, J.; Ishii, S.-I.; Mitani, M.; Matsui, S.; Tohi, Y.; Makio, H.; Matsukawa, N.; Tanaka, H.; Fujita, T. Ethylene and propylene polymerization behavior of a series of bis(phenoxy–imine)titanium complexes. J. Mol. Catal. A Chem. 2003, 200, 31–42. [Google Scholar] [CrossRef]
- Mason, A.F.; Coates, G.W. New phenoxyketimine titanium complexes: combining isotacticity and living behavior in propylene polymerization. J. Am. Chem. Soc. 2004, 126, 16326–16327. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.; Mazzeo, M.; Pappalardo, D.; Zambelli, A.; Pellecchia, C. Polymerization of propene by post-metallocene catalysts. Macromol. Symp. 2004, 213, 235–252. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–315. [Google Scholar] [CrossRef] [PubMed]
- Budagumpi, S.; Kim, K.H.; Kim, I. Catalytic and coordination facets of single-site non-metallocene organometallic catalysts with N-heterocyclic scaffolds employed in olefin polymerization. Coord. Chem. Rev. 2011, 255, 2785–2809. [Google Scholar] [CrossRef]
- Yuan, S.F.; Yan, Y.; Solan, G.A.; Ma, Y.P.; Sun, W.H. Recent advancements in N-ligated group 4 molecular catalysts for the (co)polymerization of ethylene. Coord. Chem. Rev. 2020, 411, 213254. [Google Scholar] [CrossRef]
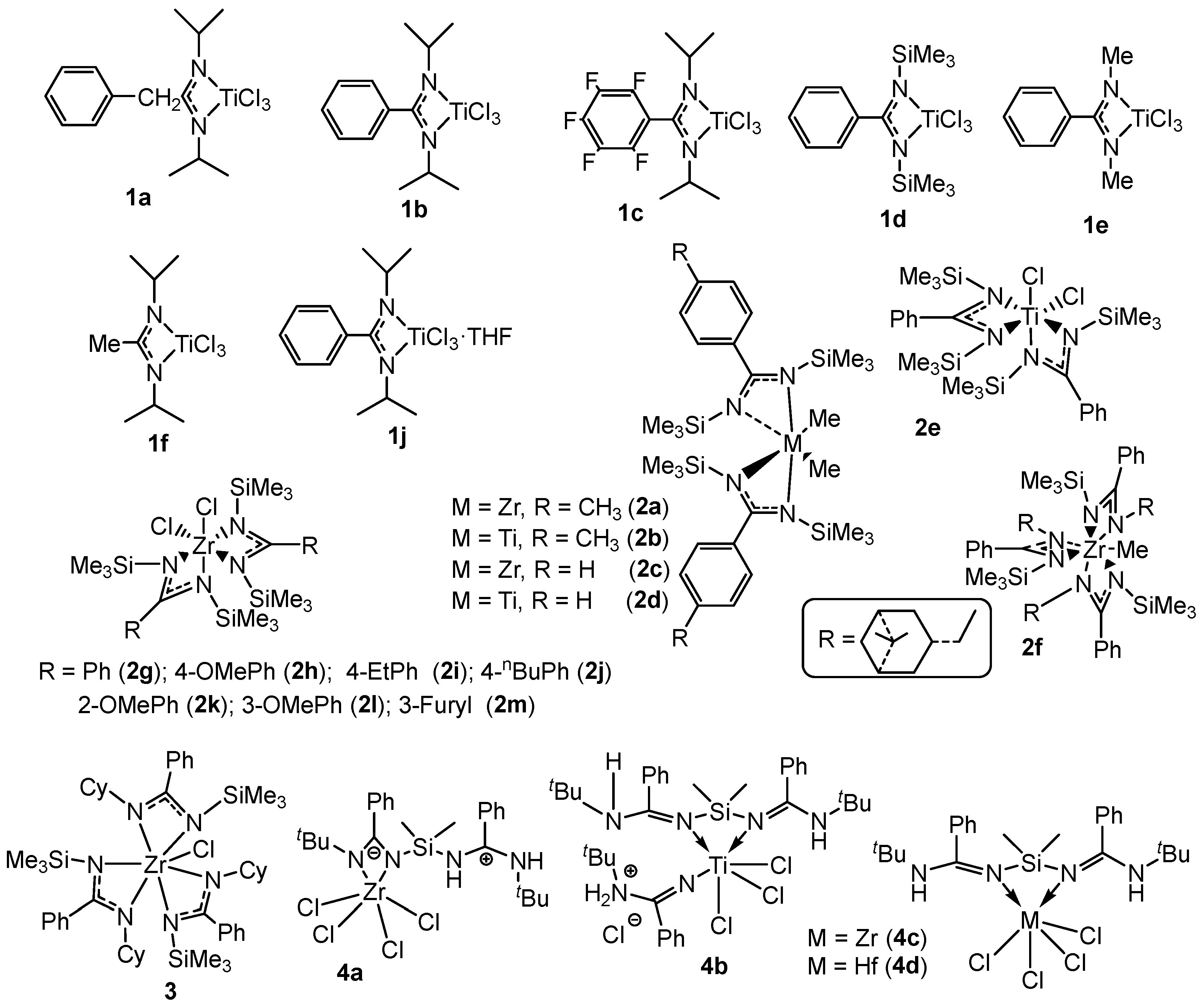
- Liguori, D.; Centore, R.; Tuzi, A.; Grisi, F.; Sessa, I.; Zambelli, A. Titanium monoamidinate-MAO catalysts: Some information about active species and stereochemical polymerization mechanisms. Macromolecules 2003, 36, 5451–5458. [Google Scholar] [CrossRef]
- Liguori, D.; Grisi, F.; Sessa, I.; Zambelli, A. Low-temperature polymerization of olefins in the presence of titanium monoamidinate/MAO catalyst. Macromol. Chem. Phys. 2003, 204, 164–170. [Google Scholar] [CrossRef]
- Volkis, V.; Nelkenbaum, E.; Lisovskii, A.; Hasson, G.; Semiat, R.; Kapon, M.; Botoshansky, M.; Eishen, Y.; Eisen, M.S. Group 4 octahedral benzamidinate complexes: Syntheses, structures, and catalytic activities in the polymerization of propylene modulated by pressure. J. Am. Chem. Soc. 2003, 125, 2179–2194. [Google Scholar] [CrossRef]
- Volkis, V.; Rodensky, M.; Lisovskii, A.; Balazs, Y.; Eisen, M.S. Stereoerror formation in the polymerization of propylene catalyzed by a titanium bis(benzamidinate) dichloride complex activated by MAO. Organometallics 2006, 25, 4934–4937. [Google Scholar] [CrossRef]
- Smolensky, E.; Eisen, M.S. Design of organometallic group IV heteroallylic complexes and their catalytic properties for polymerizations and olefin centered transformations. Dalton Trans. 2007, 48, 5623–5650. [Google Scholar] [CrossRef] [PubMed]
- Aharonovich, S.; Kulkarni, N.V.; Zhang, J.S.; Botoshansky, M.; Kapon, M.; Eisen, M.S. Polymerization of propylene promoted by zirconium benzamidinates. Dalton Trans. 2013, 42, 16762–16772. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yang, Q.; Tong, H.; Yuan, S.; Jia, B.; Guo, D.; Zhou, M.; Liu, D. Synthesis and structural study of an unsymmetrical aliphatic benzamidinato ligand and its use in the formation of selected Cu(I), Mg(II) and Zr(IV) complexes. RSC Adv. 2012, 2, 6599–6605. [Google Scholar] [CrossRef]
- Bai, S.-D.; Liu, R.-Q.; Wang, T.; Guan, F.; Wu, Y.-B.; Chao, J.-B.; Tong, H.-B.; Liu, D.-S. An alkyl-ended ansa-bis(amidine) and solvent-influenced complexation modes of its group IV metal derivatives. Polyhedron 2013, 65, 161–169. [Google Scholar] [CrossRef]
- Volkis, V.; Averbuj, C.; Eisen, M.S. Reactivity of group 4 benzamidinate complexes towards mono- and bis-substituted silanes and 1,5-hexadiene. J. Organomet. Chem. 2007, 692, 1940–1950. [Google Scholar] [CrossRef]
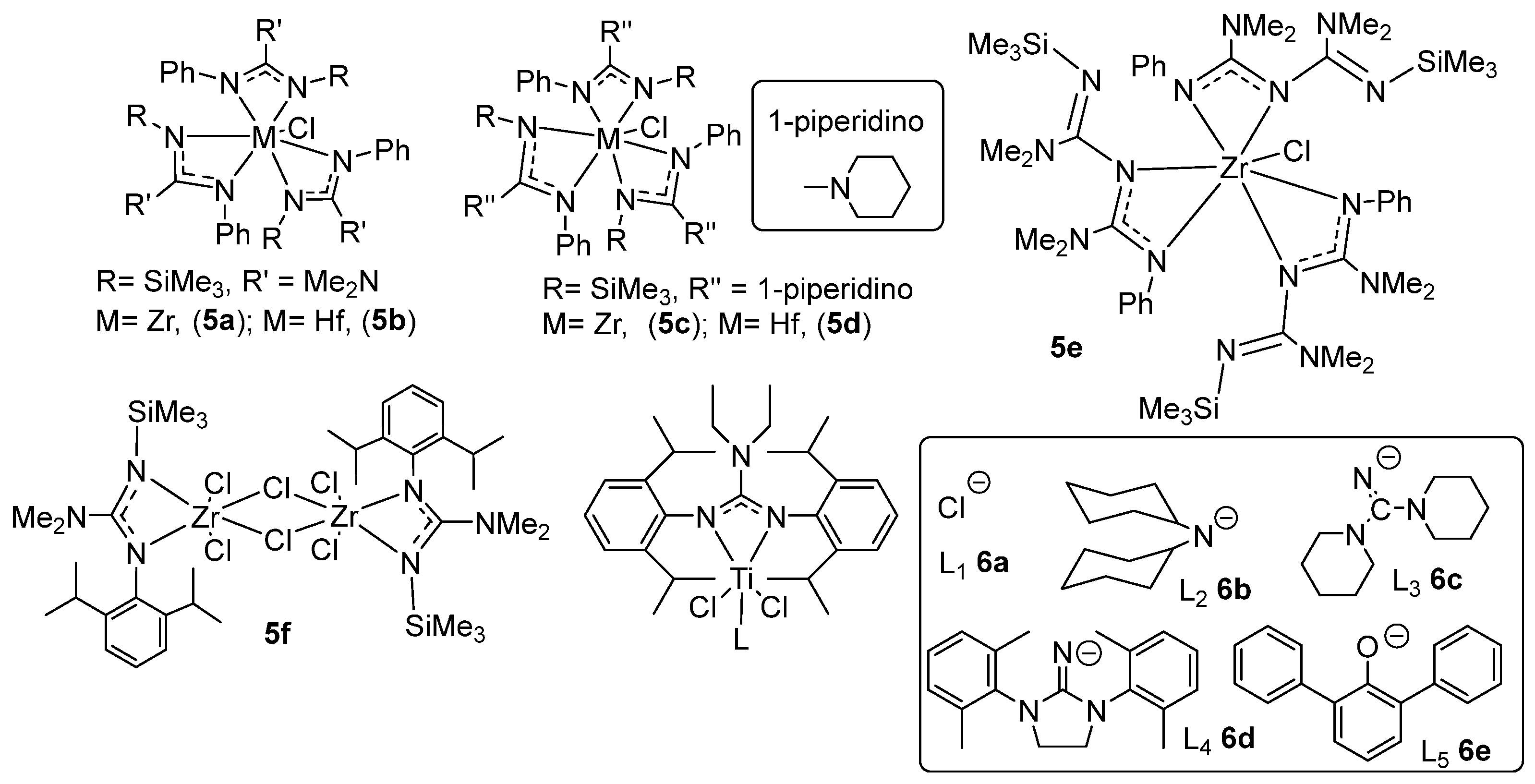
- Zhou, M.; Tong, H.; Wei, X.; Liu, D. Syntheses and structures of non-symmetric guanidinato zirconium and hafnium complexes and their catalytic behavior for ethylene polymerization. J. Organomet. Chem. 2007, 692, 5195–5202. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, S.; Tong, H.B.; Sun, W.H.; Liu, D.S. Synthesis, structure and catalytic properties of a novel zirconium guanidinato complex [Zr{ArNC(NMe2)N(SiMe3)} (μ2-Cl)Cl2]2[Ar = 2, 6-iPr2-C6H3]. Inorg. Chem. Commun. 2007, 10, 1262–1264. [Google Scholar] [CrossRef]
- Zhou, M.S.; Yang, Q.K.; Tong, H.B.; Yan, L.; Wang, X.Y. Organoamido zirconium(IV) and titanium(IV) complexes and their catalysis towards ethylene polymerization. RSC Adv. 2015, 5, 105292–105298. [Google Scholar] [CrossRef]
- Haas, I.; Hubner, C.; Kretschmer, W.P.; Kempe, R. A highly efficient titanium catalyst for the synthesis of ultrahigh-molecular-weight polyethylene (UHMWPE). Chem. Eur. J. 2013, 19, 9132–9136. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Qian, C. Dramatic electronic effect of fluoro substituents on the olefin polymerization activity of mono β-diiminato titanium complexes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 211–217. [Google Scholar] [CrossRef]
- Li, T.C.; Song, W.; Ai, H.T.; You, Q.L.; Zhang, A.Q.; Xie, G.Y. β-diiminato titanium complexes with varying fluorine substitution patterns on the N-aryl moiety: Probing the effect of ligand substitution on ethylene polymerization. J. Polym. Res. 2015, 22, 631. [Google Scholar] [CrossRef]
- Yuan, S.F.; Zhang, L.P.; Liu, D.S.; Sun, W.H. Synthesis and ethylene polymerization by β-diketiminato zirconium chlorides. Macromol. Res. 2010, 18, 690–694. [Google Scholar] [CrossRef]
- Hamaki, H.; Takeda, N.; Nabika, M.; Tokitoh, N. Catalytic activities for olefin polymerization: Titanium(III), titanium(IV), zirconium(IV), and hafnium(IV) β-diketiminato, 1-aza-1,3-butadienyl-imido, and 1-aza-2-butenyl-imido complexes bearing an extremely bulky substituent, the Tbt group (Tbt = 2,4,6-[(Me3Si)2CH]3C6H2). Macromolecules 2012, 45, 1758–1769. [Google Scholar]
- Xie, G.Y.; Liu, G.Y.; Li, L.; Li, T.C.; Zhang, A.Q.; Feng, J.W. Tandem catalysis of iron and titanium non-metallocene catalysts for the production of branched polyethylene. Catal. Commun. 2014, 45, 7–10. [Google Scholar] [CrossRef]
- Xiao, X.; Hao, X.M.; Bai, J.L.; Chao, J.B.; Cao, W.; Chen, X. Synthesis, mechanism and ethylene polymerization catalysis of Ge(IV), Sn(II) and Zr(IV) complexes derived from substituted β-diketiminates. RSC Adv. 2016, 6, 60723–60728. [Google Scholar] [CrossRef]
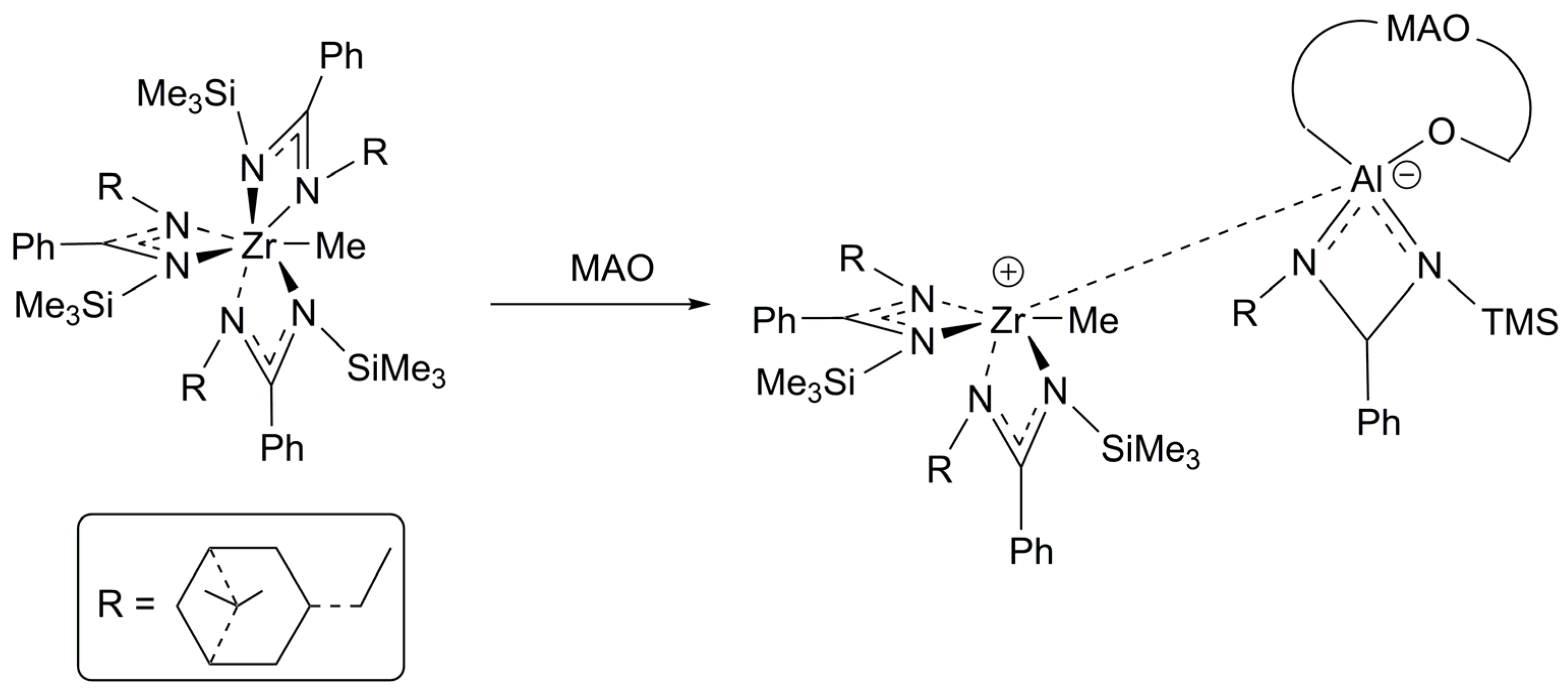
- Tsurugi, H.; Ohnishi, R.; Kaneko, H.; Panda, T.K.; Mashima, K. Controlled benzylation of α-diimine ligands bound to zirconium and hafnium: An alternative method for preparing mono- and bis(amido)M(CH2Ph)n (n=2, 3) complexes as catalyst precursors for isospecific polymerization of α-olefins. Organometallics 2009, 28, 680–687. [Google Scholar] [CrossRef]
- Qi, C.H.; Zhang, S.B. Titanium and zirconium complexes with aminoiminophosphorane ligands. Appl. Organomet. Chem. 2006, 20, 70–73. [Google Scholar] [CrossRef]
- Krajete, A.; Steiner, G.; Kopacka, H.; Ongania, H.K.; Wurst, K.; Kristen, M.O.; Preishuber-Pflugl, P.; Bildstein, B. Iminohydroxamato early and late transition metal halide complexes—New precatalysts for aluminoxane-cocatalyzed olefin insertion polymerization. Eur. J. Inorg. Chem. 2004, 2004, 1740–1752. [Google Scholar] [CrossRef]
- Wang, X.Y.; Guo, Z.L.; Tong, H.B.; Zhou, M.S. Synthesis and structures of titanium(IV) imido complexes with dianionic ligands of triazapentadienyl derivatives. J. Organomet. Chem. 2017, 828, 10–15. [Google Scholar] [CrossRef]
- Yuan, S.F.; Wei, X.H.; Tong, H.B.; Zhang, L.P.; Liu, D.S.; Sun, W.H. μ2,η1-N-[(N,N-dimethylamino)dimethylsilyl]-2,6-diisopropylanilido metal (Li, Zr, Hf) compounds and the catalytic behaviors of the IVB compounds in ethylene (co)polymerization. Organometallics 2010, 29, 2085–2092. [Google Scholar] [CrossRef]
- Li, W.; Bai, S.D.; Su, F.; Yuan, S.F.; Duan, X.E.; Liu, D.S. Zirconium complexes based on an ethylene linked amidinate-amido ligand: Synthesis, characterization and ethylene polymerization. New J. Chem. 2017, 41, 661–670. [Google Scholar] [CrossRef]
- Narayana, G.V.; Xu, G.J.; Wang, D.R.; Frey, W.; Buchmeiser, M.R. Access to ultra-high-molecular weight poly(ethylene) and activity boost in the presence of cyclopentene with group 4 bis-amido complexes. ChemPlusChem 2014, 79, 151–162. [Google Scholar] [CrossRef]
- Duan, X.E.; Xing, Q.F.; Dong, Z.M.; Chao, J.B.; Bai, S.D.; Liu, D.S.; Sun, W.H. Trichlorozirconium η2-hydrazonides: Synthesis, characterization and their catalytic behavior toward ethylene polymerization. Chin. J. Polym. Sci. 2016, 34, 390–398. [Google Scholar] [CrossRef]
- Shafir, A.; Arnold, J. Zirconium complexes incorporating diaryldiamidoferrocene ligands: Generation of cationic derivatives and polymerization activity towards ethylene and 1-hexene. Inorganica Chim. Acta 2003, 345, 216–220. [Google Scholar] [CrossRef]
- Shafir, A.; Arnold, J. Ferrocene-based olefin polymerization catalysts: activation, structure, and intermediates. Organometallics 2003, 22, 567–575. [Google Scholar] [CrossRef]
- Tsurugi, H.; Yamagata, T.; Tani, K.; Mashima, K. Unusual enhancement of ethylene polymerization activity of benzyl zirconium complexes by benzylation of the imino moiety of 2-(N-aryliminomethyl)pyrrolyl ligand. Chem. Lett. 2003, 32, 756–757. [Google Scholar] [CrossRef]
- Matsui, S.; Yoshida, Y.; Takagi, Y.; Spaniol, T.P.; Okuda, J. Pyrrolide-imine benzyl complexes of zirconium and hafnium: Synthesis, structures, and efficient ethylene polymerization catalysis. J. Organomet. Chem. 2004, 689, 1155–1164. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mohri, J.; Ishii, S.; Mitani, M.; Saito, J.; Matsui, S.; Makio, H.; Nakano, T.; Tanaka, H.; Onda, M.; et al. Living copolymerization of ethylene with norbornene catalyzed by bis(pyrrolide-imine) titanium complexes with MAO. J. Am. Chem. Soc. 2004, 126, 12023–12032. [Google Scholar] [CrossRef]
- Yoshida, Y.; Matsui, S.; Fujita, T. Bis(pyrrolide-imine) Ti complexes with MAO: A new family of high performance catalysts for olefin polymerization. J. Organomet. Chem. 2005, 690, 4382–4397. [Google Scholar] [CrossRef]
- Zuo, W.; Sun, W.-H.; Zhang, S.; Hao, P.; Shiga, A. Highly active ethylene polymerization and copolymerization with norbornene using bis(imino-indolide) titanium dichloride–MAO system. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3415–3430. [Google Scholar] [CrossRef]
- Ohta, S.; Kasai, Y.; Toda, T.; Nishii, K.; Okazaki, M. Ethylene polymerization and ethylene/1-octene copolymerization with a titanium complex supported by a bis(indolyl) ligand. Polym. J. 2019, 51, 345–349. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, S.; You, F.; Shi, X. Synthesis and characterization of the titanium catalysts supported by pyrrolide-benzoxazole ligands and their application in ethylene polymerization. Polyhedron 2022, 219, 115791. [Google Scholar] [CrossRef]
- Smolensky, E.; Kapon, M.; Woollins, J.D.; Eisen, M.S. Formation of elastomeric polypropylene promoted by a dynamic octahedral titanium complex. Organometallics 2005, 24, 3255–3265. [Google Scholar] [CrossRef]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.K.; Murphy, V.; Shoemaker, J.A.W.; Turner, H.; Rosen, R.K.; et al. Nonconventional catalysts for isotactic propene polymerization in solution developed by using high-throughput-screening technologies. Angew. Chem. Int. Ed. 2006, 45, 3278–3283. [Google Scholar] [CrossRef]
- Duan, X.E.; Yuan, S.F.; Tong, H.B.; Bai, S.D.; Wei, X.H.; Liu, D.S. Metal (Mg, Fe, Co, Zr and Ti) complexes derived from aminosilyl substituted aminopyridinato ligand: Synthesis, structures and ethylene polymerization behaviors of the group 4 complexes. Dalton Trans. 2012, 41, 9460–9467. [Google Scholar] [CrossRef]
- Yan, L.; Wang, X.Y.; Zhou, M.S. Synthesis, structural characterization and catalytic properties of a N-functionalized organoamide zirconium complex. Inorg. Chem. Commun. 2016, 65, 32–34. [Google Scholar] [CrossRef]
- Sanz, M.; Mosquera, M.E.G.; Cuenca, T.; de Arellano, C.R.; Schormann, M.; Bochmann, M. Bis(3,5-dimethylpyrazol-1-ato) zirconium complexes as precursors for ethylene polymerisation upon activation with MAO: Syntheses, characterisation and X-ray molecular structure of [Zr(η2-3,5-Me2Pz)2Cl2(η1-3,5-Me2PzH)2]·(3,5-Me2PzH) and [Zr(η2-3,5-Me2Pz)2(CH2Ph)2] (3,5-Me2Pz = 3,5-dimethylpyrazol-1-ato). Polyhedron 2007, 26, 5339–5348. [Google Scholar]
- Diamond, G.M.; Hall, K.A.; LaPointe, A.M.; Lecerc, M.K.; Longmire, J.; Shoemaker, J.A.W.; Sun, P. High-throughput discovery and optimization of hafnium heteroaryl-amido catalysts for the isospecific polymerization of propylene. ACS Catal. 2011, 1, 887–900. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Ueligger, S.; Klosin, J.; Hazari, A.; Daller, J.; Hou, J.B. Development of improved amidoquinoline polyolefin catalysts with ultrahigh molecular weight capacity. Organometallics 2015, 34, 1354–1363. [Google Scholar] [CrossRef]
- Han, B.; Liu, Y.; Feng, C.; Liu, S.; Li, Z. Development of group 4 metal complexes bearing fused-ring amido-trihydroquinoline ligands with improved high-temperature catalytic performance toward olefin (co)polymerization. Organometallics 2021, 40, 242–252. [Google Scholar] [CrossRef]
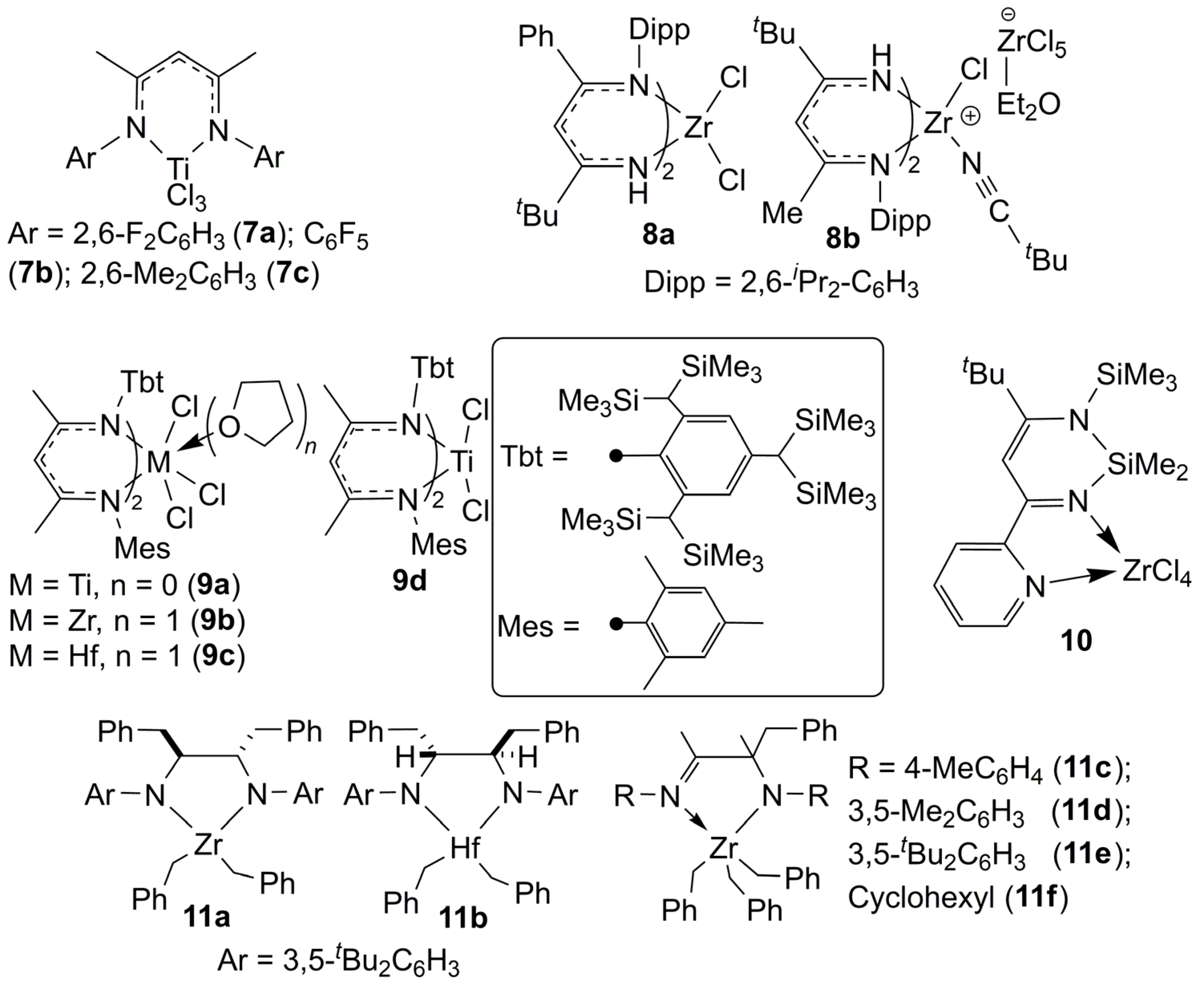
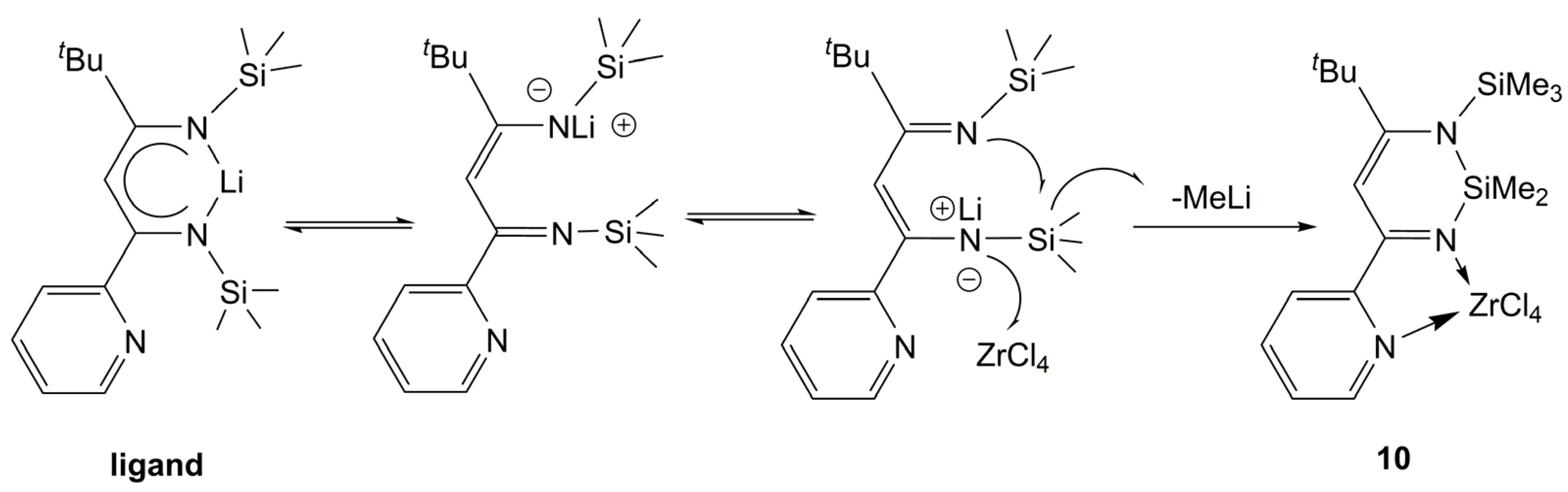

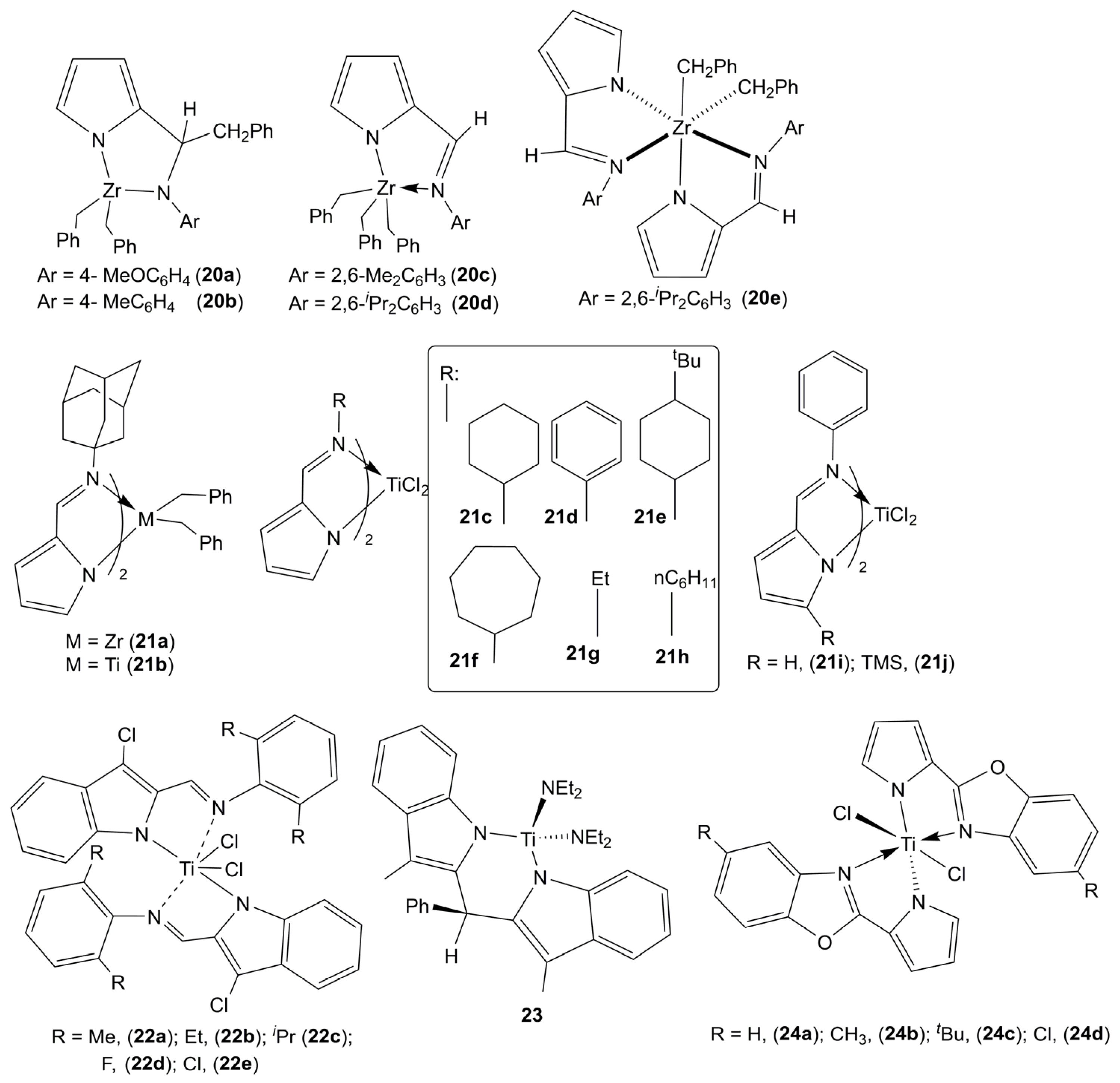


| Complex | Solvent | [Propene] (mol·L−1) | [mm] (%) | [mr] (%) | [rr] (%) | Vicinal Methyl Fraction (%) | Time (h) | Yield (g) | Mw (×104) | Mw/Mn | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | Toluene | 5.9 | 5 | 42 | 53 | 2.5 | 16 | 0.40 | 1.2 | 1.76 | [19] |
| 1b | Toluene | 5.9 | 4 | 41 | 55 | 2.3 | 17 | 0.63 | [19] | ||
| 1c | Toluene | 5.9 | 5 | 43 | 52 | 3.2 | 17 | 1.10 | 1.5 | 1.71 | [19] |
| 1d | Toluene | 5.9 | 7 | 40 | 53 | 4.8 | 16 | 0.32 | [19] | ||
| 1e | Toluene | 5.9 | 39 | 32 | 29 | 1.8 | 16 | 0.05 | [19] | ||
| 1f | Toluene | 5.9 | 41 | 39 | 20 | 1.7 | 16 | 0.05 | [19] | ||
| 1g | Toluene | 5.9 | 9 | 40 | 51 | 2.5 | 16 | 0.30 | [19] |
| Monomer b | Complex | Amount (μmol) | Al:M | Activity c | Temp. (°C) | [mmmm] % | Time (h) | Mw (×104) | Mw/Mn | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| P | 1a | 20 | 200 | 50.0 × 101 | −60 | 16 | 1.20 | 1.76 | [19] | |
| P | 1c | 20 | 200 | 12.9 × 102 | −60 | 17 | 1.50 | 1.71 | [19] | |
| P | 1c | 50 | 200 | 31.3 × 103 | 20 | 2 | [19] | |||
| P | 1a | 50 | 200 | 30.0 × 103 | 20 | 2 | [19] | |||
| S | 1a | 50 | 200 | 20.0 × 102 | 50 | 1 | [19] | |||
| BD | 1c | 50 | 200 | 13.3 × 102 | −20 | 3 | [19] | |||
| P | 2a | 7.4 | 250 | 11.0 × 104 | 25 | 86.0 | 44.00 | 1.69 | [23] | |
| P d | 2a | 7.4 | 1000 | 79.0 × 104 | 25 | 98.0 | 8.30 | 1.42 | [23] | |
| P | 2d | 20 | 1000 | 28.5 × 105 | 25 | 17.0 | 31.01 | 2.48 | [21] | |
| HD | 2c | 7.9 | 400 | 68.0 × 102 | 25 | 1.35 | 2.62 | [27] | ||
| HD | 2f | 7.2 | 800 | 11.0 × 104 | 50 | 0.47 | 1.50 | [27] | ||
| P | 2g | 15.5 | 1000 | 19.0 × 102 | 25 | 63.5 | 3 | 4.76 | 6.80 | [24] |
| P | 2k | 15.5 | 1000 | 36.0 × 102 | 25 | 71.0 | 3 | 379.32 | 2.33 | [24] |
| P | 2l | 15.5 | 1000 | 13.7 × 103 | 25 | 66.1 | 3 | 1.54 | 2.57 | [24] |
| P | 2m | 15.5 | 1000 | 93.0 × 102 | 25 | 65.0 | 3 | 5.12 | 5.12 | [24] |
| Monomer | Complex | Amount (μmol) | Al:M | Pressure (atm) | Temp. (°C) | Time (min) | Activity b | Mw | MWD | H/% c | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E | 7a | 1 | 2000 | 1 | 30 | 5 | 76.8 × 105 | 50.27 × 102 | 2.30 | [32] | |
| E | 7c | 5 | 2000 | 1 | 30 | 60 | 88.0 × 102 | 13.13 × 104 | 6.53 | [32] | |
| E/H d | 7a | 1 | 2000 | 1 | 30 | 5 | 26.1 × 106 | 15.48 × 103 | 1.82 | 32.3 | [32] |
| E/H d | 7c | 5 | 2000 | 1 | 30 | 60 | 13.2 × 103 | 21.13 × 104 | 13.35 | 7.4 | [32] |
| E | 8a | 5 | 1000 | 10 | 70 | 30 | 10.0 × 105 | 26.00 × 105 | 4.10 | [34] | |
| E | 8b | 5 | 1000 | 10 | 70 | 10 | 11.0 × 105 | 79.00 × 104 | 7.20 | [34] | |
| E | 9a | 0.1 | 400 | 6 | 40 | 20 | 65.0 × 105 | [35] | |||
| E | 9d | 0.1 | 400 | 6 | 40 | 20 | 39.0 × 105 | [35] | |||
| E/H e | 9a | 0.1 | 400 | 6 | 40 | 20 | 51.0 × 105 | [35] | |||
| E | 10 | 5 | 500 | 1 | 50 | 30 | 22.4 × 104 | 12.40 × 105 | 6.70 | [37] |
| Monomer | Complex | Amount (μmol) | Al:M | Pressure (atm) | Temp. (°C) | Time (min) | Activity b | Mw (g/mol) | MWD | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| E | 13a | 2.7 | excess | 40 | 70 | 45 | 30.7 × 104 | [40] | ||
| E | 13b | 3.6 | excess | 40 | 70 | 15 | 11.2 × 104 | [40] | ||
| E | 14a | 5 | 1000 | 10 | 70 | 30 | 56.6 × 103 | [41] | ||
| E | 14b | 5 | 1000 | 10 | 70 | 30 | 45.0 × 103 | [41] | ||
| E | 15a | 5 | 1000 | 10 | 70 | 30 | 69.2 × 104 | 25.30 × 104 | 4.20 | [42] |
| E | 15b | 5 | 1000 | 10 | 70 | 30 | 97.6 × 104 | 41.10 × 104 | 11.00 | [42] |
| E/H c | 15b | 5 | 1000 | 10 | 70 | 30 | 10.3 × 105 | 33.40 × 104 | 26.00 | [42] |
| E | 15c | 5 | 1000 | 10 | 70 | 30 | 17.3 × 105 | 33.70 × 104 | 8.70 | [42] |
| E | 16 | 5 | 1000 | 10 | 50 | 30 | 48.6 × 104 | 95.10 × 104 | 10.08 | [43] |
| E | 17a | 1 | 2000 | 4 | 90 | 60 | 18.8 × 104 | 40.00 × 105 | 7 | [44] |
| E | 17b | 1 | 2000 | 4 | 90 | 60 | 18.5 × 104 | 90.00 × 104 | 8 | [44] |
| E/CPE d | 17b | 1 | 2000 | 4 | 50 | 60 | 75.0 × 105 | 50.00 × 103 | 2.5 | [44] |
| E | 18a | 2 | 1000 | 10 | 30 | 30 | 36.0 × 103 | 20.50 × 105 | 1.49 | [45] |
| H e | 19a | 47 | f | 30 | 20.00 × 103 | 1.30~1.40 | [46] | |||
| E | 19b | 46 | f | 5 | 10.2 × 104 | [47] |
| M b | Complex | Amount (μmol) | MAO (equiv.) | Pressure (atm) | Temp. (°C) | Time (min) | Activity c | Mw (kg/mol) | MWD | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| E | 22a | 5 | 500 | 10 | 80 | 30 | 40.0 × 103 | 1760 | 8,1 | [52] |
| E | 22b | 5 | 500 | 10 | 80 | 30 | 52.0 × 103 | 2800 | 7.3 | [52] |
| E | 22c | 5 | 500 | 10 | 80 | 30 | 56.0 × 103 | 1980 | 5.6 | [52] |
| E | 22d | 5 | 500 | 10 | 80 | 30 | 96.0 × 103 | 660 | 7.7 | [52] |
| E | 22e | 5 | 500 | 10 | 80 | 30 | 94.0 × 103 | 560 | 7.6 | [52] |
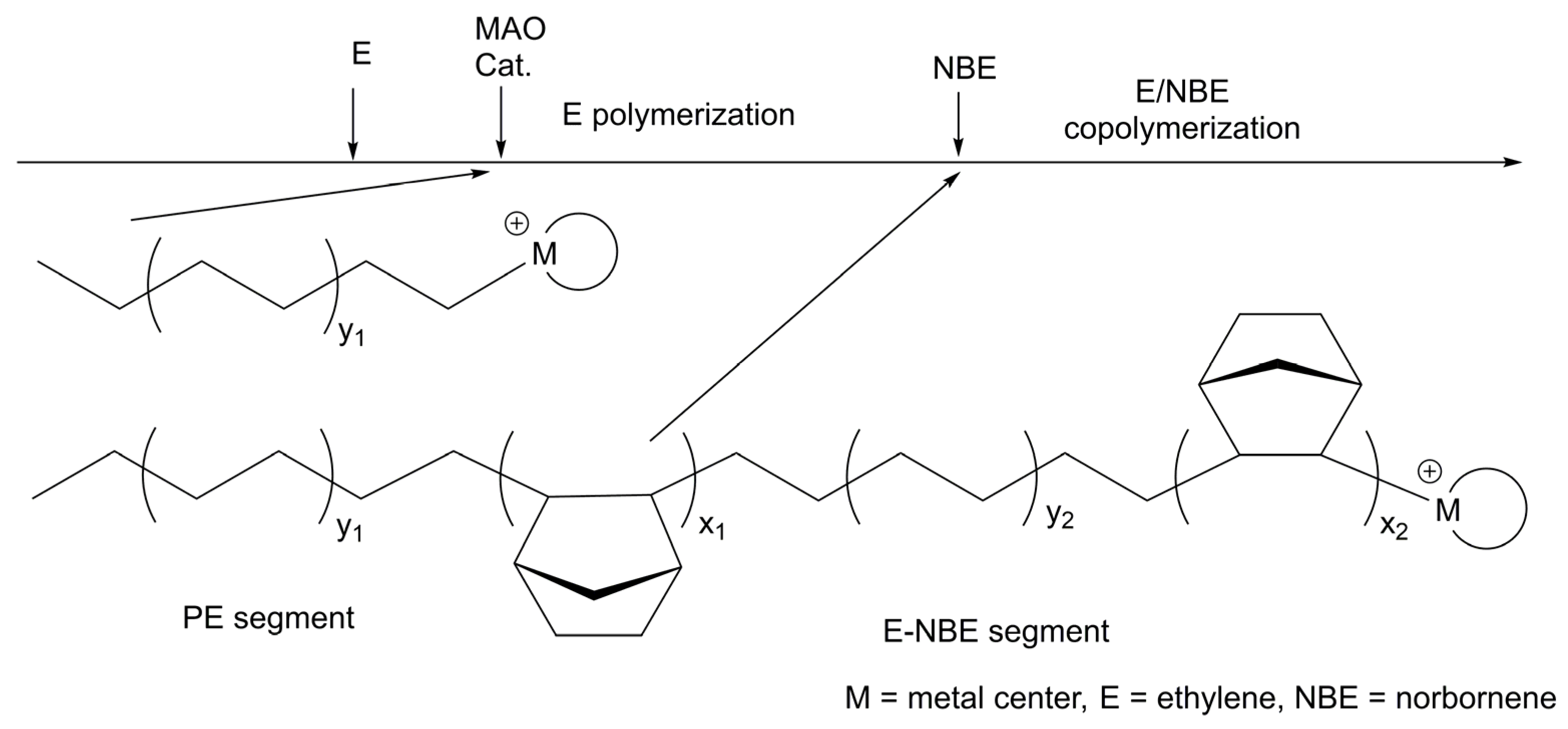
| E/NBE d | 22d | 7.5 | 500 | 10 | 80 | 10 | 20.0 × 105 | 90 | 6.0 | [52] |
| E/NBE e | 22d | 7.5 | 500 | 5 | 80 | 10 | 17.0 × 105 | 55 | 4.2 | [52] |
| Monomer | Complex | Amount (μmol) | Al:M | Pressure (atm) | Temp. (°C) | Time (min) | Activity b | Mw (g/mol) | MWD | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| P | 25 | 600 | 25 | 600 | 13.1 × 103 | 11.34 × 104 | 3.60 | [55] | ||
| P | 26 | 0.5 | 8 c | 6.9 | 90 | 3.3 | 18.0 × 107 | d | 3.20 | [56] |
| E | 27 | 5 | 2000 | 10 | 60 | 30 | 12.4 × 104 | 20.30 × 104 | 10.70 | [57] |
| E | 28 | 5 | 2000 | 10 | 50 | 30 | 14.7 × 104 | 29.60 × 104 | 60.00 | [58] |
| E | 29 | 10 | 1000 | 4 | 25 | 15 | 38.0 × 103 | 25.30 × 104 | 4.20 | [59] |
| P | 30a | 130 | 78.0 × 106 | [60] | ||||||
| P | 30b | e | 6.8 | 110 | 66.0 × 106 | d | [60] | |||
| P | 31b | e | 6.8 | 110 | 24.6 × 105 | d | 13.2 | [60] | ||
| P | 31d | e | 6.8 | 75 | 40.9 × 107 | d | [60] | |||
| E/O f | 32a | 0.6 | 10 g | 19.6 | 140 | 10 | 46.7 × 106 | 46.04 × 104 | 3.38 h | [61] |
| E/O f | 32b | 2.5 | 10 g | 19.6 | 140 | 10 | 61.0 × 105 | 28.52 × 104 | 3.68 h | [61] |
| E | 33a | 5 | i | 5 | 100 | 2 | 88.8 × 105 | 14.63 × 105 | 3.80 | [62] |
| E | 33c | 5 | i | 5 | 100 | 2 | 48.0 × 105 | 63.00 × 104 | 2.20 | [62] |
| E | 33d | 5 | i | 5 | 100 | 2 | 30.0 × 105 | 61.40 × 104 | 4.50 | [62] |
| E | 33c | 5 | j | 5 | 100 | 2 | 13.8 × 106 | 93.10 × 104 | 3.70 | [62] |
| E/O f | 33a | 5 | j | 5 | 100 | 2 | 38.6 × 106 | 33.80 × 104 | 2.50 h | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Z.; Wu, C.; Chen, J.; Qu, S.; Li, X.; Wang, W. Homogeneous Non-Metallocene Group 4 Metals Ligated with [N,N] Bidentate Ligand(s) for Olefin Polymerization. Polymers 2024, 16, 406. https://doi.org/10.3390/polym16030406
Wen Z, Wu C, Chen J, Qu S, Li X, Wang W. Homogeneous Non-Metallocene Group 4 Metals Ligated with [N,N] Bidentate Ligand(s) for Olefin Polymerization. Polymers. 2024; 16(3):406. https://doi.org/10.3390/polym16030406
Chicago/Turabian StyleWen, Zhao, Changjiang Wu, Jian Chen, Shuzhang Qu, Xinwei Li, and Wei Wang. 2024. "Homogeneous Non-Metallocene Group 4 Metals Ligated with [N,N] Bidentate Ligand(s) for Olefin Polymerization" Polymers 16, no. 3: 406. https://doi.org/10.3390/polym16030406
APA StyleWen, Z., Wu, C., Chen, J., Qu, S., Li, X., & Wang, W. (2024). Homogeneous Non-Metallocene Group 4 Metals Ligated with [N,N] Bidentate Ligand(s) for Olefin Polymerization. Polymers, 16(3), 406. https://doi.org/10.3390/polym16030406





