Nanocomposites Based on Thermoplastic Acrylic Resin with the Addition of Chemically Modified Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Surface Modification of MWCNTs
2.1.2. Preparation of Acrylic Resin/MWCNT Mixtures
2.2. Methods
3. Results
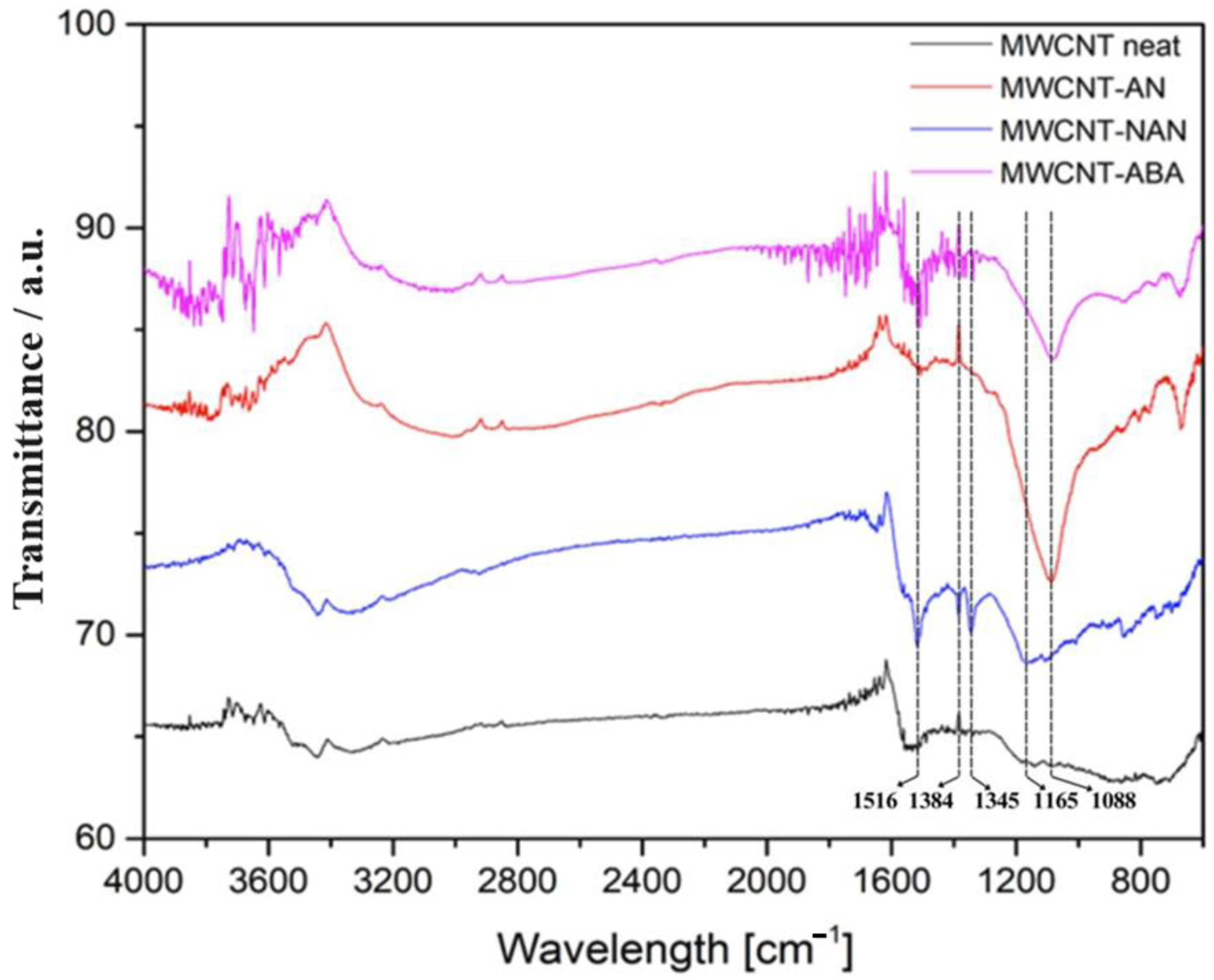
3.1. FT-IR Spectroscopy
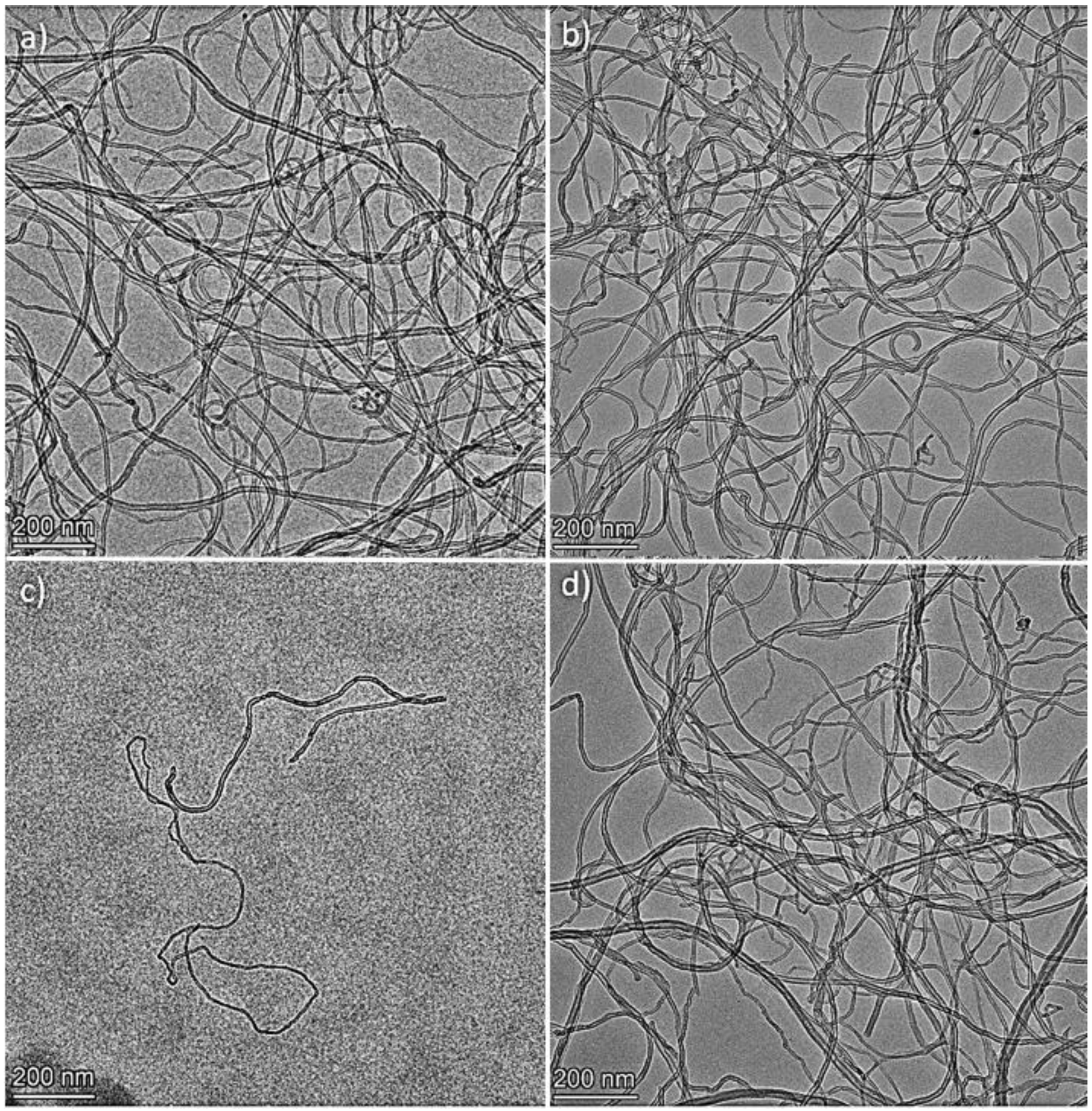
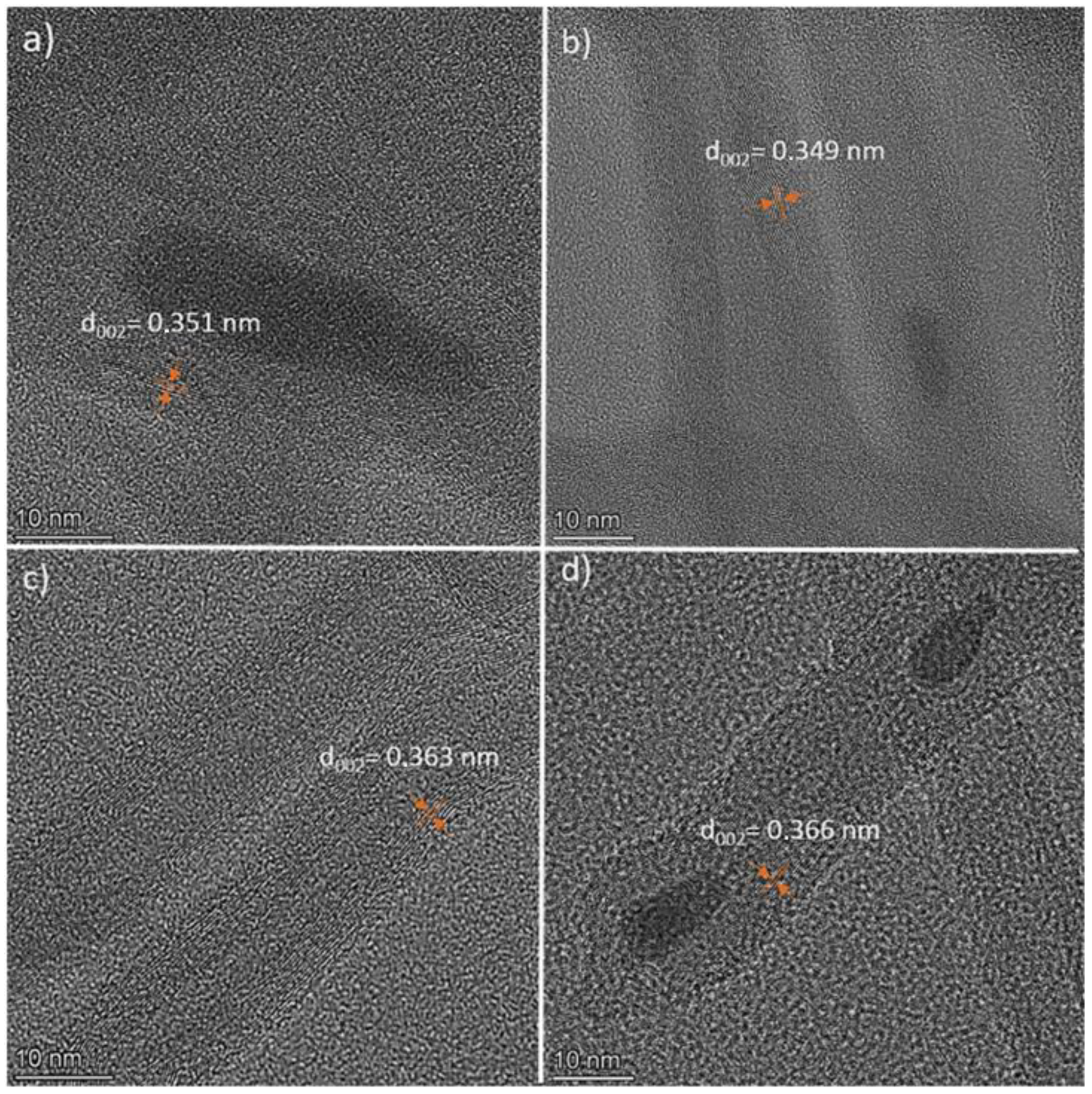
3.2. Transmission Electron Microscopy
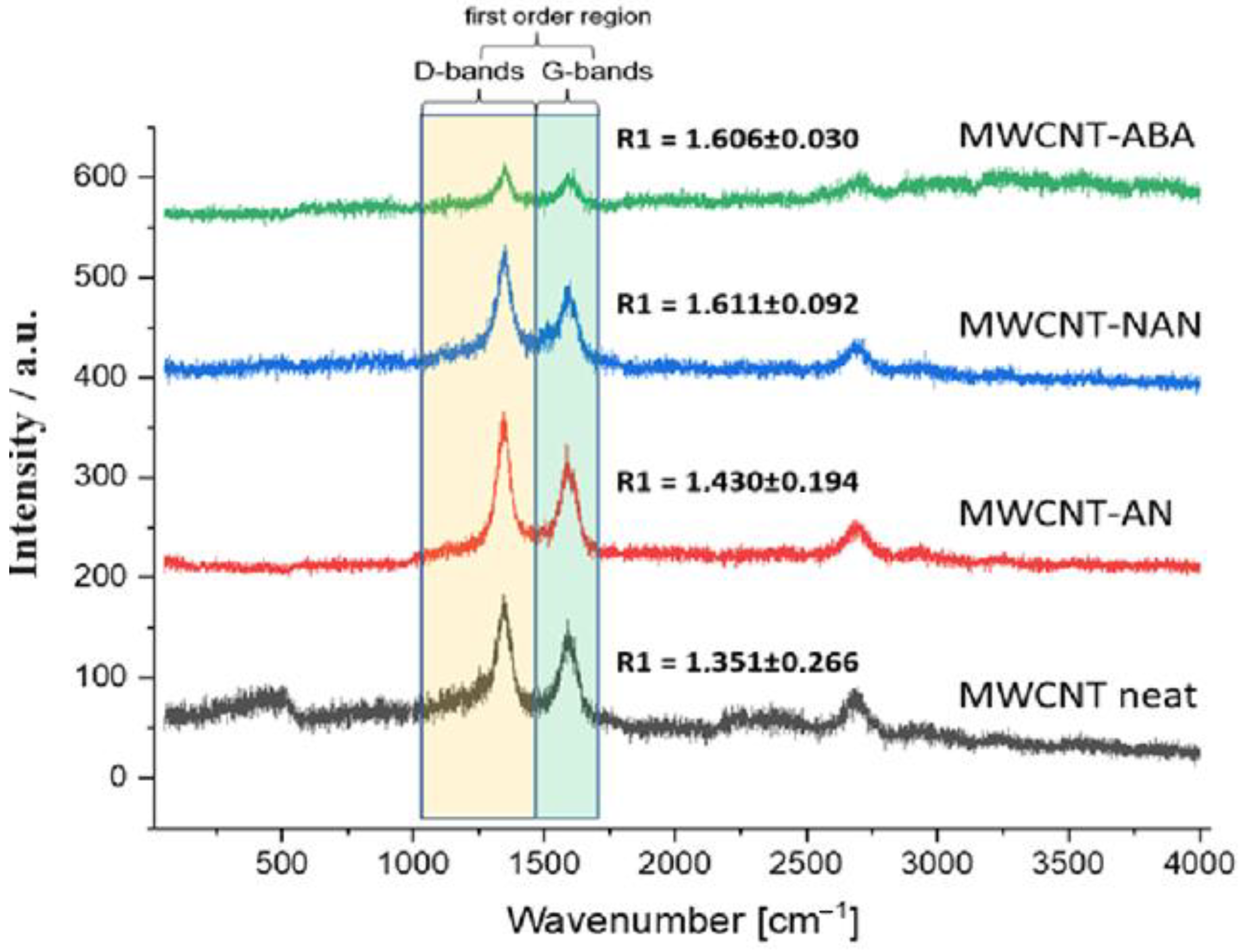
3.3. Raman Spectroscopy
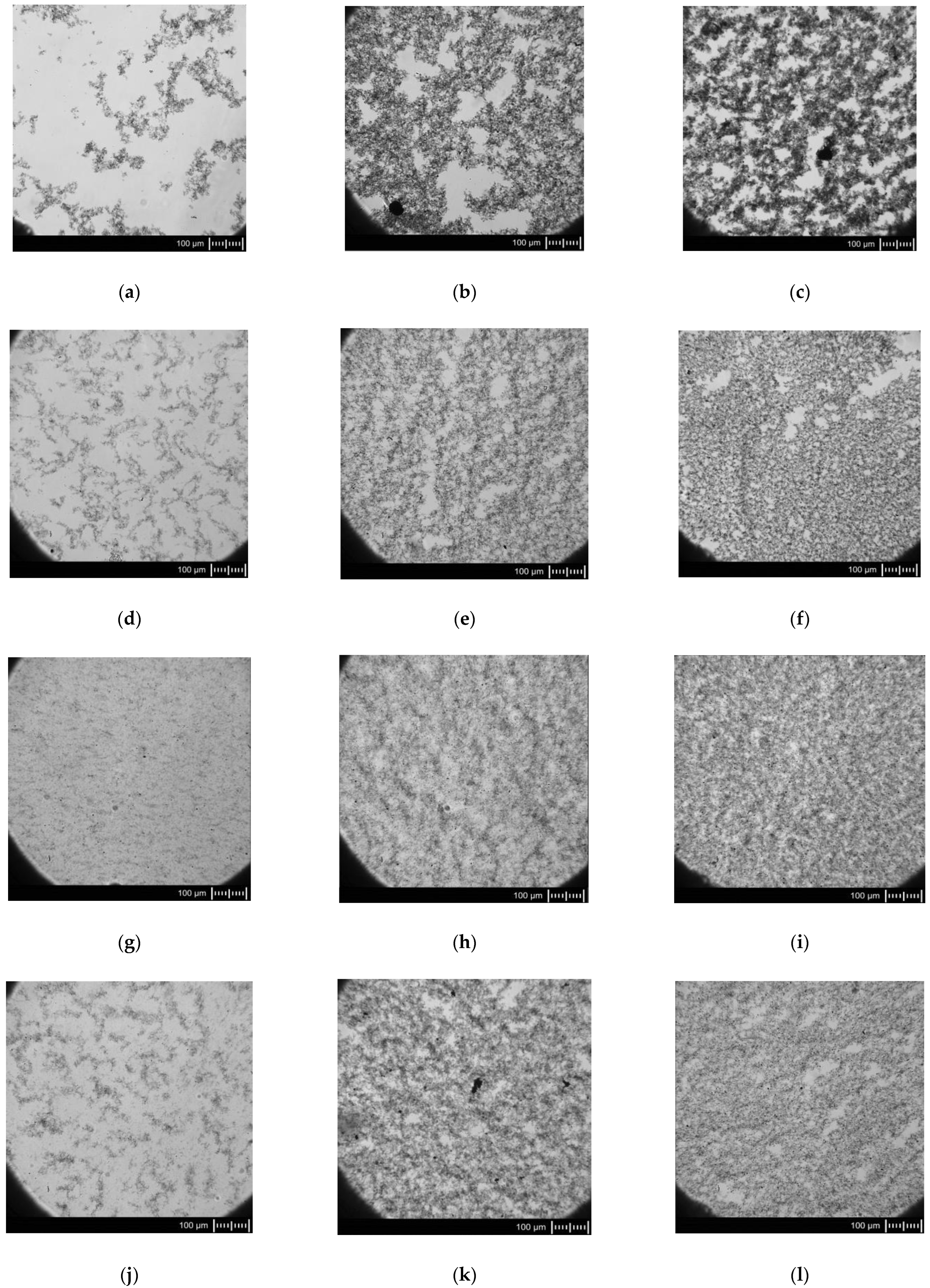
3.4. Microscopy Observations
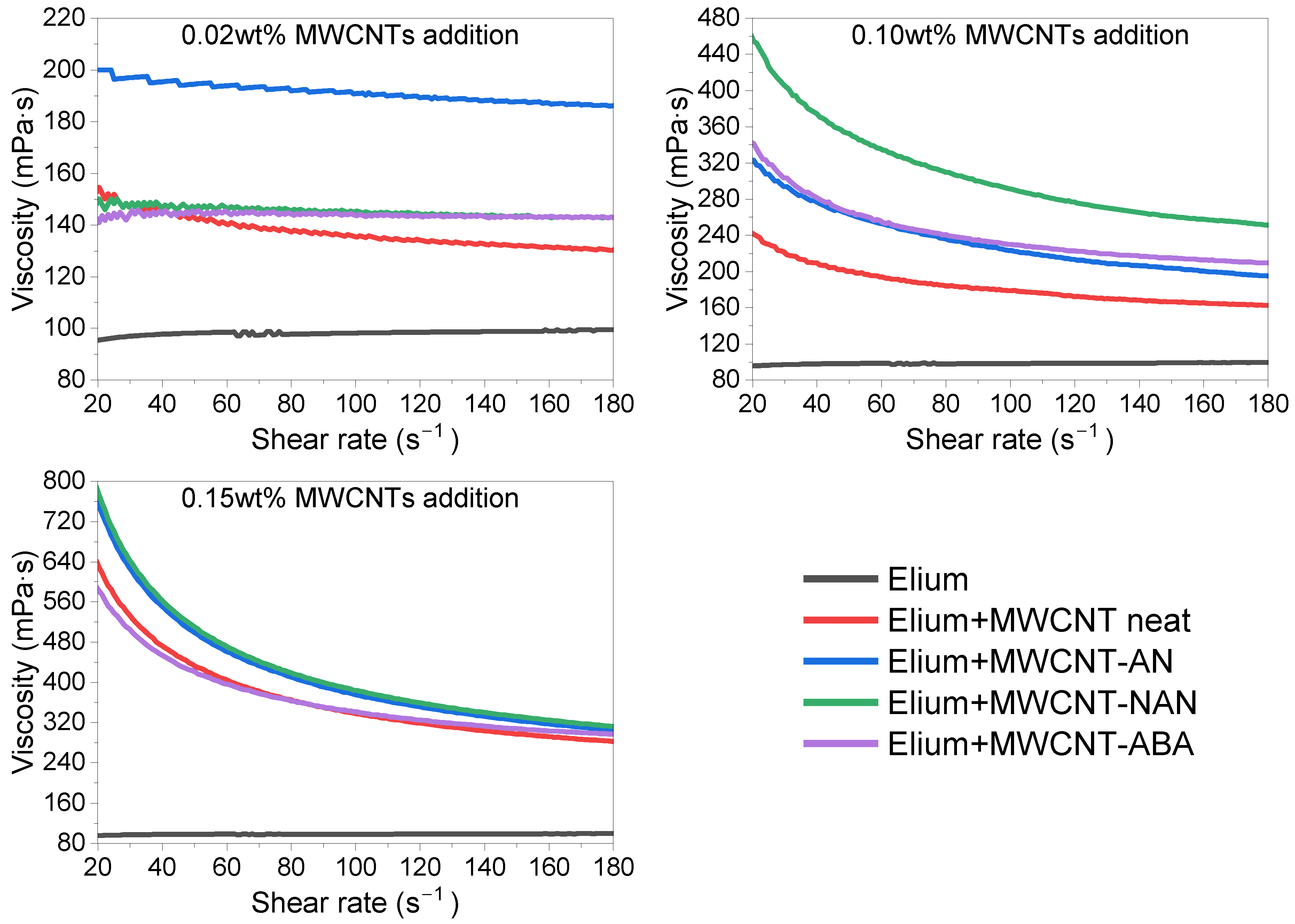
3.5. Viscosity
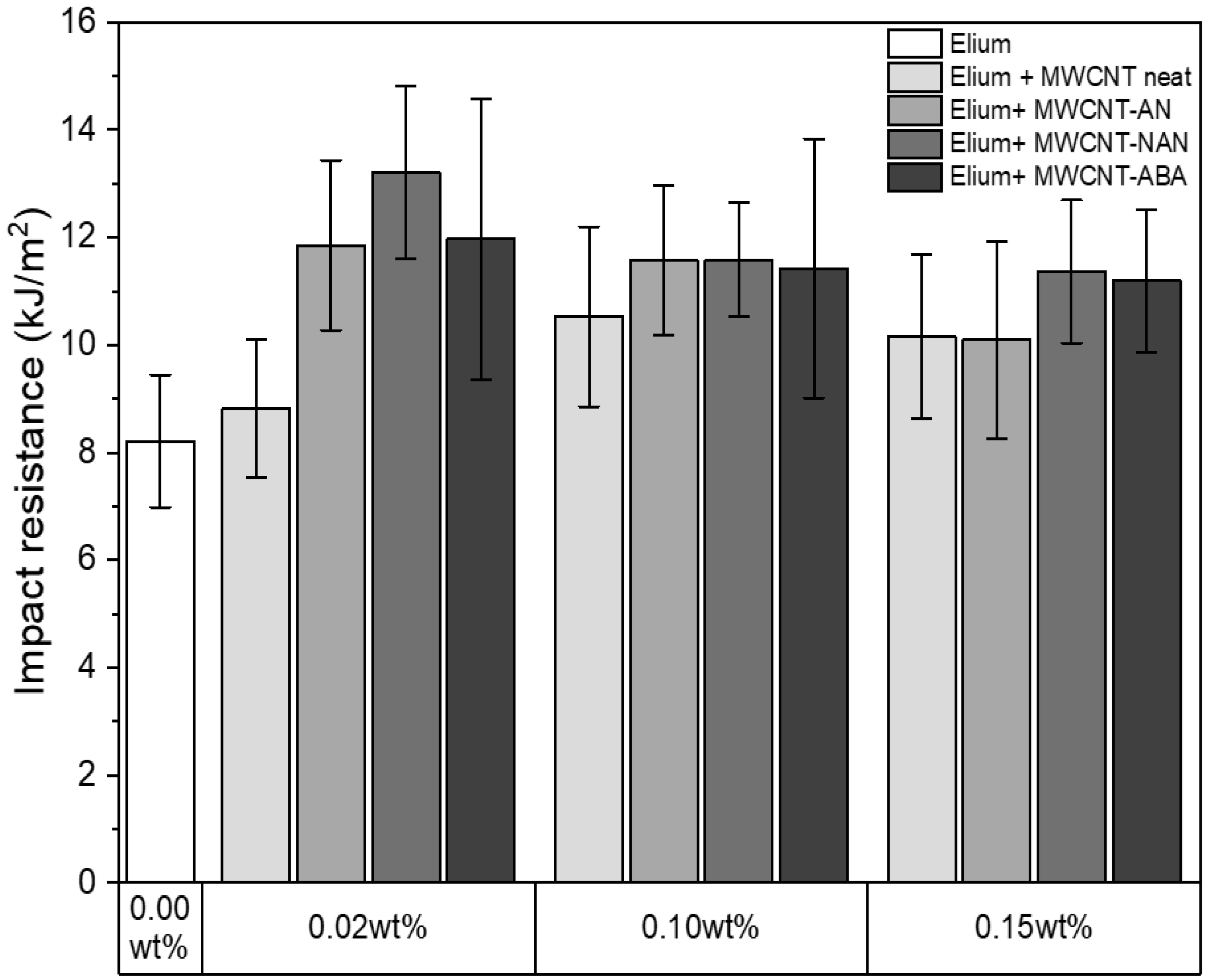
3.6. Impact Resistance
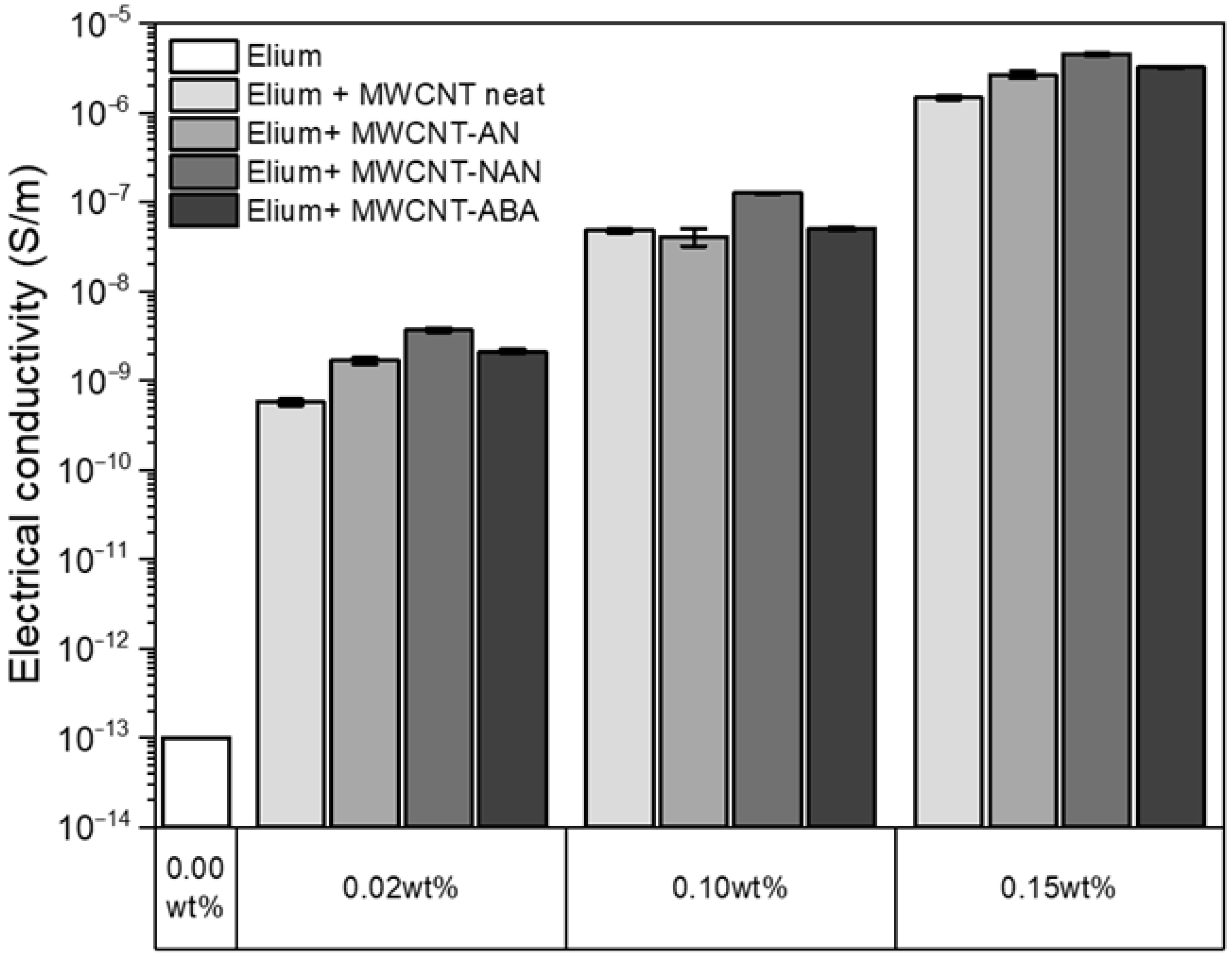
3.7. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moisala, A.; Li, Q.; Kinloch, I.; Windle, A. Thermal and electrical conductivity of single- and multi-walled carbon nanotube-epoxy composites. Compos. Sci. Technol. 2006, 66, 1285–1288. [Google Scholar] [CrossRef]
- Murray, R.E.; Beach, R.; Barnes, D.; Snowberg, D.; Berry, D.; Rooney, S.; Jenks, M.; Gage, B.; Boro, T.; Wallen, S.; et al. Structural validation of a thermoplastic composite wind turbine blade with comparison to a thermoset composite blade. Renew. Energy 2021, 164, 1100–1107. [Google Scholar] [CrossRef]
- Humbe, S.S.; Joshi, G. Chapter 17—Polyether ether ketone high-performance composites and blends present trends: A review. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Altalhi, T., Inamuddin, Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 373–392. [Google Scholar] [CrossRef]
- Hong, J.; Kwon, J.; Im, D.; Ko, J.; Nam, C.Y.; Yang, H.G.; Shin, S.H.; Hong, S.M.; Hwang, S.S.; Yoon, H.G.; et al. Best practices for correlating electrical conductivity with broadband EMI shielding in binary filler-based conducting polymer composites. Chem. Eng. J. 2022, 455, 140528. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Gudkov, N.; Lomov, S.; Akhatov, I.; Abaimov, S. Conductive CNT-polymer nanocomposites digital twins for self-diagnostic structures: Sensitivity to CNT parameters. Compos. Struct. 2022, 291, 115617. [Google Scholar] [CrossRef]
- Yanagi, K.; Udoguchi, H.; Sagitani, S.; Oshima, Y.; Takenobu, T.; Kataura, H.; Ishida, T.; Matsuda, K.; Maniwa, Y. Transport Mechanisms in Metallic and Semiconducting Single-Wall Carbon Nanotube Networks. ACS Nano 2010, 4, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, W.; Pan, Z.; Chang, B.; Sun, L. Mechanical and physical properties on carbon nanotube. J. Phys. Chem. Solids 2000, 61, 1153–1158. [Google Scholar] [CrossRef]
- Beyou, E.; Akbar, S.; Chaumont, P.; Cassagnau, P. Polymer Nanocomposites Containing Functionalised Multiwalled Carbon NanoTubes: A Particular Attention to Polyolefin Based Materials; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Atif, M.; Afzaal, I.; Naseer, H.; Abrar, M.; Bongiovanni, R.M. Review—Surface Modification of Carbon Nanotubes: A Tool to Control Electrochemical Performance. ECS J. Solid State Sci. Technol. 2020, 9, 041009. [Google Scholar] [CrossRef]
- Yao, X.; Wu, H.; Wang, J.; Qu, S.; Chen, G. Carbon Nanotube/Poly(methyl methacrylate) (CNT/PMMA) Composite Electrode Fabricated by In Situ Polymerization for Microchip Capillary Electrophoresis. Chem. A Eur. J. 2007, 13, 846–853. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kim, D.-A.; Patel, K.D.; Shin, U.S.; Kim, H.-W.; Lee, J.-H.; Lee, H.-H. Carbon nanotube incorporation in PMMA to prevent microbial adhesion. Sci. Rep. 2019, 9, 4921. [Google Scholar] [CrossRef]
- Pande, S.; Singh, B.P. Properties, and Applications, Properties of PMMA/Carbon Nanotubes Nanocomposites Polymer Nanotube Nanocomposites; Scrivener Publishing LLC: Beverly, MA, USA, 2010; pp. 177–220. [Google Scholar]
- Pérez, E.M.; Martín, N. π–π interactions in carbon nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yi, Z.; Jing, Q.; Yue, R.; Jiang, W.; Lin, D. Sonication-assisted dispersion of carbon nanotubes in aqueous solutions of the anionic surfactant SDBS: The role of sonication energy. Chin. Sci. Bull. 2013, 58, 2082–2090. [Google Scholar] [CrossRef]
- Atif, R.; Inam, F. Reasons and remedies for the agglomeration of multilayered graphene and carbon nanotubes in polymers. Beilstein J. Nanotechnol. 2016, 7, 1174–1196. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, O.; Sierra, G.; Tobón, J.I. Influence of super plasticizer and Ca(OH)2 on the stability of functionalized multi-walled carbon nanotubes dispersions for cement composites applications. Constr. Build. Mater. 2013, 47, 771–778. [Google Scholar] [CrossRef]
- Choudhary, V.; Singh, B.P.; Mathur, R.B.; Choudhary, V.; Singh, B.P.; Mathur, R.B. Carbon Nanotubes and Their Composites; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Bahr, J.L.; Tour, J.M. Highly Functionalized Carbon Nanotubes Using in Situ Generated Diazonium Compounds. Chem. Mater. 2001, 13, 3823–3824. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Castell, P.; Bespín-Gascón, S.; Ansón-Casaos, A.; Díez-Pascual, A.M.; Gómez-Fatou, M.A.; Benito, A.M.; Maser, W.K.; Martínez, M.T. Covalent functionalization of MWCNTs with poly(p-phenylene sulphide) oligomers: A route to the efficient integration through a chemical approach. J. Mater. Chem. 2012, 22, 21285–21297. [Google Scholar] [CrossRef]
- Mesnage, A.; Lefèvre, X.; Jégou, P.; Deniau, G.; Palacin, S. Spontaneous Grafting of Diazonium Salts: Chemical Mechanism on Metallic Surfaces. Langmuir 2012, 28, 11767–11778. [Google Scholar] [CrossRef]
- Hetemi, D.; Noël, V.; Pinson, J. Grafting of Diazonium Salts on Surfaces: Application to Biosensors. Biosensors 2020, 10, 4. [Google Scholar] [CrossRef]
- Raponi, O.d.A.; de Souza, B.R.; Barbosa, L.C.M.; Junior, A.C.A. Thermal, rheological, and dielectric analyses of the polymerization reaction of a liquid thermoplastic resin for infusion manufacturing of composite materials. Polym. Test. 2018, 71, 32–37. [Google Scholar] [CrossRef]
- Raponi, O.d.A.; Barbosa, L.C.M.; de Souza, B.R.; Junior, A.C.A. Study of the influence of initiator content in the polymerization reaction of a thermoplastic liquid resin for advanced composite manufacturing. Adv. Polym. Technol. 2018, 37, 3579–3587. [Google Scholar] [CrossRef]
- Barbosa, L.C.M.; Santos, M.; Oliveira, T.L.L.; Gomes, G.F.; Junior, A.C.A. Effects of moisture absorption on mechanical and viscoelastic properties in liquid thermoplastic resin/carbon fiber composites. Polym. Eng. Sci. 2019, 59, 2185–2194. [Google Scholar] [CrossRef]
- Bhudolia, S.K.; Gohel, G.; Kantipudi, J.; Leong, K.F.; Gerard, P. Mechanical performance and damage mechanisms of thin rectangular carbon/Elium® tubular thermoplastic composites under flexure and low-velocity impact. Thin-Walled Struct. 2021, 165, 107971. [Google Scholar] [CrossRef]
- Boumbimba, R.M.; Coulibaly, M.; Khabouchi, A.; Kinvi-Dossou, G.; Bonfoh, N.; Gerard, P. Glass fibres reinforced acrylic thermoplastic resin-based tri-block copolymers composites: Low velocity impact response at various temperatures. Compos. Struct. 2017, 160, 939–951. [Google Scholar] [CrossRef]
- Chen, S.J.; Zou, B.; Collins, F.; Zhao, X.L.; Majumber, M.; Duan, W.H. Predicting the influence of ultrasonication energy on the reinforcing efficiency of carbon nanotubes. Carbon 2014, 77, 1–10. [Google Scholar] [CrossRef]
- Krause, B.; Mende, M.; Pötschke, P.; Petzold, G. Dispersability and particle size distribution of CNTs in an aqueous surfactant dispersion as a function of ultrasonic treatment time. Carbon 2010, 48, 2746–2754. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- PN-EN ISO 179-1:2010; Plastics Determination of Charpy Impact Properties. ISO Standards: Geneva, Switzerland, 2010.
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, A.; Hatakeyama, K.; Kano, E.; Higuchi, K.; Sugiyama, S. One-pot generation of benzynes from 2-aminophenylboronates via a Rh(II)-catalyzed N–H amination/oxidation/elimination cascade process. Org. Chem. Front. 2019, 7, 64–68. [Google Scholar] [CrossRef]
- Wiklund, P.; Bergman, J. The Chemistry of Anthranilic Acid. Curr. Org. Synth. 2006, 3, 379–402. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Filho, A.G.S.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 5355–5377. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Polymer Nanotubes Nanocomposites: Synthesis, Properties and Applications, 2nd ed.; Wiley-Scrivener: Austin, TX, USA, 2014; Available online: https://www.wiley.com/en-us/Polymer+Nanotubes+Nanocomposites%3A+Synthesis%2C+Properties+and+Applications%2C+2nd+Edition-p-9781118945933 (accessed on 2 January 2023).
- Pan, Y.; Li, L.; Chan, S.H.; Zhao, J. Correlation between dispersion state and electrical conductivity of MWCNTs/PP composites prepared by melt blending. Compos. Part A Appl. Sci. Manuf. 2010, 41, 419–426. [Google Scholar] [CrossRef]
- Chopra, S.; Ramanadham, V.; Vullengala, S.P.; Tiwari, S.; Lad, K.; Deshmukh, K.A.; Peshwe, D. Outcome of using olive oils for MWCNT functionalization and the influence of –OH modified MWCNTs on PA and PBT nano-composites. Mater. Today Proc. 2020, 28, 408–419. [Google Scholar] [CrossRef]
- Nobile, M.R. 15—Rheology of polymer–carbon nanotube composites melts. In Polymer–Carbon Nanotube Composites; McNally, T., Pötschke, P., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 428–481. [Google Scholar] [CrossRef]
- Meier, R.; Kirdar, C.; Rudolph, N.; Zaremba, S.; Drechsler, K. Investigation of the shear thinning behavior of epoxy resins for utilization in vibration assisted liquid composite molding processes. AIP Conf. Proc. 2014, 1593, 458–462. [Google Scholar]
- Vyas, A.; Garg, V.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S. Photopolymerizable resin-based 3D printed biomedical composites: Factors affecting resin viscosity. Mater. Today Proc. 2022, 62, 1435–1439. [Google Scholar] [CrossRef]
- Wladyka-Przybylak, M.; Wesolek, D.; Gieparda, W.; Boczkowska, A.; Ciecierska, E. The effect of the surface modification of carbon nanotubes on their dispersion in the epoxy matrix. Pol. J. Chem. Technol. 2011, 13, 62–69. [Google Scholar] [CrossRef]
- Yetgin, S.H. Effect of multi walled carbon nanotube on mechanical, thermal and rheological properties of polypropylene. J. Mater. Res. Technol. 2019, 8, 4725–4735. [Google Scholar] [CrossRef]
- Spasojevic, P.M. Chapter 15—Thermal and Rheological Properties of Unsaturated Polyester Resins-Based Composites. In Unsaturated Polyester Resins; Thomas, S., Hosur, M., Chirayil, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–406. [Google Scholar] [CrossRef]
- Salvetat, J.P.; Briggs, G.A.D.; Bonard, J.M.; Basca, R.R.; Kulik, A.J.; Stockli, T. Elastic and Shear Moduli of Single Walled Nanotube Ropes. Phys. Rev. Lett. 1999, 82, 944–947. [Google Scholar] [CrossRef]
- Godara, A.; Gorbatikh, L.; Kalinka, G.; Warrier, A.; Rochez, O.; Mezzo, L.; Luizi, F.; van Vuure, A.; Lomov, S.; Verpoest, I. Interfacial shear strength of a glass fiber/epoxy bonding in composites modified with carbon nanotubes. Compos. Sci. Technol. 2010, 70, 1346–1352. [Google Scholar] [CrossRef]
- Ash, B.; Eitan, A.; Schadler, L. Polymer Nanocomposites with Nanoparticle and Carbon Nanotube Fillers. In Dekker Encyclopedia of Nanoscience and Nanotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 3760–3772. [Google Scholar]
- Laurenzi, S.; Pastore, R.; Giannini, G.; Marchetti, M. Experimental study of impact resistance in multi-walled carbon nanotube reinforced epoxy. Compos. Struct. 2013, 99, 62–68. [Google Scholar] [CrossRef]
- Zheng, W.; Wong, S.-C. Electrical conductivity and dielectric properties of PMMA/expanded graphite composites. Compos. Sci. Technol. 2003, 63, 225–235. [Google Scholar] [CrossRef]
- Lee, J.-H.; Rhee, K.Y.; Park, S.J. Silane modification of carbon nanotubes and its effects on the material properties of carbon/CNT/epoxy three-phase composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 478–483. [Google Scholar] [CrossRef]
- Blokhin, A.; Stolyarov, R.; Burmistrov, I.; Gorshkov, N.; Kolesnikov, E.; Yagubov, V.; Tkachev, A.; Zaytsev, I.; Tarov, D.; Galunin, E.; et al. Increasing electrical conductivity of PMMA-MWCNT composites by gas phase iodination. Compos. Sci. Technol. 2021, 214, 108972. [Google Scholar] [CrossRef]


| Aniline Modifier Derivative | Aniline Modifier Structure | MWCNTs System Abbreviation |
|---|---|---|
| None | - | MWCNT neat |
| Aniline | 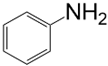 | MWCNT-AN |
| p-nitroaniline | 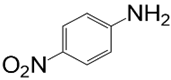 | MWCNT-NAN |
| anthranilic acid (o-aminobenzoic acid) | 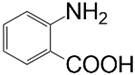 | MWCNT-ABA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demski, S.; Brząkalski, D.; Gubernat, M.; Dydek, K.; Czaja, P.; Żochowski, K.; Kozera, P.; Krawczyk, Z.; Sztorch, B.; Przekop, R.E.; et al. Nanocomposites Based on Thermoplastic Acrylic Resin with the Addition of Chemically Modified Multi-Walled Carbon Nanotubes. Polymers 2024, 16, 422. https://doi.org/10.3390/polym16030422
Demski S, Brząkalski D, Gubernat M, Dydek K, Czaja P, Żochowski K, Kozera P, Krawczyk Z, Sztorch B, Przekop RE, et al. Nanocomposites Based on Thermoplastic Acrylic Resin with the Addition of Chemically Modified Multi-Walled Carbon Nanotubes. Polymers. 2024; 16(3):422. https://doi.org/10.3390/polym16030422
Chicago/Turabian StyleDemski, Szymon, Dariusz Brząkalski, Maciej Gubernat, Kamil Dydek, Paweł Czaja, Konrad Żochowski, Paulina Kozera, Zuzanna Krawczyk, Bogna Sztorch, Robert Edward Przekop, and et al. 2024. "Nanocomposites Based on Thermoplastic Acrylic Resin with the Addition of Chemically Modified Multi-Walled Carbon Nanotubes" Polymers 16, no. 3: 422. https://doi.org/10.3390/polym16030422
APA StyleDemski, S., Brząkalski, D., Gubernat, M., Dydek, K., Czaja, P., Żochowski, K., Kozera, P., Krawczyk, Z., Sztorch, B., Przekop, R. E., Marczak, M., Ehrlich, H., & Boczkowska, A. (2024). Nanocomposites Based on Thermoplastic Acrylic Resin with the Addition of Chemically Modified Multi-Walled Carbon Nanotubes. Polymers, 16(3), 422. https://doi.org/10.3390/polym16030422












