Exploring Functional Polymers in the Synthesis of Luminescent ZnO Quantum Dots for the Detection of Cr6+, Fe2+, and Cu2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Copolymers via RAFT Photo-Polymerization
2.3. Synthesis of Fluorescent ZnO QDs
2.4. Characterization and Properties
3. Results and Discussion
3.1. Synthesis of Functional Copolymers
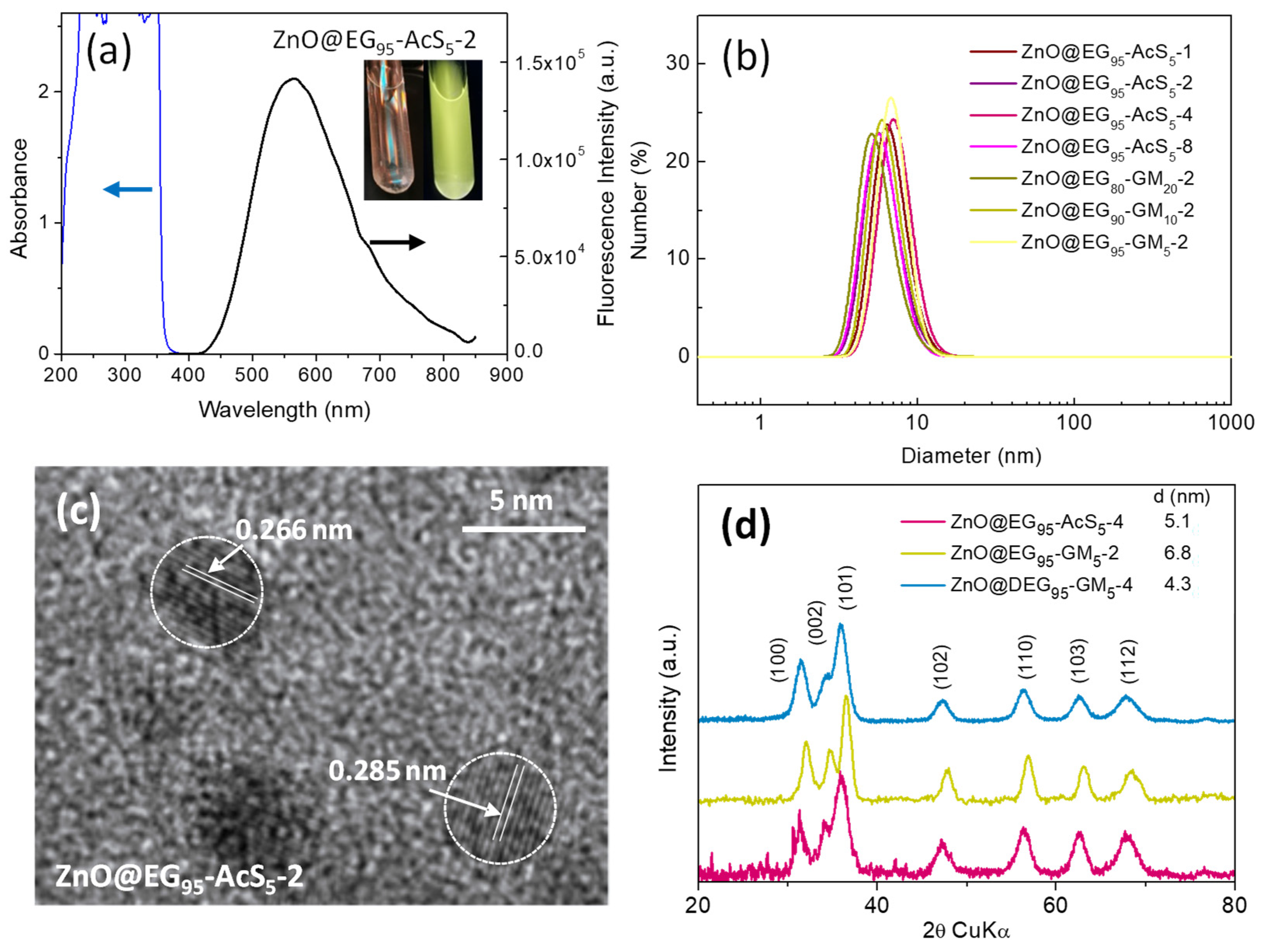
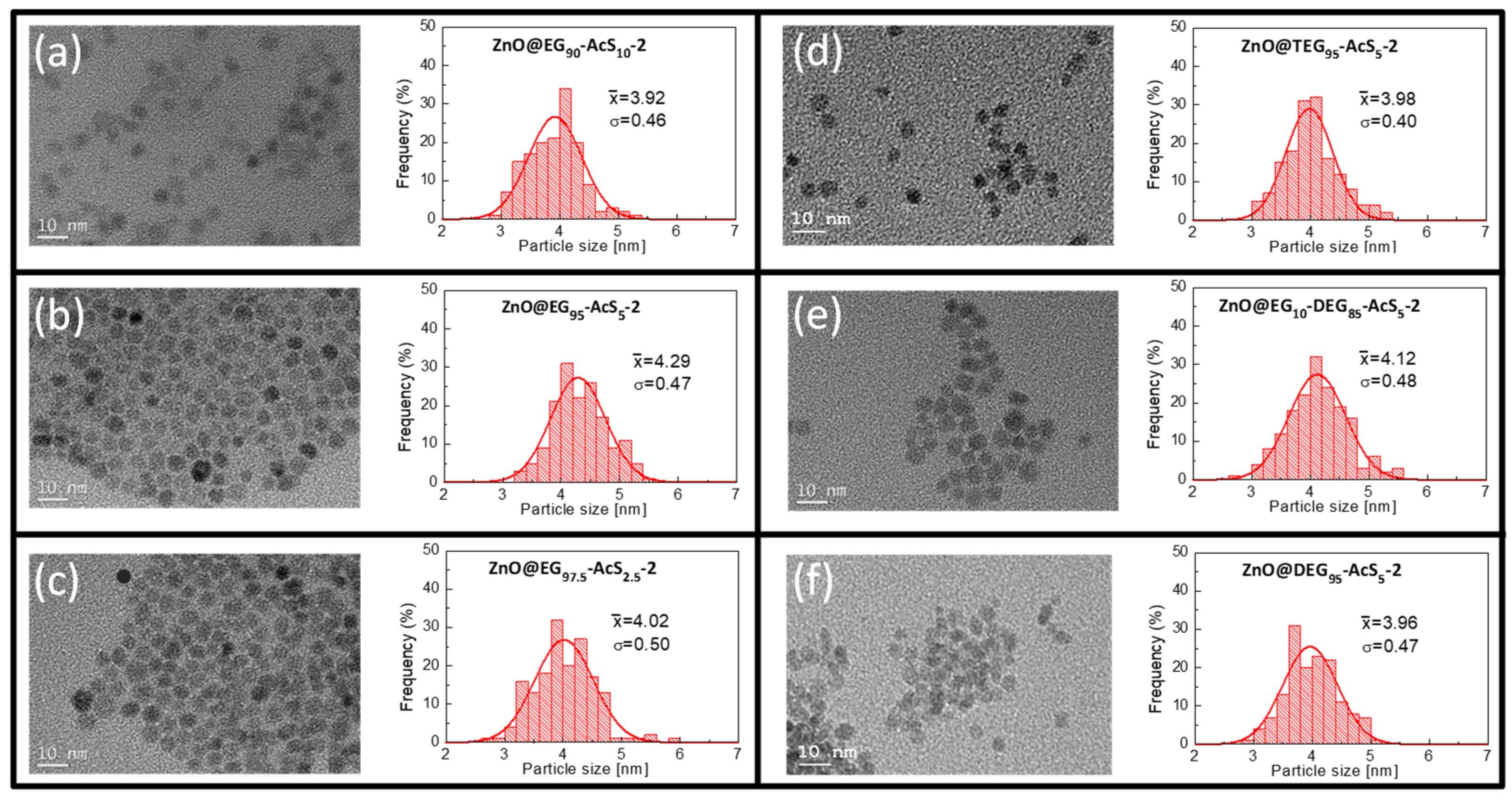
3.2. Synthesis of Hybrid ZnO QDs Using Functional Copolymers as Templates
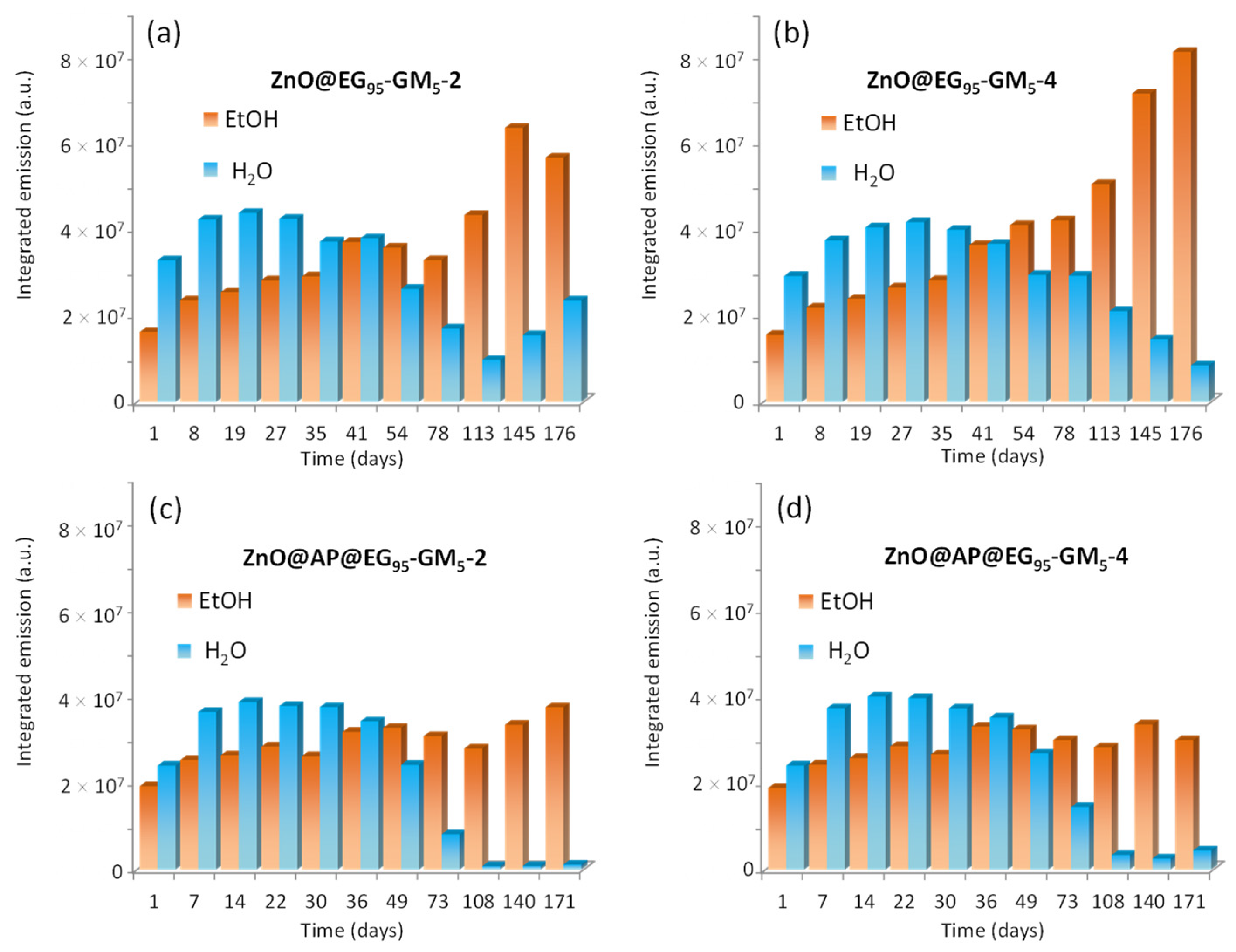
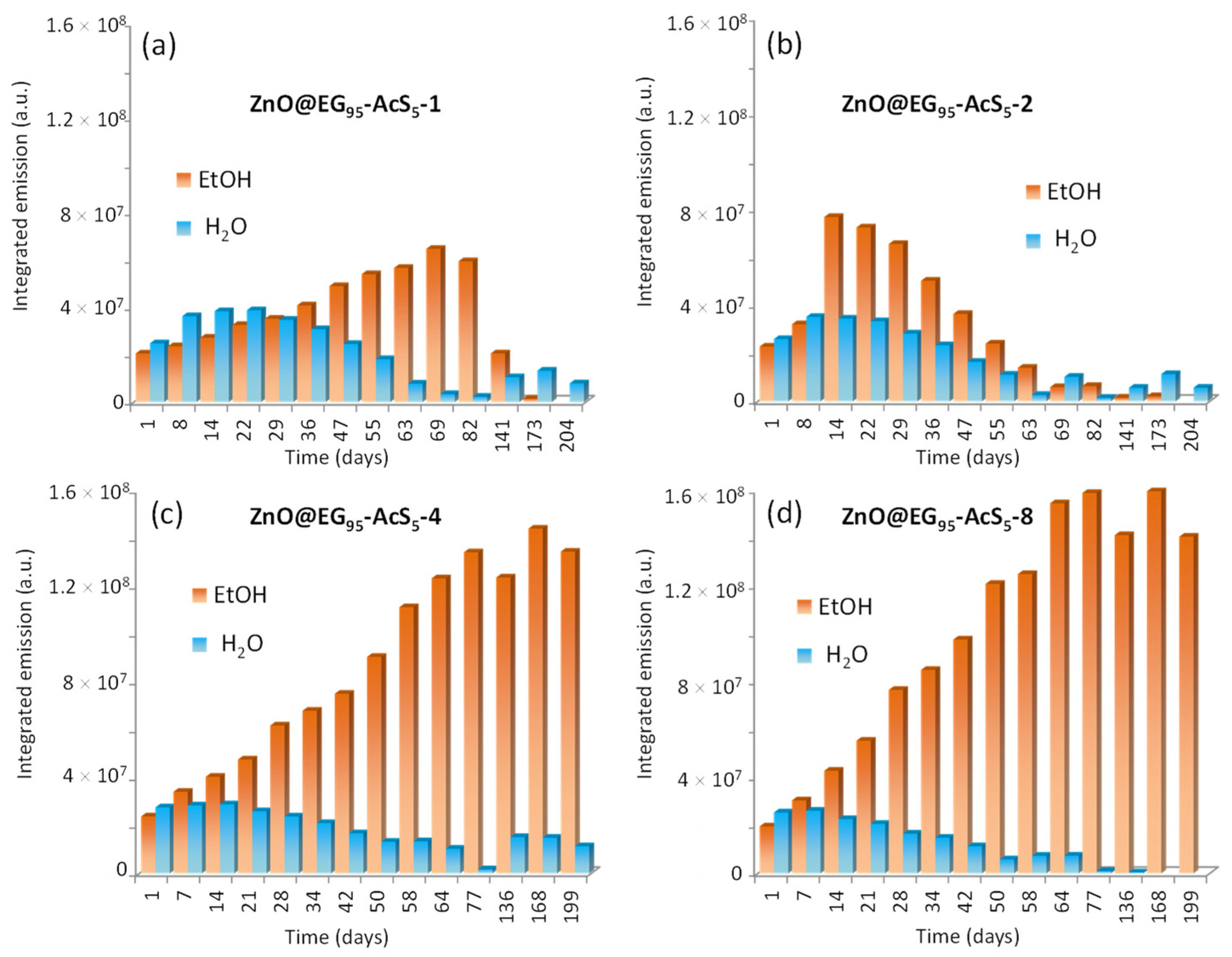
3.3. Stability of ZnO QDs Hybrids in Aqueous Medium
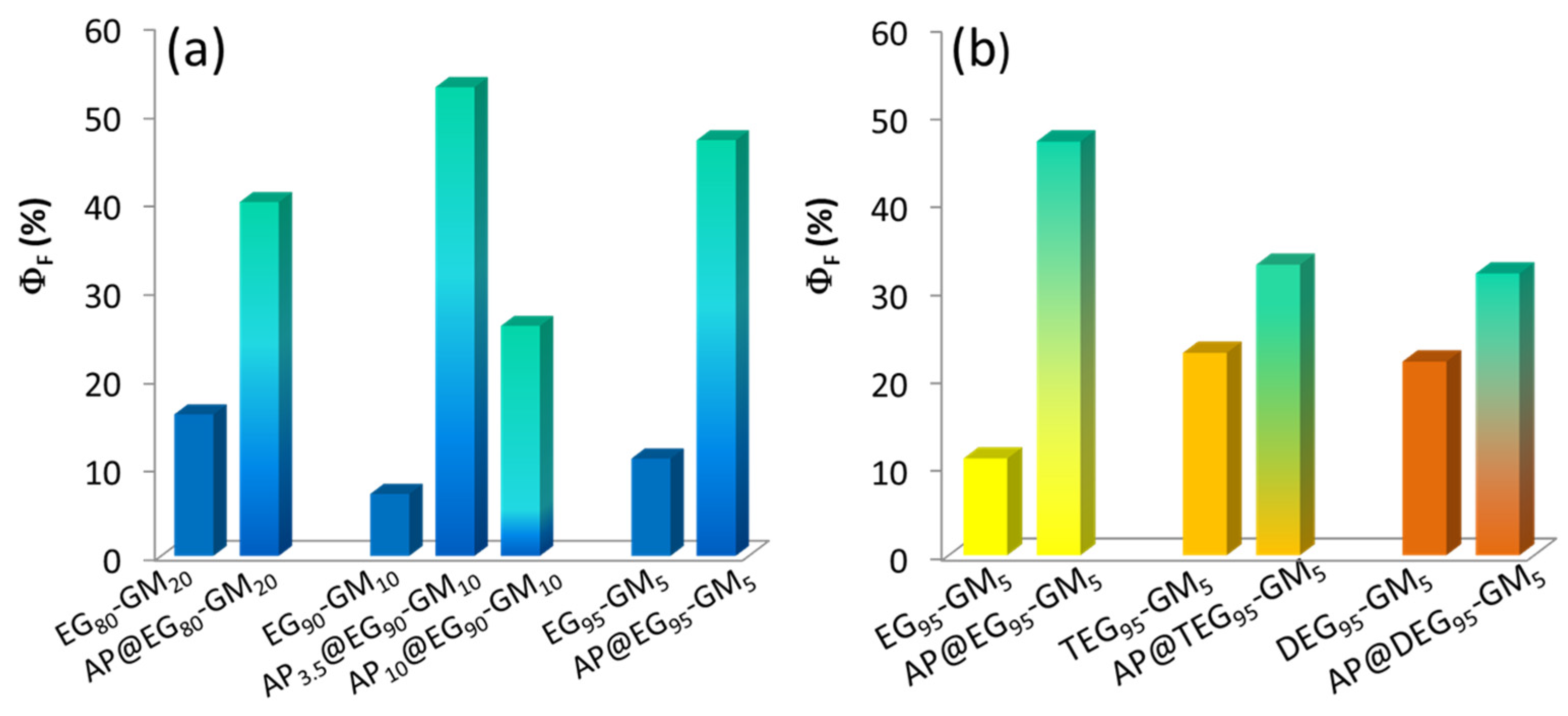
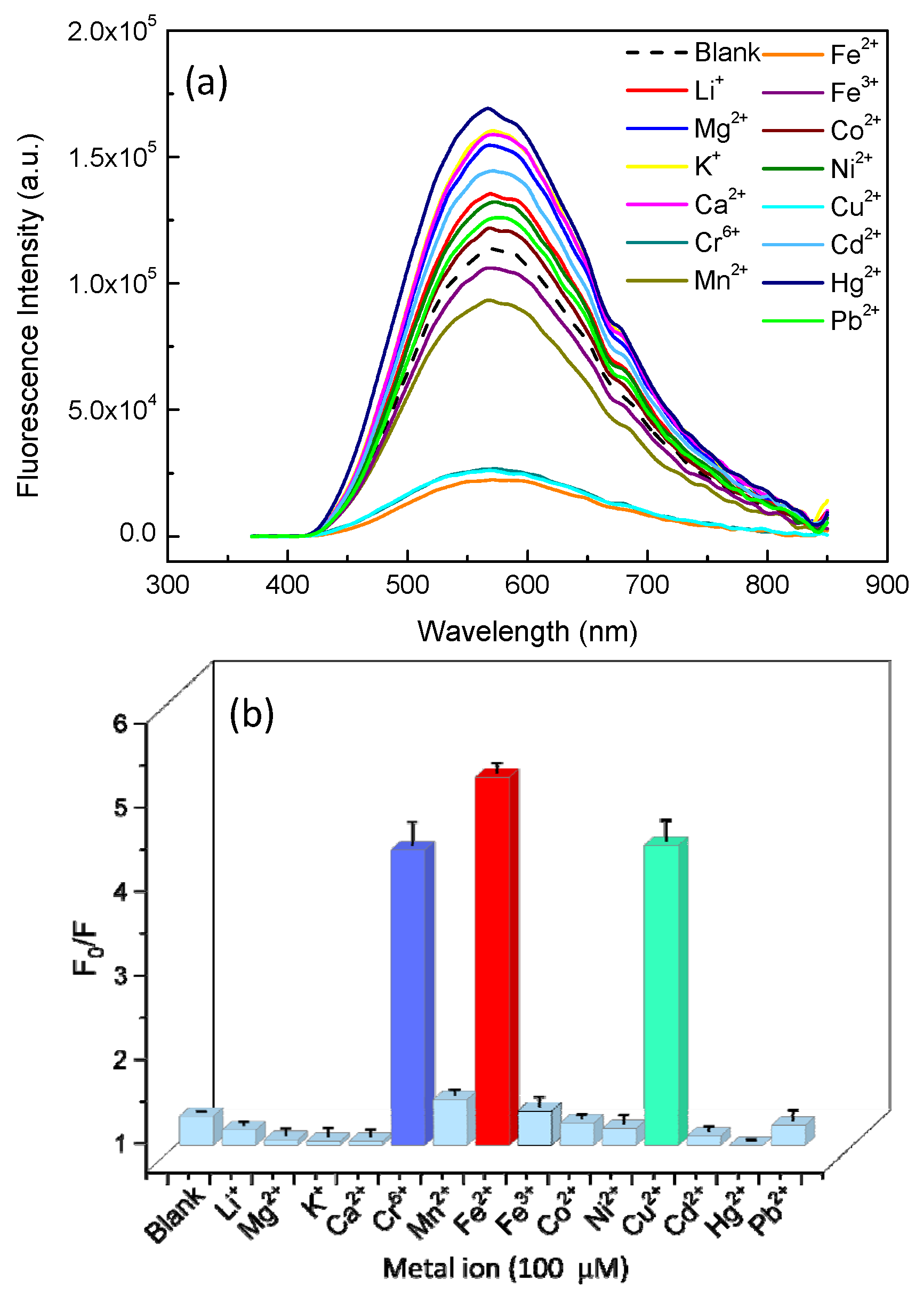
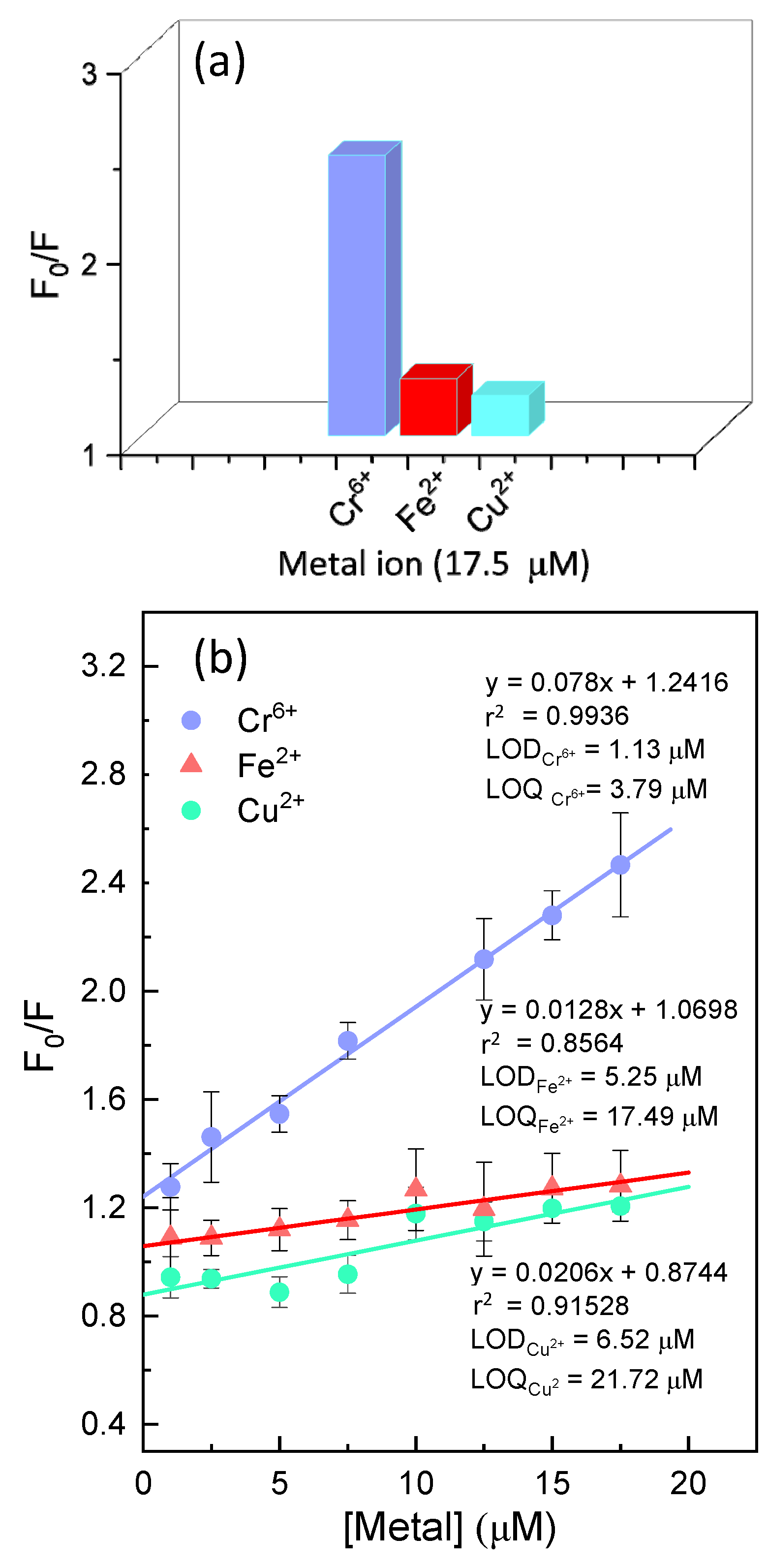
3.4. ZnO QDs Hybrids for Metal Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.Y.; Xiong, H.M. Photoluminescent ZnO nanoparticles and their biological applications. Materials 2015, 8, 3101–3127. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Xiong, H.-M.; Ren, Q.-G.; Xia, Y.-Y.; Kong, J.-L. ZnO@silica core–shell nanoparticles with remarkable luminescence and stability in cell imaging. J. Mater. Chem. 2012, 22, 13159–13165. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots–Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Lin, S.M.; Shi, Y.; Li, N.B.; Luo, H.Q. Determination of cobalt(II) using β-cyclodextrin-capped ZnO quantum dots as a fluorescent probe. Microchim. Acta 2017, 184, 2533–2539. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, T.; Tao, T. ZnO quantum dots for fluorescent detection of environmental contaminants. J. Environ. Chem. Eng. 2021, 9, 106800–106809. [Google Scholar] [CrossRef]
- Zou, T.; Xing, X.; Yang, Y.; Wang, Z.; Wang, Z.; Zhao, R.; Zhang, X.; Wang, Y. Water-soluble ZnO quantum dots modified by (3-aminopropyl)triethoxysilane: The promising fluorescent probe for the selective detection of Cu2+ ion in drinking water. J. Alloys Compd. 2020, 825, 153904–153914. [Google Scholar] [CrossRef]
- Kadam, V.V.; Balakrishnan, R.M.; Ettiyappan, J.P. Fluorometric detection of bisphenol A using β-cyclodextrin-functionalized ZnO QDs. Environ. Sci. Pollut. Res. 2021, 28, 11882–11892. [Google Scholar] [CrossRef]
- Fonoberov, V.A.; Balandin, A.A. ZnO Quantum Dots: Physical Properties and Optoelectronic Applications. J. Nanoelectron. Optoelectron. 2006, 1, 19–38. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Mukherjee, S.; Mukherjee, S.; Singh, S.P.; Prasad, T. ZnO Quantum Dots: Broad Spectrum Microbicidal Agent Against Multidrug Resistant Pathogens E. coli and C. albicans. Front. Nanotechnol. 2020, 2, 576342–576353. [Google Scholar] [CrossRef]
- Ikram, M.; Shahid, H.; Haider, J.; Haider, A.; Naz, S.; Ul-Hamid, A.; Shahzadi, I.; Naz, M.; Nabgan, W.; Ali, S. Nb/Starch-Doped ZnO Nanostructures for Polluted Water Treatment and Antimicrobial Applications: Molecular Docking Analysis. ACS Omega 2022, 7, 39347–39361. [Google Scholar] [CrossRef]
- Schmitz, F.; Silva de Albuquerque, M.B.; Alberton, M.D.; Riegel-Vidotti, I.C.; Zimmermann, L.M. Zein films with ZnO and ZnO:Mg quantum dots as functional nanofillers: New nanocomposites for food package with UV-blocker and antimicrobial properties. Polym. Test. 2020, 91, 106709. [Google Scholar] [CrossRef]
- Puspasari, V.; Ridhova, A.; Hermawan, A.; Amal, M.I.; Khan, M.M. ZnO-based antimicrobial coatings for biomedical applications. Bioprocess Biosyst. Eng. 2022, 45, 1421–1445. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Z.; Li, R.; Dong, M.; Zhang, L.; Qi, W.; Zhang, X.; Wang, H. ZnO Nanocomposites Modified by Hydrophobic and Hydrophilic Silanes with Dramatically Enhanced Tunable Fluorescence and Aqueous Ultrastability toward Biological Imaging Applications. Sci. Rep. 2015, 5, 8475–8482. [Google Scholar] [CrossRef]
- San José, L.; García, O.; Quijada-Garrido, I.; López-González, M. RAFT Hydroxylated Polymers as Templates and Ligands for the Synthesis of Fluorescent ZnO Quantum Dots. Nanomaterials 2022, 12, 3441–3458. [Google Scholar] [CrossRef]
- Dazzazi, A.; Coppel, Y.; In, M.; Chassenieux, C.; Mascalchi, P.; Salomé, L.; Bouhaouss, A.; Kahn, M.L.; Gauffre, F. Oligomeric and polymeric surfactants for the transfer of luminescent ZnO nanocrystals to water. J. Mater. Chem. C 2013, 1, 2158–2165. [Google Scholar] [CrossRef]
- Laopa, P.; Vilaivan, T. Cationic-Polymer-Functionalized Zinc Oxide Quantum Dots: Preparation and Application to Iron(II) Ion Detection. ChemistrySelect 2019, 4, 4251–4257. [Google Scholar] [CrossRef]
- Lei, G.; Yang, S.; Cao, R.; Zhou, P.; Peng, H.; Peng, R.; Zhang, X.; Yang, Y.; Li, Y.; Wang, M.; et al. In situ preparation of amphibious zno quantum dots with blue fluorescence based on hyperbranched polymers and their application in bio-imaging. Polymers 2020, 12, 144. [Google Scholar] [CrossRef]
- Liras, M.; García, O.; Guarrotxena, N.; Palacios-Cuesta, M.; Quijada-Garrido, I. Versatile thiolated thermosensitive polymers synthesized by ATRP of MEO2MA and AcSEMA, a new methacrylic monomer with a protected thiol group. Polym. Chem. 2013, 4, 5751–5759. [Google Scholar] [CrossRef]
- Adnan, N.N.M.; Ahmad, S.; Kuchel, R.P.; Boyer, C. Exploring the potential of linear polymer structures for the synthesis of fluorescent gold nanoclusters. Mater. Chem. Front. 2017, 1, 80–90. [Google Scholar] [CrossRef]
- Quijada-Garrido, I.; García, O. How a family of nanostructured amphiphilic block copolymers synthesized by RAFT-PISA take advantage of thiol groups to direct the in situ assembly of high luminescent CuNCs within their thermo-responsive core. Eur. Polym. J. 2021, 160, 110806. [Google Scholar] [CrossRef]
- Liras, M.; Quijada-Garrido, I.; Palacios-Cuesta, M.; Muñoz-Durieux, S.; García, O. Acetyl protected thiol methacrylic polymers as effective ligands to keep quantum dots in luminescent standby mode. Polym. Chem. 2014, 5, 433–442. [Google Scholar] [CrossRef]
- Borah, R.; Ninakanti, R.; Nuyts, G.; Peeters, H.; Pedrazo-Tardajos, A.; Nuti, S.; Vande Velde, C.; De Wael, K.; Lenaerts, S.; Bals, S.; et al. Selectivity in the Ligand Functionalization of Photocatalytic Metal Oxide Nanoparticles for Phase Transfer and Self-Assembly Applications. Chem. Eur. J. 2021, 27, 9011–9021. [Google Scholar] [CrossRef] [PubMed]
- Arslan, O.; Singh, A.P.; Belkoura, L.; Mathur, S. Cysteine-functionalized zwitterionic ZnO quantum dots. J. Mater. Res. 2013, 28, 1947–1954. [Google Scholar] [CrossRef]
- Muşat, V.; Tăbăcaru, A.; Vasile, B.Ş.; Surdu, V.-A. Size-dependent photoluminescence of zinc oxide quantum dots through organosilane functionalization. RSC Adv. 2014, 4, 63128–63136. [Google Scholar] [CrossRef]
- Shi, H.-Q.; Li, W.-N.; Sun, L.-W.; Liu, Y.; Xiao, H.-M.; Fu, S.-Y. Synthesis of silane surface modified ZnO quantum dots with ultrastable, strong and tunable luminescence. Chem. Commun. 2011, 47, 11921–11923. [Google Scholar] [CrossRef] [PubMed]
- Lovett, J.R.; Ratcliffe, L.P.D.; Warren, N.J.; Armes, S.P.; Smallridge, M.J.; Cracknell, R.B.; Saunders, B.R. A Robust Cross-Linking Strategy for Block Copolymer Worms Prepared via Polymerization-Induced Self-Assembly. Macromolecules 2016, 49, 2928–2941. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Kumar, R. Journey of ZnO quantum dots from undoped to rare-earth and transition metal-doped and their applications. RSC Adv. 2021, 11, 2512–2545. [Google Scholar] [CrossRef]
- Tong, Z.; Xing, X.; Yang, Y.; Hong, P.; Wang, Z.; Zhao, R.; Zhang, X.; Peng, S.; Wang, Y. Fluorescent ZnO quantum dots synthesized with urea for the selective detection of Cr6+ ion in water with a wide range of concentrations. Methods Appl. Fluoresc. 2019, 7, 035007. [Google Scholar] [CrossRef]

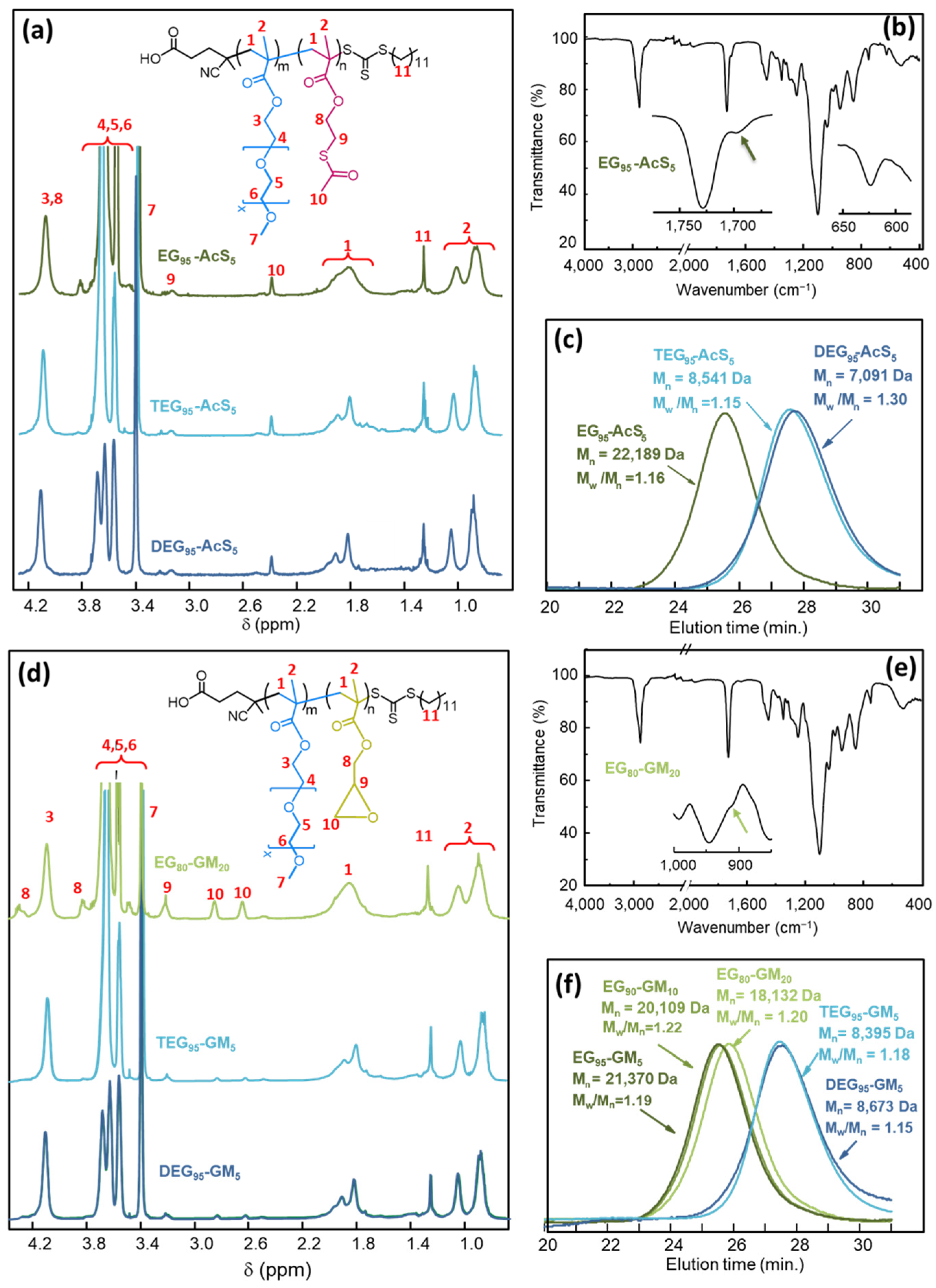



| Statistical Copolymer a | [M]0/ [CDTPA]0 | Time (h) | M2(theor.) b (% mol) | M2 (exp.) c (% mol) | MW d (g mol−1) | Mn d (g mol−1) | Ð d | Conv. e (%) | Mn (theor.) f (g mol−1) |
|---|---|---|---|---|---|---|---|---|---|
| EG90-AcS10 a | 50 | 2 | 10 | 9 | 31,609 | 26,764 | 1.18 | 83 | 19,339 |
| EG95-AcS5 | 50 | 2 | 5 | 5 | 25,720 | 22,189 | 1.16 | 81 | 19,716 |
| EG95-AcS5-L | 17 | 2 | 5 | 6 | 9636 | 8084 | 1.19 | 77 | 6341 |
| EG97.5-AcS2.5 | 50 | 2 | 2.5 | 3 | 26,166 | 22,046 | 1.19 | 79 | 19,442 |
| TEG95-AcS5 | 50 | 2 | 5 | 6 | 9823 | 8541 | 1.15 | 65 | 7512 |
| DEG95-AcS5 | 50 | 2 | 5 | 5 | 9244 | 7091 | 1.30 | 70 | 6541 |
| DEG95-AcS5-L | 25 | 2 | 5 | 5 | 4866 | 3790 | 1.28 | 77 | 3623 |
| EG10—DEG85-AcS5 | 50 | 2 | 5 | 4.6 | 13,164 | 10,589 | 1.24 | 74 | 8118 |
| EG80-GM20 | 50 | 2 | 20 | 23 | 21,843 | 18,132 | 1.20 | 69 | 14,781 |
| EG90-GM10 | 50 | 2 | 10 | 12 | 24,439 | 20,109 | 1.22 | 73 | 16,944 |
| EG95-GM5 | 50 | 2 | 5 | 5 | 25,504 | 21,370 | 1.19 | 80 | 19,284 |
| TEG95-GM5 | 50 | 2 | 5 | 5 | 9936 | 8395 | 1.18 | 73 | 8314 |
| DEG95-GM5 | 50 | 2 | 5 | 5 | 9993 | 8673 | 1.15 | 77 | 7158 |
| Sample | GlyMA/(µmol) a | Main Monomer | GlyMA (mol %) b | APTES (mol %) c | Size (nm) DLS d | SD (nm) | λmax EtOH | ΦF (%) | λmax H2O |
|---|---|---|---|---|---|---|---|---|---|
| ZnO@EG80-GM20-2 | 2 | PEGMEMA | 20 | 0 | 5.7 | 0.31 | 556 | 16 | 568 |
| ZnO@AP-EG80-GM20-2 | 2 | 3.5 | 6.0 | 0.41 | 554 | 40 | 567 | ||
| ZnO@EG90-GM10-2 | 2 | 10 | 0 | 6.5 | 0.24 | 561 | 7 | 567 | |
| ZnO@AP-EG90-GM10-2 | 2 | 3.5 | 6.2 | 0.26 | 563 | 53 | 567 | ||
| ZnO@ AP10-EG90-GM10-2 | 2 | 10 | 5.7 | 0.29 | 562 | 26 | 569 | ||
| ZnO@EG95-GM5-2 | 2 | 5 | 0 | 7.0 | 0.43 | 543 | 11 | 567 | |
| ZnO@EG95-GM5-2 | 2 | 3.5 | 6.0 | 1.35 | 554 | 47 | 566 | ||
| ZnO@EG95-GM5-4 | 4 | 5 | 0 | 5.5 | 0.93 | 551 | 5 | 568 | |
| ZnO@AP-EG95-GM5-4 | 4 | 3.5 | 7.1 | 1.16 | 541 | 38 | 565 | ||
| ZnO@TEG95-GM5-2 | 2 | MEO3MA | 5 | 0 | 6.3 | 0.56 | 567 | 23 | 570 |
| ZnO@AP-TEG95-GM5-2 | 2 | 3.5 | 5.9 | 0.34 | 557 | 33 | 566 | ||
| ZnO@DEG95-GM5-2 | 2 | MEO2MA | 5 | 0 | 6.0 | 0.43 | 567 | 22 | 570 |
| ZnO@AP-DEG95-GM5-2 | 2 | 3.5 | 5.9 | 0.34 | 560 | 32 | 572 |
| Sample | Thiol (µmol) a | Main Monomer | AcSEMA (mol %) b | Size (nm) DLS c | SD (nm) | λmax EtOH | ΦF (%) | λmax H2O |
|---|---|---|---|---|---|---|---|---|
| ZnO@EG90-AcS10-2 | 2 | PEGMEMA | 10 | 6.2 | 0.41 | 556 | 17 | 568 |
| ZnO@EG95-AcS5-1 | 1 | 5 | 6.9 | 0.37 | 552 | 10 | 566 | |
| ZnO@EG95-AcS5-2 | 2 | 5 | 6.2 | 0.37 | 562 | 36 | 567 | |
| ZnO@EG95-AcS5-4 | 4 | 5 | 7.4 | 0.68 | 548 | 42 | 568 | |
| ZnO@EG95-AcS5-8 | 8 | 5 | 6.0 | 0.58 | 556 | 33 | 563 | |
| ZnO@EG95-AcS5-L-2 | 2 | 5 | 5.5 | 0.35 | 560 | 33 | 569 | |
| ZnO@EG97.5-AcS2.5-2 | 2 | 2.5 | 6.2 | 0.34 | 560 | 20 | 567 | |
| ZnO@TEG95-AcS5-2 | 2 | MEO3MA | 5 | 7.8 | 0.24 | 544 | 37 | 564 |
| ZnO@TEG95-AcS5-4 | 4 | 5 | 6.2 | 1.31 | 549 | 30 | 570 | |
| ZnO@DEG95-AcS5-2 | 2 | MEO2MA | 5 | 7.8 | 1.5 | 549 | 52 | 572 |
| ZnO@DEG95-AcS5-4 | 4 | 5 | 7.5 | 0.47 | 544 | 27 | 569 | |
| ZnO@DEG95-AcS5-L-2 | 2 | 5 | 5.8 | 0.42 | 558 | 36 | 569 | |
| EG10—DEG85-AcS5 | PEGMEMA/MEO2MA | 5 | 7.1 | 0.43 | 560 | 22 | 568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San José, L.; Yuriychuk, N.; García, O.; López-González, M.; Quijada-Garrido, I. Exploring Functional Polymers in the Synthesis of Luminescent ZnO Quantum Dots for the Detection of Cr6+, Fe2+, and Cu2+. Polymers 2024, 16, 429. https://doi.org/10.3390/polym16030429
San José L, Yuriychuk N, García O, López-González M, Quijada-Garrido I. Exploring Functional Polymers in the Synthesis of Luminescent ZnO Quantum Dots for the Detection of Cr6+, Fe2+, and Cu2+. Polymers. 2024; 16(3):429. https://doi.org/10.3390/polym16030429
Chicago/Turabian StyleSan José, Leire, Nastasiya Yuriychuk, Olga García, Mar López-González, and Isabel Quijada-Garrido. 2024. "Exploring Functional Polymers in the Synthesis of Luminescent ZnO Quantum Dots for the Detection of Cr6+, Fe2+, and Cu2+" Polymers 16, no. 3: 429. https://doi.org/10.3390/polym16030429





